Abstract
Phosphorylation of eukaryotic initiation factor-2 (eIF2) is an important mechanism mitigating cellular injury in response to diverse environmental stresses. While all eukaryotic organisms characterized to date contain an eIF2 kinase stress response pathway, the composition of eIF2 kinases differs, with mammals containing four distinct family members and the well-studied lower eukaryote Saccharomyces cerevisiae expressing only a single eIF2 kinase. We are interested in the mechanisms by which multiple eIF2 kinases interface with complex stress signals and elicit response pathways. In this report we find that in addition to two previously described eIF2 kinases related to mammalian HRI, designated Hri1p and Hri2p, the yeast Schizosaccharomyces pombe expresses a third eIF2 kinase, a Gcn2p ortholog. To delineate the roles of each eIF2 kinase, we constructed S. pombe strains expressing only a single eIF2 kinase gene or deleted for the entire eIF2 kinase family. We find that Hri2p is the primary activated eIF2 kinase in response to exposure to heat shock, arsenite, or cadmium. Gcn2p serves as the primary eIF2 kinase induced during a nutrient downshift, treatment with the amino acid biosynthetic inhibitor 3-aminotriazole, or upon exposure to high concentrations of sodium chloride. In one stress example, exposure to H2O2, there is early tandem activation of both Hri2p and Gcn2p. Interestingly, with extended stress conditions there is activation of alternative secondary eIF2 kinases, suggesting that eukaryotes have mechanisms of coordinate activation of eIF2 kinase in their stress remediation responses. Deletion of these eIF2 kinases renders S. pombe more sensitive to many of these stress conditions.
IN response to diverse environmental stresses, phosphorylation of eukaryotic initiation factor-2 (eIF2) induces a program of gene expression designed to mitigate cellular injury. A family of eIF2 kinases that share sequence and structural features in their catalytic domains has been identified, but have unique flanking regulatory domains, allowing each to recognize distinct stress conditions (Chen 2000; Kaufman 2000; Dever 2002; Harding et al. 2002; Wek et al. 2004). For example, uncharged tRNA that accumulates in response to amino acid starvation associates with a domain in the eIF2 kinase GCN2 that is homologous to histidyl-tRNA synthetases (Hinnebusch 2000; Wek et al. 2004). The resulting conformational change enhances GCN2 phosphorylation of the α-subunit of eIF2 at serine-51, leading to reduced activity of this initiation factor and lowered general protein synthesis accompanied by enhanced translation of specific mRNAs encoding proteins that remediate stress. In addition to GCN2, three other eIF2 kinases have been identified in mammals: HRI that is mainly expressed in the erythroid lineage and couples protein synthesis, predominantly globin in these tissues, to the availability of heme (Chen 2000); PEK/Perk that modulates gene expression in response to protein misfolding in the endoplasmic reticulum (Harding et al. 2002); and PKR that participates in an antiviral pathway mediated by interferon (Kaufman 2000).
We have been interested in the mechanisms by which eIF2 kinases interface with complex stress signals and elicit stress response pathways. As highlighted for the four mammalian eIF2 kinases, it is proposed that there is a primary eIF2 kinase containing a distinct regulatory domain functioning to recognize each stress condition. By contrast in lower eukaryotes, there appear to be fewer eIF2 kinases contributing to stress remediation. In the yeast Saccharomyces cerevisiae, the sole eIF2 kinase Gcn2p is activated not only by nutrient limitations, including amino acid, purine, and glucose deprivation, but also by high concentrations of sodium, the immunosuppressant rapamycin, and methyl methanesulfonate (MMS; Hinnebusch 2000; Yang et al. 2000; Goosens et al. 2001; Valenzuela et al. 2001; Cherkasova and Hinnebusch 2003; Narasimhan et al. 2004; Wek et al. 2004). Thus, this single eIF2 kinase recognizes a diverse set of stress conditions. In the yeast Schizosaccharomyces pombe, two HRI-related enzymes were identified (Zhan et al. 2002). An important question is whether these S. pombe eIF2 kinases have been adapted to recognize a broad spectrum of environmental stress as described for Gcn2p in S. cerevisiae.
In this report, we find that, in addition to two HRI-related enzymes, S. pombe stress response includes a third eIF2 kinase, a Gcn2p ortholog. To delineate the roles of each S. pombe eIF2 kinase, we constructed S. pombe strains expressing only a single eIF2 kinase gene or deleted for their entire eIF2 kinase family. We find that Hri2p and Gcn2p serve as primary eIF2 kinases in response to different sets of environmental stresses, and in one example, exposure to H2O2, there is early tandem activation of these eIF2 kinases. Interestingly, with extended stress conditions there is activation of secondary eIF2 kinases, suggesting that eukaryotes have mechanisms of coordinate activation of eIF2 kinase in their stress remediation responses.
MATERIALS AND METHODS
Yeast strains and growth conditions:
S. pombe strains used in this study are derived from related strains CHP428 (h+ ade6-210 leu1-32 ura4-D18 his7-366) and SP223 (h− ade6-216 leu1-32 ura4-D18; Zhan et al. 2002), including strains WY743 (h+ ade6-210 leu1-32 ura4-D18 his7-366 gcn2::ura4+), WY458 (h− ade6-216 leu1-32 ura4-D18 hri2::leu1+), WY758 (h− ade6-216 leu1-32 ura4-D18 hri2::leu1+ gcn2::ura4+), WY459 (h− ade6-216 leu1-32 ura4-D18 hri1::ura4+ hri2::leu1+), WY764 (h− ade6-216 leu1-32 ura4-D18 leu1+ ura4+), WY766 (h− ade6-216 leu1-32 ura4-D18 his7-366 hri1::ura4+ hri2::leu1+ gcn2::ura4+), WY767 (h− ade6-216 leu1-32 ura4-D18 his7-366 hri1:: ura4+ gcn2::ura4+), and WY761 (h− ade6-216 leu1-32 ura4-D18 gcn2::ura4+ leu1+). S. pombe strains were grown in yeast extract plus supplements (YES) or Edinburgh minimum medium (EMM), supplemented with 3% glucose and with 225 μg/ml of adenine, 225 μg/ml of uracil, and amino acids as indicated. S. pombe strains were grown at 30° on agar plates or in liquid culture with agitation. Deletion of gcn2+ from the S. pombe strains was generated in one step through homologous recombination by transforming linear DNA containing ura4+ substituted for the entire coding region of gcn2+. Transformants were selected for growth in the absence of uracil supplement in the medium. The gcn2− knockout was confirmed by PCR analysis, and the gcn2− strain was mated with hri1− and hri1− hri2− strains with opposing mating types. The hri1− gcn2− strain and hri1− hri2− gcn2− strains were obtained after sporulation and tetrad analysis, and genotypes were confirmed by PCR analysis.
Isolation of gcn2+ cDNA:
To obtain a gcn2+ cDNA, total RNA was isolated from S. pombe as described (Schmitt et al. 1990), and purified poly(A)+ was prepared by using a MicroPoly(A) Pure isolation kit (Ambion, Austin, TX). Subsequent reverse transcription-PCR was carried out using the Titan One Tube RT-PCR system (Roche, Indianapolis). Due to the long length of gcn2+, we carried out the RT-PCR in two steps using primers derived from the S. pombe genomic sequence listed in the overlapping sequencing in GenBank, accession nos. SBP18G and SPB36B7. The gcn2+ cDNA fragments of 2.2 and 2.7 kb were obtained by RT-PCR amplification and sequenced by the dideoxy method.
