Summary
Oxidative stress, through the production of reactive oxygen species (ROS), has been proposed as the root cause underlying the development of insulin resistance, β-cell dysfunction, impaired glucose tolerance and type 2 diabetes mellitus (T2DM). It has also been implicated in the progression of long-term diabetes complications, including microvascular and macrovascular dysfunction. Excess nourishment and a sedentary lifestyle leads to glucose and fatty acid overload, resulting in production of ROS. Additionally, reaction of glucose with plasma proteins forms advanced glycation end products, triggering production of ROS. These ROS initiate a chain reaction leading to reduced nitric oxide availability, increased markers of inflammation and chemical modification of lipoproteins, all of which may increase the risk of atherogenesis. With the postulation that hyperglycaemia and fluctuations in blood glucose lead to generation of ROS, it follows that aggressive treatment of fasting and postprandial hyperglycaemia is important for prevention of micro and macrovascular complications in T2DM.
Keywords: Type 2 diabetes, oxidative stress, reactive oxygen species, cardiovascular
Introduction
Worldwide, there were approximately 194 million adults aged 20–79 years with diagnosed diabetes mellitus (DM) in 2003 (with type 2 diabetes accounting for 90–95% of all diagnosed cases), and that number is expected to increase to 333 million over the next 20 years (1). Diabetes is associated with increased coronary artery, cerebrovascular and peripheral vascular disease, with up to 80% of deaths in people with diabetes caused by cardiovascular disease (1). Diabetes is also largely responsible for blindness, amputations and end stage renal disease in the developed world (1). Because of this, it is important to recognise and treat this devastating disease early in its progression to postpone or even prevent the serious complications associated with it.
A currently favoured hypothesis is that oxidative stress, through a single unifying mechanism of superoxide production, is the common pathogenic factor leading to insulin resistance, β-cell dysfunction, impaired glucose tolerance (IGT) and ultimately to type 2 DM (T2DM) (2). Furthermore, this mechanism has been implicated as the underlying cause of both the macrovascular and microvascular complications associated with T2DM (3). It follows that therapies aimed at reducing oxidative stress would benefit patients with T2DM and those at risk for developing diabetes.
To optimise the treatment of these patients, it is necessary to understand the root cause of oxidative stress. Excess nourishment, combined with a sedentary lifestyle, results in overabundance of glucose and fatty acid accumulation within muscle, adipose tissue and pancreatic cells. This leads to the generation of excess reactive oxygen species (ROS), particularly superoxide anion, through the mitochondrial electron-transport chain (3, 4). Recently, it has been suggested that fluctuating blood glucose concentrations, like those observed during postprandial glycaemic excursions in people with IGT or T2DM, may contribute significantly to oxidative stress – perhaps even more so than chronically elevated blood glucose (5, 6).
This review will focus on the factors leading to the generation of ROS in diabetes and the effects of oxidative stress on vascular function and cardiac risk factors. Additionally, practical considerations for earlier identification and treatment of persons at risk for developing T2DM will be discussed.
The importance of blood glucose control in minimising oxidative stress
Achieving Target HbA1c Values may not be Sufficient
Evidence now exists to suggest that maintaining tight blood glucose control, by reducing the frequency and magnitude of glucose excursions, may be as important to the reduction in long-term complications of diabetes as achieving a target HbA1c value (5, 6). Attaining HbA1c values <7% has generally been considered a benchmark of successful diabetes therapy. It is important to note, however, that while HbA1c values have been accepted as biomarkers for glycaemic control, they represent an average measure of glycaemic exposure over time. Because of this, individuals who have identical HbA1c values may have experienced widely varying blood glucose ranges over the period of time reflected by the HbA1c value.
Results from a subgroup analysis 2 years after the completion of the Diabetes Complications and Control Trial (DCCT) demonstrated in patients with type 1 diabetes that, despite having similar HbA1c levels, participants in the intensive treatment group showed a marked reduction in the risk of development of diabetic retinopathy compared with their counterparts in the conventional treatment group (7). Some have hypothesised that there was a greater frequency and magnitude of glycaemic excursions in the conventionally treated patients compared with patients in the intensive treatment group, and that this increased glycaemic variability generated more ROS, leading to vascular damage (5).
