Summary
Risk factors for invasive pneumococcal disease (IPD) include young and old age, comorbidities (such as splenic dysfunction, immunodeficiencies, chronic renal disease, chronic heart or lung disease or cerebral spinal fluid leak), crowded environments or poor socioeconomic conditions. Universal use of the 7-valent pneumococcal conjugate (7vPncCRM) vaccine for infants and young children has led to significant decreases in IPD in the vaccinated population (direct protection), and there has also been a decrease in the incidence of IPD among the nonvaccinated population (indirect immunity; herd protection). While 7vPncCRM vaccine is administered universally to children in USA, many countries of the European Union have chosen to target children with comorbidities. This review aims to highlight individual risk factors for IPD, describe studies that evaluated pneumococcal conjugate vaccines in at-risk groups and estimate the proportion of at-risk children who may have been vaccinated in the European Union since the 7vPncCRM vaccine was introduced, using UK as an example. Although immunisation targeting only children with comorbidities may achieve satisfactory results for a few, many otherwise healthy children at risk simply because of their age will be neglected, and herd protection might not be established.
Keywords: Pneumococcal disease, risk factors, conjugate vaccine, herd protection, immunisation programmes, USA, UK, European Union, epidemiology, infant
Introduction
Streptococcus pneumoniae (pneumococcus) is a leading cause of bacterial illnesses among children throughout the world, responsible for invasive pneumococcal disease (IPD) (e.g. meningitis, sepsis, bacteraemic pneumonia and bacteraemia) and non-IPD (e.g. pneumonia, acute otitis media and sinusitis). A 20-year (1980–1999) surveillance study conducted in Nottingham, UK, for instance, found that the mean annual incidence of IPD was 47.1 per 100,000 in infants younger than 1 year (1). The expansion in recent years of pneumococcal strains with diminished sensitivity to antibiotics has complicated the treatment of IPD.
The 23-valent pneumococcal polysaccharide (23vPncPS) vaccines, available since the 1980s, are licensed only for at-risk individuals older than 2 years of age. With the advent of the pneumococcal conjugate vaccine, the focus of disease management for children below 2 years of age has shifted to prevention. In spite of this advance in vaccinology, from the polysaccharide to the conjugate vaccine, the concept that pneumococcal vaccination would only be meant for at-risk individuals has persisted in the minds of many public health officials and practitioners.
Children younger than 2 years are one of the highest risk groups for IPD; therefore, a 7-valent pneumococcal conjugate (7vPncCRM) vaccine was developed that protects against the important paediatric serotypes, 4, 6B, 9V, 14, 18C, 19F and 23F. Although young age is a major risk factor for IPD, factors such as crowded living conditions and lower socioeconomic status may further increase the likelihood of developing pneumococcal disease (2) among otherwise healthy children.
Two 7vPncCRM vaccine immunisation approaches have been utilised to date: universal vaccination of all infants and children, or vaccination targeted to certain vulnerable groups. The USA adopted the former approach in August 2000, when the Advisory Committee on Immunization Practices (Centers for Disease Control and Prevention) recommended vaccination for every infant and child younger than 2 years of age and also recommended catch-up vaccination targeted for all children 2–5 years of age with particular comorbidities (e.g. sickle cell anaemia, splenic dysfunction, human immunodeficiency virus (HIV) infection, chronic disease or an immuno-compromising condition). Furthermore, US authorities underscored that vaccination also should be considered for all other children 2–5 years of age, with priority given to those who are 2–3 years of age, those of African-American, Native American or Alaskan native descent or those who attend out-of-home childcare.
By contrast, the countries in the European Union have, to date, adopted an approach targeted uniquely towards at-risk children. The UK approach, for instance, is based on the January 2002 recommendations of the Joint Committee on Vaccination and Immunisation, which were subsequently broadened in August 2004 to include additional comorbid conditions.
