Summary
Angiotensin receptor blockers (ARBs) have become established as a major class of antihypertensive on the basis of their powerful effects on blood pressure (BP), excellent tolerability and pleiotropic end-organ-protective effects. However, individual ARBs vary in antihypertensive efficacy, which may be important to clinical outcome. Several strategies are available to ensure that BP reductions with ARBs are at least as great as that which can be achieved with other antihypertensive classes. Firstly, several newer ARBs, including irbesartan, candesartan, telmisartan and olmesartan, have been reported to provide equivalent antihypertensive efficacy to amlodipine and greater efficacy than either losartan, valsartan or both. Secondly, increases in dose may improve the antihypertensive efficacy of agents such as valsartan, although clinical studies are necessary to provide characterisation of new, higher-dose monotherapies. Thirdly, fixed dose combinations with hydrochlorothiazide (HCTZ) increase the antihypertensive effect of all ARBs. It is likely that differences in efficacy between newer and older ARBs will in some cases be sustained in combination therapy, such that the most potent ARBs and HCTZ will provide another tier of control. The future use of ARBs is likely to involve a growing emphasis on compound-specific data, with regard to the antihypertensive efficacy and pleiotropic protective actions of agents.
Keywords: Angiotensin II receptor blockers, antihypertensive therapy, blood pressure monitoring
Introduction
Angiotensin receptor blockers (ARBs) are powerful antihypertensives that have rapidly become established as one of the leading therapeutic classes in the management of hypertension. In addition to their antihypertensive efficacy, their rapid ascendancy reflects two cardinal strengths which distinguish these agents from previous classes of antihypertensive. Firstly, several large clinical trials have reported that ARBs have pleiotropic effects that are protective of end-organ function independently of their effects on blood pressure (BP) (1–6). Secondly, ARBs have excellent tolerability, which supports improved patient persistence with medication and thereby improved long-term BP control (7, 8).
During the past year, there has been considerable discussion of the strategies by which aggressive BP control can be optimised using ARBs. Several newer ARBs have been reported to reduce BP more effectively than losartan or valsartan, while the availability of fixed dose hydrochlorothiazide (HCTZ) combinations provide further options for optimising the basic antihypertensive power that can be achieved with ARB-based therapy. Here, we review the clinical role of the ARBs in their core indication of BP control and consider the available strategies for ensuring that the full range of benefits delivered by these agents – powerful BP reductions, end-organ protection and good tolerability – are optimised.
Aggressive BP Control: a Challenge for Antihypertensives
It is well established that achieving ambitious BP targets improves long-term clinical outcomes in the management of hypertension (9–11). In particular, aggressive lowering of systolic BP (SBP) has been identified as a key goal of antihypertensive therapy (12). The importance of aggressive BP control with ambitious targets is now entrenched in clinical guidelines in both the United States and Europe (13, 14). The importance of promptly achieving and then sustaining aggressive BP targets was recently illustrated in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial, which evaluated the importance of the long-term treatment of hypertension with either valsartan or amlodipine, with regard to cardiovascular events and death (15). A major factor in the outcome of VALUE was the superior antihypertensive efficacy of amlodipine, at the trial dose of 5–10 mg, in comparison with the valsartan dose of 80–160 mg/day used in this study. At these doses, amlodipine reduced BP more rapidly than valsartan during the first 6 months, with a 1–2 mmHg difference in achieved BP sustained thereafter. Thus, amlodipine's greater antihypertensive efficacy appeared to compensate for its more modest protective effects against end-organ dysfunction and adverse effects (16).
