Summary
Heart rate, a major determinant of angina in coronary disease, is also an important predictor of cardiovascular mortality. Lowering heart rate is therefore one of the most important therapeutic approaches in the treatment of stable angina pectoris. To date, β-blockers and some calcium-channel antagonists reduce heart rate, but their use may be limited by adverse reactions or contraindications. Heart rate is determined by spontaneous electrical pacemaker activity in the sinoatrial node controlled by the If current. Ivabradine is the first specific heart rate-lowering agent that has completed clinical development for stable angina pectoris. It is selective for the If current, lowering heart rate at concentrations that do not affect other cardiac ionic currents. Specific heart-rate lowering with ivabradine reduces myocardial oxygen demand, simultaneously improving oxygen supply. Ivabradine has no negative inotropic or lusitropic effects, preserving ventricular contractility, and does not change any major electrophysiological parameters unrelated to heart rate. Randomised clinical studies in patients with stable angina show that ivabradine effectively reduces heart rate, improves exercise capacity and reduces the number of angina attacks. It has superior anti-anginal and anti-ischaemic activity to placebo and is non-inferior to atenolol and amlodipine. Ivabradine therefore offers a valuable approach to lowering heart rate exclusively and provides an attractive alternative to conventional treatment for a wide range of patients with confirmed stable angina.
Keywords: Ivabradine, heart rate, angina, If current, If channel
Introduction
The most prevalent cardiovascular disease in Western society is atherosclerotic coronary artery obstruction, of which angina pectoris is the primary symptom. Angina is a symptom of myocardial ischaemia, which occurs when insufficient oxygen is supplied to the heart muscle. Heart rate is a primary determinant of myocardial oxygen demand and may also affect myocardial perfusion. Lowering heart rate increases the duration of diastole relative to cardiac cycle length, allowing more time for effective left ventricular (LV) filling and coronary perfusion. Therefore, lowering the heart rate may improve both the aspects of myocardial oxygen balance.
Heart rate may also be involved in the progression of atherosclerosis in patients with coronary heart disease (1). High heart rate is associated with coronary plaque disruption, independent of blood pressure, possibly as a result of increased haemodynamic stress (2). Taken together, these effects predict that lowering heart rate may improve myocardial efficiency.
There is considerable evidence that survival is inversely related to heart rate, both for the general population and in several specific disease states (3–18), including angina (19). Thus, the modulation of heart rate is important in patients with angina.
Role of the if Current
In the normal, non-diseased state, heart rate is controlled by the sinoatrial node, the origin of cardiac pacemaker activity. Sinoatrial myocytes, the pacemaker cells in the heart, have the unique capacity to spontaneously generate slow diastolic depolarisation, driving the membrane voltage away from the hyperpolarised level reached at the completion of one action potential towards the threshold level for initiating a subsequent action potential. The rhythmic action potentials generated in this way propagate through the conducting systems of the heart and trigger myocardial contraction.
Pacemaker activity involves interplay between several ionic currents that influence spontaneous diastolic depolarisation of the sinoatrial node, including the If current (20). The ‘f’ denotes ‘funny’, so called because it had unusual properties compared with other current systems known at the time of its discovery. The If current is carried by both sodium and potassium ions across the sarcolemma; it is inward at voltages in the diastolic range, is activated on hyperpolarisation (within the diastolic range of voltages regularly observed in cardiac pacemaker tissue) (21) and is characterised by unusually low single-channel conductance and slow activation kinetics. The If current is directly activated by intracellular cyclic adenosine monophosphate (cAMP), not linked to cAMP-dependent phosphorylation activity (22) and is carried by the hyperpolarisation-activated cyclic nucleotide-gated family of ion channels (23). These form the naturally occurring If channels in cardiac pacemaker cells and the related Ih channels in certain neuronal structures.
The If and Ih channels open and close in response to both ambient voltage and local intracellular cAMP concentrations. Adrenergic agonists activate adenylate cyclase, increasing local cAMP concentrations and thus increasing cAMP binding to the If channel (24). Conversely, cholinergic transmitters decrease local cAMP concentrations by inhibiting adenylate cyclase, thereby decreasing cAMP binding to the If channel. An If channel bound to cAMP is more likely to open, increasing the rate of slow diastolic depolarisation, whereas an unbound channel is more likely to remain closed, lowering the heart rate (25).