The goal of this article is to compare the two approaches: universal vaccination of all infants and children and targeted vaccination for certain at-risk groups. Specifically, we seek to i) highlight the risk factors for IPD; and ii) describe studies that have evaluated pneumococcal conjugate vaccines in at-risk groups. Using UK as an example, we have tried to estimate the proportion of at-risk infants and young children who may have been vaccinated between the introduction of 7vPncCRM vaccine and the broadening of recommendations in August 2004.
Risk factors for ipd
Predisposing factors, such as genetic factors, comorbidities or other pathologies, may place an individual at risk of developing IPD. Multiple genetic factors, for instance, are almost certainly associated with an individual's risk of IPD, and some recognised phenotypic bases for risk include hypogammaglobulinaemia, impaired opsonophagocytosis activity, complement defects, or poor splenic clearance of intravascular bacteria. Anatomic abnormalities (e.g. skull fracture/cerebrospinal fluid leak, cochlear implant or congenital heart disease), immunosuppressive therapy, bone marrow and solid organ transplantation, chronic disease (pulmonary, neurological or hepatic), diabetes mellitus and renal conditions (renal insufficiency or nephrotic syndrome) are other pathologies that increase risk (Table 1). The possibility of developing IPD is further complicated by the impact of socioeconomic factors, perinatal factors and age – individuals younger than 2 years or older than 65 years are particularly at risk (Table 1) (3–10).
Table 1.
Some risk factors for invasive pneumococcal disease (IPD)
| Condition | Incidence/Risk | Reference |
|---|---|---|
| <2 years of age | 34.3/100,000 | USA population (3) |
| ≥65 years of age | 41.6/100,000 | |
| Group day care (defined as spending≥4 h/week with ≥2 unrelatedchildren under adult supervision) | Two to threefold greater risk | Vs. children not in group day care (4) |
| Low birth weight | 2.6-fold greater risk | Vs. normal birth weight (5) |
| Pre-term <38 weeks gestation | 1.6-fold greater risk | Vs. full term (5) |
| HIV-positive/AIDS | 6100 cases/100,000 | HIV-infected children <7 years (6) |
| 11,300 cases/100,000 | HIV-infected children <3 years (6) | |
| Sickle-cell disease | 5500–6500 cases/100,000 | Children with sickle cell disease <5 years (6) |
| Socioeconomic factors | Rate of IPD >threefold greater | Canadian Aboriginals vs. Canadiannon-Aboriginals (7, 8) |
| Pneumococcal bacteraemia and pneumococcal meningitis rates are >fourfold greater | Alaskan Native children <5 years comparedwith non-Alaskan Native/non-Native American children (9) | |
| IPD rates are 1.6-fold greater | African-American children <2 years comparedwith white children (10) |
Nonetheless, most children hospitalised for IPD do not belong to any recognised at-risk group. In a US surveillance study, only 27% of hospitalised children had an underlying condition (11), while Canadian, French, Spanish and Finnish studies found that 23.2% (12), 16.7% (13), 10% (14) and 16% (15) of hospitalised children, respectively, had underlying conditions.
Risk-based recommendations throughout europe
In the European Union, the 7vPncCRM vaccine is licensed for children from 2 months to 5 years of age. The national recommendations, however, for the use of 7vPncCRM vaccine vary by country (Table 2). In Germany, Italy, Spain and UK, for instance, the vaccine is currently only recommended for high-risk individuals.
Table 2.