Aggressive Therapy: the Role of Newer Arbs
One potential approach to optimising aggressive antihypertensive therapy with ARBs is to select a high potency agent and an agent with the most effective starting dose. Since the introduction of losartan and valsartan, several newer ARBs have been reported to reduce BP to a greater degree than these two agents. A meta-analysis of 51 trials reported diastolic BP (DBP)/SBP reductions of 8.0/5.5 and 7.5/4.0 mmHg for losartan 50–100 mg and valsartan 80–160 mg, respectively, compared with 10.0/6.5 mmHg for irbesartan 150–300 mg, 10.0/6.0 mmHg for candesartan 8–32 mg and 9.5/6.0 mmHg for telmisartan 20–80 mg (17). Studies such as VALUE underline the importance of these differences in efficacy and the need to achieve timely and sustained BP targets in the management of hypertension.
Several newer agents have been reported to have equivalent antihypertensive efficacy to amlodipine at its standard 5–10 mg dose range. In a study of 60 hypertensives with left ventricular hypertrophy, the antihypertensive efficacy of irbesartan 150–300 mg/day was found to be at least as effective as amlodipine 5–10 mg/day across the dose range (18), and two further studies, including the IDNT clinical trial and a short-term comparison of irbesartan 150 mg and amlodipine 5 mg in 181 patients, also reported comparable antihypertensive efficacy of irbesartan and amlodipine (5, 19). In a study of 251 patients with mild hypertension, candesartan 16–32 mg daily was reported to have equivalent antihypertensive efficacy to amlodipine 5–10 mg daily (20). A study in 150 patients with telmisartan 40–80 mg daily detected no differences in 24-h BP control with amlodipine 5–10 mg daily (21). Similar BP control was also reported in a study of 440 patients with mild-to-moderate hypertension for starting doses of olmesartan 20 mg/day and amlodipine 5 mg/day (22).
Comparisons with Losartan and Valsartan
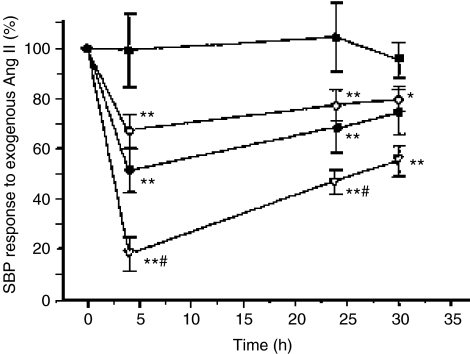
The differential effects of standard starting doses of irbesartan 150 mg, valsartan 80 mg and losartan 50 mg on the SBP response to exogenous angiotensin II in 12 healthy normotensive subjects are shown in Figure 1 (23). The irbesartan-induced blockade was significantly more pronounced than that achieved by losartan or valsartan for 24 h after dosing, indicating greater and more sustained AT1 receptor blockade with irbesartan. This pharmacologic difference needs to be confirmed by comparative clinical trials.
Figure 1.
Differential effects of losartan, valsartan and irbesartan in normotensive subjects. Time course of the in vivo angiotensin II receptor blockade induced by 50 mg of losartan (◊), 80 mg of valsartan (•), 150 mg of irbesartan (˚), and placebo (▪). Values are mean ± SEM. *p < 0.05. **p < 0.01 vs placebo. #p < 0.05 vs other antagonists (Reproduced with permission from Mazzolai L.et al Hypertension 1999; 33: 850(23)).
Greater efficacy compared with losartan has been reported in the clinical setting for irbesartan and several other newer ARBs. In a double-blind, placebo-controlled trial involving 567 patients with mild-to-moderate hypertension, irbesartan 300 mg provided superior and more rapid BP control compared with losartan 100 mg (24). Significant differences were evident as early as the first week – a notable finding in light of the growing emphasis on rapid BP control. A further study has confirmed the superior antihypertensive effect and more rapid control of BP achieved with irbesartan 150–300 mg compared with losartan 50–100 mg (25). In a 6-week, double-blind, placebo-controlled study in 223 patients with mild-to-moderate hypertension, telmisartan at doses of 40 and 80 mg/day was found to achieve superior 24-h BP control at 6 weeks to losartan, although losartan was given at a low 50 mg daily dose (26). In an 8-week study of clinical and ambulatory hypertension in 268 patients, candesartan 8–16 mg/day was reported to reduce SBP and DBP to a significantly greater degree than losartan 50–100 mg after both 4 and 8 weeks (27). Similar results were reported in an 8-week study of 332 patients by Gradman et al. (28). Also, in a 12-week study of 316 patients with mild-to-moderate hypertension, olmesartan 10–20 mg/day was reported to reduce BP to a significantly greater degree than losartan 50 mg/day (29) All ARBs were well tolerated.