Lowering the Heart Rate in Angina
Lowering heart rate reduces cardiac work, thereby diminishing myocardial oxygen demand. This mechanism is the primary basis for the anti-ischaemic and anti-anginal effects of heart rate-lowering drugs. Heart-rate lowering might also increase coronary blood flow and, thus, myocardial oxygen supply, mitigating ischaemia by increasing diastolic perfusion time, during which coronary flow proceeds against relatively low resistance (26, 27). This effect is most pronounced in the highly vulnerable subendocardium, where contraction-induced resistance is greatest. In theory, the disruption of atherosclerotic plaques is partly due to mechanical perturbation of the plaque by foreshortening and twisting of large epicardial arteries during systole, which is diminished by heart-rate lowering (2).
Heart-rate modulation is part of standard angina-prevention strategies. However, under current treatments, almost two-thirds of patients continue to experience an average of two angina episodes per week despite simultaneous use of multiple anti-anginal drugs (28).
Current Therapeutic Options
Although β-adrenergic blocking drugs lower heart rate and prevent angina in patients with coronary artery disease, β-blockade may be associated with adverse events, including hypotension, psychological depression, erectile dysfunction and worsening of intrinsic atrioventricular node disease (29), obstructive pulmonary diseases (30), diabetes mellitus (31), hyperlipidaemia (32) and intermittent claudication and related symptoms in patients with occlusive peripheral arterial disease (33), a relatively common concomitant of coronary artery disease. In addition, ‘rebound’ effects, sometimes fatal, have been reported when short-acting β-blockers are stopped abruptly (34).
In addition, β-blockers and certain heart rate-lowering calcium antagonists have negative inotropic effects and may be inappropriate in patients with heart failure or with atrioventricular node dysfunction. Calcium antagonists can also cause constipation or peripheral oedema (35).
Long-acting nitrates, which do not lower heart rate but cause reflex cardio-acceleration (36), can produce headaches or light-headedness. Continual administration of these agents can lead to pharmacological tolerance to therapeutic effects (37) and can be associated with rebound angina and vasoconstriction when stopped.
In the light of these complications, alternative approaches to angina prevention have been sought, including the lowering of heart rate through novel mechanisms.
The if Current as a Pharmacological Target
More than three decades ago, the search began for pure heart rate-lowering agents that would prevent angina without the adverse effects of β-blockers. The discovery of the If current and If channels offered a possible approach to developing pure heart rate-lowering agents. Ivabradine has a considerable selectivity for If channel blockade (38), which allows the administration of doses that cause relatively pronounced If current inhibition and substantial heart-rate lowering. Currently, ivabradine is the only If current inhibitor in the late-stage clinical development.
Ivabradine reduces the firing rate of the pacemaker cells in the sinoatrial node without affecting the duration of the action potential (20, 39), whilst acting at concentrations that have no effect on other ionic currents, making ivabradine a selective If inhibitor (40). Ivabradine blocks If channels in a concentration-dependent manner by entering the channel pore from the intracellular side (40, 41). Blockade is only possible when the If channel is open, and the magnitude of If inhibition is directly related to the frequency of channel opening. Unlike other heart rate-lowering mechanisms, direct If blockade depends on the current driving force, as block dramatically increases across the voltage interval, and on sodium concentration in the surrounding milieu (41). Thus, ivabradine would be expected to be most effective at higher heart rates, where its clinical usefulness would also be greatest.
Animal Studies With Ivabradine
Several experimental studies in animals, including dogs and pigs, have clarified the different beneficial effects that may be associated with pure heart-rate lowering with ivabradine. Although ivabradine and the β-blocker propranolol reduced both tachycardia during exercise and ST-segment shift to the same extent, ivabradine, unlike propranolol, does not reduce LV contractility and preserves systolic shortening fraction in ischaemic regions to a greater degree than propranolol (42). Furthermore, the recovery of contractility in ischaemic LV contractile dysfunction is significantly more rapid with ivabradine than with another β-blocker, atenolol (43, 44). Heart-rate lowering with ivabradine also dose-dependently increases diastolic time and reduces myocardial oxygen consumption, giving a linear relationship between heart rate and oxygen consumption (27). In contrast, the negative inotropic action of atenolol leads to a prolonged ejection time and, consequently, a smaller increase in diastolic time for the same reduction in heart rate compared with ivabradine (26). Ivabradine, unlike atenolol, does not depress the physiological exercise-induced acceleration of LV relaxation, so ivabradine does not show the negative lusitropic effects associated with β-blockade (45).