Recommendations for 7-valent pneumococcal conjugate vaccine use in individuals by selected countries of Europe (reviewed January 2006)
| Risk-based recommendations | AUT | BEL | DEU* | DNK | ESP | FIN | FRA | GBR† | GRC | IRL | ISL | ITA‡ | LUX | NLD | NOR | PRT | SWE§ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medical comorbidities | |||||||||||||||||
| Splenic dysfunction** | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Immunodeficiency †† | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓‡‡ | ✓ | |||||||
| Chronic disease§§ | ✓ | ✓ | ✓¶¶ | ✓ | ✓¶¶ | ✓¶¶ | ✓ | ✓ | ✓¶¶ | ✓¶¶ | |||||||
| CSF leak | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Diabetes mellitus | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Cochlear implant | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Previous IPD infection | ✓ | ✓ | ✓ | ||||||||||||||
| ‘Any other at-risk pathology’ | ✓ | ✓ | |||||||||||||||
| Predisposing conditions | |||||||||||||||||
| Developmental delay | ✓ | ✓ | |||||||||||||||
| Failure to thrive | ✓ | ✓ | |||||||||||||||
| Premature | ✓ | ✓ | |||||||||||||||
| Low birth weight | ✓ | ✓ | |||||||||||||||
| Other considerations | |||||||||||||||||
| Day care | ✓*** | ✓ | |||||||||||||||
| Family with >2 children | ✓ | ||||||||||||||||
| Breast feeding <2 months | ✓ | ||||||||||||||||
| Age-based policy (children <5 years old) – private physicians | ✓††† | ✓††† | ✓††† | ✓††† | ✓††† | ✓††† | ✓††† | ✓††† | ✓††† | ✓‡‡‡ | ✓††† | ✓††† | ✓††† | ||||
| National (regional) immunisation programs | ✓ | ✓ | (✓) | ✓ | ✓ | (✓) | ✓ | ✓ | |||||||||
AUT, Austria; BEL, Belgium; DEU, Germany; DNK, Denmark; ESP, Spain; FIN, Finland; FRA, France; GBR, United Kingdom; GRC, Greece; IRL, Ireland; ISL, Iceland; ITA, Italy; LUX, Luxembourg; NLD, Netherlands; PRT, Portugal; SWE, Sweden.
The German STIKO extends the risk-based recommendations to children <5 years of age. (July 2005).
Universal recommendations are anticipated in 2006 in Great Britain.
Three regions have universal recommendations (Liguria, Sicily and Puglia), five have risk-based vaccination programs (Veneto, Lombardia, Lazio, Toscana and Friuli) and one has extended vaccination program (active offer) to day-care subjects (Emilia-Romagna). National Vaccines Committee has approved, in principle, universal recommendations from National Authority, through an offer to all children <24 months of age and a three-dose schedule for newborns.
One region (Örebro) has issued risk-based recommendations.
Including causes of splenic dysfunction such as homozygous sickle cell disease, thallassaemia, asplenia or coeliac disease.
Primary or secondary immunodeficiency (e.g. malignancy such as lymphoma, Hodgkin's disease or leukaemia, immunosuppressive therapy, transplantation or HIV/AIDS. Immunosuppressive therapy is defined in GBR as on or likely to be on systemic steroids for more than a month at a dose equivalent to prednisolone at 20 mg or more per day, any age or for children under 20 kg at a dose of 1 mg or more per kg per day).
Excluding chronic granulomatous disease.
Renal disease including nephrotic syndrome, chronic renal failure, renal transplantation, chronic heart disease such as congenital heart disease or heart failure, lung disease, liver disease including cirrhosis, or neurological disease.
Excluding asthma, apart from asthmas under chronic corticosteroid therapy. [In GBR, ‘chronic lung disease’ includes asthma requiring continuous or repeated use of inhaled or systemic steroids (or with previous exacerbations requiring hospital admission) and includes children who have previously been admitted to hospital for lower respiratory tract infection].
Defined as ‘children kept for more than 4 h/week with more than two children not counting siblings’.
By private physicians, nationwide.
By private physicians, particular regions of the country.
In most countries, the schedule for paediatric 7vPncCRM vaccine vaccination is a three-dose primary series followed by a booster dose in the second year of life. There are exceptions. In UK, a three-dose primary series only is recommended (i.e. no booster). In the Nordic countries and in Italy, the standard paediatric vaccine regimen consists of a two-dose primary series and a booster dose at about 12 months of age.
Efficacy of pneumococcal conjugate vaccines in at-risk groups
Safety and immunogenicity studies in at-risk groups aim to demonstrate that the immune response to a pneumococcal conjugate vaccine is comparable to that seen in healthy subjects, for which vaccine efficacy may have been demonstrated. Some of the groups studied include children with sickle cell disease, HIV-infected adults and children, adults with Hodgkin's disease or patients undergoing bone marrow transplantation.
