Abstract
A substantial fraction of insects and other terrestrial arthropods are infected with parasitic, maternally transmitted endosymbiotic bacteria that manipulate host reproduction. In addition to imposing direct selection on the host to resist these effects, endosymbionts may also have indirect effects on the evolution of the mtDNA with which they are cotransmitted. Patterns of mtDNA diversity and evolution were examined in Drosophila recens, which is infected with the endosymbiont Wolbachia, and its uninfected sister species D. subquinaria. The level of mitochondrial, but not nuclear, DNA diversity is much lower in D. recens than in D. subquinaria, consistent with the hypothesized diversity-purging effects of an evolutionarily recent Wolbachia sweep. The dN/dS ratio in mtDNA is significantly greater in D. recens, suggesting that Muller's ratchet has brought about an increased rate of substitution of slightly deleterious mutations. The data also reveal elevated rates of synonymous substitutions in D. recens, suggesting that these sites may experience weak selection. These findings show that maternally transmitted endosymbionts can severely depress levels of mtDNA diversity within an infected host species, while accelerating the rate of divergence among mtDNA lineages in different species.
INNUMERABLE species of insects and other arthropods are infected with maternally transmitted endosymbionts. Among the most prevalent of these are bacteria that manipulate host reproduction in a variety of ways to increase the relative transmission rates of infected lineages (Stouthamer et al. 1999). The two most widely reported modes of reproductive manipulation are cytoplasmic incompatibility (CI), in which matings between infected males and uninfected females result in high levels of offspring mortality, and male killing, in which the male offspring of infected females suffer high rates of embryonic mortality (Stouthamer et al. 1999). The substantial mortality caused by these infections is likely to favor the evolution of countermeasures by the host species. These endosymbionts may also affect the evolution of their hosts in more subtle ways, having specific effects on the population genetics and molecular evolution of host mitochondrial DNA (Turelli and Hoffmann 1991; Turelli et al. 1992; Johnstone and Hurst 1996). Because of the maternal transmission of both elements, patterns of molecular evolution of endosymbionts and mtDNA are more strongly correlated with each other than with nuclear alleles within a species.
The spread of a maternally transmitted microorganism will result in the hitchhiking of all maternally inherited organelles, including mitochondria, associated with the initially infected female (Turelli and Hoffmann 1991; Solignac et al. 1994; Turelli 1994; Turelli and Hoffmann 1995; Hoffmann et al. 1998). The mtDNA will be forced through a bottleneck of one host female, from which all mtDNA haplotypes in the population will be descended. Consequently, infected populations may have lower mtDNA diversity (Caspari and Watson 1959; Fine 1978; Turelli 1994). Because the expected coalescent time of mtDNA in an uninfected species is 2Nf, where Nf is the effective population size of females (Avise 2000), a decrease in mtDNA diversity is expected to be evident for ∼2Nf generations following a Wolbachia sweep or strain replacement. Given this expectation and the large population sizes of many insect species, mtDNA diversity may be reduced for millions of generations.
Another possible effect of these infections on mtDNA arises from the small effective population size that organelles pass through as a consequence of recurrent endosymbiont sweeps. Several species of asexual endosymbionts have experienced accelerated rates of molecular evolution relative to their free-living relatives (Moran 1996; Brynnel et al. 1998; Peek et al. 1998; Clark et al. 1999; Wernegreen and Moran 1999; Thao et al. 2000; Abbot and Moran 2002; Woolfit and Bromham 2003). This has been interpreted as a result of Muller's ratchet—the increased probability of fixation of slightly deleterious mutations in species such as endosymbionts with small effective population sizes. Consider an endosymbiont that undergoes a series of variant replacements within a host species, due perhaps to the evolution of new CI interaction types (Turelli 1994; Hurst and McVean 1996) or simply as a result of adaptation to the intracellular environment of the host. For each such turnover within the endosymbiont population, the associated mtDNA in a host species will be taken through an effective population size of one host female. Thus, Muller's ratchet is expected to affect the mtDNA of an endosymbiont-infected species, and such effects may be evident in elevated rates of substitution of slightly deleterious mutations.