These beneficial effects of ivabradine compared with β-blockers may be because, unlike ivabradine, β-blockers reduce If activation by decreasing sympathetic activity and cAMP formation, thereby lowering heart rate. Although the negative inotropic effects of β-blockers contribute directly to the diminution of myocardial oxygen consumption, they can also limit the increases in coronary flow otherwise associated with heart-rate lowering (26, 27). Also, myocardial relaxation occurs more slowly with β-blockade than with If current inhibition, which also minimises the impact of β-blockers on flow (45).
Heart-rate lowering with If inhibition, unlike that with β-blockade, results in increased stroke volume, supporting cardiac output and coronary flow (46). If inhibition may improve LV function and ventricular remodelling (47).
Clinical Studies of Ivabradine in the Prevention of Angina
Preclinical and clinical studies show that therapeutic doses of ivabradine are likely to be well tolerated in clinical use. Ivabradine at these doses has no negative inotropic effects in healthy volunteers (48) or in patients with LV dysfunction (49), causes no peripheral vasodilation and has no effect on the cardiac conduction system (50). Furthermore, no rebound effects with drug cessation or pharmacological tolerance with long-term use have been observed following ivabradine treatment (51). Because of their mechanism of action, If inhibitors are contraindicated in patients with intrinsic sinoatrial node disease (e.g. sick sinus syndrome); such patients were excluded from ivabradine trials.
The efficacy of ivabradine monotherapy in patients with stable angina has been evaluated in two published large-scale clinical studies. The anti-anginal, anti-ischaemic effect of ivabradine, alone and in combination with other drugs, and its tolerability have been assessed in a clinical programme involving more than 5000 patients. Ivabradine 5 mg and 7.5 mg twice daily (bid) are the licensed dosages for the treatment of stable angina.
Ivabradine Monotherapy
A randomised, placebo-controlled, double-blind, multicentre, multinational study in 360 patients with stable angina for at least 3 months and documented coronary artery disease evaluated ivabradine in a short dose-ranging phase and in longer-term use (52). Initially, participants received either placebo or ivabradine orally twice daily for 2 weeks. The primary efficacy criteria were time to 1-mm ST-segment depression (a measure of ischaemia) and time to limiting angina during exercise tolerance testing (ETT). Patients recorded angina attacks and nitroglycerin consumption in diaries.
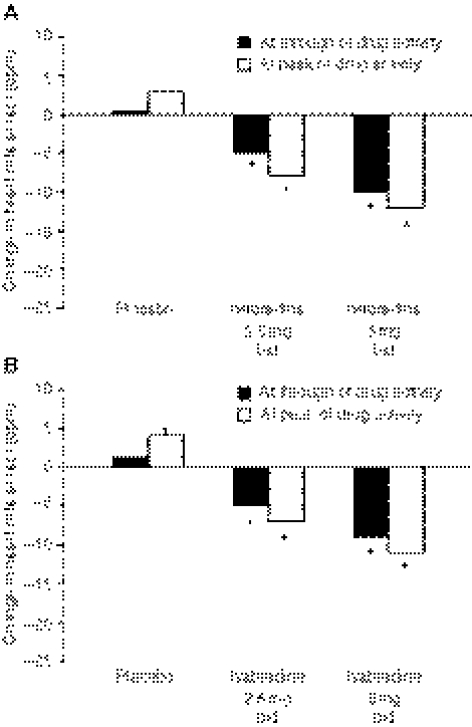
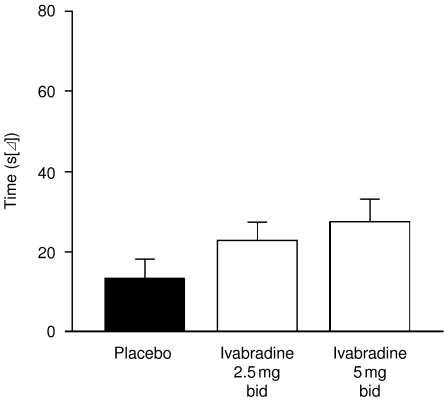
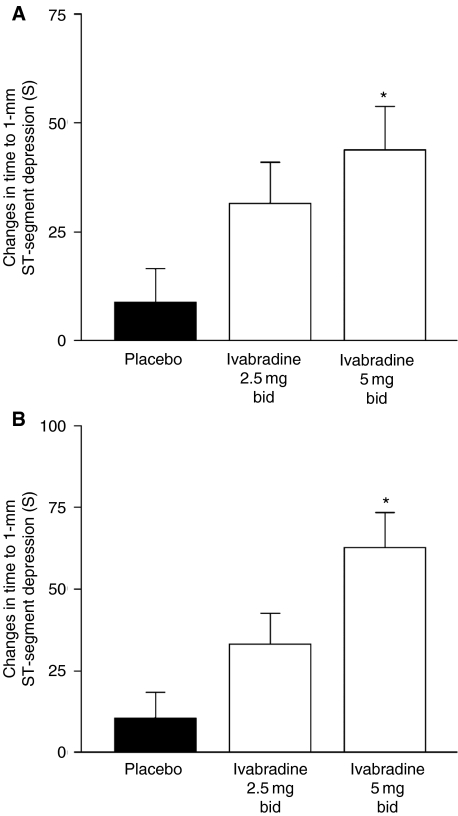
After 2 weeks of treatment, resting heart rate was significantly slower with ivabradine compared with placebo at both peak and trough drug activities, and this reduction increased significantly with increasing dose (−4.5 and −9.5 bpm at 2.5 and 5.0 mg, respectively) (Figure 1). Heart rate rose slightly, but not significantly, on placebo. Maximal heart rate at peak exercise was lowered to the same extent as resting heart rate. The reduction in heart rate during exercise was associated with a significant