The immunologic response in children with sickle cell disease is at least as substantial as that found in healthy patients (16). Moreover, toddlers first immunised with a 9-valent pneumococcal conjugate vaccine candidate followed by a 23vPncPS vaccine had significantly higher antibody levels for the serotypes tested than those vaccinated with the 23vPncPS vaccine alone (17). In children 2–6 years of age with sickle cell disease who were previously given the 23vPncPS vaccine, higher antibody concentrations were achieved with 7vPncCRM vaccine compared with no subsequent conjugate vaccine dose (18). It has also been shown that one conjugate vaccine dose can prime toddlers for immunologic memory, indicating that 7vPncCRM vaccine may confer protection after one dose in children 2–5 years of age (19).
The immunogenicity of the 7vPncCRM vaccine has also been demonstrated in HIV-infected adults (20) and children (21, 22), in adults with Hodgkin's disease (23) and in patients undergoing bone marrow transplantation (24). In one study of HIV-infected infants, 7vPncCRM vaccine induced geometric mean concentrations of serotype-specific serum antibody to levels >0.15 µg/ml in 95% of HIV-infected infants. Although concentrations declined at 24 months, they remained well above preimmunisation levels (25). In adults with Hodgkin's disease, priming with a 7vPncOMPC vaccine candidate resulted in a significant increase in antibody concentration values after a subsequent dose of 23vPncPS vaccine compared with nonprimed individuals (23). In patients undergoing bone-marrow transplantation, post-transplantation antibody responses after the first two vaccine doses were greater among patients whose donors had initially received 7vPncCRM vaccine. By the third dose, >60% of patients had antibody concentrations for each vaccine serotype considered by these investigators to indicate protection (i.e. ≥0.5 µg/ml) (24).
The immune response in individuals who are nonresponders to polysaccharide vaccine has also been shown to be comparable to that seen in healthy subjects. In children and adolescents with recurrent infections, two doses of 7vPncCRM vaccine resulted in a successful vaccination (defined in this study as postvaccination titres of >1 µg/ml for ≥5 of 7 serotypes) in 50% of patients; 80% responded to ≥2 serotypes (26). In a separate study, 7vPncCRM vaccine elicited only low responses in the recurrent infections patient group; nevertheless, antibody levels were superior to those observed with the 23vPncPS vaccine (27).
In contrast to these safety and immunogenicity studies, there are a few efficacy studies in at-risk individuals. Studies conducted to date show that 7vPncCRM vaccine is highly efficacious in low birth weight and premature infants and is protective for children of the Navajo Nation in North America (5, 28). Furthermore, in USA, where vaccination with 7vPncCRM vaccine is recommended for every infant and child younger than 2 years, and catch-up programmes are targeted towards at-risk children younger than 5 years, the racial disparity in IPD incidence, of children younger than 2 years, between African-Americans and whites has decreased from 3.3-fold to 1.6-fold (8). Protection from IPD has also been reported in children in the Republic of South Africa with HIV infection (29).
ESTIMATED USE OF 7vPncCRM VACCINE IN AT-RISK CHILDREN IN UK
The number of distributed doses was obtained from UK Wyeth sales records. It was assumed that all 7vPncCRM vaccine administered in UK up to the issue of the broadened recommendations in August 2004 had been given to at-risk infants and children younger than 2 years, and that they had only received, on average, two doses. (For an unvaccinated English child aged 6–12 months, only two doses are necessary, while after the first year only one dose is indicated). The estimates of the number of infants and children in UK at risk for IPD January 2002 to August 2004 were obtained by speaking with senior paediatric specialists in each clinical area (gastro, haem, cardio, CF, liver, renal, neonatal, HIV) and from their knowledge of the number of patients in their own clinics and similar clinics throughout UK, estimating numbers for UK. The numbers are likely to be underestimates.