Stress assays:
Yeast cells were grown to early exponential phase (A600 < 0.5) and subjected to stress by adding 100 μm NaAsO2, 100 μm CdCl2, 1 m NaCl, or 1 mm H2O2 to the YES medium for the indicated times or by shifting cell cultures from 30° to 48° for 10, 20, or 30 min. Addition of all 20 amino acids to the YES medium ensured minimal phosphorylation of eIF2α under nonstressed conditions. To elicit nutrient stress, cells were transferred from YES medium to EMM or shifted from EMM supplemented with all 20 amino acids to EMM containing all amino acids except histidine and 15 mm 3-aminotriazole. Given that 3-aminotriazole is an inhibitor of histidine biosynthesis, only cells expressing a functional his7 gene product were used in this stress analysis. Cells were resuspended in solution A [20 mm Tris-HCl (pH 7.8), 500 mm NaCl, 10% glycerol] supplemented with protease inhibitors (100 μm phenylmethylsulfonyl fluoride, 1 μm pepstatin, 1 μm leupeptin, and 0.15 μm aprotinin) and were broken with acid-washed glass beads and vortexing. The lysate preparation was clarified by centrifugation and stored in aliquots at −80°. A total of 20 μg of each protein sample was separated by electrophoresis in a SDS-12.5% polyacrylamide gel. Proteins were transferred to nitrocellulose filters, and the filters were blocked with TBS-T solution [20 mm Tris-HCl (pH 7.6), 150 mm NaCl, 0.1% Tween-20, and 4% nonfat milk powder]. Filters were then incubated with affinity-purified antibody that specifically recognizes eIF2α phosphorylated at serine-51 (Research Genetics, Birmingham, AL) or antisera prepared against an eIF2α polypeptide that recognized either phosphorylated or nonphosphorylated eIF2α (BioSource, Camarillo, TX). To measure cell growth following heat shock, cell cultures were exposed to 48° for 20 min and then monitored for growth at 600 nm at 30° for 30 hr. To determine growth resistance in response to 1 mm H2O2 or 100 μm sodium arsenite, strains were treated with these stress agents for 1 hr and then collected by centrifugation, rinsed, and resuspended in the same volume of liquid YES medium. Cell cultures were then incubated with agitation at 30° and growth was monitored at A600. To determine growth in response to amino acid limitation, 15 mm 3-aminotriazole was added to cells cultured in EMM with required supplements, and growth at 30° was monitored at 600 nm.
RESULTS
Identification of a GCN2 ortholog in S. pombe:
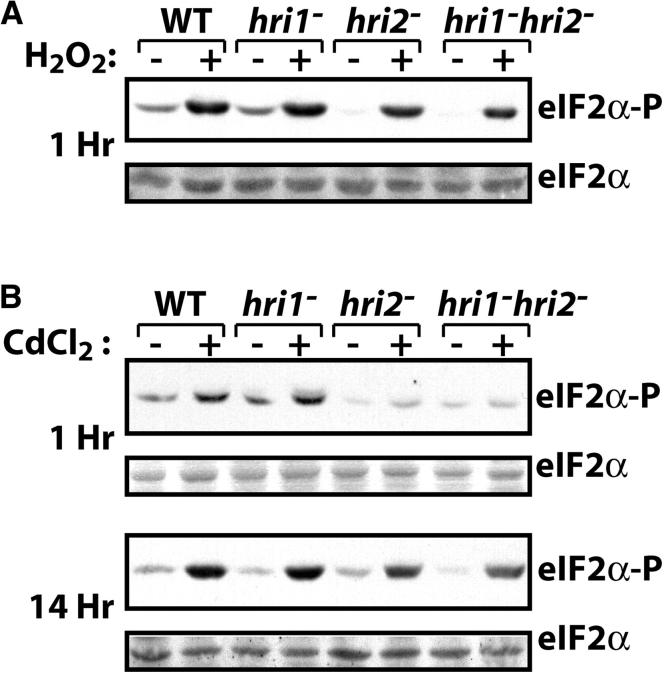
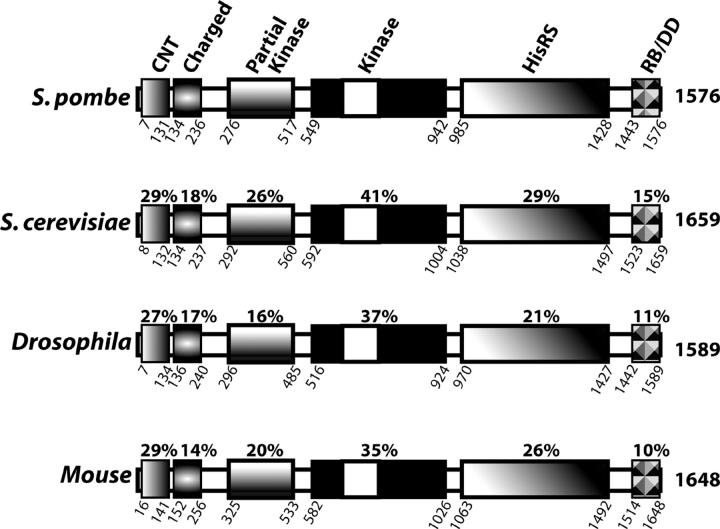
The mutant strain with the combined deletions of hri1+ and hri2+ showed a modest, but reproducible, level of eIF2α phosphorylation within 1 hr of exposure to arsenite (Zhan et al. 2002). We also found high levels of phosphorylation of eIF2α, as judged by immunoblot using antibody specific to eIF2α phosphorylated at serine-51, in the hri1− hri2− cells in response to treatment with H2O2 or during prolonged exposure to cadmium (Figure 1). Levels of total eIF2α were measured by immunoblot using polyclonal antibody that recognizes both phosphorylated and nonphosphorylated forms of this initiation factor and found to be unchanged in response to either stress condition. These studies suggest that one or more additional eIF2 kinases contribute to translation control in response to stress conditions. To identify such protein kinases, we used the mammalian eIF2 kinase GCN2, PKR, and PEK sequences as queries and the Blast program to search for related eIF2 kinases encoded in the S. pombe genome. We found a third eIF2 kinase most closely related to GCN2 that was encoded in adjacent cosmids SPBP18G and SPB36B7 derived from chromosome II. The sum probability of random correspondence of the pairwise segments (i.e., the blast score) between S. pombe Gcn2p and the orthologs from S. cerevisiae and Neurospora crassa were e-176 and e-163, respectively. To obtain a gcn2+ cDNA, we isolated poly(A)+ RNA from S. pombe strain SP223 and used RT-PCR analysis and oligonucleotide primers complementary to the gcn2+ coding region. The cloned S. pombe cDNA was sequenced by the dideoxy method and was found to be identical with the 4.7-kb genomic sequence, with the exception of the predicted intron located near the 5′ end of the gcn2+ sequence that was absent in the cDNA. The putative S. pombe Gcn2p ortholog is 1576 amino acids in length and has the domains conserved among the many GCN2 orthologs, including the conserved N-terminal domain (CNT) that facilitates interaction with the Gcn1p regulatory protein, protein kinase domain, partial kinase domain, HisRS-regulatory domain, and the carboxy terminus that facilitate yeast Gcn2p dimerization and ribosomal association (Figure 2; Garcia-Barrio et al. 2000; Kubota et al. 2000; Sattlegger and Hinnebusch 2000; Sood et al. 2000b; Narasimhan et al. 2004; Wek et al. 2004). We did not find any additional sequences in the S. pombe genome that are related to eIF2 kinases PEK or PKR. This bioinformatics analysis, combined with our earlier report on two HRI-related enzymes, suggests that there are three eIF2 kinases in S. pombe. The conserved domain arrangement of the S. pombe GCN2 ortholog suggests that it functions analogous to that described for this eIF2 kinase in S. cerevisiae and mammals.