There are fewer studies comparing the newer ARBs with valsartan. However, an 8-week study of 426 patients with mild-to-moderate hypertension found that irbesartan at its standard starting dose of 150 mg once daily was associated with superior 24-h ambulatory BP control to valsartan at a comparable starting dose of 80 mg (30). Mean reductions in DBP at trough were 6.7 mmHg for irbesartan 150 mg compared with 4.8 mmHg for valsartan 80 mg (p = 0.035), while mean reductions in SBP at trough were 11.6 mmHg for irbesartan compared with 7.5 mmHg for valsartan, respectively (p < 0.01). In addition, significantly more patients treated with irbesartan achieved normalised BP (DBP < 90 mmHg; 52.5% vs. 38.2%; p = 0.004). A starting-dose study of olmesartan 20 mg in essential hypertensives was reported to have equivalent efficacy to irbesartan 150 mg on most parameters, but greater efficacy than losartan 50 mg or valsartan 80 mg (31). After initial studies reported conflicting results between telmisartan and valsartan (32, 33), a combined analysis of two further studies comparing telmisartan 40–80 mg with valsartan 80–160 mg involving a total of over 800 patients found significantly greater reductions in both SBP and DBP at 8 weeks in the telmisartan group (34).
These direct comparisons between ARBs must be evaluated with care. The studies are of varying size and duration; not all use like-for-like dosing comparisons and they have been carried out across a range of patient populations with a diversity of BP measurement techniques and endpoints. Moreover, subtle aspects of the methodology may in some cases impact on the reported results. However, it is apparent that at their current standard doses, four ARBs – irbesartan 150–300 mg, candesartan 8–32 mg, olmesartan 20–40 mg and telmisartan 40–80 mg – all reduce BP more effectively than losartan 50–100 mg. In addition, irbesartan 150 mg, olmesartan 20 mg and telmisartan 60–80 mg have all been reported to reduce BP to a greater degree than valsartan at comparable doses. These agents clearly strengthen the ability of the ARB class to achieve aggressive BP control with ARB-based therapy.
Higher Potency or Higher Doses?
The potency of individual ARBs is a function of pharmacokinetic factors, including bioavailability, volume of distribution and elimination half-life (35) and differences in the nature and potency of interaction with the AT1 receptor, including binding affinity, dissociation and whether the inhibition is insurmountable or competitive (Table 1) (35–39). On the basis of elimination half-lives, losartan, valsartan and eprosartan have been described as shorter-acting agents, with irbesartan, candesartan and telmisartan described as longer acting (35); and while candesartan and irbesartan block the AT1 receptor with maximal antagonism, losartan and valsartan have been classified as competitive antagonists (35). However, although the newer ARBs have higher potency, their reported improvements in efficacy may also be a function of more rational posology. Another option for increasing the antihypertensive efficacy of the less-potent ARBs may therefore be to administer them at higher doses. For instance, in the wake of the VALUE study, it is apparent that the dose range of 80–160 mg daily that has previously been stated to be optimal for valsartan is not appropriate for all patients (40). One issue for the introduction of high monotherapy doses, however, is the availability of supporting data. The publication of extensive trials data will be necessary to characterise in detail the efficacy and tolerability profile of compounds administered at greater maximum doses than were typical for their original trials programmes.