The estimated number of infants and young children in UK who belonged to one of the groups identified to be at increased risk for IPD during the period January 2002 to August 2004 is summarised in Table 3. Excluding patients for whome exact prevalence data were unavailable (i.e. diabetes, bone marrow transplantation, primary immunodeficiency or splenectomy), there were an estimated 4000 infants and young children in UK who were eligible for 7vPncCRM vaccine in the first 2.5 years after its recommendation. Over that period, 14,800 doses of 7vPncCRM vaccine were sold in UK. Although it is not certain that all the doses were used for this purpose (e.g. some doses may have been administered to healthy children or used in clinical trials), the number of doses distributed suggests that a large proportion of eligible patients may have been immunised.
Table 3.
Estimated number of infants and young children in UK at risk for invasive pneumococcal disease (IPD) during the period January 2002 to August 2004
| Condition | Number |
|---|---|
| Coeliac disease | 1400 |
| Sickle cell disease | 625 |
| Chronic cardiac disease | 500 |
| Cystic fibrosis | 400 |
| Chronic liver disease | 400 |
| Pre-end stage renal failure | 300 |
| Chronic lung disease of prematurity | 200 |
| Nephrotic syndrome | 140 |
| End-stage renal failure | 75 |
| HIV infection | 40* |
| Total | 4080 |
Estimate based on 400 infants and children in UK with HIV in total, approximately 10% of whom are younger than 5 years of age. The number of infants and young children in UK at risk for IPD due to diabetes, bone mar transplantation, primary immunodeficiency, or splenectomy could not be estimated.
IMPACT OF 7vPncCRM VACCINE ON US POPULATION
Clinical evaluation of 7vPncCRM vaccine for prevention of IPD was obtained in a large-scale trial from October 1995 to April 1999 involving 37,868 healthy infants in the Northern California Kaiser Permanente (NCKP) population (30). In this trial, the vaccine was 97% efficacious in the prevention of IPD caused by 7vPncCRM vaccine serotypes in children vaccinated with at least three doses, and 94% efficacious in children receiving at least one dose of the vaccine. Furthermore, the vaccine was 89% effective in reducing all IPD, regardless of serotype. A postlicensure analysis of 7vPncCRM vaccine that included the entire NCKP population (vaccinated and nonvaccinated) showed that from April 2002 to March 2003, no cases of vaccine serotype disease were seen in children younger than 1 year, which compares favourably with a former incidence ranging from 51.5 to 98.2 cases per 100,000 person-years in the years before vaccine licensure. Additionally, there was no evidence of a concomitant increase in IPD caused by nonvaccine serotypes (31), although close surveillance continues.
Pneumococcal conjugate vaccines reduce pneumococcal nasopharyngeal carriage among vaccinated individuals, which can decrease the likelihood of transmission to unvaccinated persons, with less risk of infection (referred to as indirect immunity or herd protection). In a community-randomised, 7vPncCRM vaccine trial among Native Americans (28), nasopharyngeal carriage was studied in family members living in the same household as a child vaccinated with the 7vPncCRM vaccine (32). Although adult family members of vaccinated children had the same overall pneumococcal nasopharyngeal rate as adult family members of nonvaccinated children, adult family members of vaccinated children had a significantly lower carriage rate of vaccine-type pneumococci (p = 0.02). Similarly, nonvaccinated children in the same household as vaccinated children were less likely to carry a vaccine-type strain than those living with nonvaccinated children (relative risk = 0.8; 95% confidence interval 0.7, 1.0) (32).
Recently, there has been further evidence that universal immunisation of children younger than 2 years of age may positively affect nonvaccinated populations. According to Centers for Disease Control and Prevention's Active Bacterial Core Surveillance system, statistically significant reductions in IPD incidence are observed in adults 20–39 years of age and in elderly adults >65 years of age (31, 33).