Figure 1.—
Loss of hri1+ and hri2+ does not reduce eIF2α phosphorylation in response to oxidative stress. (A) Four isogenic strains with the relevant genotypes indicated were treated with H2O2 for 1 hr (+) or no stress (−). Equal amounts of nonstressed and stressed lysates were loaded on the basis of protein content. Immunoblots were carried out by using antibody that specifically recognizes phosphorylated eIF2α or antisera that measures total eIF2α levels. (B) This collection of isogenic strains was incubated in the presence of CdCl2 (+) for 1 hr (top) or 14 hr (bottom) or no stress (−). Levels of phosphorylated eIF2α were measured by immunoblot.
Figure 2.—
Alignment between Gcn2 orthologs from S. pombe, S. cerevisiae, Drosophila, and mouse. The Gcn2 sequences are represented as a bar with highlights of the conserved N-terminal domain (CNT, also designated G1), which is flanked by charged residues, partial kinase, protein kinase, HisRS related, and the carboxy terminus that facilitates ribosomal binding and GCN2 dimerization (RB/DD). The white portion of the kinase domain represents the insert between subdomains IV and V that is a hallmark feature shared among eIF2 kinases. The length of each GCN2 sequence is shown to the right of the illustrated GCN2 sequence, and the amino and carboxy terminal residues that form the boundary for each region are designated below. Pairwise comparisons were carried out using the Clustal W algorithm (Higgins et al. 1996). The percentage identity between S. pombe and each of the five regions of the GCN2 orthologs is shown above each bar.
Deletion of the gcn2+ gene from the S. pombe genome:
To determine the function of gcn2+ in the S. pombe stress response, we introduced a linear gcn2::ura4+ DNA knockout cassette into strain CHP428 (h+ ade6-210 leu1-32 ura4-D18 his7-366) and a related hri2::leu1+ mutant WY458 and selected for uracil prototrophy. The entire gcn2+ coding region was deleted in each of these strains, which was confirmed by PCR using genomic DNA. To generate S. pombe strains deleted for all three eIF2 kinases or each pairwise combination, the gcn2− strain WY743 (h+) was crossed with strain WY459 (h− hri1− hri2−), and the resulting diploid strain was induced to undergo sporulation. The resulting haploid spores were dissected and analyzed to determine their genotype. Strain WY766 was confirmed to be deleted for all three of the eIF2 kinase genes, and gcn2+ was deleted in combination with hri2+ in strain WY758 and with hri1+ in WY767. As described below, no eIF2α phosphorylation was detected in strain WY766 in response to many different environmental stresses, consistent with the idea that there are only three eIF2 kinases in S. pombe.
eIF2 kinase Gcn2p is activated in response to a nutrient downshift or elevated NaCl levels:
To characterize the role of each eIF2 kinase in a range of different stress conditions, we studied five isogenic strains, including a wild-type strain encoding the full complement with eIF2 kinases (WT), hri1− hri2− gcn2− (none), and strains deleted for two eIF2 kinase genes, thereby expressing only Gcn2p, Hri1p, or Hri2p. In S. cerevisiae, the Gcn2p eIF2 kinase is activated by nutritional deprivation or exposure to high concentrations of NaCl (Hinnebusch 2000; Goosens et al. 2001; Natarajan et al. 2001; Narasimhan et al. 2004; Wek et al. 2004). We therefore shifted this collection of strains from rich medium, YES, to minimal medium, EMM, and cultured these cells for 1 hr at 30°. The nutrient downshift elicited eIF2α phosphorylation predominantly by Gcn2p, with minimal levels of eIF2α phosphorylation found in the strains expressing only Hri1p or Hri2p and no phosphorylation in strain WY766 devoid of eIF2 kinases (Figure 3A). Furthermore, we measured phosphorylation of eIF2α in response to treatment with 3-aminotriazole (3AT), an inhibitor of histidine biosynthesis. Within 1 hr of exposure to 15 mm 3AT, there was a significant induction of eIF2α phosphorylation in the wild-type strain (Figure 3B). High levels of eIF2α phosphorylation were also seen in cells expressing only Gcn2p, while cells expressing only Hri1p or both Hri1p and Hri2p (strain WY761) had minimal levels of phosphorylation in response to 3AT treatment. These observations indicate that Gcn2p is activated in S. pombe in response to a shift to reduced nutritional conditions.
Figure 3.—
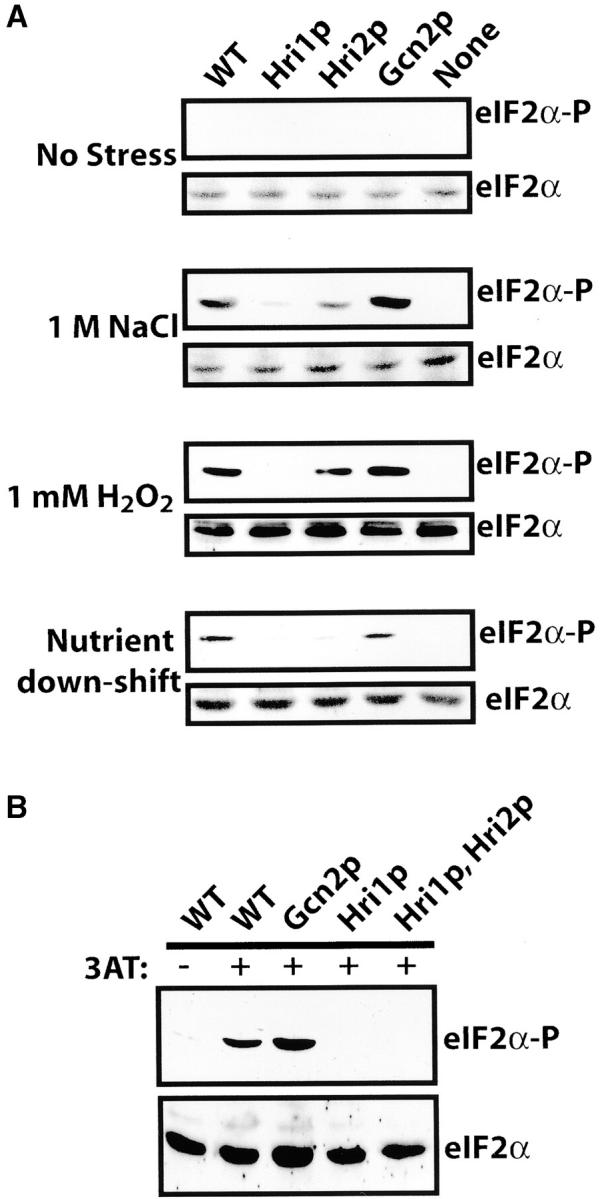
eIF2 kinases respond to different stress conditions in S. pombe. (A) Strains WY764 (WT), WY758 (Hri1p), WY767 (Hri2p), WY459 (Gcn2p), and WY766 (none) were grown to early exponential phase in YES medium supplemented with all 20 amino acids at 30° and subjected for 1 hr to 1 m NaCl, 1 mm H2O2, or nutritional deprivation involving a shift from rich YES medium to minimal medium EMM. Alternatively, cells were grown in YES medium in the absence of stress. Lysates were prepared from the stressed cells, and immunoblots were carried out using polyclonal antibody that specifically recognizes eIF2α phosphorylated at serine-51 (eIF2α-P) or total eIF2α protein (eIF2α). (B) Strains WY764 (WT), WY459 (Gcn2p), WY758 (Hri1p), and WY761 (Hri1p, Hri2p), were grown in EMM containing all amino acids except histidine and 15 mm 3AT for 1 hr (+). Under nonstressed conditions, wild-type cells were grown in EMM containing all 20 amino acids in the absence of 3AT (−). Mutant cells displayed similar low levels of eIF2α phosphorylation under these nonstressed culture conditions (data not shown).
We next examined eIF2 kinase activity in the strain collection in response to exposure to 1 m NaCl and again found that Gcn2p was the predominant eIF2 kinase (Figure 3A). Phosphorylation of eIF2α in the strain expressing only Gcn2p was near that measured in wild-type cells similarly treated with the elevated concentration of NaCl. By contrast, modest levels of eIF2α phosphorylation were detected in the strain expressing only Hri2p. Together these results suggest that Gcn2p is the primary activated eIF2 kinase in S. pombe subjected to nutritional stress or elevated NaCl conditions as described for Gcn2p in S. cerevisiae.