Table 1.
| Standard dose range (mg) | Half-life (h) | Volume of distribution (l) | Bioavailability (%) | Receptor binding | |
|---|---|---|---|---|---|
| Losartan | 50–100 | 2 (6–9)* | 34 (12)* | 33 | Competitive (insurmountable)* |
| Valsartan | 80–160 (maximum 320) | 6 | 17 | 25 | Competitive |
| Irbesartan | 150–300 | 11–15 | 53–93 | 60–80 | Insurmountable |
| Candesartan | 8–32 | 9–12 | 0.13 L/kg | 15 | Insurmountable |
| Telmisartan | 20–80 | 24 | 500 | 42–58 | Insurmountable |
| Olmesartan | 20–40 | 13 | 17 | 26 | Insurmountable |
| Eprosartan | 400–800 | 5–7 | 13 | 13 | Insurmountable |
EXP 3171 active metabolite of losartan.
Combination Therapy and Antihypertensive Power
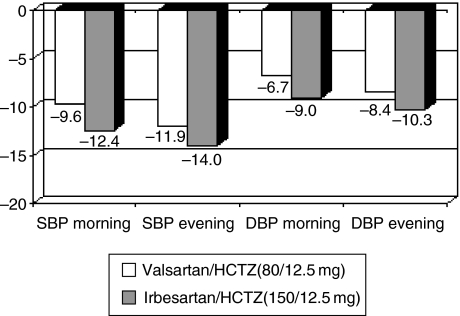
ARBs have enhanced efficacy when combined with 12.5–25 mg dose of the diuretic HCTZ, providing an additional option for tighter BP control. An important question arising from the availability of fixed dose combination products is whether the efficacy advantages reported for some of the newer ARBs compared with valsartan and losartan are reduced by the addition of HCTZ in the combination therapy. In the case of irbesartan and valsartan, the difference in efficacy seen in monotherapy (30) appears to be sustained: a recent 8-week study of prospective, randomised, open-label, blinded-endpoint design in 414 patients comparing irbesartan 150 mg/HCTZ 12.5 mg with valsartan 80 mg/HCTZ 12.5 mg reported that the irbesartan/HCTZ combination was associated with greater mean reductions of 2.4 mmHg (p = 0.0094) and 2.0 mmHg (p = 0.0007), respectively, in-home SBP and DBP compared with valsartan/HCTZ; (Figure 2) (41). Similarly, significantly more patients treated with irbesartan had their BP normalised (<135/85 mmHg; 50.2% vs. 33.2%; p < 0.0001). The overall safety was similar in the irbesartan/HCTZ and valsartan/HCTZ groups.
Figure 2.
Differences in antihypertensive efficacy between irbesartan 150 mg and valsartan 80 mg in monotherapy are sustained in hydrochlorothiazide (HCTZ) combination therapy: results from the COSIMA study (Reproduced with permission from Bobrie G, et al. Am J Hypertens 2005; 18: 1482–8(41)). Mean morning and evening BP decrease (mm Hg) measured by Home BP Measurements. p < 0.01 for all values except SBP evening (p = 0.065). Intent-to-treat analysis.
Results for some of the other ARB fixed dose combinations have been more variable. In a 6-week study involving nearly 400 patients, telmisartan 40 mg or 80 mg plus HCTZ 12.5 mg was found to reduce BP to a significantly greater degree than losartan/HCTZ 50 mg/12.5 mg (42), but a larger 6-week study of 682 patients found that telmisartan 80 mg/HCTZ 12.5 mg did not achieve greater reductions in BP than losartan 50 mg/HCTZ 12.5 mg (43). A study comparing candesartan/HCTZ 16 mg/12.5 mg with losartan/HCTZ 50 mg/12.5 mg in 299 patients with mild-to-moderate hypertension reported significantly greater reductions in the candesartan group (44). All of the ARB fixed dose combinations were well tolerated. Further comparative studies are therefore required, but on the basis of the irbesartan data, it appears that the combination of the most potent ARBs with high-dose HCTZ provides the option of a further level of highly aggressive BP control for difficult-to-treat patients.