Variable Glucose: Effects on Markers of Oxidative Stress and Inflammation
Several in vitro studies have demonstrated increased expression of markers of oxidative stress in cells exposed to fluctuating glucose concentrations (8–10). One such study examined the effects of variable glucose concentrations vs. constant high or normal glucose conditions on cultured human umbilical vein endothelial cells (8). The investigators monitored the generation of ROS by measuring levels of nitrotyrosine and showed higher levels of nitrotyrosine in cells exposed to variable glucose concentrations than for cells exposed to either constant normal or elevated glucose concentrations (8).
Owing to the ability to monitor ROS production via measurement of nitrotyrosine, there are now data in patients with T2DM that make evident the existence of increased oxidative stress in response to postprandial hyperglycaemia (11). In a study comparing T2DM patients with matched healthy controls, nitrotyrosine levels were significantly higher in diabetic individuals in the fasting state and were further elevated in the postprandial state. No such postprandial elevation in nitrotyrosine was observed in healthy control patients (11).
Markers of inflammation, a well-recognised manifestation of oxidative stress, have also been observed to increase in response to intermittent elevated glucose levels (10). In a study comparing the effects of inconsistent vs. constant glycaemic conditions on cultured human kidney cells, the authors noted that production of the inflammatory cytokines, transforming growth factor β (TGF-β) and insulin-like growth factor binding protein (IGFBP)-3, increased to a greater extent when exposed to variable glucose concentrations compared with constant hyperglycaemic conditions. The authors concluded that while maintenance of normal blood glucose levels would result in the smallest degree of oxidative stress and inflammation in the tubulointerstitium, variable glycaemic control would likely be even more damaging than constant hyperglycaemia (10).
Effects of oxidative stress on vascular function and cardiac risk factors
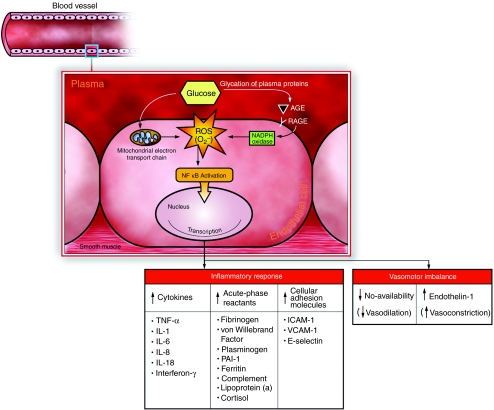
The adverse effects of oxidative stress on the cardiovascular system are numerous and varied but may be generally categorised into effects on nitric oxide availability, inflammatory response and lipid and lipoprotein modifications. (Figure 1, a summary of the effects of hyperglycaemia and oxidative stress on vascular function.)
Figure 1.
Glucose in the plasma undergoes non-enzymatic reaction with circulating proteins (including lipoproteins) to form AGEs. AGEs bind with RAGE on the surface of endothelial cells lining blood vessels, triggering the production of ROS, in particular super oxide anion, by NADPH oxidase. ROS are also produced as a result of glucose overload within the mitochondria. Once formed, ROS activate NFκB, which results in the transcriptional activation of genes relevant for inflammation, immunity and atherosclerosis. AGE, advanced glycation/glycoxidation endproduct; RAGE, receptor for AGE; ROS, reactive oxygen species; O2–, super oxide anion; NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); NFκB, nuclear factor κB; TNF-α, tumour necrosis factor α; IL, interlukin; PAI-1, plasminogen activator inhibitor 1; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule; NO, nitric oxide.
Reduction of Nitric Oxide Availability
Paradoxically, hyperglycaemic conditions result simultaneously in both increased NO production and decreased NO availability (12–16). However, reduction in NO availability is the primary pathogenic factor that appears responsible for endothelial dysfunction and diabetic angiopathy (12). The molecular mechanisms behind this apparent paradox are as follows: superoxide anions, resulting from hyperglycaemia, activate nuclear factor-κB (NF-κB), which causes increased expression of inducible nitric oxide synthase (iNOS) (16). This increase in iNOS results in amplified generation of NO. However, when superoxide anions are present at high concentration, they rapidly react with the newly created NO to form the strong oxidant peroxynitrite (15). The net result is an overall decline in the availability of NO to the endothelium and the formation of peroxynitrite, which is itself toxic to endothelial cells. Peroxynitrite exerts its toxic effect through oxidation of proteins, initiation of lipid peroxidation and nitration of amino acids (15). Another consequence of hyperglycaemia-induced production of superoxide anions is the inhibition of endothelial NOS, reducing the generation of NO and contributing to the universal NO deficiency (14). The ultimate outcome of this reduction in NO availability is defective endothelial-dependent vasodilation, leading to microvascular and macrovascular complications.
