Summary
Developments in acute stroke therapy have followed advances in the understanding of the evolving pathophysiology in both ischaemic stroke and intracerebral haemorrhage (ICH). In ischaemic stroke, rapid reperfusion of the ischaemic penumbra with thrombolysis within 3 h of symptom onset is of proven benefit, but few patients currently receive therapy, mainly due to the short-time window and lack of stroke expertise. In ICH, a recent study indicated that a haemostatic agent can limit ongoing bleeding and improve outcomes when administered within 4 h of stroke onset. These advances in acute stroke therapy underlie the concept that ‘time is brain’ and that urgent intervention can limit cerebral damage. Neuroprotective therapy could offer the prospect of a greater proportion of stroke patients receiving treatment, potentially before imaging and even in the ambulance setting. Virtually all stroke patients would benefit from receiving multidisciplinary care in acute stroke units.
Keywords: Ischaemic stroke, emergency, neuroprotection, multidisciplinary
Introduction
Stroke is a medical emergency, with a mortality rate higher than most forms of cancer. It is the second leading cause of death in developed countries (1) and is the most common cause of serious, long-term disability in adults (2). In addition to the impact on patients and families, there are major economic consequences (2, 3): the total cost of stroke in the USA is estimated to exceed US $56 billion in 2005 (4). In addition, the incidence of stroke is increasing with the ageing of populations and is therefore a major challenge to health planners (5).
Important evidence-based advances in acute stroke have included proof of the benefit of organised care in stroke units, modern brain imaging, thrombolytic therapy, the modest benefit of acute aspirin in ischaemic stroke and the potential use of haemostatic therapy with recombinant factor VIIa (rFVIIa) in intracerebral haemorrhage (ICH). Currently, intravenous recombinant tissue plasminogen activator (rt-PA) is the only pharmacological treatment licensed for treatment of acute ischaemic stroke in most Western countries, but the uptake of rt-PA has been disappointingly low, and >95% of patients with ischaemic stroke do not receive any specific pharmacological therapy (6–9). Of particular concern is that, while ≤15% of ischaemic stroke patients receive rt-PA in some well-organised stroke centres (8), <2–3% of patients receive such treatment in community hospitals (7). Why are the majority of ischaemic stroke patients unable to access acute therapy? Clearly, a lack of awareness of the common symptoms of stroke remains a major educational challenge, and the urgency of stroke treatment is still poorly appreciated. Despite the proven benefit of stroke units, the majority of patients in most countries cannot access specialised stroke care. In this paper, we review current treatment guidelines and new therapeutic prospects, emphasising the importance of early intervention and the need for a multidisciplinary approach to the management of stroke patients.
Pathophysiology of Acute Stroke Poses an Emergency
The two main developments underlying therapeutic advances in stroke are the delineation of the ischaemic penumbra in ischaemic stroke and the observation of haematoma growth in ICH.
Ischaemic Stroke
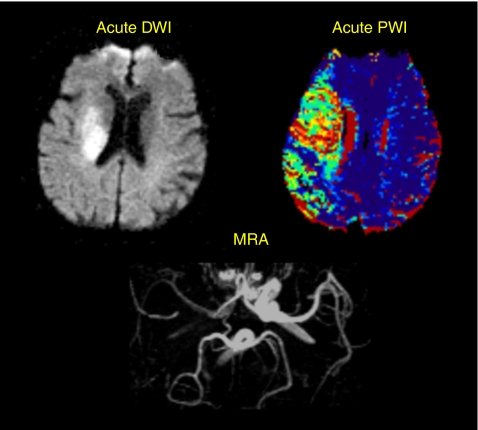
The severity of an acute ischaemic stroke depends on the degree of impairment of cerebral blood flow and the time to reperfusion. As the ischaemic process evolves, most commonly due to thromboembolic arterial occlusion, there is a progressive decrease in cerebral blood flow. When this falls from normal levels of approximately 50 to <10 ml/100 g/min, neuronal cell death rapidly occurs. However, between the ischaemic core and the normally perfused brain at the periphery lies the ischaemic penumbra, a zone of moderately reduced cerebral blood flow, dependent on the proximal arterial occlusion and collateral supply (Fig. 1). Within the ischaemic penumbra, the neurones are hypoxic, functionally inactive, but still viable, and this is the region targeted by acute stroke therapies. The penumbra is a dynamic, time-based region in which brain tissue will undergo necrosis over hours to days due to perfusion failure and a secondary cascade of damaging biochemical events.
Figure 1.
Patient with an acute right middle cerebral territory infarct, demonstrating a large ischaemic penumbra perfusion-weighted imaging (PWI) (perfusion lesion) > diffusion-weighted imaging (DWI) (diffusion lesion). The magnetic resonance angiogram (MRA) shows an occluded right middle cerebral artery. The penumbra represents threatened tissue, which is a target for acute stroke therapies such as thrombolysis
These neurotoxic processes include release of glutamate, activation of N-methyl-d-aspartate (NMDA) and other cell receptors, influx of sodium and calcium into cells, release of free-radical species and, ultimately, cellular destruction. The critical time for effective reperfusion, based on magnetic resonance-imaging studies, may be around 4.5 h, with earlier restoration leading to greater tissue salvage (10). However, the therapeutic time windows in ischaemia remain uncertain. Later, pathophysiological processes include inflammatory reactions and free-radical release (11). A limited therapeutic window of opportunity underlies the concept that ‘time is brain’, and current approaches to therapy are aimed at limiting stroke damage and improving functional outcomes.