Coupling of Wolbachia and mtDNA dynamics has been demonstrated in a variety of species, including Drosophila simulans (Turelli and Hoffmann 1991; Hoffmann et al. 1994; Solignac et al. 1994; Ballard 2000; Ballard et al. 2002), D. recens (Shoemaker et al. 1999), the mosquitoes Aedes albopictus (Kambhampati et al. 1992) and Culex pipiens (Guillemaud et al. 1997), the fire ant Solenopsis invicta (Shoemaker et al. 2000, 2003), the oak gallwasp Biorhiza pallida (Rokas et al. 2001), and the isopods Porcellionides pruinosus (Marcade et al. 1999) and Armadillidium vulgare (Rigaud et al. 1999). By far, the most thorough studies have been conducted on D. simulans. Indeed, the first study documenting the spread of a Wolbachia infection (and associated mtDNA haplotype) in nature was conducted in D. simulans (Turelli and Hoffmann 1991). Subsequent studies have demonstrated that this species is infected with four genetically distinct strains of Wolbachia, presumably representing four independent invasions across three distinct clades of mitochondrial haplotypes (James and Ballard 2000; Ballard 2004). There is very little sequence variation within each of the three defined haplotype clades, but substantial differences among them (Ballard 2000). Furthermore, each mitochondrial lineage is characterized by an excess of nonsynonymous relative to synonymous substitutions, consistent with a possible effect of Muller's ratchet. Much of the groundbreaking work on the effects of Wolbachia on mtDNA dynamics has focused on D. simulans, including studies demonstrating reduced mtDNA diversity within lineages (Turelli and Hoffmann 1991, 1995; Turelli et al. 1992; Ballard et al. 1996, 2002; Ballard 2000, 2004; James and Ballard 2000; James et al. 2002; Dean et al. 2003). However, because D. simulans and all of its close relatives are infected with Wolbachia, this has precluded a comparative analysis of rates of molecular evolution in Wolbachia-infected and uninfected sister taxa. Thus, the specific hypothesis that a Wolbachia infection leads to an accelerated rate of molecular evolution of the mtDNA genome remains to be tested.
In this article, we address three questions:
Does a Wolbachia-infected species harbor lower levels of mtDNA diversity than a closely related but uninfected species?
Does the mtDNA within an infected species show evidence of having experienced a greater rate of nucleotide substitution than that within an uninfected species?
If the infected species exhibits an elevated rate of mtDNA substitution, are the patterns of substitution consistent with expected effects of Muller's ratchet?
To address these issues, we exploit a trio of Drosophila species. D. recens is a mycophagous member of the quinaria species group whose range encompasses cool forested regions of eastern North America and is thought to have high rates of gene flow among populations (Shoemaker and Jaenike 1997). All populations are infected at a very high frequency (98–99%) with CI-causing Wolbachia, with the rare uninfected individuals (1–2%) resulting from incomplete maternal transmission of the endosymbiont, as they harbor mitochondrial haplotypes identical to those found in infected individuals. D. subquinaria is a mycophagous species found in forests of western North America and is the closest known relative of D. recens. Of several hundred wild-caught individuals of D. subquinaria surveyed from throughout the range of this species, none have been found to be infected with Wolbachia. D. quinaria is a closely related outgroup species that breeds in decaying vegetation and is found in eastern North America (Perlman et al. 2003).
MATERIALS AND METHODS
Samples:
D. recens were collected from Rochester, New York (n = 9), Big Moose, New York (10), Brunswick, Maine (10), Great Smoky Mountains National Park, Tennessee (17), and Edmonton, Alberta (12). D. subquinaria were collected from Deary, Idaho (3), Seattle, Washington (4), Port Hardy, British Columbia (9), and Peachland, British Columbia (10). One D. quinaria was used as an outgroup for molecular evolutionary analyses.
A previous study showed that neither D. quinaria nor D. palustris was infected with Wolbachia (Werren and Jaenike 1995). These two species represent two closely related, successive outgroups to the recens-subquinaria clade (Perlman et al. 2003). More extensive surveys of D. quinaria and D. palustris lead to the conclusion that the common ancestor of these four species was uninfected with Wolbachia (K. A. Dyer and J. Jaenike, unpublished data). Therefore, the Wolbachia infection in D. recens is a derived state within this species.
DNA sequencing:
Wolbachia variation within D. recens was surveyed by sequencing a portion of the wsp gene (∼590 bp) from 33 Wolbachia-infected D. recens using the primers wsp 81F and wsp 691R (Zhou et al. 1998; Shoemaker et al. 2003). For all sequencing, PCR amplicons were cleaned using Agencourt magnetic beads or QIAGEN (Chatsworth, CA) columns and sequenced directly using ABI Prism Big Dye terminator chemistry.
To contrast patterns of molecular evolution between D. recens and D. subquinaria, the entire mtDNA cytochrome oxidase I (COI) gene was amplified and sequenced from 58 individuals of D. recens, 26 of D. subquinaria, and one of the outgroup D. quinaria. PCR was carried out using the primers TY-J-1460 and TL2-N-3014 (Simon et al. 1994). These as well as two internal primers (DR-CO I internal forward, 5′-AATAATATCTACAGATGAGTTAG-3′; DR-CO I internal reverse, 5′-AGCAATTTTTTCTTTACATTTAG-3′) were used to sequence both strands. In addition to sequencing COI, we generated sequences for a much larger portion of the mtDNA genome for 12 D. recens, one D. subquinaria, and one D. quinaria. This included a total 7248–8757 bp representing portions of 12 of the 14 mitochondrial protein-coding genes (primers available upon request).