Increased Markers of Inflammation – Implications for Accelerated Atherosclerosis
Cytokines. Inflammation is involved in the pathogenesis of every stage of atherosclerosis (17, 18). It has been proposed that T2DM is a disease of the immune system, involving a cytokine-mediated acute-phase inflammatory response (19). In T2DM, there is an accelerated rate of atherosclerosis, which is thought to be due, in part, to the irreversible formation and deposition of molecules known as advanced glycation end products (AGEs) (20). Elevated blood glucose levels contribute to the glycation of proteins and lipids, resulting in the formation of AGEs (20).
Receptors for AGEs (RAGE) are expressed in many different tissues and cell types, including endothelial cells, vascular smooth muscle cells and macrophages (21–23). The binding of AGEs to RAGE leads to the intracellular generation of ROS (24, 25), which, in turn, activate NF-κB. As a consequence of NF-κB activation, expression of a variety of cytokines is increased, including tumour necrosis factors (TNF-α and TNF-β), interleukins (IL) 1, 6, 8and 18 and interferon-γ (26, 27).
A recent clinical study suggests that acute hyperglycaemia can result in elevated levels of circulating inflammatory cytokines, in particular TNF-α, IL-6 and IL-18 (27). The study demonstrated that increased cytokine levels were more pronounced in response to intermittent plasma glucose spikes compared with constant hyperglycaemia. These results were observed in both nondiabetic patients and patients with IGT. However, cytokine elevations were greater and lasted longer in patients with IGT compared with nondiabetic patients. Moreover, administration of glutathione (a powerful antioxidant) in control and IGT patients completely suppressed cytokine elevation in response to intermittent glucose pulses, supporting the concept that an oxidative stress mechanism mediates the inflammatory effect of hyperglycaemia in humans (27).
Acute-phase reactants. One of the many sequelae to the generation of ROS is cytokine-induced stimulation of acute-phase reactant synthesis by the liver (28). Cytokines – primarily TNF-α and IL-6 – stimulate the synthesis of C-reactive protein, complement, serum amyloid A and fibrinogen, to name a few (29). Other examples of acute-phase reactants that are known to be elevated in T2DM include plasminogen activator inhibitor 1 (PAI-1), von Willebrand factor, lipoprotein(a) and cortisol (29).
Cellular adhesion molecules. Another adverse effect of hyperglycaemia-induced oxidative stress on vascular function is increased expression of cellular adhesion molecules on the endothelial cell surface (30). These molecules attach to circulating leucocytes, leading to the formation of the atherosclerotic plaque.
Plasma from diabetic individuals contains elevated levels of some soluble forms of these cellular adhesion molecules, specifically intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 and E-selectin (31). Although the exact mechanism is unclear, recent data strongly suggest that, in humans, postpr and ial hyperglycaemia and hypertriglyceridemia generate increased expression of cellular adhesion molecules through a pathway mediated by oxidative stress (30). Specifically, nitrotyrosine levels fluctuate in concert with plasma ICAM-1, VCAM-1 and E-selectin concentrations in response to consumption of high fat and /or glucose. Furthermore, short-term treatment with simvastatin, a drug proposed to have intracellular antioxidant properties, reduced postpr and ial plasma concentrations of nitrotyrosine, as well as ICAM-1, VCAM-1 and E-selectin (30). It should be noted that it is currently unknown whether this is a property observed across all medications in this class, or specific only to certain statins. Thus, postprandial acute elevations in plasma glucose and lipids, and the ensuing generation of ROS, likely result in amplified circulating levels of cellular adhesion molecules, increasing the risk of atherogenesis.
Modification of Lipoproteins and Lipids – Contributions to Atherosclerosis
Lipoprotein glycation and glycoxidation. In addition to the primary dislipidemia of T2DM, chemical modifications to lipoproteins [e.g., low-density lipoprotein (LDL) and high-density lipoprotein (HDL)] may contribute to diabetic cardiovascular complications. One such modification, glycation/glycoxidation of lipoprotein, has been observed early in the progression of diabetes and has been shown to be correlated with other measures of glycaemia such as HbA1c (32, 33).