Network of eIF2 kinases recognizes stress in S. pombe:
We surveyed a series of stress conditions previously linked to eIF2α phosphorylation to establish the contribution of each of the three eIF2 kinases in the S. pombe stress response. When the collection of strains was exposed to 1 mm H2O2 for 1 hr, eIF2α phosphorylation was induced to a similar magnitude in the cells expressing Hri2p or Gcn2p (Figure 3A). Minimal eIF2α phosphorylation was observed in the strain expressing only Hri1p. These results suggest that the eIF2 kinase functions of Hri2p and Gcn2p overlap in S. pombe in response to oxidative stress.
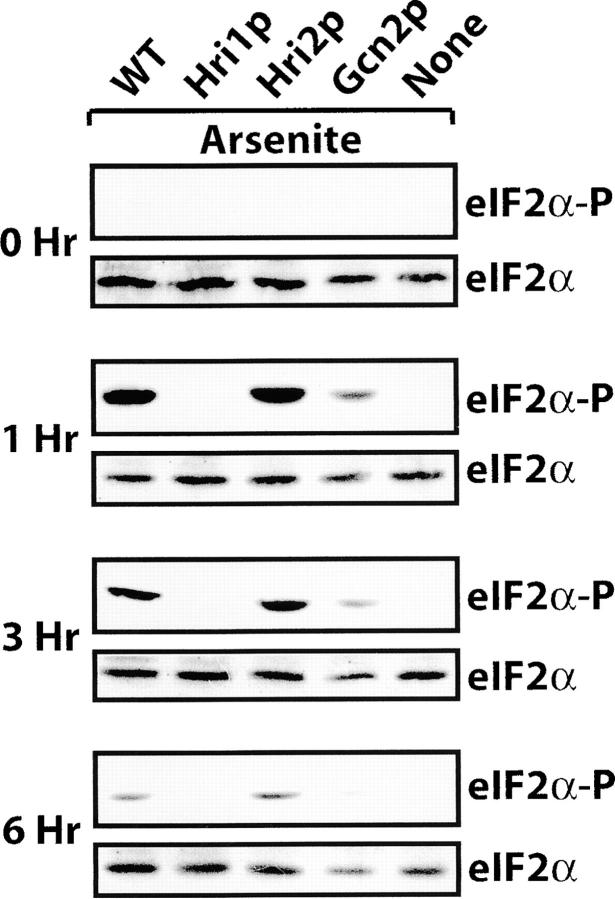
Two stress conditions previously linked to Hri2p were arsenite exposure and heat shock (Zhan et al. 2002). We treated the strain collection with 100 μm sodium arsenite. Following 1 hr of exposure to arsenite there was a significant induction of eIF2α phosphorylation in the Hri2p-expressing cells, with only moderate levels in the Gcn2p-expressing cells (Figure 4). Phosphorylation of eIF2α was absent in the strain containing only Hri1p. A similar pattern of eIF2α phosphorylation was detected in cells after 3 and 6 hr of arsenite exposure, although following 6 hr of treatment the levels were reduced in these mutant cells as well as in the wild-type strain compared to the shorter exposure times (Figure 4). These results support the idea that Hri2p is the primary eIF2 kinase in S. pombe treated with arsenic stress, with Gcn2p providing a secondary role.
Figure 4.—
Hri2p is the predominant eIF2 kinase responding to arsenite stress. Five isogenic strains expressing the entire collection of eIF2 kinases (WT), the indicated eIF2 kinases, or devoid of all three eIF2 kinases (none) were incubated with sodium arsenite for 1, 3, or 6 hr or no stress (0 hr). Cells were collected and washed and equal amounts of lysate based on protein content were analyzed by immunoblot using antibody that specifically recognizes phosphorylated eIF2α (eIF2α-P) or antiserum that recognizes total eIF2 (eIF2α).
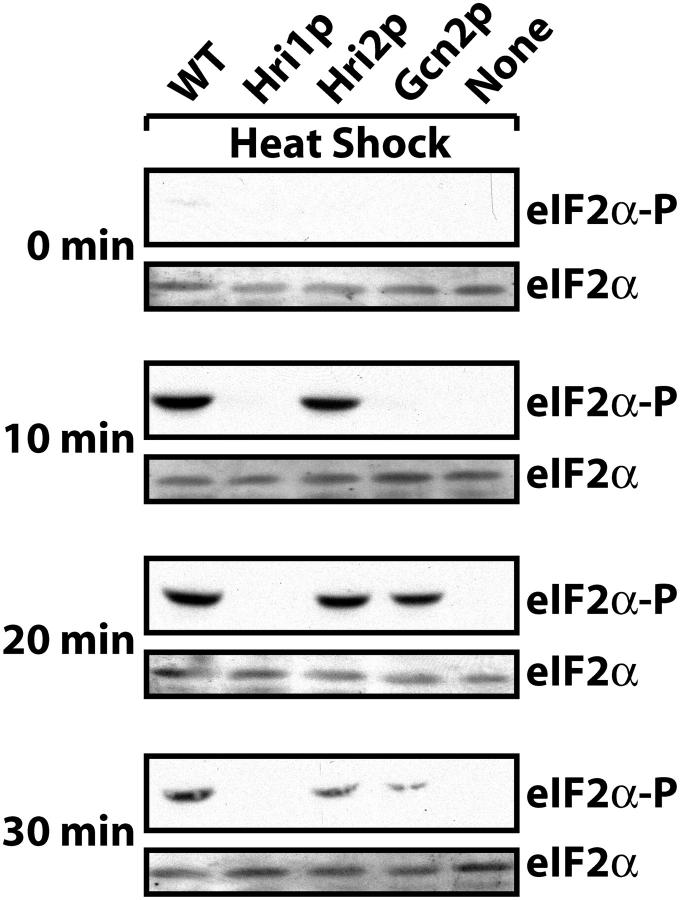
The strain collection was next exposed to heat shock at 48° for 10, 20, or 30 min. After 10 min of heat shock, the strain expressing only Hri2p exhibited levels of phosphorylated eIF2α that were similar to that measured in the wild-type cells (Figure 5). No eIF2α phosphorylation was detected in the strains expressing only Hri1p or Gcn2p or the mutant cells devoid of all three eIF2α kinases. Following 20 or 30 min of heat shock, significant levels of eIF2α phosphorylation were observed in both the Hri2p- and the Gcn2p-expressing cells. It is noteworthy with the longer 30-min exposure that there were reduced levels of eIF2α phosphorylation in both the Hri2p- and the Gcn2p-expressing cells, as well as in the wild-type cells. These results suggest that Hri2p is the predominant eIF2 kinase in response to heat shock, but Gcn2p has a compensatory role with longer periods of heat stress.
Figure 5.—
Hri2p is the primary and Gcn2p is the secondary eIF2 kinase to respond to heat shock in S. pombe. The wild-type (WT) strain or mutant versions expressing Hri1p, Hri2p, Gcn2p, or no eIF2 kinases (none) were incubated at 48° for 10, 20, or 30 min or no stress (0 min). Equal amounts of lysates based on protein content were analyzed by immunoblot using antibody that specifically recognizes phosphorylated eIF2α (eIF2α-P) or antiserum that recognizes total eIF2 (eIF2α).