To distinguish effects specific to mtDNA from factors that affect the entire genome, we sequenced three nuclear gene regions: period, adhr (alcohol-dehydrogenase-related protein), and tpi (triose phosphate isomerase). All three genes have been used to investigate patterns of divergence and polymorphism in other Drosophila, and the patterns for at least synonymous sites are consistent with the standard neutral model (Wang and Hey 1996; Gleason and Powell 1997; Andolfatto and Przeworski 2000; Rand et al. 2000; Weinrich and Rand 2000; Begun 2001; Przeworski et al. 2001; Machado et al. 2002). period is an X-linked gene in D. recens and in other species of Drosophila. A 642-bp coding portion was amplified and sequenced on both strands using two primers that we designed (5′-GAACGTCAACCCCAGGCGGAAGG-3′ and 5′-ACAAGGAGAAGTCCAGGAAGAAG-3′) from 36 of D. recens, 11 of D. subquinaria, and one individual of D. quinaria. Only male flies were used because they carry a single X chromosome and thus only one period allele. adhr and tpi are autosomal, with the latter likely occurring on the second chromosome in D. recens (Patterson and Stone 1952). A 516-bp portion of adhr was amplified and sequenced from 7 D. recens, 8 D. subquinaria, and one individual of D. quinaria, using the two previously published primers D1 and D4 (Betran and Ashburner 2000). A 381-bp coding portion of tpi was amplified and sequenced using two primers that we designed (5′-CAACTGGAAGATGAAYGGIGACC-3′ and 5′-TTCTTGGCATAGGCGCACATYTG-3′) from 13 D. recens, 12 D. subquinaria, and one individual of D. quinaria. For both adhr and tpi we inferred heterozygous sites by manually examining the chromatograms for double peaks; we did not attempt to infer haplotypes. We sequenced a total of 14 and 26 alleles from D. recens for adhr and tpi, respectively.
Levels and patterns of diversity:
To visualize relationships and relative abundances of mtDNA haplotypes within D. recens, a median-joining network was generated using the program Network (Figure 1; http://www.fluxus-engineering.com/sharenet.htm). One individual of D. quinaria was included to root the network.
Figure 1.—
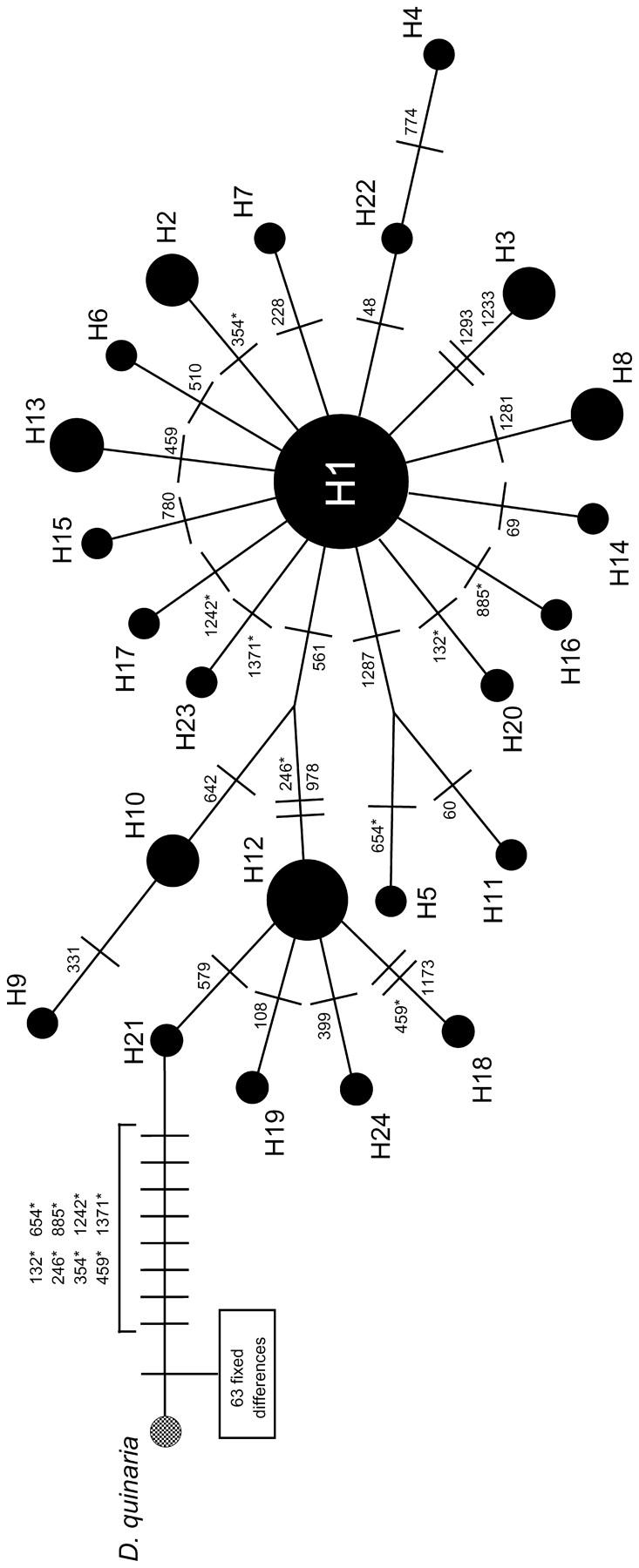
Median-joining network of mtDNA haplotypes within D. recens. The frequency of each haplotype is proportional to its area. The predominant haplotype is indicated as H1. D. quinaria is included as an outgroup. Numbers on the network indicate the base-pair positions of mutations within the mtDNA sequences. Asterisks indicate the eight inferred back mutations within D. recens.
Levels of polymorphism estimated included nucleotide diversity (π; Tajima 1983; Nei 1987), number of segregating sites (θw; Watterson 1975; Nei 1987), haplotype number, and haplotype diversity (Rozas and Rozas 1999). Unless otherwise noted, the program DnaSP v.3.53 (26) was used to estimate parameters and to perform statistical analyses. We then asked whether the mtDNA has a disproportionate reduction in diversity in D. recens; thus, we used an HKA test to contrast levels of polymorphism and sequence divergence in 7248 bp of mtDNA with the three nuclear genes in D. recens and D. subquinaria (Hudson et al. 1987). For mtDNA, we included either all sites or silent sites only and for period, adhr, and tpi we used only silent sites. The difference in effective population sizes among the loci was taken into account in assessing statistical significance (Hudson et al. 1987).