Glycated and glycoxidised lipoproteins are formed through a non-enzymatic process in which sugars bind to free amino groups of the lipoprotein (34, 35). These compounds undergo further rearrangement to yield irreversible AGE structures. Lipoprotein modification takes place in the absence (glycation) and presence (glycoxidation) of oxygen (36). These chemical modifications can alter lipoprotein structure and function (37). For example, pro-atherogenic properties have been observed for glycated/glycoxidised-LDL (33, 38). Additionally, glycated/glycoxidised-LDL has been shown to increase PAI-1 production and decrease tissue plasminogen activator in cultured human vascular endothelial cells, suggestive of a prothrombotic effect (39).
Lipid peroxidation and lipoxidation. Although the two terms appear similar, lipid peroxidation and lipoxidation are two distinct processes. Recent evidence suggests a central role for both of these chemical reactions in the development of cardiovascular disease in diabetes (40).
Lipid peroxidation is the formation of lipid peroxides via enzymatic and/or non-enzymatic mechanisms. ROS resulting from hyperglycaemia are thought to contribute to the initiation of lipid peroxidation (41). Once formed, lipid peroxides undergo a series of complex reactions, ultimately binding chemically to proteins and yielding advanced lipoxidation end products (ALEs) (42–44). Thus, lipoxidation is the covalent binding of products of lipid peroxidation reactions to proteins (36).
Studies of oxidised lipoproteins and vascular cells have demonstrated numerous pro-atherogenic effects of oxidised LDL and ALE-containing LDL. These include, but are not limited to, increased smooth muscle cell proliferation, increased apoptosis in endothelial cells, induction of macrophage-derived foam cell formation, activation of protein kinase-C and transforming growth factor-beta (TGF-β), increased matrix production, increased endothelin-1, decreased nitric oxide bio-availability, pro-inflammatory effects, pro-clotting effects and inhibition of antioxidant enzymes (37).
That many of the effects on vascular endothelial cells caused by ALE-containing lipoproteins are similar to those observed for AGE–RAGE interactions may be explained by the fact that several AGEs and ALEs share common reactive intermediates, formed during glycoxidation or lipid peroxidation reactions, respectively (45). For this reason, it has been suggested that the chronic excess of substrate (whether lipid or carbohydrate) present in T2DM results in creation of an abundance of reactive intermediates, leading to increased chemical modification of proteins (AGEs and ALEs) and persistent saturation of receptors such as RAGE, contributing to the atherogenic process (36).
Practical considerations for the clinician
Early Diagnosis and Intervention
The results from the DCCT and Epidemiology of Diabetes Interventions and Complications (EDIC) studies highlight the need for early diagnosis and treatment of diabetes for the prevention of long-term complications. For patients at high risk for development of T2DM, performance of an oral glucose tolerance test is important for detection of an impairment in early phase insulin release, a condition which is always present in type 2 diabetic patients, and occurs early in the development of the disease (46). Unfortunately, depending solely on fasting blood glucose measurement for the diagnosis of diabetes may miss individuals who have isolated postprandial hyperglycaemia but a normal fasting plasma glucose (47). Prospective studies have shown that this abnormality is not only common but it doubles the mortality risk (47, 48).
Patients diagnosed with T2DM require close monitoring and intensified therapy when appropriate. It has been demonstrated that nonfasting plasma glucose is a better indicator of overall glycaemic control than fasting glucose in these patients (49), and an recent study established the utility of casual postprandial glucose measurements in determining the appropriate time for intensified therapy (50). Casual postprandial plasma glucose (cPPG) was defined simply as the plasma glucose concentration determined 1–4 h after consumption of a meal, regardless of the meal size or composition (50). A cutoff cPPG value of >150 mg/dL had a positive predictive value of 88% for an HbA1c level >6.5% (50). While acquisition of an HbA1c level is still recommended for a thorough analysis, the 150 mg/dL cutoff for cPPG can provide a convenient indicator of the need for intensified therapy in type 2 diabetic patients when home blood glucose monitoring records or current HbA1c levels are not available (50).