A final stress condition that was characterized was exposure to the heavy metal cadmium. Cells were grown to early exponential phase in YES medium and then exposed to 100 μm CdCl2 for between 1 and 6 hr. With increased time of exposure to cadmium there was enhanced eIF2α phosphorylation in the wild-type cells that was absent in the mutant strain devoid of eIF2 kinases (Figure 6). Following 1 hr of cadmium treatment there was significant phosphorylation of eIF2α in the Hri2p-expressing cells, and this level of phosphorylation was retained for up to 6 hr of exposure to the heavy metal. By comparison, in the Gcn2p-expressing cells there were modest levels of phosphorylation of eIF2α following 3 hr of CdCl2 exposure, with a further enhancement after 6 hr of this stress. Interestingly, the levels of eIF2α phosphorylation in the Hri1p-expressing cells were significantly elevated following 6 hr of heavy metal exposure. Together these results support a model of compensatory eIF2 kinase activation outlined above. In the example of this heavy metal, Hri2p is the primary eIF2 kinase; however, with longer periods of exposure to cadmium, secondary eIF2 kinases Gcn2p and Hri1p are induced and contribute significantly to the levels of eIF2α phosphorylation.
Figure 6.—
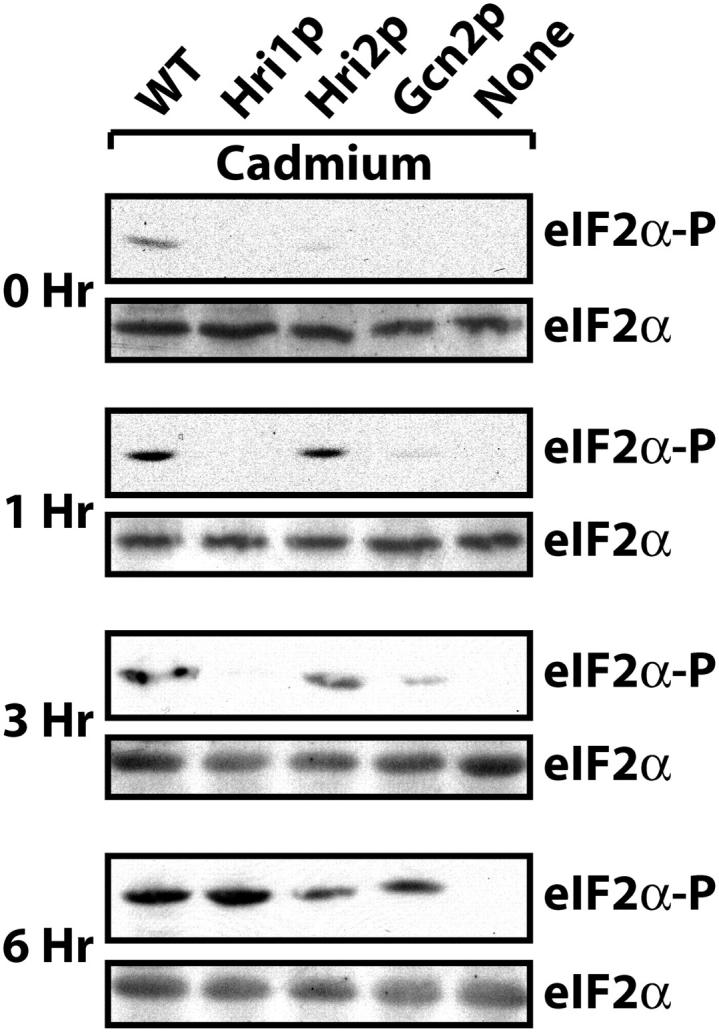
eIF2 kinases, Hri1p, Hri2p, and Gcn2p, are differentially activated in response to cadmium exposure. The isogenic collection of strains expressing all three eIF2 kinases (WT), no eIF2 kinases (none), or Hri1p, Hri2p, or Gcn2p as indicated were incubated with CdCl2 for 1, 3, or 6 hr or no stress (0 hr). Equal amounts of lysate were loaded on the basis of protein content, and immunoblots were carried out using antibody that specifically recognizes phosphorylated eIF2α (eIF2α-P) and antiserum that recognizes total eIF2α (eIF2α).
eIF2 kinases facilitate growth resistance to environmental stresses:
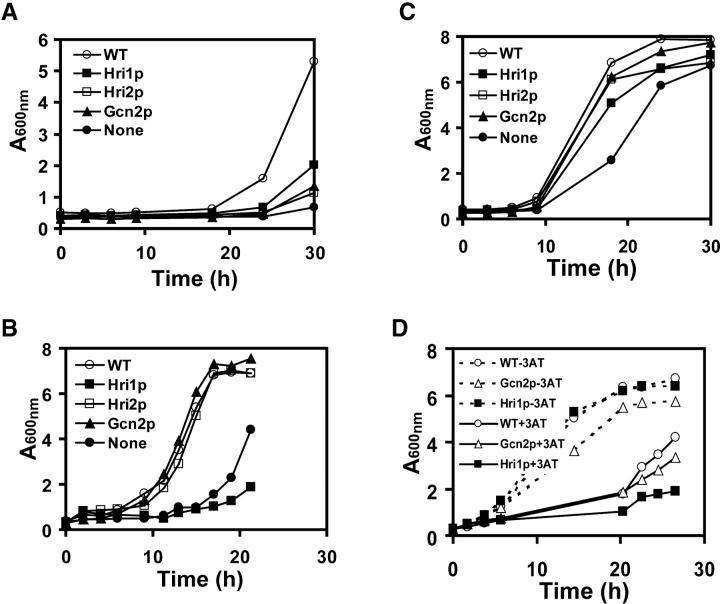
Given the activity differences between eIF2 kinases in response to stress conditions, we next wished to address the contribution of Hri1p, Hri2p, and Gcn2p to growth resistance in response to these different environmental insults. The strain collection was subjected to heat shock for 20 min. After 20-hr recovery from the heat shock, wild-type cells achieved exponential growth and approached stationary phase at A600 = 5.5 (Figure 7A). By comparison, cells expressing only Hri1p, Hri2p, or Gcn2p did not resume appreciable growth until 24 hr after heat shock, and strain WY766, devoid of eIF2 kinase activity, showed minimal growth over the 30-hr time course.
Figure 7.—
eIF2 kinases facilitate growth in S. pombe subject to environmental stresses. S. pombe strains WY764 (○, WT), WY758 (▪, Hri1p), WY767 (□, Hri2p), WY459 (▴, Gcn2p), and WY766 (•, none of the eIF2 kinase genes expressed) were monitored for growth by measuring absorbance at 600 nm. Strains were inoculated in YES medium supplemented with all 20 amino acids and subjected to a 20-min heat shock at 48° (A), 1-hr exposure to 1 mm H2O2 (B), or 1-hr exposure to 100 μm sodium arsenite (C). Following the stress conditions, cells were grown in the YES medium in the absence of stress. (D) Strains WY764 (WT), WY758 (Hri1p), and WY459 (Gcn2p) were grown in EMM medium in the absence or presence of 15 mm 3AT. Solid lines represent cell cultures treated with 3AT, and dashed lines indicate these strains grown in the absence of stress.
S. pombe cells resumed growth more rapidly following a 1-hr exposure to H2O2 or arsenite stresses compared with heat shock (Figure 7, B and C). The wild-type strain showed appreciable growth following 8 hr of the H2O2 treatment. Strain WY766, devoid of eIF2 kinases, showed growth 15 hr after the introduction of this stress condition. Interestingly, while the cells expressing either Hri2p or Gcn2p displayed near wild-type growth upon H2O2 exposure, strain WY758 (hri1+ hri2− gcn2−) showed growth sensitivity that in fact exceeded strain WY766 (Figure 7B). These results are consistent with Hri2p and Gcn2p serving as tandem primary eIF2 kinases in response to this oxidative stress, while the Hri1p-expressing cells appeared devoid of eIF2α phosphorylation (Figure 3A).