The future
Immunogenicity trials in individuals with HIV, sickle cell disease or Hodgkin's disease, as well as in haematopoietic cell transplant recipients, show that the 7vPncCRM vaccine is both safe and appropriately immunogenic; however, further research may be needed in these and other high-risk groups to establish the optimum schedule for the 7vPncCRM vaccine. Moreover, several specific actions can be taken, both at the level of the individual and in the general paediatric population, to improve protection for at-risk groups. At the individual level, systemic and mucosal immune responses to pneumococci might be enhanced using an adjuvant, such as interleukin-12 (34) or LT-K63 (35), or administering the vaccine by the mucosal route. In addition, practical considerations should be implemented for children at elevated individual risk of infection (i.e. anatomic defects, immunosuppressive conditions or comorbidities), such as increasing the number of outpatient visits for preventive care or improving the provision of prophylactic antibiotics (36).
The diversity of recommendations for administration of 7vPncCRM vaccine to at-risk groups in countries of the European Union is detailed in Table 2, and the lack of consistency seems to reflect uncertainty among national authorities as to who exactly is at risk. Clearly, the paediatric group at highest risk for IPD includes all children younger than 2 years of age; however, most of these children are not classified as at-risk and therefore currently remain unprotected. As the identification of at-risk groups remains problematic, measures implemented in the general paediatric population can benefit both identified and unidentified at-risk groups.
An effective means of protecting at-risk individuals is to reduce the chance of transmission by decreasing pneumococcal nasopharyngeal carriage in the general paediatric population. Thus, universal vaccination of children younger than 2 years protects immunised children and, by means of herd protection, protects non-immunised individuals who might have contact with them. Extensive data support the safety, immunogenicity and efficacy of 7vPncCRM vaccine against IPD in infants and young children (30), and the most recent studies indicate substantial indirect beneficial effects from universal vaccination of all children younger than 2 years (28, 31, 33). In USA, where a universal recommendation is established, postlicensure data show that the incidence of disease has decreased on a nationwide level (31, 33), among the vaccinated and the nonvaccinated, in small groups identified to be at greater risk, as well as in members of the general population who do not belong to one of these specific groups. This is in contrast with the targeted approach used currently in the European Union, where unrecognised at-risk individuals, as well as unvaccinated members of the general population, remain vulnerable to pneumococcal disease. Consequently, universal vaccination as a consistent approach across the European Union may be helpful to obtain the benefits of herd protection.
Conclusions
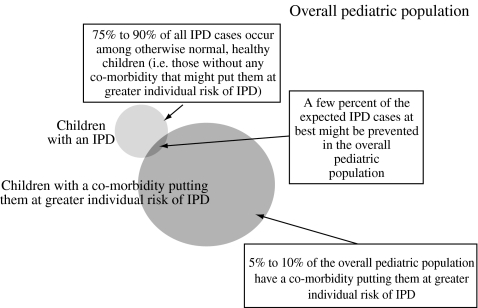
There are at least three arguments favouring universal vaccination. First, herd protection (for unvaccinated infants, parents and grandparents) cannot be achieved without broad vaccine coverage. Second, it is impossible to identify most of the infants who will be ‘at risk’. At the age when an infant would receive 7vPncCRM vaccination series to ensure protective immunity (children 6–24 months of age), few children can be identified as belonging to an at-risk group. Third, a small percentage of hospitalised IPD cases will be avoided by a targeted approach. Only about 5–10 percent of the overall paediatric population has a comorbidity that health authorities may recognise as a risk factor that is important enough for IPD that 7vPncCRM vaccination is recommended. Consequently, a targeted vaccination approach might lower the rate of IPD among the overall paediatric population by no more than a few percent (Figure 1).
Figure 1.
Illustration representing proportion of children with invasive pneumococcal disease (IPD) who have a risk factor
The identification of high-risk individuals is often difficult, and vaccination programmes that target only certain subpopulations will miss individuals who would develop pneumococcal disease. Based on the success of the US experience, universal vaccination appears to be the most effective in protecting all children, who are at risk simply because of their young age.