Pharmacological Therapy
Effective treatment of patients with T2DM requires the combination of diet, exercise, oral agents, incretin hormones and insulin and should be aimed at maintaining blood glucose levels as close to normal as possible, while minimising blood glucose fluctuations and hypoglycaemia. There are five classes of oral agents currently available: sulphonylureas; biguanides (metformin); α-glucosidase inhibitors (acarbose, miglitol); thiazolidinediones (pioglitazone, rosiglitazone); and meglitinides (nateglinide, repaglinide). With the exception of the α-glucosidase inhibitors and the meglitinides, the majority of the oral antihyperglycaemic agents act by lowering fasting plasma glucose levels via increased insulin secretion or increased insulin sensitivity (51). The meglitinides are rapid-acting insulin secretagogues and are therefore targeted to control postprandial glucose excursions. Similarly, α-glucosidase inhibitors lower postprandial glucose spikes by inhibiting the enzymatic break down of carbohydrates in the small intestine, thereby slowing carbohydrate absorption and blunting the increase in plasma glucose. Reviews of oral agent therapy, treatment algorithms and guidelines for dosing have previously been published (51–53).
Because T2DM is a disease characterised by the progressive loss of pancreatic β-cell function, most patients will eventually require insulin replacement therapy (54). Unfortunately, clinicians often wait too long to initiate insulin therapy, resulting in excessive glycaemic exposure that may go unchecked for months or even years (55). The guidelines for glycaemic control published by the American Diabetes Association (ADA) and American College of Endocrinology (ACE) are helpful in determining which patients should be started on insulin. The goals for glycaemic control recommended by ADA include an HbA1c level <7%, a fasting plasma glucose (FPG) concentration of 90–130 mg/dL, and a peak postprandial plasma glucose (PPG)(1–2 h after meal) concentration <180 mg/dL (56). The comparable values from ACE are an HbA1c = 6.5%, a FPG concentration <110 mg/dL and a 2-h PPG concentration <140 mg/dL (57).
The ideal insulin regimen is the basal-bolus regimen (long- or intermediate-acting basal insulin in combination with rapid-acting insulin analogues at each meal), because it closely mimics the normal physiologic insulin profile. However, basal-bolus therapy requires a great deal of motivation on the part of both the patient and the physician and necessitates thorough training in food/insulin matching and insulin adjustment (58). This level of complexity has led to the widespread use of therapies based only on basal insulin which, although simple to initiate, only treat the fasting blood glucose and fail to correct the postprandial blood glucose excursions and which, as described above, may lead to increased oxidative stress. Therefore, insulin therapies aimed at improving blood glucose should target both postprandial and fasting glycaemia. A twice-daily regimen using a premixed preparation of rapid-acting insulin analogs is a good alternative for patients who are reluctant to start a basal-bolus regimen initially (58). A comprehensive, yet practical guide to determining the most appropriate time for initiating insulin therapy and the most effective insulin regimen was recently published by Hirsch et al. (58).
Conclusions
Poorly controlled blood glucose levels can lead to numerous pathological conditions that ultimately result in long-term microvascular and macrovascular complications. It is now understood that a mechanism underlying hyperglycaemia-induced effects on inflammation and vascular dysfunction is the action of ROS within the cell nucleus. This action initiates a cascade of transcription events, ultimately leading to changes in the levels of NO, cytokines, acute-phase reactants and cellular adhesion molecules. Generation of ROS can be reduced by avoiding hyperglycaemia and by minimising fluctuations in blood glucose levels. A major contributor to these fluctuations in blood glucose is postprandial glycaemia. Furthermore, the earlier in the progression of T2DM that tight control of blood glucose can be achieved, the greater will be the reduction in long-term complications. Therefore, we propose that it is of utmost importance to diagnose patients at risk for developing T2DM as early as possible and to aggressively treat those already diagnosed by appropriately treating both fasting and postprandial glycaemia.
Acknowledgments
The authors thank Dr. Antonio Ceriello, University of Udine, Italy, for his scientific guidance and critical review of this paper. We also thank Ms. Heather Fox, Eli Lilly and Company, Indiana, for her editorial assistance in preparation of this manuscript.
Disclosures
Dr. Eugene Wright has served on advisory panels and received consulting fees or honoraria from Eli Lilly and Company, Amylin Pharmaceuticals and Eyetel. Dr. Jamie Scism-Bacon is an employee of Eli Lilly and Company and owns stock in Lilly. Dr. Leonard Glass is an employee of Eli Lilly and Company and owns stock in Lilly. Eli Lilly and Company manufactures pharmaceutical products for the treatment of diabetes.