ICH
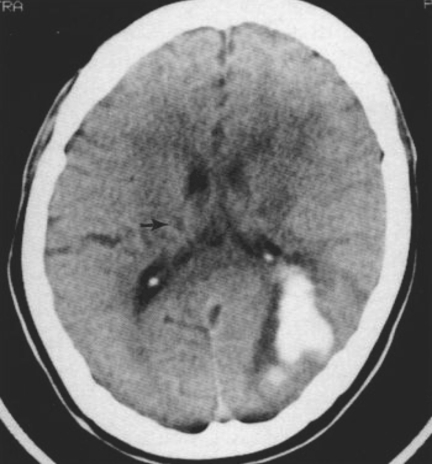
More recent studies have shown that ICH is also a dynamic process and potentially amenable to therapeutic intervention. A prospective study in ICH showed that 38% of patients exhibited substantial haematoma growth (greater than one-third increase in volume) if imaged with computerised tomography (CT) within 3 h of onset of stroke and repeated 24 h later (12) (Fig. 2). Most of this growth (26%) occurred within 1 h of the first scan. This expansion is probably due to continued bleeding or re-bleeding. This observation led to the hypothesis that haemostatic therapy could reduce the volume of the haematoma and result in improved outcomes (13).
Figure 2.
Computerised tomography scan of haemorrhagic transformation
Diagnosis and Treatment of Acute Ischaemic Stroke
Successful care of acute stroke patients relies on a four-step process: (i) prompt recognition and reaction to warning signs; (ii) immediate use of emergency services; (iii) priority transport with notification of the receiving hospital and (iv) rapid and accurate diagnosis and intervention at the hospital (14, 15). This ‘chain of recovery’ has also been described as a five-stage process, comprising the five Rs of successful stroke management: recognition (of symptoms), reaction (emergency services are called), response (medical assessment), reveal (brain imaging) and Rx (treatment initiation) (16).
Emergency Department Assessment
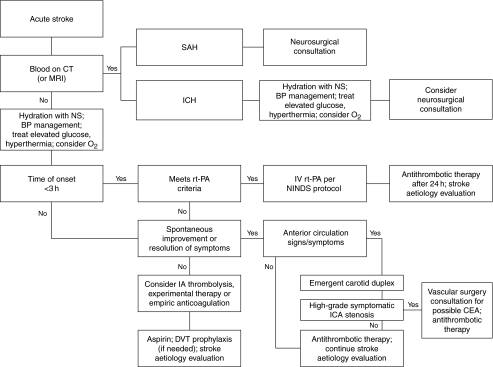
Once a diagnosis of acute ischaemic stroke is suspected, the duration since symptom onset should be determined as accurately as possible, as time from onset is the single most important determinant of therapeutic options. Patients arriving at hospital with a symptom onset of <3 h should be evaluated for potential treatment with rt-PA, although a ‘door to needle time’ of around 60 min usually means a hospital arrival time within 2 h for rt-PA candidates. A treatment algorithm is shown in Fig. 3 (2).
Figure 3.
Algorithm for the management of acute stroke [adapted with permission from Zweifler (2)]. BP, blood pressure, CEA, carotid endarterectomy, CT, computerised tomography; DVT, deep vein thrombosis; IA, intra-arterial; ICA, internal carotid artery; ICH, intracerebral haemorrhage, IV, intravenous; MRI, magnetic resonance imaging; NINDS, National Institute of Neurological Disorders and Stroke; NS, normal saline, rt-PA, recombinant tissue plasminogen activator; SAH, subarachnoid haemorrhage
Patients with acute stroke should be investigated with an emergency CT brain scan, to differentiate ischaemic stroke from ICH and other pathological processes such as subdural haematoma, tumour and abscess (2). Other emergency investigations should include blood glucose, electrolytes, full blood examination and an electrocardiogram.
Stroke Unit Management
Rapid triage of patients to a stroke unit has been shown to reduce mortality by ≥20% (17) and improve functional outcomes (18), reducing disability and the need for institutional care compared with treatment in a general medical ward (14). A stroke unit is a geographically localised treatment facility involving a multidisciplinary stroke team of medical, nursing and allied health staff, incorporating best-practice stroke treatment protocols (14, 19, 20). Adherence to guidelines reduces costs with shorter hospital stays (21). However, access to stroke unit care remains a problem, even in countries with well-developed stroke systems (22–25).
Stroke units should use integrated care pathways, shown to reduce the risk of complications (20). An Australian study correlated improved outcomes in stroke units compared with general medical wards with adherence to processes of care (26). Stroke units can be supervised by a neurologist or a general physician with stroke training and expertise (27, 28).
Physiological monitoring is an important aspect of stroke unit management. Hyperglycaemia and fever should be avoided or treated vigorously, as they are independent adverse prognostic factors. Most stroke patients are hypertensive on admission, but blood pressure often spontaneously subsides. The management of acute hypertension is controversial, and randomised clinical trials are being performed to resolve this uncertainty (29).
Optimal management of acute stroke patients should include continuous respiratory and cardiac monitoring, fluid and metabolic replacement (usually intravenous normal saline), and treatment of hyperglycaemia, seizures and fever. Prophylaxis for complications such as deep vein thrombosis, pulmonary embolism, aspiration pneumonia and other infections are also essential (2). Antiembolic stockings should be routine, and low-dose heparin/heparinoid is widely recommended (19).