References
- 1.Ispahani P, Slack RC, Donald FE, et al. Twenty year surveillance of invasive pneumococcal disease in Nottingham: serogroups responsible and implications for immunisation. Arch Dis Child. 2004;89:757–62. doi: 10.1136/adc.2003.036921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melegaro A, Gay NJ, Medley GF. Estimating the transmission parameters of pneumococcal carriage in households. Epidemiol Infect. 2004;132:433–41. doi: 10.1017/s0950268804001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) report emerging infections program network Streptococcus pneumoniae. [October 15 2004]; Available at: http://www.cdc.gov/ncidod/dbmd/abcs/reports.htm.
- 4.Levine OS, Farley M, Harrison LH, et al. Risk factors for invasive pneumococcal disease in children: a population-based case-control study in North America. Pediatrics. 1999;103:E28. doi: 10.1542/peds.103.3.e28. [DOI] [PubMed] [Google Scholar]
- 5.Shinefield H, Black S, Ray P, et al. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr Infect Dis J. 2002;21:182–6. doi: 10.1097/00006454-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Overturf GD, American Academy of Pediatrics Committee on Infections Diseases Technical report: prevention of pneumococcal infections, including the use of pneumococcal conjugate and polysaccharide vaccines and antibiotic prophylaxis. Pediatrics. 2000;106:367–76. doi: 10.1542/peds.106.2.367. [DOI] [PubMed] [Google Scholar]
- 7.National Advisory Committee on Immunization. Statement on recommended use of pneumococcal conjugate vaccine. Can Commun Dis Rep. 2002;28:1–32. [PubMed] [Google Scholar]
- 8.Flannery B, Schrag S, Bennett NM, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA. 2004;291:2197–203. doi: 10.1001/jama.291.18.2197. [DOI] [PubMed] [Google Scholar]
- 9.Marrie TJ. Streptococcus pneumoniae is the most common cause of community-acquired pneumonia in North American aboriginals in Canada. 2002. p. 29. 3rd International Symposium on Pneumococci and Pneumococcal Disease Program and Abstracts Book.
- 10.Van Beneden CA, Whitney CG, Levine OS. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2000;49:1. [PubMed] [Google Scholar]
- 11.Kaplan SL, Mason EO, Jr, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics. 2004;113:443–9. doi: 10.1542/peds.113.3.443. [DOI] [PubMed] [Google Scholar]
- 12.Bjornson GL, Scheifele DW, Halperin SA. Population-based epidemiology of invasive pneumococcal infection in children in nine urban centers in Canada, 1994 through 1998. Pediatr Infect Dis J. 2002;21:947–50. doi: 10.1097/00006454-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Laurichesse H, Romaszko JP, Nguyen LT, et al. Clinical characteristics and outcome of patients with invasive pneumococcal disease, Puy-de-Dome, France, 1994–1998. Eur J Clin Microbiol Infect Dis. 2001;20:299–308. doi: 10.1007/pl00011269. [DOI] [PubMed] [Google Scholar]
- 14.Perez A, Sala P, Gimenez M, et al. Pneumococcal bacteremia in children: an 8-year review in two hospitals in Barcelona. Eur J Clin Microbiol Infect Dis. 2004;23:677–81. doi: 10.1007/s10096-004-1197-2. [DOI] [PubMed] [Google Scholar]
- 15.Eskola J, Takala AK, Kela E, et al. Epidemiology of invasive pneumococcal infections in children in Finland. JAMA. 1992;268:3323–7. [PubMed] [Google Scholar]
- 16.O'Brien KL, Swift AJ, Winkelstein JA, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM(197) among infants with sickle cell disease. Pneumococcal Conjugate Vaccine Study Group. Pediatrics. 2000;106:965–72. doi: 10.1542/peds.106.5.965. [DOI] [PubMed] [Google Scholar]
- 17.Goldblatt D, Akoto OY, Ashton L, et al. Immunogenicity and the generation of immune memory following 9-valent pneumococcal conjugate vaccination in Ghanaian infants with sickle cell disease. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000; Abstract 245 presented at the. [Google Scholar]