increase in time to 1-mm ST-segment depression during ETT in a dose-dependent manner, and there was a statistical trend to improvement in time to limiting angina after just 2 weeks. Analysis indicated that time to limiting angina increased compared with placebo although not reaching statistical significance (Figure 2). A similar increase was seen in time to 1-mm ST-segment depression, although this reached significance at ivabradine doses of 5 mg (Figure 3). The frequency of angina attacks and consumption of short-acting nitrates tended to fall with ivabradine use, which reached significance by the end of the 2–3-month open-label phase.
Figure 1.
Changes in heart rate in the different treatment groups during the double-blind, dose-ranging phase at rest (A) and at peak exercise (B). *p < 0.05 vs. placebo in pairwise comparisons. bid = twice daily. Adapted from (52)
Figure 2.
Effect of ivabradine on time to angina sufficient to limit continued bicycle exercise among protocol-compliant patients with coronary artery disease. Measurements were obtained after 2 weeks of double-blind randomised therapy. A dose–response relationship is apparent. Adapted from (52)
Figure 3.
Changes in time to 1-mm ST-segment depression at trough (A) and peak (B) drug activity. *p < 0.05 vs. placebo. bid = twice daily. Adapted from (52)
Blood pressure was little affected by the drug, with no hypotension at peak or trough drug effect with any dose. The only reported adverse reactions were dosage-related visual symptoms which were reported in less than 2% of patients with 5 mg orally twice daily. Such effects were generally transient, always reversible and very seldom severe enough to cause patients to voluntarily stop the drug.
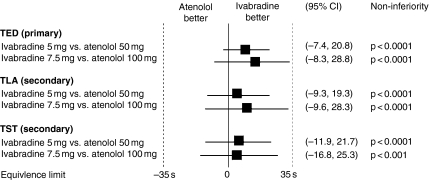
The results of the International Trial on the Treatment of angina with Ivabradine vs. Atenolol (INITIATIVE) have just been published (53). This randomised, double-blind study compared ivabradine with atenolol over 4 months in 939 patients with stable angina pectoris and documented coronary artery disease. Patients received either ivabradine 5 mg twice daily for 4 weeks increased to 7.5 mg twice daily for a further 3 months, or atenolol 50 mg once daily for 4 weeks increased to 100 mg once daily for a further 3 months. At 4 months, total exercise duration on a treadmill ETT at trough drug activity increased by 86.8 s with ivabradine 7.5 mg and 78.8 s with atenolol 100 mg (Figure 4). At trough drug activity, ivabradine 7.5 mg was non-inferior to atenolol for all primary and secondary analyses including time to limiting angina, time to angina onset and time to 1-mm ST-segment depression at month 4 (p < 0.0001). Ivabradine was non-inferior to atenolol at peak drug activity at month 1 for all parameters. By month 4, non-inferiority of ivabradine 7.5 mg to atenolol was retained for all parameters except time to 1-mm ST-segment depression. Visual symptoms occurred at a similar rate to that seen in the first study. These results indicate that by lowering heart rate, ivabradine is at least as effective as atenolol in patients with stable angina pectoris. Extension of the analysis in patients aged over 65 years confirmed that the efficacy of ivabradine is maintained in older patients (54).