Neutrality tests were performed on all the genes to determine if the frequency spectrum of polymorphisms conformed to the predictions of the neutral model of molecular evolution. At equilibrium with selective neutrality, both Tajima's D (32) and Fu and Li's D (33) are expected to be zero. A value significantly less than zero indicates a higher-than-expected number of low-frequency variants and can result from a recent selective sweep or population expansion (Tajima 1989; Aris-Brosou and Excoffier 1996).
Rates of molecular evolution:
Rates of molecular evolution of mtDNA in D. recens and D. subquinaria were compared using a relative-rates test, as implemented in the program RRTree (Robinson-Rechavi and Huchon 2000). We estimated substitution rates for synonymous and nonsynonymous sites within the 7248-bp mtDNA sequences of the two species. We used a closely related species, D. quinaria, as the outgroup to increase the statistical power of the relative-rates test (Muse and Weir 1992).
To test whether differences in substitution rates were limited to mtDNA or were genome-wide, relative-rate tests were also carried out on four nuclear genes, period, adhr, tpi, and R1, the latter being a retrotransposable element that inserts into the rDNA locus. R1 sequences, representing 781 bp of the 3′-untranslated region of the element, were obtained from one individual each of D. recens (GenBank accession no. AF248076), D. subquinaria (W. Burke and T. Eickbush, unpublished data), and D. quinaria (AF24874). Previous analyses have demonstrated that R1 elements are passed vertically within species and evolve at rates similar to those of nuclear genes (Gentile et al. 2001).
Characterization of substitution patterns:
To address whether Muller's ratchet has affected mtDNA evolution in D. recens, we asked whether the dN/dS ratio (ω) of the 7248 bp of mtDNA is elevated in this species relative to D. subquinaria and D. quinaria. The parameter ω was estimated in three ways: (1) constraining all branches in the phylogeny to have the same value, (2) allowing ω to differ between the D. recens and the D. subquinaria + D. quinaria branches of the tree, and (3) allowing ω to vary among all three species. Likelihood-ratio tests were used to determine if a D. recens-specific value of ω significantly improved the fit to the data. These analyses were performed using the codeml program in the PAML package, with expected codon frequencies estimated from the average nucleotide frequencies in the three positions and one ratio for dN/dS among codons (Yang 1997, 2000). The data set comprises 12 mtDNA sequences from D. recens and thus includes both fixed and polymorphic mutations. Because only one sequence per species is available for D. subquinaria and D. quinaria, all mutations identified in D. recens (some of which may be polymorphic) were used to estimate its dN/dS ratio.