- 18.Alexander EM, Telfer P, Goldblatt D, et al. Immunogenicity of pneumococcal conjugate vaccine in children with sickle cell disorder. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 30 October – 2 November 2004; Washington DC, USA. Abstract G-529 presented at the. [Google Scholar]
- 19.Goldblatt D, Akoto OY, Ashton L, et al. Does one dose of pneumococcal conjugate vaccine induce immunological memory in toddlers?. 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000; Abstract 235 presented at the. [Google Scholar]
- 20.Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine. 2001;20:545–53. doi: 10.1016/s0264-410x(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 21.King JC, Vink PE, Chang I, et al. Antibody titers eight months after three doses of a five-valent pneumococcal conjugate vaccine in HIV and non-HIV-infected children less than two years of age. Vaccine. 1998;16:361–5. doi: 10.1016/s0264-410x(97)80914-0. [DOI] [PubMed] [Google Scholar]
- 22.King JC, Vink PE, Farley JJ, et al. Safety and immunogenicity of three doses of a five-valent pneumococcal conjugate vaccine in children younger than two years with and without human immunodeficiency virus infection. Pediatrics. 1997;99:575–80. doi: 10.1542/peds.99.4.575. [DOI] [PubMed] [Google Scholar]
- 23.Chan CY, Molrine DC, George S, et al. Pneumococcal conjugate vaccine primes for antibody responses to polysaccharide pneumococcal vaccine after treatment of Hodgkin's disease. J Infect Dis. 1996;173:256–8. doi: 10.1093/infdis/173.1.256. [DOI] [PubMed] [Google Scholar]
- 24.Molrine DC, Antin JH, Guinan EC, et al. Donor immunization with pneumococcal conjugate vaccine and early protective antibody responses following allogeneic hematopoietic cell transplantation. Blood. 2003;101:831–6. doi: 10.1182/blood-2002-03-0832. [DOI] [PubMed] [Google Scholar]
- 25.Nachman S, Kim S, King J, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants with human immunodeficiency virus type 1 infection. Pediatrics. 2003;112:66–73. doi: 10.1542/peds.112.1.66. [DOI] [PubMed] [Google Scholar]
- 26.Zielen S, Buhring I, Strnad N, et al. Immunogenicity and tolerance of a 7-valent pneumococcal conjugate vaccine in nonresponders to the 23-valent pneumococcal vaccine. Infect Immun. 2000;68:1435–40. doi: 10.1128/iai.68.3.1435-1440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen RU, Leiva LE, Giangrosso PA, et al. Response to a heptavalent conjugate Streptococcus pneumoniae vaccine in children with recurrent infections who are unresponsive to the polysaccharide vaccine. Pediatr Infect Dis J. 1998;17:685–91. doi: 10.1097/00006454-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362:355–61. doi: 10.1016/S0140-6736(03)14022-6. [DOI] [PubMed] [Google Scholar]
- 29.Klugman KP, Madhi SA, Huebner RE, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 30.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485–9. doi: 10.1097/01.inf.0000129685.04847.94. [DOI] [PubMed] [Google Scholar]
- 32.Watt JP, O'Brien KL, Bronsdon MA, et al. Impact of pneumococcal conjugate (PnCRM7) vaccination of children on pneumococcal carriage in adult family members. 2002. pp. 23–4. 3rd International Symposium on Pneumococci and Pneumococcal Disease Program and Abstracts Book.
- 33.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 34.Lynch JM, Briles DE, Metzger DW. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect Immun. 2003;71:4780–8. doi: 10.1128/IAI.71.8.4780-4788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakobsen H, Jonsdottir I. Mucosal vaccination against encapsulated respiratory bacteria-new potentials for conjugate vaccines? Scand J Immunol. 2003;58:119–28. doi: 10.1046/j.1365-3083.2003.01292.x. [DOI] [PubMed] [Google Scholar]
- 36.Sox CM, Cooper WO, Koepsell TD, et al. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA. 2003;290:1057–61. doi: 10.1001/jama.290.8.1057. [DOI] [PubMed] [Google Scholar]