Antiplatelet therapy with aspirin is advised in ischaemic stroke unless there are contraindications or the patient is being treated with rt-PA (15). For every 1000 patients treated with acute aspirin, about nine deaths or nonfatal strokes are prevented (30, 31). Full anticoagulation is now rarely advocated for most patients in the acute stage, based on results from a series of negative trials (32). Heparin is frequently recommended for patients with cerebral vein thrombosis, arterial dissection and small infarcts, where there is a very high risk of recurrence. However, it should be emphasised that adequately powered randomised trials have not been performed for these indications.
Thrombolysis
Randomised trials of intravenous rt-PA in acute ischaemic stroke (33–38) and subsequent meta-analyses (32, 39, 40) have demonstrated an overall benefit for rt-PA treatment, with a significantly increased probability of excellent recovery and no increase in mortality rate (32). Overall, the rate of symptomatic haemorrhagic transformation is increased threefold, but this is greatly outweighed by the therapeutic benefits.
Phase IV studies have subsequently established the relative safety of rt-PA in appropriate settings (41). The Canadian Alteplase for Stroke Effectiveness study (42) found that the rate of symptomatic ICH was low (4.6%) and that the 3-month outcomes were comparable with the National Institute of Neurological Disorders and Stroke (NINDS) study data (42, 43). Centres in Germany, the USA and Australia have published similar findings (44–46). Stroke guidelines therefore recommend the use of rt-PA in carefully selected patients within 3 h of the onset of ischaemic stroke (14, 19).
Optimal treatment outcomes with rt-PA are seen in specialist stroke centres. Failure to follow the NINDS guidelines and protocol violations (9) can lead to an increased risk of haemorrhage and a poorer patient prognosis (47). Hence, rapid referral of eligible patients to a specialist stroke centre should be facilitated (48), where there is expertise in neuroimaging, stroke evaluation and treatment in a co-ordinated stroke unit (19).
As mentioned previously, use of rt-PA remains disappointingly low in most countries. This reflects a number of factors, including not only the short time window, the relative lack of expertise and stroke emergency systems, and also professional pessimism. A vocal minority of emergency department physicians have argued that additional trial evidence is required (41, 49), but most experts, including European regulatory authorities, acknowledge that it is time to explore extended indications for rt-PA in stroke. For example, the European licensing approval of rt-PA for acute ischaemic stroke within 3 h of symptom onset after prior exclusion of intracranial haemorrhage stipulated that a randomised placebo-controlled trial of alteplase 3–4.5 h after symptom onset (ECASS III) be undertaken and that every treated patient must be entered onto a postmarketing registry (SITS-MOST). ECASS-III is a randomised trial to investigate the safety and efficacy of rt-PA when administered between 3 and 4 h after symptom onset (50).
Treatment of ICH
ICH is less common than ischaemic stroke (15 vs. 85% in most Western populations) but is associated with substantially worse outcomes. Mortality rates approach 50%, and there is little effective treatment. A recent trial of more than 1000 patients failed to demonstrate any benefit of early surgery for supratentorial ICH (51). In this trial, the overall mortality rate was 64%, and only about 25% of patients had a favourable outcome. The role of surgical evacuation is controversial, although there is a consensus for surgery in selected cases of cerebellar ICH.
The finding of haematoma growth in a significant proportion of patients with ICH led to the trials of the haemostatic agent rFVIIa. In a dose-ranging, proof-of-concept trial (52), haematoma growth was attenuated by nearly 50%. This was associated with a 40% reduction in mortality in the pooled rFVIIa-treated groups and improved functional outcomes. There was a small increase in myocardial and cerebral ischaemic events with rFVIIa, but this was substantially outweighed by the benefits. Based on these results, a second confirmatory trial is underway.
Optimal Practice and Drawbacks of Current Practices
Failure to recognise the stroke symptoms is an important challenge. Up to 70% of patients having a stroke are unaware of it, because of lack of knowledge of symptoms or because they are asleep during onset (53). Furthermore, family members and/or carers do not always recognise the common signs of stroke, compounded by a lack of general awareness of the urgency of the condition. This underlies the need for public awareness campaigns to increase knowledge of stroke symptoms and facilitate patients being able to access urgent treatment.
Immediate ambulance transfer to a stroke centre should be facilitated (14). Accurate diagnosis remains a challenge in many settings, emphasising the importance of professional education. Rapid triage and prioritisation of stroke patients require the development of effective stroke-management systems (14).
Future Opportunities
Despite these important advances in stroke treatment, it is clear that there is an urgent need for more widely applicable pharmacological therapy with a wider therapeutic window and an improved safety profile.
Reperfusion Approaches
Beyond intravenous rt-PA, other reperfusion strategies in various stages of clinical trial evaluation include intra-arterial thrombolytic therapy, new thrombolytic agents such as desmoteplase, intravenous antiplatelet therapies such as abciximab, enhancement of thrombolysis with ultrasound and mechanical clot retrieval devices such as the recently licensed MERCI Retriever device (54) (Table 1). The benefits of reperfusion therapies always have to be balanced against the increased risk of bleeding complications. Ultrasound-enhanced thrombolysis was promising in one study (55) but associated with increased bleeding complications in another (56). An abciximab trial has been halted due to a high rate of bleeding complications.
Table 1.