We also determined whether mutational changes in these mtDNA sequences were from either G or C to either A or T or in the other direction. In D. recens, both polymorphic and apparently fixed mutations were identified. Because only one comparable D. subquinaria sequence has been obtained, polymorphic and fixed mutations in this lineage are not distinguished.
RESULTS AND DISCUSSION
Levels and patterns of diversity:
All 33 isolates of Wolbachia from wild-caught D. recens had identical wsp sequences, including the hypervariable regions (GenBank AY154399). Consistent with earlier studies, the Wolbachia strain present in D. recens falls within the A clade of Wolbachia and is closely related to the Wolbachia strains found in D. simulans and D. melanogaster (Dyer and Jaenike 2004). Because wsp is the most rapidly evolving gene known in Wolbachia (Zhou et al. 1998) and because Wolbachia are vertically transmitted, this lack of sequence variation indicates that all extant Wolbachia and mtDNA haplotypes within D. recens are descended from a single ancestral infected female. The D. recens mtDNA haplotype network (Figure 1) shows that this particular infection has been in the species long enough for a considerable number of mutations to accumulate. The discrepancy between the number of polymorphisms within the mtDNA and wsp presumably is due to a higher mutation rate in the mtDNA. While we cannot pinpoint the ancestral mtDNA haplotype, it is not the one that is currently at highest frequency (H1), as this is a derived state (Shimodaira-Hasegawa test, P = 0.021, rejecting an alternative tree with H1 placed ancestral to all other D. recens haplotypes).
Does this pattern of mtDNA variation within D. recens differ from its uninfected sister species D. subquinaria, and more importantly, from the rest of the D. recens genome? Analyses of polymorphism (Table 1) show that mitochondrial DNA variation is much lower in D. recens than in D. subquinaria by all measures, including the number of segregating sites (θw), nucleotide diversity (π), intraspecific sequence divergence, and haplotype diversity. The numbers of segregating sites and nucleotide diversity at the COI gene were, respectively, 4-fold and 10-fold lower in D. recens than in D. subquinaria, whether all sites or only silent sites were considered. In contrast, the nuclear genes period, adhr, and tpi have higher levels of diversity in D. recens than in D. subquinaria (Table 1).
TABLE 1.
Nucleotide diversity estimates and tests for departure from neutrality for mtDNA and nuclear genes fromD. recens andD. subquinaria
| Gene species | N | L | h | S | θw (total) |
θw (silent) |
π (total) |
π (silent) |
Tajima's D | Fu and Li's D |
|---|---|---|---|---|---|---|---|---|---|---|
| COI | ||||||||||
| D. recens | 58 | 1411/329 | 23 | 25/24 | 0.0038 | 0.0158 | 0.0014 | 0.0058 | −2.040* | −3.422* |
| D. subquinaria | 26 | 1411/329 | 25 | 83/81 | 0.0149 | 0.0630 | 0.0140 | 0.0592 | −0.297 | −0.390 |
| mtDNAa | ||||||||||
| D. recens | 12 | 8757/1827 | 12 | 63/49 | 0.0024 | 0.0087 | 0.0013 | 0.0047 | −2.120** | −2.470* |
| period | ||||||||||
| D. recens | 36 | 624/138 | 34 | 66/44 | 0.0254 | 0.0735 | 0.0250 | 0.0717 | −0.308 | −0.901 |
| D. subquinaria | 11 | 624/138 | 8 | 20/13 | 0.0110 | 0.0307 | 0.0119 | 0.0385 | 0.158 | 0.300 |
| adhr | ||||||||||
| D. recens | 14 | 516/114 | — | 56/18 | 0.0305 | 0.0367 | 0.0266 | 0.0376 | −0.938 | −0.880 |
| D. subquinaria | 18 | 516/114 | — | 39/14 | 0.0197 | 0.0356 | 0.0216 | 0.0271 | 0.189 | 0.322 |
| tpi | ||||||||||
| D. recens | 26 | 381/95 | — | 28/26 | 0.0192 | 0.0477 | 0.0120 | 0.0467 | −1.682 | −2.218 |
| D. subquinaria | 24 | 381/95 | — | 8/8 | 0.0056 | 0.0224 | 0.0047 | 0.0187 | −0.533 | −1.0527 |
N, the number of sequences; L, average length of sequences from each species (entire sequence/synonymous sites only); h, number of different haplotypes; and S, the number of polymorphic (segregating) sites for all sites/synonymous sites only.
P < 0.05,
P < 0.01.
Represents portions of 12 mitochondrial protein-encoding genes.