Figure 4.
Comparison of total exercise duration (TED), time to limiting angina (TLA) and time to 1-mm ST-segment depression (TST) with ivabradine compared with atenolol. CI = confidence interval. Adapted from (53)
Preliminary data have been presented from a trial involving 1195 patients (55). In this double-blind, randomised study, patients received either amlodipine or ivabradine for 3 months. Ivabradine 7.5 mg bid significantly increased total exercise duration by 27.6 s. In addition, ivabradine also improved both angina, as measured by time to limiting angina and time to angina onset, and ischaemia, as measured by time to 1-mm ST-segment depression. Formal statistical testing indicated that ivabradine was non-inferior to amlodipine in preventing angina (p < 0.0001). Ivabradine significantly decreased the number of angina attacks by two-thirds and reduced use of short-acting nitrates.
Ivabradine Combination Therapy
In a 12-month, double-blind study, 386 patients receiving nitrates or calcium blockers for the treatment of angina were randomised to receive ivabradine 5.0 mg or 7.5 mg twice daily concomitant to their existing therapy (56). Ivabradine reduced heart rate by 10 and 12 bpm, respectively, for 5.0 and 7.5 mg doses. Moreover, the number of angina attacks was significantly reduced from baseline to month 12 in patients receiving ivabradine.
Tolerability Studies
Ivabradine reduces heart rate without any observed effects on myocardial contractility and does not alter the cardiac conduction system (49, 50). In one study, 14 patients received a single intravenous administration of ivabradine (0.2 mg/kg). Resting heart rate was lower by approximately 14 bpm, but no major electrophysiological parameters other than those related to the heart rate were altered (57). As expected, the QT interval was prolonged by 37.5 s. However, when QT was corrected for heart rate (QTc), there was no QTc prolongation. Ivabradine did not modify the PR and QRS intervals or the conductivity and refractoriness of the atrium, atrioventricular node, His-Purkinje system and ventricles.
In a randomised, placebo-controlled study in 44 patients with LV dysfunction, a single intravenous infusion of ivabradine 0.2–0.3 mg/kg lowered the resting heart rate by more than 17%, whilst preserving fractional shortening (49).
Although ivabradine is selective for If channels, it can also interact with the structurally similar retinal Ih channel. Data from clinical trials demonstrated that visual symptoms were reported in less that 2% of patients receiving the 5 mg bid dosage of ivabradine and that there were few withdrawals (<1%). Furthermore, ivabradine was not associated with any alteration of ocular structures or permanent visual disorders. In addition, most visual effects reported were mild and only occurred as occasional brief episodes, often associated with abrupt changes in light intensity. These episodes had minimal impact on patients' daily activities with no deleterious effects on their quality of life (54).
Conclusion
‘Pure’ heart-rate lowering via If inhibition is clinically feasible and can effectively prevent angina with acceptable tolerability. Ivabradine effectively prevents angina and concomitantly reduces ischaemia. Ivabradine is currently the only agent shown to clinically lower heart rate with no negative inotropism or effects on conduction and contractility.