Reperfusion strategies for the treatment of acute ischaemic stroke
| Strategy | Agent | Comments |
|---|---|---|
| Antithrombotic agents | Heparin | Heparin is widely used in acute stroke, but no randomised clinical trials support its use |
| Tinzaparin Low molecular weight heparin | Ongoing study of eptifibatide in combination with aspirin, tinzaparin and standard alteplase therapy | |
| Antiplatelet agents | Aspirin | Two trials showed a small (∼1%) but significant effect with early aspirin use in acute stroke (30, 31) |
| Abciximab | Ongoing Phase III studies | |
| Thrombolytics | rt-PA | Approved |
| Ongoing studies to investigate extension of treatment window to 6 h, intra-arterial administration and as combination therapy(e.g. with ultrasound) | ||
| Pro-urokinase | PROACT III trial planned | |
| Desmoteplase | Dose-ranging Phase III trial planned | |
| Urokinase | MELT ongoing in Japan | |
| Mechanical clot retrieval device | MERCI Retriever | Licensed |
| In a study of 141 patients, efficacy appeared similar to that of rt-PA (54) |
rt-PA, recombinant tissue plasminogen activator; PROACT, prolyse in acute cerebral thromboembolism; MELT, MCA-Embolism Local fibrinolytic intervention Trial.
Ongoing clinical trials using rt-PA are aimed at prolonging the currently restrictive 3-h window. These include ECASS III (rt-PA vs. placebo 3–4 h after stroke onset) (50) and IST 3 (rt-PA vs. placebo 0–6 h after onset) (57). To date, there has only been one Phase III trial of intra-arterial thrombolysis. The Prolyse in Acute Cerebral Thromboembolism trial used a 6-h time window and focused on patients with middle cerebral artery (MCA)-territory infarction (58). The trial was positive, but a confirmatory trial is needed. Intra-arterial thrombolytic therapy is used in expert centres in highly selected patients, such as those with basilar artery thrombosis, where substantial Phase II evidence suggests a correlation between recanalisation and reduced mortality (59). However, one study indicated equally good results from intravenous thrombolytic therapy (60).
There are also several magnetic resonance imaging-based trials using combined perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI), aimed at determining whether PWI > DWI mismatch, as a signature of the ischaemic penumbra, can be used to select treatment responders beyond 3 h (61). Intravenous desmoteplase was shown to enhance reperfusion with associated clinical benefits up to 9 h in a recent study (62).
Neuroprotective Strategies
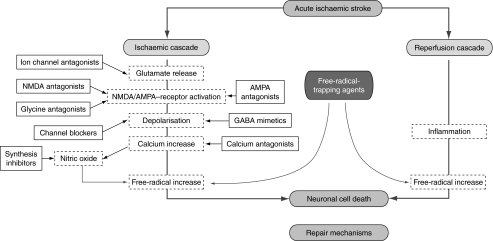
Ideally, acute therapy for ischaemic stroke would not require a preliminary CT scan so that therapy could potentially be administered by paramedical personnel in the prehospital setting. A safe therapy would also facilitate treatment outside of specialist stroke centres. An optimal neuroprotective strategy should be administrable intravenously, have a longer window of opportunity than rt-PA, be well tolerated and improve functional recovery after stroke. The proposed sites of action of various neuroprotective strategies within the ischaemic cascade are illustrated in Fig. 4.
Figure 4.
Schematic showing sites of action of neuroprotectants in the ischaemic cascade. AMPA, α-amino-3-hydroxy-methyl-4-isoxazolyl-propionic acid; GABA, γ-amino-butyric acid; NMDA, N-methyl-d-aspartate
To date, a large number of neuroprotective strategies have proved effective in animal models, but have generally failed translation to the clinical setting. However, the field has been revitalised with the positive results of the first trial of NXY-059 in acute ischemic stroke. Possible reasons for the many failed trials include difficulty with more complex and heterogeneous human stroke compared with the animal models, dosing issues with poor penetration of neuroprotective drugs into the ischaemic penumbra, relatively long therapeutic time windows used in many neuroprotective trials, adverse effects, inadequate sample size and other trial design issues. To improve the prospects of translational success, the Stroke Therapy Academic Industry Roundtable (STAIR) devised recommendations for clinical development of acute ischaemic stroke therapies. These criteria included human trials of agents that have been proven effective in more than one animal model, improved trial design with adequate sample sizes and valid outcome measures that are easy to measure, reproducible, valid, clinically meaningful and resistant to bias (63, 64).
Several novel neuroprotective compounds are currently in clinical development. These include NXY-059 (free-radical-trapping agent), DP-b99 (calcium chelator), magnesium (NMDA channel blocker) (65), ONO-2506 (astrocyte-modulating agent) (66), traxoprodil (NR2B-selective NMDA-receptor antagonist) (67) and citicoline (membrane stabiliser) (68).
Of these compounds, NXY-059 was the first to meet the STAIR criteria and is the most advanced in development (69, 70). NXY-059 is a novel free-radical-trapping neuroprotectant that limits infarct size and improves functional outcomes in animal models (69–71). Preclinical studies showed that NXY-059 reduces infarct size in both transient (72, 73) and permanent (73, 74) MCA occlusion (MCAO) stroke models in the rat. Furthermore, when administered 4 h after permanent MCAO in a primate model, NXY-059 produced improvements in motor paresis and spatial neglect (75).