HKA tests were used to contrast patterns of polymorphism and divergence among the mtDNA sequences encompassing 12 protein-coding genes and the nuclear sequences encoding per, adhr, and tpi in D. recens and D. subquinaria (Hudson et al. 1987). In the contrasts comparing silent sites among mtDNA and the three nuclear genes, the results were significant or nearly so for all three comparisons (mtDNA-per, χ2 = 6.06 and P = 0.014; mtDNA-adhr, χ2 = 3.78 and P = 0.052; mtDNA-tpi, χ2 = 5.82 and P = 0.016). However, none of the comparisons among the nuclear genes was significant (P > 0.5 in every case). Again, these data support an mtDNA-specific reduction in variation within D. recens.
Comparing D. recens with another ecologically and phylogenetically related species bolsters the conclusion that this species has disproportionately low levels of mtDNA variation. D. falleni has a geographical range size and population density similar to those of D. recens yet it is not infected with Wolbachia (Shoemaker et al. 1999). Average heterozygosity across 18 allozyme loci is very similar between the two species [0.191 for D. recens and 0.185 for D. falleni (Lacy 1982)]. Furthermore, two autosomal loci (tpi and Adhr), whose patterns of variation are consistent with neutral expectations, exhibit very similar levels of silent-site nucleotide diversity, whether measured by π (mean = 0.043 for both D. recens and D. falleni) or θ (mean = 0.042 for D. recens and 0.055 for D. falleni). The data for D. falleni were obtained from Table 4 of Dyer and Jaenike (2004). In striking contrast, mtDNA nucleotide diversity, estimated from RFLP variation, is five times greater in D. falleni than in D. recens (Shoemaker et al. 1999).
Next, we ask whether this reduction in mtDNA diversity in D. recens is the result of a Wolbachia-driven selective sweep or is, instead, consistent with demographic effects that shape both mtDNA and nuclear genes. Statistical tests of departure from neutral expectations are presented in Table 1. Both Tajima's D and Fu and Li's D were significantly less than zero for mtDNA of D. recens, indicating an excess of rare variants. In contrast, the nuclear genes in D. recens did not deviate significantly from neutral expectations, nor did either the COI or the nuclear genes of D. subquinaria. Thus, an excess of rare variants is specific to the mtDNA of D. recens, consistent with a recent selective spread of a particular mtDNA haplotype.
Population genetic data provide little evidence that an mtDNA sweep is currently occurring in D. recens. First, the most abundant haplotype, H1, is not at a significantly higher frequency than would be expected under neutrality [Hudson's haplotype test (31), P = 0.06, P = 0.50 if H1 and haplotypes one derived mutational step away are considered]. Furthermore, Fay and Wu's H (Fay and Wu 2000), which tests for an ongoing sweep by examining the frequency of derived mutations, is not significantly greater than zero for the mitochondrial COI gene of D. recens (H = 0.619; P > 0.10, accounting for backmutations and using D. quinaria as the outgroup; see Figure 1). Thus, the most recent mtDNA sweep in D. recens appears to be in a recovery phase that is expected to last up to 2Nf generations or until the next sweep occurs (Avise 2000).
In D. recens, decreased diversity and departure from neutrality of the mtDNA are most likely the result of hitchhiking with a Wolbachia infection during CI-driven sweep. This is consistent with the observed pattern of decreased diversity of the mtDNA relative to the rest of the genome shown in other Wolbachia-infected organisms (Ballard et al. 2002; Jiggins 2003). Given the high incidence of endosymbiont infection among insects and other arthropods, our results support the notion that low levels of mtDNA diversity within a species may not indicate a small effective population size overall.
Rates of molecular evolution:
Because the mtDNA in an infected host species will be taken through an effective population size of one host female with each Wolbachia sweep, Muller's ratchet is expected to affect the mtDNA of an endosymbiont-infected species. Such effects may be evident in elevated rates of substitution. To compare rates of evolution between D. recens and D. subquinaria, we used a relative-rates test. This test requires identification of an appropriate outgroup to the taxa being compared. All regions sequenced, including four nuclear gene regions and the ∼8-kb mtDNA sequence encompassing 12 protein coding genes within the mtDNA genome, are consistent with an earlier study by Perlman et al. (2003) that showed that D. recens and D. subquinaria are the most similar and that D. quinaria is a very closely related outgroup (Table 2).
TABLE 2.