Cells expressing Hri1p, Hri2p, or Gcn2p displayed an intermediate growth resistance to arsenic stress compared to the wild-type strain, with WY758 (hri1+ hri2− gcn2−) showing the most growth sensitivity (Figure 7C). Treatment with 3AT delayed growth of the wild-type strain compared to nonstressed cells (Figure 7D), with appreciable growth following ∼20 hr of 3AT exposure. This growth pattern was also observed in Gcn2p-expressing cells, while Hri1p-expressing cells showed enhanced sensitivity to 3AT. Together, these results are consistent with this Gcn2p being the primary eIF2 kinase in response to nutrient limitation. Following a 2-hr exposure to 1 m NaCl, wild-type cells resumed exponential growth after a 3-hr delay compared to untreated cells. The growth response to 1 m NaCl for the cells expressing an individual eIF2 kinase or deleted for each of the three protein kinases was comparable to wild-type cells (data not shown).
DISCUSSION
Coordinate eIF2 kinase stress pathways in S. pombe:
Each eIF2 kinase in S. pombe recognizes a different subset of stress signals with a unique timing for activation. Typically one eIF2 kinase has a predominant role in response to a specific cellular stress condition. For example when exposed to arsenite, cells expressing only Hri2p showed significant eIF2α phosphorylation within 1 hr of exposure (Figure 4). By comparison Gcn2p provides a compensatory role during arsenite stress, with reduced levels of eIF2 kinase function compared with Hri2p. Hri2p is also the primary eIF2 kinase in response to heat shock and cadmium stress (Figures 5 and 6). In the example of cadmium stress, cells expressing Gcn2p showed only modest amounts of eIF2α phosphorylation following 3 hr of exposure. After 6 hr of cadmium treatment, eIF2α phosphorylation in the Gcn2p-expressing cells was similar to that measured in the Hri2p strain. At this time point, Hri1p-expressing cells also showed significant phosphorylation of eIF2α. Overall, Hri1p appears to provide largely a supportive role with the homologous Hri2p being the more active eIF2 kinase.
Gcn2p is the primary eIF2 kinase in response to a different set of stress conditions. Upon exposure to elevated levels of NaCl or in response to nutrient downshift, there were high levels of eIF2α phosphorylation in the cells expressing Gcn2p, with little eIF2 kinase activity in the strains expressing Hri1p or Hri2p (Figure 3). Therefore, there are stress conditions in which Gcn2p is the predominant eIF2 kinase. Interestingly, in the case of H2O2 exposure, both Gcn2p and Hri2p are activated in tandem. Loss of both eIF2 kinases significantly reduces the growth resistance to H2O2, emphasizing the importance of eIF2α phosphorylation for S. pombe to overcome this environmental insult (Figure 7).
Comparisons between nutrient stress responses from S. pombe and S. cerevisiae:
In S. cerevisiae starved for amino acids, phosphorylation of eIF2α by Gcn2p increases the translational expression of Gcn4p, a transcriptional activator of hundreds of genes involved in the metabolism of amino acids, nucleotides, and vitamins and the biogenesis of peroxisomes (Hinnebusch and Natarajan 2002). Uncharged tRNA that accumulates in response to amino acid deprivation binds to the HisRS-related sequence of S. cerevisiae Gcn2p, leading to a conformational change that enhances eIF2 kinase activity (Wek et al. 1995; Dong et al. 2000; Qiu et al. 2001, 2002; Wek et al. 2004). The observation that S. pombe Gcn2p is activated in response to histidine limitation (Figure 3B) and has homology with both the kinase and the HisRS-related domains of S. cerevisiae Gcn2p (Figure 2) suggests that common mechanisms activate these Gcn2p orthologs. Further supporting this common regulation model, S. pombe Gcn2p also has the conserved N-terminal domain (CNT) that is important for S. cerevisiae Gcn2p binding to the Gcn1p/Gcn20p complex. Gcn1p/Gcn20p associates with ribosomes and is proposed to transfer uncharged tRNA evicted from the A site of ribosomes to the HisRS-related domain of Gcn2p (Marton et al. 1997; Garcia-Barrio et al. 2000; Kubota et al. 2000; Sattlegger and Hinnebusch 2000; Anthony et al. 2004; Nameki et al. 2004; Sattlegger et al. 2004). Bioinformatic analysis of the S. pombe genome sequence indicates the presence of Gcn1p (NP_595837) and Gcn20p (NP_593669) orthologs, supporting the idea that S. pombe Gcn2p is presented with uncharged tRNA by a similar regulatory scheme.
It is curious that no Gcn4p ortholog is present in S. pombe, despite the conservation of this basic zipper (bZIP) transcriptional activator among a range of fungi, including S. cerevisiae, N. crassa, Candida albicans, and Aspergillus nidulans (Paluh et al. 1988; Hoffmann et al. 2001; Tripahti et al. 2002). In mammals, a related bZIP transcriptional activator, ATF4, is induced by eIF2α phosphorylation through a mechanism of translation reinitiation as described for Gcn4p (Harding et al. 2000a; Harding et al. 2003; Vattem and Wek 2004). In addition to genes involved in metabolism, ATF4 activates transcription of those regulating the redox status of the cell and apoptosis. Such conservation of translational control of bZIP transcriptional regulators from yeast to mammals suggests that a related transcriptional activator is also controlled by eIF2α kinases in S. pombe. A number of bZIP transcription factors are present in S. pombe, and interestingly two bZIP proteins, Pap1p and Atf1p, are regulated in response to environmental stresses, including UV irradiation, oxidative damage, and heat shock (Wilkinson et al. 1996; Degols and Russell 1997; Nguyen et al. 2000).
Conservation of eIF2 kinase stress responses among eukaryotes:
The pattern of primary and secondary eIF2 kinases, which differentially contribute to eIF2α phosphorylation depending on the specific stress condition and length of exposure time, is also inferred from studies of mammalian cells. Similar to that measured for S. pombe Hri2p, HRI in mouse fetal liver cells is the primary eIF2 kinase in response to arsenite or heat stress and is a secondary eIF2 kinase in response to exposure to high concentrations of NaCl (Lu et al. 2001). GCN2 is the primary eIF2 kinase in mammalian cells in response to nutrient limitation (Hinnebusch 2000; Wek et al. 2004). Following 1 hr of leucine limitation in mouse embryo fibroblast (MEF) cells, there is significant induction of eIF2α phosphorylation that is absent in GCN2-deficient cells (Jiang et al. 2003, 2004). However, following 6 hr of amino acid deprivation, eIF2α phosphorylation levels approached that measured in GCN2+/+ cells. These results suggest that many of the stress recognition properties of eIF2 kinases are conserved from yeast to mammals. Primary and secondary eIF2 kinases also function in response to accumulation of misfolded protein in the endoplasmic reticulum. Deletion of PEK (PERK) blocks eIF2α phosphorylation in MEF cells in response to this ER stress (Harding et al. 2000b; Jiang et al. 2003, 2004). However, there are near wild-type levels of eIF2α phosphorylation in PEK−/− MEF cells subjected to an extended 6-hr exposure to 1 μm thapsigargin, a standard ER stress agent (Jiang et al. 2004). The secondary eIF2 kinase is suggested to be GCN2, as combined deletions of PEK and GCN2 in MEF cells diminish eIF2α phosphorylation in response to ER stress to levels observed in nonstressed cells. Together, these mammalian studies support the proposed S. pombe model whereby there is a predominant eIF2 kinase that responds to a given stress condition. With extended stress conditions, secondary eIF2 kinases exhibit appreciable activity.