Conclusions: Balancing Power, Protection and Tolerability
The first and major objective of antihypertensive therapy is to achieve effective BP control, using therapy that rapidly achieves ambitious targets. While this goal is supported by all ARBs, the ability of the class to achieve them has been enhanced by the introduction of newer, more potent molecules such as irbesartan, candesartan and olmesartan, and by the availability of fixed dose HCTZ combinations for all agents. Developments in the posology of some of the older ARBs may further increase the antihypertensive efficacy of these agents in monotherapy, although these will need to be supported by detailed clinical characterisation. The availability of HCTZ fixed dose combinations provides increased antihypertensive efficacy for all ARBs, and it is likely that the differences in antihypertensive efficacy revealed between some of the newer and older agents in monotherapy will be sustained in this context, providing an additional level of control.
The emerging differences among the members of the ARB class should not obscure the fact that all ARBs are powerful reducers of BP, and that, as a class, ARBs are unique among antihypertensives in their very good tolerability. Furthermore, the end-organ-protective properties of ARBs remain a major rationale for their use. In this context, major long-term data on end-organ protection are still lacking for some of the most recently introduced ARBs. Those agents that will set a benchmark for the class are those that provide a strong evidence-based foundation not for BP-lowering efficacy alone but for all three goals of antihypertensive therapy – powerful BP control, end-organ protection and tolerability. It is by providing patients with this triad of benefits, on a compound-specific and evidential basis, that the ARBs will continue to improve standards of clinical hypertension management.
References
- 1.Brenner BM, Cooper ME, de Zeeuw D, et al. for the RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 2.Dahlof B, Devereux RB, Kjeldsen SE, et al. LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 3.Cohn JN, Tognoni G Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, McMurray JJV, Velazquez EJ, et al. for the Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Clarke WR, et al. for the Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 6.Granger CB, McMurray JJV, Yusuf S, et al. CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–6. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 7.Gerth WC. Compliance and persistence with newer antihypertensive agents. Curr Hypertens Rep. 2002;4:424–33. doi: 10.1007/s11906-002-0021-6. [DOI] [PubMed] [Google Scholar]
- 8.Hasford J, Mimran A, Simons WR. A population-based European cohort study of persistence in newly diagnosed hypertensive patients. J Hum Hypertens. 2002;16:569–75. doi: 10.1038/sj.jhh.1001451. [DOI] [PubMed] [Google Scholar]
- 9.Hansson L, Zanchetti A, Carruthers SG, et al. for the HOT Study Group. Effects of intensive blood pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 10.ALLHAT Officers and Coordinators. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 11.Staessen JA, Thijs L, Fagards R, et al. Systolic Hypertension in Europe (Syst-Eur) Trial Investigators Effects of immediate versus delayed antihypertensive therapy on outcome in the Systolic Hypertension in Europe Trial. J Hypertens. 2004;22:847–57. doi: 10.1097/00004872-200404000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Black HR. The paradigm has shifted to systolic blood pressure. J Hum Hypertens. 2004;18(Suppl. 2):S3–7. doi: 10.1038/sj.jhh.1001795. [DOI] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute National High Blood Pressure Education Program Coordinating Committee: the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 14.European Society of Hypertension. European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–54. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Julius S, Kjeldsen SE, Weber M, et al. VALUE Trial Group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–31. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 16.Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–51. doi: 10.1016/S0140-6736(04)16456-8. [DOI] [PubMed] [Google Scholar]