NXY-059 has been shown to be well tolerated in stroke patients at doses that are neuroprotective in both transient and permanent MCAO models in animals (76, 77). A pharmacokinetic study of NXY-059 in acute stroke patients (78) showed that an initial loading dose, followed by a maintenance infusion (individualised by creatinine clearance), resulted in the early achievement of target plasma concentrations. NXY-059 is being evaluated in two Phase III Stroke Acute Ischaemic NXY-059 Treatment trials, one of which has been completed. The results of this trial of NXY-059 in 1699 patients showed that administration of this drug within 6 hours of the onset of ischemic stroke significantly improved the primary outcome, namely disability at 90 days. The drug also had an excellent safety profile. Although neurological functioning was not improved using a prespecified measure of change in the National Institutes of Health Stroke Scale (NIHSS), there were encouraging trends to benefit in the proportion of patients with excellent neurological recovery and in other outcome functional measures. In a further analysis, co-administration of NXY-059 and tPA was associated with a reduced rate of hemorrhagic transformation. A second pivotal trial of NXY-059 is well underway. Confirmation of the results of the initial trial should lead to availability of a treatment that could be widely applied and safely used alone or in patients co-treated with thrombolysis. These trial designs were based on the STAIR criteria (64). In addition, the Cerebral Hemorrhage and NXY Treatment (CHANT) trial is a double-blind, randomised trial aimed at assessing the safety and tolerability of intravenous infusion of NXY-059 in ICH.
ONO-2506 progressed to clinical development after demonstrating neuroprotective effects in rodent and primate models. ONO-2506 was shown to reduce infarct expansion in a rat model up to 48 h after permanent MCAO (80). When administered 6 h after permanent MCAO in a primate model (cynomolgus monkeys), ONO-2506 significantly reduced neurological symptoms up to 14 days after the onset of ischaemia (81). However, a Phase II study in the USA involving approximately 1300 patients was recently halted because of futility in an interim analysis (82). A Phase II/III study is currently ongoing in Japan.
Although intravenous magnesium sulphate was not effective within 12 h in a large Phase III trial (83), pilot studies in the USA have shown the feasibility of an ambulance-based therapy for stroke (84). A large Phase III trial is now underway with a 2-h time window, involving prehospital therapy (FAST-MAG) (85). There also remains a possibility that later administration of magnesium may be of benefit in lacunar stroke (83).
Conclusions
Stroke is a clinical emergency requiring urgent medical intervention. Thrombolytic therapy with rt-PA is available for the treatment of acute ischaemic stroke, but its use is currently suboptimal. Recent advances in the organisation of acute stroke care, improved understanding of the evolving pathophysiology of both acute ischaemic stroke and ICH, together with advances in acute pharmacological therapy including neuroprotective approaches, are changing the way that acute stroke is managed around the world. Further advances in drug therapy are likely to occur in the next few years, with the potential for prehospital treatment of stroke patients. All of these treatment advances are based on immediate intervention, underlining the urgency of stroke recognition and treatment.
Acknowledgments
We thank Michaela Cain from Complete Medical Communications, who provided editing assistance on behalf of AstraZeneca.
References
- 1.World Health Organization. The World Heath Report. Geneva: the World Health Organization; 2004. The World Health Report 2004. [Google Scholar]
- 2.Zweifler RM. Management of acute stroke. South Med J. 2003;96:380–5. doi: 10.1097/01.SMJ.0000063467.75456.7A. [DOI] [PubMed] [Google Scholar]
- 3.Holmqvist LW, von Koch L, de Pedro-Cuesta J. Use of healthcare, impact on family caregivers and patient satisfaction of rehabilitation at home after stroke in southwest Stockholm. Scand J Rehabil Med. 2000;32:173–9. doi: 10.1080/003655000750060922. [DOI] [PubMed] [Google Scholar]
- 4.American Heart Association. Dallas, Texas: American Heart Association; 2005. Heart Disease and Stroke Statistics – 2005 update. [Google Scholar]
- 5.Payne KA, Huybrechts KF, Caro JJ, Craig Green TJ, Klittich WS. Long term cost-of-illness in stroke. An international review. Pharmacoeconomics. 2002;20:813–25. doi: 10.2165/00019053-200220120-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Brott T, Caplan L, et al. Thrombolysis in acute ischemic stroke: controlled trials and clinical experience. Neurology. 1999;53(Suppl. 4):S3–S14. [PubMed] [Google Scholar]
- 7.Katzan IL, Furlan AJ, Lloyd LE, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA. 2000;283:1151–8. doi: 10.1001/jama.283.9.1151. [DOI] [PubMed] [Google Scholar]
- 8.Grotta JC, Burgin WS, El-Mitwalli A, et al. Intravenous tissue-type plasminogen activator therapy for ischemic stroke. Houston experience 1996 to 2000. Arch Neurol. 2001;58:2009–13. doi: 10.1001/archneur.58.12.2009. [DOI] [PubMed] [Google Scholar]
- 9.Furlan AJ, Katzan IL, Caplan LR. Thrombolytic therapy in acute ischemic stroke. Curr Treat Options Cardiovasc Med. 2003;5:171–80. doi: 10.1007/s11936-003-0001-4. [DOI] [PubMed] [Google Scholar]
- 10.Butcher K, Parsons M, Baird T, et al. Perfusion thresholds in acute stroke thrombolysis. Stroke. 2003;34:2159–64. doi: 10.1161/01.STR.0000086529.83878.A2. [DOI] [PubMed] [Google Scholar]
- 11.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 12.Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Mayer SA. Ultra-early hemostatic therapy for intracerebral hemorrhage. Stroke. 2003;34:224–9. doi: 10.1161/01.str.0000046458.67968.e4. [DOI] [PubMed] [Google Scholar]
- 14.The European Stroke Initiative Executive Committee and the EUSI Writing Committee. European stroke initiative recommendations for stroke management – update 2003. Cerebrovasc Dis. 2003;16:311–37. doi: 10.1159/000072554. [DOI] [PubMed] [Google Scholar]
- 15.EUSI Executive Committee and EUSI Writing Committee. European stroke initiative recommendations 2003: Ischaemic stroke: prophylaxis and treatment. Information for Doctors in Hospital and in Practice. 2003.