References
- 1.International Diabetes Federation. Diabetes e-Atlas. [July 14 2005]; Available at: http://www.eatlas.idf.org.
- 2.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–23. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 4.Maddux BA, See W, Lawrence JC, et al. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes. 2001;50:404–10. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 5.Hirsh IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178–81. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Hirsh IB. Intensifying insulin therapy in patients with type 2 diabetes mellitus. Am J Med. 2005;118(Suppl. 5A):21S–6S. doi: 10.1016/j.amjmed.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial (DCCT) Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–83. [PubMed] [Google Scholar]
- 8.Quagliaro L, Piconi L, Assalone R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-Oxidase activation. Diabetes. 2003;52:2795–804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 9.Schiekofer S, Andrassy M, Chen J, et al. Acute hyperglycemia causes intracellular formation of CML and activation of ras, p42/44 MAPK, and nuclear factor ?B in PBMCs. Diabetes. 2003;52:621–33. doi: 10.2337/diabetes.52.3.621. [DOI] [PubMed] [Google Scholar]
- 10.Jones SC, Saunders HJ, Qi W, et al. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia. 1999;42:1113–9. doi: 10.1007/s001250051279. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A, Quagliaro L, Catone B, et al. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25:1439–43. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- 12.Santilli F, Cipollone F, Mezzetti A, et al. The role of nitric oxide in the development of diabetic angiopathy. Horm Metab Res. 2004;36:319–35. doi: 10.1055/s-2004-814489. [DOI] [PubMed] [Google Scholar]
- 13.Giuglian D, Marfella R, Coppola L, et al. Vascular effects of acute hyperglycemia in humans are reversed by 1-arginine: evidence for reduced availability of nitric oxide during hyperglycemia. Circulation. 1997;95:1783–90. doi: 10.1161/01.cir.95.7.1783. [DOI] [PubMed] [Google Scholar]
- 14.Du XL, Edelstein D, Dimmeler S, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 16.Spitaler MM, Graier WF. Vascular targets of redox signaling in diabetes mellitus. Diabetologia. 2002;45:476–94. doi: 10.1007/s00125-002-0782-0. [DOI] [PubMed] [Google Scholar]
- 17.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 18.Binder CJ, Chang M-K, Shaw PX, et al. Innate and acquired immunity in atherosclerosis. Nat Med. 2002;8:1218–26. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 19.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 20.Basta G, Schmidt AM, DeCaterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–92. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation endproducts of proteins. J Biol Chem. 1992;267:14998–5004. [PubMed] [Google Scholar]
- 22.Brett J, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–712. [PMC free article] [PubMed] [Google Scholar]
- 23.Wendt T, Bucciarelli L, Qu W, et al. Receptor for Advanced Glycation Endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep. 2002;4:228–37. doi: 10.1007/s11883-002-0024-4. [DOI] [PubMed] [Google Scholar]
- 24.Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–97. [PubMed] [Google Scholar]
- 25.Wautier MP, Chappey O, Corda S, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 26.Basta G, Lazzerini G, Massaro M, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–22. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 27.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 28.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 29.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 30.Ceriello A, Quagliaro L, Piconi L, et al. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004;53:701–10. doi: 10.2337/diabetes.53.3.701. [DOI] [PubMed] [Google Scholar]
- 31.Marfella R, Esposito K, Giunta R, et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101:2247–51. doi: 10.1161/01.cir.101.19.2247. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins AJ, Rowley KG, Lyons TJ, et al. Lipoproteins and diabetic microvascular complications. Curr Pharm Des. 2004;10:3395–418. doi: 10.2174/1381612043383188. [DOI] [PubMed] [Google Scholar]
- 33.Lyons TJ, Klein RL, Baynes JW, et al. Stimulation of cholesteryl ester synthesis in human monocyte-derived macrophages by low-density lipoproteins from type 1 (insulin-dependent) diabetic patients: the influence of non-enzymatic glycosylation of low-density lipoproteins. Diabetologia. 1987;30:916–23. doi: 10.1007/BF00295874. [DOI] [PubMed] [Google Scholar]