- 17.Conlin PR. Angiotensin II antagonists in the treatment of hypertension: more similarities than differences. J Clin Hypertens. 2000;2:253–7. [PubMed] [Google Scholar]
- 18.Gaudio C, Ferri FM, Giovannini M, et al. Comparative effects of irbesartan versus amlodipine on left ventricular mass index in hypertensive patients with left ventricular hypertrophy. J Cardiovasc Pharmacol. 2003;42:622–8. doi: 10.1097/00005344-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Neutel J, Germino W, Smith D, et al. The antihypertensive efficacy and safety of irbesartan compared with amlodipine for the treatment of mild-to-moderate hypertension [Abstract no. D068] Am J Hypertens. 1999;12(Pt 2):128A. [Google Scholar]
- 20.Kloner RA, Weinberger M, Pool JL, et al. Comparison of Candesartan and Amlodipine for Safety, Tolerability and Efficacy (CASTLE) Study Investigators. Comparative effects of candesartan cilexetil and amlodipine in patients with mild systemic hypertension. Comparison of Candesartan and Amlodipine for Safety, Tolerability and Efficacy (CASTLE) Study Investigators. Am J Cardiol. 2001;87:727–31. doi: 10.1016/s0002-9149(00)01491-0. [DOI] [PubMed] [Google Scholar]
- 21.Lacourciere Y, Lenis J, Orchard R, et al. A comparison of the efficacies and duration of action of the angiotensin II receptor blockers telmisartan and amlodipine. Blood Press Monit. 1998;3:295–302. [PubMed] [Google Scholar]
- 22.Chrysant SG, Marbury TC, Robinson TD. Antihypertensive efficacy and safety of olmesartan medoxomil compared with amlodipine for mild-to-moderate hypertension. J Hum Hypertens. 2003;1:425–32. doi: 10.1038/sj.jhh.1001577. [DOI] [PubMed] [Google Scholar]
- 23.Mazzolai L, Maillard M, Rossat J, et al. Angiotensin II receptor blockade in normotensive subjects: a direct comparison of three AT1 receptor antagonists. Hypertension. 1999;33:850–5. doi: 10.1161/01.hyp.33.3.850. [DOI] [PubMed] [Google Scholar]
- 24.Kassler-Taub K, Littlejohn T, Elliott W, et al. Comparative efficacy of two angiotensin II receptor antagonists, irbesartan and losartan in mild-to-moderate hypertension. Irbesartan/Losartan Study Investigators. Am J Hypertens. 1998;11:445–53. doi: 10.1016/s0895-7061(97)00491-3. [DOI] [PubMed] [Google Scholar]
- 25.Oparil S, Guthrie R, Levin AJ, et al. An elective-titration study of the comparative effectiveness of two angiotensin II-receptor blockers, irbesartan and losartan. Irbesartan/Losartan Study Investigators. Clin Ther. 1998;20:398–409. doi: 10.1016/s0149-2918(98)80051-9. [DOI] [PubMed] [Google Scholar]
- 26.Mallion J, Siche J, Lacourciere Y. ABPM comparison of the antihypertensive profiles of the selective angiotensin II receptor antagonists telmisartan and losartan in patients with mild-to-moderate hypertension. J Hum Hypertens. 1999;13:657–64. doi: 10.1038/sj.jhh.1000925. [DOI] [PubMed] [Google Scholar]
- 27.Lacourciere Y, Asmar R. A comparison of the efficacy and duration of action of candesartan cilexetil and losartan as assessed by clinic and ambulatory blood pressure after a missed dose, in truly hypertensive patients: a placebo-controlled, forced titration study. Am J Hypertens. 1999;12:1181–7. doi: 10.1016/s0895-7061(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 28.Gradman AH, Lewin A, Bowling BT, et al. Comparative effects of candesartan cilexetil and losartan in patients with systemic hypertension. Candesartan versus Losartan Efficacy Comparison (CANDLE) Study Group. Heart Dis. 1999;1:52–7. [PubMed] [Google Scholar]
- 29.Ball KJ, Williams PA, Stumpe KO. Relative efficacy of an angiotensin II antagonist compared with other antihypertensive agents. Olmesartan medoxomil versus antihypertensives. J Hypertens Suppl. 2001;19(Suppl. 1):S49–S56. doi: 10.1097/00004872-200106001-00007. [DOI] [PubMed] [Google Scholar]