- 16.Kennedy J, Ma C, Buchan AM. Organization of regional and local stroke resources: methods to expedite acute management of stroke. Curr Neurol Neurosci Rep. 2004;4:13–8. doi: 10.1007/s11910-004-0005-9. [DOI] [PubMed] [Google Scholar]
- 17.Stroke Unit Trialists' Collaboration. Collaborative systematic review of the randomised trials of organised inpatient (stroke unit) care after stroke. BMJ. 1997;314:1151–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Stroke Unit Trialists' Collaboration. How do stroke units improve patient outcomes? A collaborative systematic review of the randomized trials. Stroke. 1997;28:2139–44. doi: 10.1161/01.str.28.11.2139. [DOI] [PubMed] [Google Scholar]
- 19.Adams HP, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke: a scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–83. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 20.Kwan J, Hand P, Sandercock P. Improving the efficiency of delivery of thrombolysis for acute stroke: a systematic review. Q J Med. 2004;97:273–9. doi: 10.1093/qjmed/hch054. [DOI] [PubMed] [Google Scholar]
- 21.Quaglini S, Cavallini A, Gerzeli S, Micieli G, GLADIS Study Group Economic benefit from clinical practice guideline compliance in stroke patient management. Health Policy. 2004;69:305–15. doi: 10.1016/j.healthpol.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Negri M, Martignoni A, Baccheschi J, Santilli G, Marchesi E. [Management of stroke in a ward of internal medicine. Limits and Prospects] Recenti Prog Med. 2004;95:137–43. [PubMed] [Google Scholar]
- 23.Kapral MK, Laupacis A, Phillips SJ, et al. Stroke care delivery in institutions participating in the Registry of the Canadian Stroke Network. Stroke. 2004;35:1756–62. doi: 10.1161/01.STR.0000130423.50191.9f. [DOI] [PubMed] [Google Scholar]
- 24.Rundek T, Nielsen K, Phillips S, et al. Health care resource use after acute stroke in the Glycine Antagonist in Neuroprotection (GAIN) Americas trial. Stroke. 2004;35:1368–74. doi: 10.1161/01.STR.0000127084.26321.7a. [DOI] [PubMed] [Google Scholar]
- 25.Kwan J, Hand P, Sandercock P. A systematic review of barriers to delivery of thrombolysis for acute stroke. Age Ageing. 2004;33:116–21. doi: 10.1093/ageing/afh064. [DOI] [PubMed] [Google Scholar]
- 26.Cadilhac DA, Ibrahim J, Pearce DC, et al. Multicenter comparison of processes of care between stroke units and conventional care wards in Australia. Stroke. 2004;35:1035–40. doi: 10.1161/01.STR.0000125709.17337.5d. [DOI] [PubMed] [Google Scholar]
- 27.Lees K. Stroke is best managed by a neurologist: battle of the titans. Stroke. 2003;34:2764–5. doi: 10.1161/01.STR.0000098001.55467.D3. [DOI] [PubMed] [Google Scholar]
- 28.Donnan G, Davis SM. Neurologist, internist, or strokologist? Stroke. 2003;34:2765. doi: 10.1161/01.STR.0000098002.30955.9D. [DOI] [PubMed] [Google Scholar]
- 29.Bath P. High blood pressure as risk factor and prognostic predictor in acute ischaemic stroke. when and how to treat it? Cerebrovasc Dis. 2004;17(Suppl. 1):51–7. doi: 10.1159/000074795. [DOI] [PubMed] [Google Scholar]
- 30.CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo-controlled trial of early aspirin use in 20 000 patients with acute ischaemic stroke. Lancet. 1997;349:1641–9. [PubMed] [Google Scholar]
- 31.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19 435 patients with acute ischaemic stroke. Lancet. 1997;349:1569–81. [PubMed] [Google Scholar]
- 32.Wardlaw JM, del Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. The Cochrane Database of Systematic Reviews. 2003;3 doi: 10.1002/14651858.CD000213. CD000213. 10.1002/14651858.CD000213. [DOI] [PubMed] [Google Scholar]
- 33.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–25. [PubMed] [Google Scholar]
- 34.NINDS rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 35.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–51. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 36.Clark WM, Wissman S, Albers GW, et al. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3–5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. JAMA. 1999;282:2019–26. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 37.Clark WM, Albers GW, Madden KP, Hamilton S for the Thrombolytic Therapy in Acute Ischemic Stroke Study Investigators. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Stroke. 2000;31:811–6. doi: 10.1161/01.str.31.4.811. [DOI] [PubMed] [Google Scholar]
- 38.The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group. Effect of intravenous recombinant tissue plasminogen activator on ischemic stroke lesion size measured by computed tomography. Stroke. 2000;31:2912–9. doi: 10.1161/01.str.31.12.2912. [DOI] [PubMed] [Google Scholar]
- 39.Wardlaw JM, Sandercock PAG, Warlow CP, Lindley RI. Trials of thrombolysis in acute ischemic stroke: Does the choice of primary outcome measure really matter? Stroke. 2000;31:1133–5. doi: 10.1161/01.str.31.5.1133. [DOI] [PubMed] [Google Scholar]