References
- 1.Perski A, Olsson G, Landou C, et al. Minimum heart rate and coronary atherosclerosis: independent relations to global severity and rate of progression of angiographic lesions in men with myocardial infarction at a young age. Am Heart J. 1992;123:609–16. doi: 10.1016/0002-8703(92)90497-j. [DOI] [PubMed] [Google Scholar]
- 2.Heidland UE, Strauer BE. Left ventricular muscle mass and elevated heart rate are associated with coronary plaque disruption. Circulation. 2001;104:1477–82. doi: 10.1161/hc3801.096325. [DOI] [PubMed] [Google Scholar]
- 3.Habib G. Is heart rate a risk factor in the general population? Dialogues Cardiovasc Med. 2001;6:25–31. [Google Scholar]
- 4.Goldberg R, Larson M, Levy D. Factors associated with survival to 75 years of age in middle-aged men and women: The Framingham Study. Arch Intern Med. 1996;156:505–9. [PubMed] [Google Scholar]
- 5.Gillum R. The epidemiology of resting heart rate in a national sample of men and women: association with hypertension, coronary artery disease, blood pressure, and other cardiovascular risk factors. Am Heart J. 1988;116:163–74. doi: 10.1016/0002-8703(88)90262-1. [DOI] [PubMed] [Google Scholar]
- 6.Kjekshus J, Gullestad L. Heart rate as a therapeutic target in heart failure. Eur Heart J. 1999;1(Suppl. H):H64–9. [Google Scholar]
- 7.Kjekshus JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term myocardial intervention trials. Am J Cardiol. 1986;57:43F–49F. doi: 10.1016/0002-9149(86)90888-x. [DOI] [PubMed] [Google Scholar]
- 8.Mensink GB, Hoffmeister H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur Heart J. 1997;18:1404–10. doi: 10.1093/oxfordjournals.eurheartj.a015465. [DOI] [PubMed] [Google Scholar]
- 9.Kristal-Boneh E, Silber H, Harari G, et al. The association of resting heart rate with cardiovascular, cancer and all-cause mortality: eight-year follow-up of 3527 male Israeli employees (the CORDIS study) Eur Heart J. 2000;21:116–24. doi: 10.1053/euhj.1999.1741. [DOI] [PubMed] [Google Scholar]
- 10.Reunanen A, Karjalainen J, Ristola P, et al. Heart rate and mortality. J Intern Med. 2000;247:231–9. doi: 10.1046/j.1365-2796.2000.00602.x. [DOI] [PubMed] [Google Scholar]
- 11.Fujiura Y, Adachi H, Tsuruta M, et al. Heart rate and mortality in a Japanese general population: an 18-year follow-up study. J Clin Epidemiol. 2001;54:495–500. doi: 10.1016/s0895-4356(00)00323-1. [DOI] [PubMed] [Google Scholar]
- 12.Benetos A, Thomas F, Bean K, et al. Resting heart rate in older people: a predictor of survival to age 85. J Am Geriatr Soc. 2003;51:284–5. doi: 10.1046/j.1532-5415.2003.51080.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang M, Havlik RJ, Corti MC, et al. Relation of heart rate at rest and mortality in the Women's Health and Aging Study. Am J Cardiol. 2003;92:1294–9. doi: 10.1016/j.amjcard.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Gillman MW, Kannel WB, Belanger A, D'Agostino RB. Influence of heart rate on mortality among persons with hypertension: The Framingham Study. Am Heart J. 1993;125:1148–54. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 15.Palatini P, Thijs L, Staessen JA, et al. Predictive value of clinic and ambulatory heart rate for mortality in elderly subjects with systolic hypertension. Arch Intern Med. 2002;162:2313–21. doi: 10.1001/archinte.162.20.2313. [DOI] [PubMed] [Google Scholar]
- 16.Imai Y, Hozawa A, Ohkubo T, et al. Heart rate measurement and outcome. Blood Press Monit. 2003;8:53–5. doi: 10.1097/00126097-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Hjalmarson A, Gilpin EA, Kjekshus J, et al. Influence of heart rate on mortality after acute myocardial infarction. Am J Cardiol. 1990;65:547–53. doi: 10.1016/0002-9149(90)91029-6. [DOI] [PubMed] [Google Scholar]
- 18.Singh N. Diabetes, heart rate, and mortality. J Cardiovasc Pharmacol Ther. 2002;7:117–29. doi: 10.1177/107424840200700208. [DOI] [PubMed] [Google Scholar]
- 19.Singh BN. Impact of heart rate on cardiovascular disorders: focus on chronic stable angina. In: Fox K, editor. Selective and Specific If Channel Inhibition in Cardiology. London: Science Press Ltd; 2004. pp. 25–36. [Google Scholar]