The representation of eIF2 kinase family members varies from unicellular eukaryotes to metazoans (Figure 8). Vertebrates, including mammals, chicken, and frogs share each of the four well-characterized members of the eIF2 kinase family. By contrast, fungi, including S. cerevisiae and N. crassa, contain only a single eIF2 kinase, related to Gcn2p, which modulates stress remediation in response to a diverse set of stress conditions (Sattlegger et al. 1998; Hinnebusch 2000; Wek et al. 2004). In addition to fungi and mammals, GCN2 is represented throughout eukaryotic organisms, including plants, Arabidopsis thaliana, and invertebrates such as Drosophila melanogaster and Caenorhabditis elegans (Olsen et al. 1998; Zhang et al. 2003; Figure 8). It is curious that S. pombe has supplemented Gcn2p with two versions of HRI-related proteins, suggesting that S. pombe may encounter certain cellular stress conditions unique to this yeast. By contrast, HRI is absent in D. melanogaster and C. elegans, which express only GCN2 and PEK (Olsen et al. 1998; Sood et al. 2000a). Analysis of the recently characterized genome sequence of the mosquito Anopheles gambiae (Holt et al. 2002), important for the passage of malaria, indicates this arthropod also contains an HRI-related protein (Figure 8). Furthermore, the silk moth, Bombyx mori, expresses an HRI-related protein (designated BeK; GenBank accession no. U87236; Prasad et al. 2003) that shares near full-length sequence similarity with HRI from A. gambiae, with a blast score of e-107. This analysis suggests that HRI is distributed throughout eukaryotes, and selected organisms retained this eIF2 kinase as part of their complement of sensors for cellular stress. We do not yet understand the basis for this retention. Future comparisons of the eIF2 kinase stress response pathways between S. pombe and other yeast expressing only GCN2 should provide insight into this intriguing question.
Figure 8.—
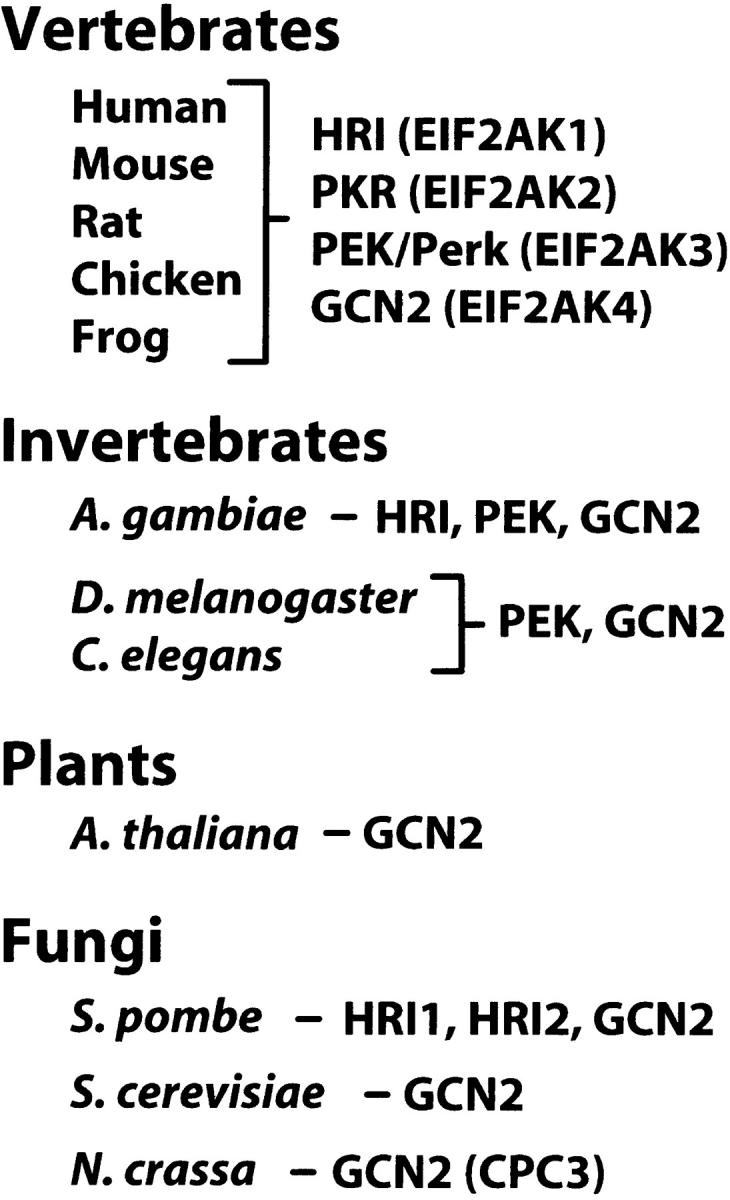
Distribution of eIF2 kinases among eukaryotes. Vertebrates contain four eIF2 kinases, which are also highlighted by the EIF2AK designation utilized in human genomics. Representative GenBank accession numbers for the eIF2 kinases are as follows (in order of HRI, PKR, PEK/Perk, and GCN2): in humans, AF181071, NM_002759, AF193339, and AB037759; in mice, AY033898, BC016422, AF076681, and AF193344; in rats, NM_013223, 229281, AF09835, and NW_047657; in chickens, AF330008, AB125660, BM490526, and BM439639; and in frog Xenopus tropicalis EST sequences suggesting portions of each eIF2 kinase, BG515260, BX732416, BX782352, and BX769528. Bioinformatics analyses were also carried out in the following invertebrate organisms: mosquito, A. gambiae (HRI, AAAB11008823; PEK, AAAB11008859; and GCN2, AAAB11008986), D. melanogaster (PEK, AF1993349 and GCN2, AF056302), and C. elegans (PEK, AF193341 and GCN2, AL034543). Plants include A. thaliana (GCN2, NP_191500). Fungi include S. pombe (HRI1, AF536223; HRI2, 536224; and GCN2 herein), S. cerevisiae GCN2 (NC_001136), and the N. crassa GCN2 ortholog designated CPC3 (X91867).
Acknowledgments
We thank the Biochemistry Biotechnology Facility at Indiana University for technical support. This study was supported in part by grants RO1GM49164 and R01GM643540 from the National Institutes of Health (R.C.W.).