- 40.Wardlaw JM, Sandercock PA, Berge E. Thrombolytic therapy with recombinant tissue plasminogen activator for acute ischemic stroke. Where do we go from here? A cumulative meta-analysis. Stroke. 2003;34:1437–42. doi: 10.1161/01.STR.0000072513.72262.7E. [DOI] [PubMed] [Google Scholar]
- 41.Graham GD. Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta-analysis of safety data. Stroke. 2003;34:2847–50. doi: 10.1161/01.STR.0000101752.23813.C3. [DOI] [PubMed] [Google Scholar]
- 42.Hill MD, Buchan AM for the Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators: Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ. 2005;172:1307–12. doi: 10.1503/cmaj.1041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gladstone DJ, Black SE. Update on intravenous tissue plasminogen activator for acute stroke: from clinical trials to clinical practice. CMAJ. 2001;165:311–7. [PMC free article] [PubMed] [Google Scholar]
- 44.Chiu D, Krieger D, Villar-Cordova C, et al. Intravenous tissue plasminogen activator for acute ischemic stroke: feasibility, safety, and efficacy in the first year of clinical practice. Stroke. 1998;29:18–22. doi: 10.1161/01.str.29.1.18. [DOI] [PubMed] [Google Scholar]
- 45.Schmulling S, Grond M, Rudolf J, Heiss W-D. One-year follow-up in acute stroke patients treated with rtPA in clinical routine. Stroke. 2000;31:1552–4. doi: 10.1161/01.str.31.7.1552. [DOI] [PubMed] [Google Scholar]
- 46.Szoeke CEI, Parsons MW, Butcher KS, et al. Acute stroke thrombolysis with intravenous tissue plasminogen activator in an Australian tertiary hospital. Med J Aust. 2003;178:324–8. doi: 10.5694/j.1326-5377.2003.tb05223.x. [DOI] [PubMed] [Google Scholar]
- 47.Bravata DM, Kim N, Concato J, Krumholz HM, Brass LM. Thrombolysis for acute stroke in routine clinical practice. Arch Intern Med. 2002;162:1994–2001. doi: 10.1001/archinte.162.17.1994. [DOI] [PubMed] [Google Scholar]
- 48.Grond M, Stenzel C, Schmulling S, et al. Early intravenous thrombolysis for acute ischemic stroke in a community-based approach. Stroke. 1998;29:1544–9. doi: 10.1161/01.str.29.8.1544. [DOI] [PubMed] [Google Scholar]
- 49.Goyal DG, Li J, Mann J, Schriger DL. Position statement on the use of intravenous thrombolytic therapy in the treatment of stroke, 2005. Am Acad Emerg Med. 2004.
- 50.Hacke W, Kaste M, Fieschi C, et al. A placebo controlled trial of aletplase (rt-PA) in acute ischemic hemispheric stroke where thrombolysis is initiated between 3 and 4 hours after stroke onset. ECASS III. American Stroke Association 28th International Stroke Conference; February 2003; Phoenix, Arizona, USA. pp. 13–5. [Google Scholar]
- 51.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 52.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–85. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 53.Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000;343:710–22. doi: 10.1056/NEJM200009073431007. [DOI] [PubMed] [Google Scholar]
- 54.Martinez H, Zoarski GH, Obuchowski AM, Stallmayer MJ, Papangelou A, Airan-Javia S. Mechanical thrombectomy of the internal carotid artery and middle cerebral arteries for acute stroke by using the retriever device. AJNR Am J Neuroradiol. 2004;25:1812–5. [PMC free article] [PubMed] [Google Scholar]
- 55.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–8. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 56.Daffertshofer M, Gass A, Ringleb P, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–6. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 57.Kane I, Sandercock P, Lindley R. Third International Stroke Trial. San Diego, California, USA: American Stroke Association 29th International Stroke Conference; 2004. [Google Scholar]
- 58.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. JAMA. 1999;282:2003–11. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell PJ, Gerraty RG, Donnan GA. Thrombolysis in the vertebrobasilar circulation: the Australian urokinase stroke trial. A pilot study. Cerebrovasc Dis. 1997;7:94–9. [Google Scholar]
- 60.Lindsberg PJ, Soinne L, Tatlisumak T, et al. Long-term outcome after intravenous thrombolysis of basilar artery occlusion. JAMA. 2004;292:1862–6. doi: 10.1001/jama.292.15.1862. [DOI] [PubMed] [Google Scholar]
- 61.Davis SM, Donnan GA, Butcher KS, Parsons M. Selection of thrombolytic therapy beyond 3 h using magnetic resonance imaging. Curr Opin Neurol. 2005;18:47–52. doi: 10.1097/00019052-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 63.Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 64.Stroke Therapy Academic Industry Roundtable II (STAIR-II) Recommendations for clinical trial evaluation of acute stroke therapies. Stroke. 2001;32:1598–606. doi: 10.1161/01.str.32.7.1598. [DOI] [PubMed] [Google Scholar]
- 65.Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies – the need for new approaches. Cerebrovasc Dis. 2004;17(Suppl. 1):153–66. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- 66.de Paulis T. ONO-2506. Ono. Curr Opin Invest Drugs. 2003;4:863–7. [PubMed] [Google Scholar]