- 20.DiFrancesco D. If current inhibitors: properties of drug–channel interaction. In: Fox K, editor. Selective and Specific If Channel Inhibition in Cardiology. London: Science Press Ltd; 2004. pp. 1–13. [Google Scholar]
- 21.DiFrancesco D. The cardiac hyperpolarizing-activated current, If: origins and developments. Prog Biophys Mol Biol. 1985;46:163–83. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- 22.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–7. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig A, Zong X, Jeglitsch M, et al. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–91. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 24.DiFrancesco D, Tromba C. Inhibition of the hyperpolarization activated current (If) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol (Lond) 1988;405:477–91. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiFrancesco D. The contribution of the ‘pace-maker’ current (If) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J Physiol. 1991;434:23–40. doi: 10.1113/jphysiol.1991.sp018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colin P, Ghaleh B, Monnet X, et al. Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am J Physiol Heart Circ Physiol. 2003;284:H676–82. doi: 10.1152/ajpheart.00564.2002. [DOI] [PubMed] [Google Scholar]
- 27.Colin P, Ghaleh B, Monnet X, et al. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther. 2004;308:236–40. doi: 10.1124/jpet.103.059717. [DOI] [PubMed] [Google Scholar]
- 28.Pepine CJ, Abrams J, Marks RG, et al. Characteristics of a contemporary population with angina pectoris: TIDES Investigators. Am J Cardiol. 1994;74:226–31. doi: 10.1016/0002-9149(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 29.Ramahi TM. Beta blocker therapy for chronic heart failure. Am Fam Physician. 2000;62:2267–74. [PubMed] [Google Scholar]
- 30.Tattersfield AE. Respiratory function in the elderly and the effects of beta blockade. Cardiovasc Drugs Ther. 1991;4(Suppl. 6):1229–32. doi: 10.1007/BF00114225. [DOI] [PubMed] [Google Scholar]
- 31.Reneland R, Alvarez E, Andersson PE, et al. Induction of insulin resistance by beta blockade but not ACE-inhibition: long-term treatment with atenolol or trandolapril. J Hum Hypertens. 2000;14:175–80. doi: 10.1038/sj.jhh.1000964. [DOI] [PubMed] [Google Scholar]
- 32.Krone W, Nagele H. Effects of antihypertensives on plasma lipids and lipoprotein metabolism. Am Heart J. 1988;116:1729–34. doi: 10.1016/0002-8703(88)90222-0. [DOI] [PubMed] [Google Scholar]
- 33.Lewis RV, Lofthouse C. Adverse reactions with beta-adrenoceptor blocking drugs: an update. Drug Saf. 1993;9:272–9. doi: 10.2165/00002018-199309040-00005. [DOI] [PubMed] [Google Scholar]
- 34.Egstrup K. Transient myocardial ischemia after abrupt withdrawal of antianginal therapy in chronic stable angina. Am J Cardiol. 1988;61:1219–22. doi: 10.1016/0002-9149(88)91158-7. [DOI] [PubMed] [Google Scholar]
- 35.Frishman WH, Sica DA. Calcium channel blockers. In: Frishman WH, et al., editors. Cardiovascular Pharmacotherapies. New York: McGraw-Hill; 2003. pp. 105–30. [Google Scholar]
- 36.Abrams J, Frishman WH. The organic nitrates and nitroprusside. In: Frishman WH, Sonnenblick EH, Sica DA, editors. Cardiovascular Pharmacotherapies. New York: McGraw-Hill; 2003. pp. 203–14. [Google Scholar]
- 37.Steering Committee, Transdermal Nitroglycerin Cooperative Study. Acute and chronic antianginal efficacy in continuous twenty-four hour application of transdermal nitroglycerin. Am J Cardiol. 1991;68:1263–70. doi: 10.1016/0002-9149(91)90229-e. [DOI] [PubMed] [Google Scholar]
- 38.Thollon C, Cambarrat C, Vian J, et al. Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea pig-cardiac preparations: comparison with UL-FS 49. Br J Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thollon C, Bidouard JP, Cambarrat C, et al. Stereospecific in vitro and in vivo effects of the new sinus node inhibitor (+)-S 16257. Eur J Pharmacol. 1997;339:43–51. doi: 10.1016/s0014-2999(97)01364-2. [DOI] [PubMed] [Google Scholar]