References
- Anthony, T. G., B. J. McDaniel, R. L. Byerley, B. C. McGrath, D. R. Cavener et al., 2004. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J. Biol. Chem. 279: 36553–35661. [DOI] [PubMed] [Google Scholar]
- Chen, J.-J., 2000 Heme-regulated eIF2α kinase, pp. 529–546 in Translational Control of Gene Expression, edited by N. Sonenberg, J. W. B. Hershey and M. Mathews. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Cherkasova, V. A., and A. G. Hinnebusch, 2003. Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev. 17: 859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols, G., and P. Russell, 1997. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol. Cell. Biol. 17: 3356–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever, T. E., 2002. Gene-specific regulation by general translation factors. Cell 108: 545–556. [DOI] [PubMed] [Google Scholar]
- Dong, J., H. Qiu, M. Garcia-Barrio, J. Anderson and A. G. Hinnebusch, 2000. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 6: 269–279. [DOI] [PubMed] [Google Scholar]
- Garcia-Barrio, M., J. Dong, S. Ulfano and A. G. Hinnebusch, 2000. Association of GCN1–GCN20 regulatory complex with the N-terminus of eIF2alpha kinase GCN2 is required for GCN2 activation. EMBO J. 19: 1887–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens, A., T. E. Dever, A. Pascual-Ahuir and R. Serrano, 2001. The protein kinase Gcn2p mediates sodium toxicity in yeast. J. Biol. Chem. 276: 30753–30760. [DOI] [PubMed] [Google Scholar]
- Harding, H. P., I. Novoa, Y. Zhang, H. Zeng, R. Wek et al., 2000. a Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6: 1099–1108. [DOI] [PubMed] [Google Scholar]
- Harding, H. P., Y. Zhang, A. Bertolotti, H. Zeng and D. Ron, 2000. b Perk is essential for translation regulation and cell survival during the unfolded protein response. Mol. Cell 5: 897–904. [DOI] [PubMed] [Google Scholar]
- Harding, H. P., M. Calfon, F. Urano, I. Novoa and D. Ron, 2002. Transcriptional and translational control in the mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 18: 575–599. [DOI] [PubMed] [Google Scholar]
- Harding, H. P., Y. Zhang, H. Zeng, I. Novoa, P. D. Lu et al., 2003. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11: 619–633. [DOI] [PubMed] [Google Scholar]
- Higgins, D. G., J. D. Thompson and T. J. Gibson, 1996. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 266: 383–402. [DOI] [PubMed] [Google Scholar]
- Hinnebusch, A. G., 2000 Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes, pp. 185–244 in Translational Control of Gene Expression, edited by N. Sonenberg, J. W. B. Hershey and M. Mathews. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Hinnebusch, A. G., and K. Natarajan, 2002. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryotic Cell 1: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, B., O. Valerius, M. Andermann and G. H. Braus, 2001. Transcriptional autoregulation and inhibition of mRNA translation of amino acid regulator gene cpcA of filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 12: 2846–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, R. A., G. M. Subramanian, A. Halpern, G. G. Sutton, R. Charlab et al., 2002. The genome sequence of the malaria mosquito Anopheles gambiae. Science 298: 129–149. [DOI] [PubMed] [Google Scholar]
- Jiang, H. Y., S. A. Wek, B. C. McGrath, D. Scheuner, R. J. Kaufmann et al., 2003. Phosphorylation of eIF2α is required for activation of NF-κB in response to diverse cellular stress. Mol. Cell. Biol. 23: 5651–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. Y., S. A. Wek, B. C. McGrath, D. Lu, T. Hai et al., 2004. Activating transcription factor 3 (ATF3) is integral to the eIF2 kinase stress response. Mol. Cell. Biol. 24: 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, R. J., 2000 Double-stranded RNA-activated protein kinase, pp. 503–528 in Translational Control of Gene Expression, edited by N. Sonenberg, J. W. B. Hershey and M. Mathews. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Kubota, H., Y. Sakaki and T. Ito, 2000. GI domain-mediated association of the eukaryotic initiation factor 2alpha kinase GCN2 with its activator GCN1 is required for general amino acid control in budding yeast. J. Biol. Chem. 275: 20243–20246. [DOI] [PubMed] [Google Scholar]
- Lu, L., A. P. Han and J.-J. Chen, 2001. Translation initiation control by heme-regulated eukaryotic initiation factor 2α kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 21: 7971–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton, M. J., C. R. Vazquez de Aldana, H. Qui, K. Chakraburtty and A. G. Hinnebusch, 1997. Evidence that GCN1 and GCN20, translational regulators of GCN4, function on elongating ribosomes in activation of eIF-2alpha kinase GCN2. Mol. Cell. Biol. 17: 4474–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nameki, N., M. Yoneyama, S. Koshiba, N. Tochio, M. Inoue et al., 2004. Solution structure of the RWD domain of the mouse GCN2 protein. Protein Sci. 13: 2089–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan, J., K. A. Staschke and R. C. Wek, 2004. Dimerization is required for activation of eIF2 kinase Gcn2 in response to diverse environmental stress conditions. J. Biol. Chem. 279: 22820–22832. [DOI] [PubMed] [Google Scholar]
- Natarajan, K., M. R. Meyer, B. M. Jackson, D. Slade, C. Roberts et al., 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21: 4347–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, A. N., A. Lee, W. Place and K. Shiozaki, 2000. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell. 11: 1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, D. S., B. Jordan, D. Chen, R. C. Wek and D. R. Cavener, 1998. Isolation of the gene encoding the Drosophila melanogaster homolog of the S. cerevisiae GCN2 eIF-2α kinase. Genetics 149: 1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh, J. L., M. J. Orback, T. L. Legerton and C. Yanofsky, 1988. The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-jun-encoded protein. Proc. Natl. Acad. Sci. USA 85: 3728–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, M. D., S. J. Han, J. Nagaraju, W. J. Lee and P. T. Brey, 2003. Cloning and characterization of an eukaryotic initiation factor-2alpha kinase from the silkworm, Bombyx mori. Biochim. Biophys. Acta 1628: 56–63. [DOI] [PubMed] [Google Scholar]
- Qiu, H., J. Dong, C. S. Francklyn and A. G. Hinnebusch, 2001. The tRNA-binding moiety in GCN2 contains a dimerization domain that interacts with the kinase domain and is required for tRNA binding and kinase activation. EMBO J. 20: 1425–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, H., C. Hu, J. Dong and A. G. Hinnebusch, 2002. Mutations that bypass tRNA binding activate the intrinsically defective kinase domain in GCN2. Genes Dev. 16: 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattlegger, E., and A. G. Hinnebusch, 2000. Separate domains in GCN1 for binding protein kinase GCN2 and ribosome are required for GCN2 activation in amino acid-starved cells. EMBO J. 19: 6622–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattlegger, E., A. G. Hinnebusch and I. B. Barthelmess, 1998. cpc-3, the Neurospora crassa homologue of yeast GCN2, encodes a polypeptide with juxtaposed eIF2alpha kinase and histidyl-tRNA synthetase-related domains required for general amino acid control. J. Biol. Chem. 273: 20404–20416. [DOI] [PubMed] [Google Scholar]
- Sattlegger, E., M. J. Swanson, E. A. Ashcraft, J. L. Jennings, R. A. Fekete et al., 2004. YIH1 is an actin-binding protein that inhibits protein kinase GCN2 and impairs general amino acid control when overexpressed. J. Biol. Chem. 279: 29952–29962. [DOI] [PubMed] [Google Scholar]
- Schmitt, M. E., T. A. Brown and B. L. Trumpower, 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18: 3091–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood, R., A. C. Porter, K. Ma, L. A. Quilliam and R. C. Wek, 2000. a Pancreatic eukaryotic initiation factor-2α kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to ER stress. Biochem. J. 346: 281–293. [PMC free article] [PubMed] [Google Scholar]
- Sood, R., A. C. Porter, D. Olsen, D. R. Cavener and R. C. Wek, 2000. b A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2α. Genetics 154: 787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripahti, G., C. Wiltshire, S. Macaskill, H. Tournu, S. Budge et al., 2002. Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J. 21: 5448–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela, L., C. Aranda and A. Gonzalez, 2001. TOR modulates GCN4-dependent expression of genes turned on by nitrogen limitation. J. Bacteriol. 183: 2331–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem, K. M., and R. C. Wek, 2004. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 101: 11269–11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek, R. C., K. A. Staschke and J. Narasimhan, 2004 Regulation of the yeast general amino acid control pathway in response to nutrient stress, pp. 171–199 in Nutrient-Induced Responses in Eukaryotic Cells, edited by J. Winderickx and P. M. Taylor. Springer-Verlag, Heidelberg, Germany.
- Wek, S. A., S. Zhu and R. C. Wek, 1995. The histidyl-tRNA synthetase-related sequence in eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell. Biol. 15: 4497–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, M. G., M. Samuels, T. Takeda, W. M. Toone, J. C. Shieh et al., 1996. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 10: 2289–2301. [DOI] [PubMed] [Google Scholar]
- Yang, R., S. A. Wek and R. C. Wek, 2000. Glucose limitation induces GCN4 translation by activation of Gcn2 protein kinase. Mol. Cell. Biol. 20: 2706–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, K., K. M. Vattem, B. N. Bauer, T. E. Dever, J.-J. Chen et al., 2002. Phosphorylation of eukaryotic initiation factor-2 by HRI-related protein kinases in Schizosaccharomyces pombe is important for resistance to environmental stresses. Mol. Cell. Biol. 22: 7134–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., J. R. Dickinson, M. J. Paul and N. G. Halford, 2003. Molecular cloning of an arabidopsis homologue of GCN2, a protein kinase involved in co-ordinated response to amino acid starvation. Planta 217: 668–675. [DOI] [PubMed] [Google Scholar]