- 30.Mancia G, Korlipara K, van Rossum P, et al. An ambulatory blood pressure monitoring study of the comparative antihypertensive efficacy of two angiotensin II receptor antagonists, irbesartan and valsartan. Blood Press Monit. 2002;7:135–42. doi: 10.1097/00126097-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Smith DH, Dubiel R, Jones M. Use of 24-hour ambulatory blood pressure monitoring to assess antihypertensive efficacy: a comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartan. Am J Cardiovasc Drugs. 2005;5:41–50. doi: 10.2165/00129784-200505010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Littlejohn T, Mroczek W, Marbury T, et al. A prospective, randomized, open-label trial comparing telmisartan 80 mg with valsartan 80 mg in patients with mild to moderate hypertension using ambulatory blood pressure monitoring. Can J Cardiol. 2000;16:1123–32. [PubMed] [Google Scholar]
- 33.Calvo C, Hermida RC, Ayala DE, Ruilope LM. Effects of telmisartan 80 mg and valsartan 160 mg on ambulatory blood pressure in patients with essential hypertension. J Hypertens. 2004;22:837–46. doi: 10.1097/00004872-200404000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Lacourciere Y, Krzesinski JM, White WB, et al. Sustained antihypertensive activity of telmisartan compared with valsartan. Blood Press Monit. 2004;9:203–10. doi: 10.1097/00126097-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Belz GG. Pharmacological differences among angiotensin II receptor antagonists. Blood Press Suppl. 2001;2:13–8. doi: 10.1080/080370501750275848. [DOI] [PubMed] [Google Scholar]
- 36.Verheijen I, Fierens FL, Debacker JP, et al. Interaction between the partially insurmountable antagonist valsartan and human recombinant angiotensin II type receptors. Fundam Clin Pharmacol. 2000;14:577–85. doi: 10.1111/j.1472-8206.2000.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 37.Olmesartan Prescribing Information, Sankyo Pharma Ltd.
- 38.Oparil S. Newly emerging pharmacologic differences in angiotensin II receptor blockers. Am J Hypertens. 2000;13(Pt 2):18S–24S. doi: 10.1016/s0895-7061(99)00250-2. [DOI] [PubMed] [Google Scholar]
- 39.Unger T. Significance of angiotensin type 1 receptor blockade: why are angiotensin II receptor blockers different? Am J Cardiol. 1999;84:9S–15S. doi: 10.1016/s0002-9149(99)00728-6. [DOI] [PubMed] [Google Scholar]
- 40.Oparil S, Guthrie R, Lewin AJ, et al. on behalf of the Irbesartan/Losartan Study Investigators The efficacy and safety of valsartan compared with placebo in the treatment of patients with essential hypertension. Clin Ther. 1996;18:797–810. doi: 10.1016/s0149-2918(96)80040-3. [DOI] [PubMed] [Google Scholar]
- 41.Bobrie G, Delonca J, Moulin C, et al. for the COSIMA Investigators. A home blood pressure monitoring study comparing the antihypertensive efficacy of two angiotensin II receptor antagonist fixed combinations. Am J Hypertens. 2005;18:1482–1488. doi: 10.1016/j.amjhyper.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Lacourciere Y, Gil-Extremera B, Mueller O, et al. Efficacy and tolerability of fixed-dose combinations of telmisartan plus HCTZ compared with losartan plus HCTZ in patients with essential hypertension. Int J Clin Pract. 2003;57:273–9. [PubMed] [Google Scholar]
- 43.Neutel JM, Kolloch RE, Pouin PF, et al. OTELLOH Study Group. Telmisartan vs losartan plus hydrochlorothiazide in the treatment of mild-to-moderate essential hypertension – a randomised ABPM study. J Hum Hypertens. 2003;17:569–75. doi: 10.1038/sj.jhh.1001592. [DOI] [PubMed] [Google Scholar]
- 44.Ohma KP, Milon H, Valnes K. Efficacy and tolerability of a combination tablet of candesartan cilexetil and hydrochlorothiazide in insufficiently controlled primary hypertension – comparison with a combination of losartan and hydrochlorothiazide. Blood Press. 2000;9:214–20. doi: 10.1080/080370500439100. [DOI] [PubMed] [Google Scholar]