- 67.Danysz W, Parsons CG. Neuroprotective potential of ionotropic glutamate receptor antagonists. Neurotox Res. 2002;4:119–26. doi: 10.1080/10298420290015872. [DOI] [PubMed] [Google Scholar]
- 68.Zweifler RM. Membrane stabilizer: citicoline. Curr Med Res Opin. 2002;18(Suppl. 2):s14–s17. doi: 10.1185/030079902125000679. [DOI] [PubMed] [Google Scholar]
- 69.Green AR, Ashwood T, Odergren T, Jackson DM. Nitrones as neuroprotective agents in cerebral ischemia, with particular reference to NXY-059. Pharmacol Ther. 2003;100:195–214. doi: 10.1016/j.pharmthera.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Wang CX, Shuaib A. NXY-059: a neuroprotective agent in acute stroke. Int J Clin Pract. 2004;58:964–9. doi: 10.1111/j.1368-5031.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- 71.Maples KR, Green AR, Floyd RA. Nitrone-related therapeutics. Potential of NXY-059 for the treatment of acute ischaemic stroke. CNS Drugs. 2004;18:1071–84. doi: 10.2165/00023210-200418150-00003. [DOI] [PubMed] [Google Scholar]
- 72.Kuroda S, Tsuchidate R, Smith ML, Maples KR, Siesjo BK. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1999;19:778–87. doi: 10.1097/00004647-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Sydserff SG, Borelli AR, Green AR, Cross AJ. Effect of NXY-059 on infarct volume after transient or permanent middle cerebral artery occlusion in the rat; studies on dose, plasma concentration and therapeutic time window. Br J Pharmacol. 2002;135:103–12. doi: 10.1038/sj.bjp.0704449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Z, Cheng M, Maples KR, Ma JY, Buchan AM. NXY-059, a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat. Brain Res. 2001;909:46–50. doi: 10.1016/s0006-8993(01)02618-x. [DOI] [PubMed] [Google Scholar]
- 75.Marshall JWB, Cummings RM, Bowes LJ, Ridley RM, Green AR. Functional and histological evidence for the protective effect of NXY-059 in a primate model of stroke when given 4 hours after occlusion. Stroke. 2003;34:2228–33. doi: 10.1161/01.STR.0000087790.79851.A8. [DOI] [PubMed] [Google Scholar]
- 76.Lees KR, Sharma AK, Barer D, et al. Tolerability and pharmacokinetics of the nitrone NXY-059 in patients with acute stroke. Stroke. 2001;32:675–80. doi: 10.1161/01.str.32.3.675. [DOI] [PubMed] [Google Scholar]
- 77.Lees KR, Barer D, Ford GA, et al. Tolerability of NXY-059 at higher target concentrations in patients with acute stroke. Stroke. 2003;34:482–7. doi: 10.1161/01.str.0000053032.14223.81. [DOI] [PubMed] [Google Scholar]
- 78.Jonsson S, Cheng YF, Edenius C, Lees KR, Odergren T, Karlsson MO. Population pharmacokinetic modelling and estimation of dosing strategy for NXY-059, a nitrone being developed for stroke. Clin Pharmacokinet. 2005;44:863–78. doi: 10.2165/00003088-200544080-00007. [DOI] [PubMed] [Google Scholar]
- 79.Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, Shuaib A, Hardemark HG, Wasiewski WW. Stroke-Acute Ischemic NXY Treatment (SAINT I) Trail Investigators. NXY-059 for acute ischemic stroke. N Eng J Med. 2006;354:588–600. [Google Scholar]
- 80.Tateishi N, Mori T, Kagamiishi Y, et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part II: suppression of astrocytic activation by a novel agent (R)-(-)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct expansion and early improvement of neurologic deficits. J Cereb Blood Flow Metab. 2002;22:723–34. doi: 10.1097/00004647-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 81.Tateishi N, Shintaku K, Kitajima S, et al. Effects of ONO-2506, an astrocyte modulating agent, on neurological symptoms after focal ischemia in cynomolgus monkeys. Society for Neuroscience 32nd Annual Meeting; November 2002; Orlando, Florida, USA. pp. 2–7. [Google Scholar]
- 82.Teal P, Selchen D, Sherman D, et al. RREACT study: rapid response with an astrocyte modulator for the treatment of acute cortical stroke. American Stroke Association 29th International Stroke Conference; February 2004; San Diego, California, USA. pp. 5–7. [Google Scholar]
- 83.Intravenous Magnesium Efficacy in Stroke (IMAGES) Study Investigators. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. Lancet. 2004;363:439–45. doi: 10.1016/S0140-6736(04)15490-1. [DOI] [PubMed] [Google Scholar]
- 84.Saver JL, Kidwell C, Eckstein M, Starkman S for the FAST MAG Pilor Trial Investigators. Prehospital neuroprotective therapy for acute stroke: results of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) pilot trial. Stroke. 2004;35:e106–e108. doi: 10.1161/01.STR.0000124458.98123.52. [DOI] [PubMed] [Google Scholar]
- 85.Saver JL, Kidwell C, Hamilton S, et al. The Field Administration of Stroke Therapy – Magnesium (FAST-MAG) Phase 3 Trial. American Stroke Association 29th International Stroke Conference; February 2004; San Diego, California, USA. pp. 5–7. [Google Scholar]