Average pairwise sequence divergence betweenD. recens,D. subquinaria, andD. quinaria, showing that for all genesD. recens andD. subquinaria are the most similar
| Species pair
|
|||
|---|---|---|---|
| Gene | D. recens-D. subquinaria | D. recens-D. quinaria | D. subquinaria-D. quinaria |
| mtDNA (∼8 kb) | 0.029 (0.114)a | 0.039 (0.156) | 0.031 (0.128) |
| period | 0.041 (0.184) | 0.123 (0.348) | 0.114 (0.336) |
| adhr | 0.011 (0.047) | 0.033 (0.113) | 0.039 (0.105) |
| tpi | 0.003 (0.046) | 0.029 (0.128) | 0.033 (0.130) |
| R1 (untranslated region) | 0.046 | 0.077 | 0.08 |
Values in parentheses represent divergence estimates at synonymous sites only.
The relative-rates tests for mtDNA indicate that D. recens has experienced a significantly greater rate of substitution than D. subquinaria at synonymous sites and an elevated, but not significantly greater, rate at nonsynonymous sites (Table 3). In contrast, the rates of molecular evolution for the four nuclear genes were very similar between D. recens and D. subquinaria, with the exception of an accelerated rate of nonsynonymous evolution at the period gene in D. recens (Table 3). This latter finding is consistent with earlier studies suggesting that nonsynonymous sites at this gene are subject to selection and evolve at rates that vary among lineages (Wang and Hey 1996; Gleason and Powell 1997; Rand et al. 2000; Machado et al. 2002).
TABLE 3.
Relative rates test for silent and replacement sites for mtDNA,period,adhr,tpi, andR1 sequences inD. recens vs.D. subquinaria, usingD. quinaria as an outgroup
| No. of substitutions
|
Probability of rate similarity |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Species | N | L | Synonymous | Nonsynonymous | Pr(Ks) | Pr(Ka) |
| mtDNAa | D. recens | 11 | 7248 | 326 | 25 | 0.002 | 0.354 |
| D. subquinaria | 1 | 7248 | 260 | 21 | |||
| period | D. recens | 36 | 633 | 83 | 32 | 0.664 | 0.007 |
| D. subquinaria | 11 | 633 | 78 | 26 | |||
| adhr | D. recens | 14 | 516 | —b | 10 | —b | 0.388 |
| D. subquinaria | 18 | 516 | —b | 12 | |||
| tpi | D. recens | 26 | 381 | 16 | 3 | 0.477 | 0.161 |
| D. subquinaria | 24 | 381 | 18 | 3 | |||
| R1 | D. recens | 1 | 781 | 64c | — | 0.74 | — |
| D. subquinaria | 1 | 781 | 66c | — | |||
N, the number of D. subquinaria and D. recens sequences for each gene; L, the length of the gene region analyzed. Pr(Ks) and Pr(Ka) represent the probabilities that the rates of evolution of silent and replacement sites, respectively, do not differ between the two lineages compared.
Represents data from portions of 12 protein-coding genes.
Pr(Ks) not calculated for adhr due to saturation of silent sites.
Noncoding region.
How might Wolbachia infection cause an increase in the rate of mtDNA evolution in D. recens? If this effect were due to Muller's ratchet, one would expect to see evidence of a greater rate of fixation of slightly deleterious mutations, one signature of which would be an elevated rate of nonsynonymous relative to synonymous substitutions. A maximum-likelihood estimate of dN/dS (Yang 1997) is a more sensitive test than the relative-rates test for nonsynonymous changes, as the former test can decompose rate variation into species-specific lineages, rather than contrasting recens + quinaria to subquinaria + quinaria lineages as does the relative-rates test. Across ∼8 kb of the mtDNA genome, the dN/dS ratio (ω) in D. recens is over twice as great as in the branches leading to D. subquinaria and D. quinaria (Table 4). A likelihood-ratio test showed that models with ω varying between the D. recens and the D. subquinaria + D. quinaria branches fit the data significantly better than a model with ω the same in all branches (P = 0.004). Allowing ω to vary between D. quinaria and D. subquinaria did not significantly improve the fit (P = 0.63). Thus, the significant heterogeneity in dN/dS ratios among these species is attributable to the elevated value in D. recens. These findings are consistent with the hypothesis that Muller's ratchet has affected the molecular evolution of mtDNA in D. recens. Further, because it is highly unlikely that a mitochondrial haplotype containing multiple nonsynonymous substitutions would spread as a consequence of a single Wolbachia sweep, the data suggest that there has been a series of Wolbachia variant replacements within D. recens.
TABLE 4.