- 40.Bois P, Bescond J, Renaudon B, et al. Mode of action of bradycardic agent, S16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol. 1996;118:1051–7. doi: 10.1111/j.1476-5381.1996.tb15505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bucchi A, Baruscotti M, DiFrancesco D. Current-dependent block of rabbit sino-atrial node If channels by ivabradine. J Gen Physiol. 2002;120:1–13. doi: 10.1085/jgp.20028593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilaine JP, Bidouard JP, Lesage L, et al. Anti-ischemic effects of ivabradine, a selective heart rate-reducing agent, in exercise-induced myocardial ischemia in pigs. J Cardiovasc Pharmacol. 2003;42:688–96. doi: 10.1097/00005344-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Monnet X, Ghaleh B, Colin P, et al. Effect of heart rate reduction with ivabradine on exercise induced myocardial ischaemia and stunning. J Pharmacol Exp Ther. 2001;299:1133–9. [PubMed] [Google Scholar]
- 44.Monnet X, Colin P, Ghaleh B, et al. Heart rate reduction during exercise-induced myocardial ischaemia and stunning. Eur Heart J. 2004;25:579–86. doi: 10.1016/j.ehj.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Colin P, Ghaleh B, Hittinger L, et al. Differential effects of heart rate reduction and beta-blockade on left ventricular relaxation during exercise. Am J Physiol Heart Circ Physiol. 2002;282:H672–9. doi: 10.1152/ajpheart.00547.2001. [DOI] [PubMed] [Google Scholar]
- 46.Simon L, Ghaleh B, Puybasset L, et al. Coronary and haemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J Pharmacol Exp Ther. 1995;275:659–66. [PubMed] [Google Scholar]
- 47.Mulder P, Barbier S, Chagraoui A, et al. Long-term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–9. doi: 10.1161/01.CIR.0000118464.48959.1C. [DOI] [PubMed] [Google Scholar]
- 48.Moore N, Joannides R, Iacob M, et al. Effects of a pure bradycardic agent, S 16257, at rest and during exercise in healthy volunteers: comparison with propranolol. Br J Clin Pharmacol. 1998;45:188–9. [Google Scholar]
- 49.Manz M, Reuter M, Lauck G, et al. A single intravenous dose of ivabradine, a novel I(f) inhibitor, lowers heart rate but does not depress left ventricular function in patients with left ventricular dysfunction. Cardiology. 2003;100:149–55. doi: 10.1159/000073933. [DOI] [PubMed] [Google Scholar]
- 50.DiFrancesco D, Camm J. Heart rate lowering by specific and selective If current inhibition with ivabradine: a new therapeutic perspective in cardiovascular disease. Drugs. 2004;64:1757–65. doi: 10.2165/00003495-200464160-00003. [DOI] [PubMed] [Google Scholar]
- 51.Tardif JC, Fox K, Tendera M, Ford I. Absence of rebound phenomenon after abrupt discontinuation of ivabradine, a new selective and specific If inhibitor, in patients with coronary artery disease. Eur Heart J. 2005;26:580. [Google Scholar]
- 52.Borer JS, Fox K, Jaillon P, et al. Anti-anginal and anti-ischemic effects of ivabradine, an If inhibitor, in stable angina: a randomized, double-blinded, multicentered, placebo-controlled trial. Circulation. 2003;107:817–23. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 53.Tardif JC, Ford I, Tendera M, Bourassa MG. Efficacy of ivabradine, a new selective If inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–36. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- 54.Fox KM, Tardif JC, Ford M. Anti-anginal and anti-ischemic efficacy of ivabradine – a selective and specific sinus node If current inhibitor – compared to atenolol in elderly patients with chronic stable angina. Heart. 2005;91(Suppl. 1):A69. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- 55.Ruzyllo W, Ford I, Tendera M, et al. Antianginal and anti-ischemic effects of the If current inhibitor ivabradine compared to amlodipine as monotherapy in patients with chronic stable angina: a 3-month randomized, controlled, double-blind, multicenter trial. Eur Heart J. 2004;25:878. (Abstract) [Google Scholar]
- 56.Lopez-Bescos L, Filipova S, Martos R, et al. Long-term safety and antianginal efficacy of the If current inhibitor ivabradine in patients with chronic stable angina. A one-year randomized, double-blind, multicentre trial. Eur Heart J. 2004;25:878. [Google Scholar]
- 57.Camm AJ, Lau CP. Electrophysiological effects of a single intravenous administration of ivabradine (S 16257) in adult patients with normal baseline electrophysiology. Drugs R D. 2003;4:83–9. doi: 10.2165/00126839-200304020-00001. [DOI] [PubMed] [Google Scholar]