- 34.Njoroge FG, Monnier VM. The chemistry of the Maillard reaction under physiological conditions: a review. Prog Clin Biol Res. 1989;304:85–107. [PubMed] [Google Scholar]
- 35.Basta G, Schmidt AM, DeCaterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–92. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med. 2000;28:1708–16. doi: 10.1016/s0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins AJ, Best JD, Klein RL, Lyons TJ. Lipoproteins, glycoxidation and diabetic angiopathy. Diabetes Metab Res Rev. 2004;20:349–68. doi: 10.1002/dmrr.491. [DOI] [PubMed] [Google Scholar]
- 38.Lopes-Virella MF, Klein RL, Lyons TJ, et al. Glycosylation of low-density lipoprotein enhances cholesteryl ester synthesis in human monocyte-derived macrophages. Diabetes. 1988;37:550–7. doi: 10.2337/diab.37.5.550. [DOI] [PubMed] [Google Scholar]
- 39.Ren S, Shen GX. Impact of antioxidants and HDL on glycated LDL-induced generation of fibrinolytic regulators from vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1688–93. doi: 10.1161/01.atv.20.6.1688. [DOI] [PubMed] [Google Scholar]
- 40.Januszewski AS, Alderson NL, Metz TO, et al. Role of lipids in chemical modification of proteins and development of complications in diabetes. Biochem Soc Trans. 2003;31:1413–6. doi: 10.1042/bst0311413. [DOI] [PubMed] [Google Scholar]
- 41.Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997;96:25–8. doi: 10.1161/01.cir.96.1.25. [DOI] [PubMed] [Google Scholar]
- 42.Esterbauer H, Gebicki J, Puhl H, Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–90. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 43.Spiteller G. Linoleic acid peroxidation – the dominant lipid peroxidation process in low density lipoprotein – and its relationship to chronic diseases. Chem Phys Lipids. 1998;95:105–62. doi: 10.1016/s0009-3084(98)00091-7. [DOI] [PubMed] [Google Scholar]
- 44.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 45.Fu MX, Requena JR, Jenkins AJ, et al. The advanced glycation end product, Nepsilon-(carboxymethyl) lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–6. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 46.Polonsky KS, Sturis J, Bell GI. Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin-dependent diabetes mellitus – a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med. 1996;334:777–83. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 47.DECODE Study Group, European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and ADA diagnostic criteria. Diabetes epidemiology collaborative analysis of diagnostic criteria in Europe. Lancet. 1999;354:617–21. [PubMed] [Google Scholar]
- 48.Shaw JE, Hodge AM, DeCourten M, et al. Isolated postchallenge hyperglycemia confirmed as a risk factor for mortality. Pacific and Indian Ocean study. Diabetologia. 1999;42:1050–4. doi: 10.1007/s001250051269. [DOI] [PubMed] [Google Scholar]
- 49.Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care. 1997;20:1822–6. doi: 10.2337/diacare.20.12.1822. [DOI] [PubMed] [Google Scholar]
- 50.El-Kebbi IM, Ziemer DC, Cook CB, et al. Utility of casual postprandial glucose levels in type 2 diabetes management. Diabetes Care. 2004;27:335–9. doi: 10.2337/diacare.27.2.335. [DOI] [PubMed] [Google Scholar]
- 51.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 52.Bell DSH. Type 2 diabetes mellitus: what is the optimal treatment regimen? Am J Med. 2004;116:23S–29S. doi: 10.1016/j.amjmed.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 53.The American Association of Clinical Endocrinologists. Medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management – 2002 update. Endocr Pract. 2002;8(Suppl. 1):S40–S82. [PubMed] [Google Scholar]
- 54.Maedler K, Donath MY. Cells in type 2 diabetes: a loss of function and mass. Horm Res. 2004;62(Suppl. 3):67–73. doi: 10.1159/000080503. [DOI] [PubMed] [Google Scholar]
- 55.Nathan DM. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342–9. doi: 10.1056/NEJMcp021106. [DOI] [PubMed] [Google Scholar]
- 56.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(Suppl. 1):S4–S36. [PubMed] [Google Scholar]
- 57.American College of Endocrinology Consensus Statement on Guidelines for Glycemic Control. Endocr Pract. 2002;8(Suppl. 1):S5–S11. [Google Scholar]
- 58.Hirsch IB, Bergenstal RM, Parkin CG, et al. A real-world approach to insulin therapy in primary care practice. Clin Diabetes. 2005;23:78–86. [Google Scholar]