Maximum-likelihood estimates of ω (dN/dS ratio) of mtDNA genes inD. recens,D. subquinaria, andD. quinaria lineages, using model F3X4 of PAML (Yang 1997)
| Model | Parameter estimates | ln L | 2(Δ ln L) |
|---|---|---|---|
| One ratio | ω = 0.020 | −13328.47 | — |
| Two ratiosa | ω = 0.012quin+subquin, ω = 0.033recens | −13324.29 | 8.36 P = 0.004, d.f. = 1 |
| Three ratiosa | ω = 0.011quinaria, ω = 0.032recens, ω = 0.015subquinaria | −13324.17 | 0.24 P = 0.63, d.f. = 1 |
In the one-ratio model all lineages have the same ω-ratio. In the two-ratio model the non-D. recens and D. recens lineages have separate ω-ratios (ωquin+subquin and ωrecens, respectively). For the three-ratio model, each species has a separate ω-ratio.
Not only is the mitochondrial dN/dS ratio significantly greater in D. recens, but also the dS is, being equal to 0.125 in D. recens and 0.078 in D. subquinaria in the model in which ω is allowed to vary among lineages. The significance of this difference is evident in the relative-rates test of synonymous-site evolution of the mtDNA from D. recens vs. D. subquinaria (P = 0.002; Table 3). If synonymous sites are indeed neutral, this finding suggests that the mtDNA of D. recens has experienced a greater mutation rate than that of D. subquinaria, as the long-term rate of neutral substitutions is expected to equal the neutral mutation rate (Kimura 1968, 1987).
Itoh et al. (2002) argued that the greater rate of molecular evolution in some endosymbiotic bacteria is due to an elevated mutation rate, caused by the loss of DNA repair enzymes, which in turn is due to massive genome reduction in obligate endosymbionts. This explanation is very unlikely to account for the higher rate of substitution in the mtDNA of D. recens, because (1) there are no known repair pathways for Drosophila mtDNA, and (2) our sequences of ∼50% of the mtDNA from both species have revealed no deletions from the mitochondrial genome of D. recens. Alternatively, it is possible that Wolbachia affect the intracellular environment in a way that increases the mutation rate of mitochondrial, but not nuclear, DNA.
An elevated rate of synonymous substitution could also arise if these mutations are not selectively equivalent. In this case, Muller's ratchet, which is driven by recurrent Wolbachia sweeps, could increase the rate of substitution of slightly deleterious synonymous mutations, such as those that result in unpreferred codons. In fact, many of the synonymous substitutions in the mtDNA of D. recens represent changes to codons that are generally relatively underutilized in Drosophila, specifically, those that end in either G or C (Clary and Wolstenholme 1985). For the ∼8-kb sequence of mtDNA, we categorized all mutations that occurred in the D. recens and D. subquinaria lineages as either A/T → G/C or G/C → A/T. On the basis of our 12 sequences from D. recens, the mutations were further categorized as polymorphic or apparently fixed within the species. Because the frequencies of A/T → G/C and G/C → A/T mutations were virtually identical between the polymorphic and fixed categories within D. recens (Wald χ2 = 0.035; P = 0.85), we combined the fixed and polymorphic mutations found within D. recens to contrast with the mutations found in D. subquinaria. The contrast between the two species is shown in Table 5. The results indicate that D. recens has experienced a significant excess of A/T → G/C mutations relative to D. subquinaria (Wald χ2 = 4.45; P = 0.035). To the extent that the low GC content of Drosophila mtDNA is a result of selection (e.g., preference for AT-ending codons), the excess of A/T → G/C mutations in D. recens may reflect the weakened effect of selection on mtDNA in this species. Alternatively, this excess may reflect mutational bias operating via an as-yet-unknown mechanism.
TABLE 5.
Frequencies of A/T → G/C and G/C → A/T mutations withinD. recens andD. subquinaria lineages across ∼8 kb of mtDNA
An mtDNA sequence from D. quinaria was used to infer direction of change. Wald χ2 = 4.45; P = 0.035.
With respect to Muller's ratchet, the dynamics of a newly arisen, favorable Wolbachia mutation will be governed by a selection coefficient that reflects the positive effects of this mutation discounted by the negative effects of any deleterious mutations in the mitochondria with which the Wolbachia are cotransmitted. The net selection coefficient for the Wolbachia mutation will be greater—and thus its spread to fixation variant more likely—if it is associated with slightly deleterious synonymous mitochondrial mutations than with more deleterious nonsynonymous mutations. The recent findings that the spontaneous mutation rates of Caenorhabditis elegans and human mtDNA are 1–2 orders of magnitude greater than the substitution rate among phylogenetic lineages (Denver et al. 2000; Howell et al. 2003) indicate that most mitochondrial mutations in these species, including those that are synonymous, are deleterious (see also Ballard and Whitlock 2004). Our finding of an elevated synonymous substitution rate in D. recens is consistent with there being a similar mutational spectrum in Drosophila and with the fact that Wolbachia evolution, through a series of variant turnovers, can accelerate the rate of fixation of slightly deleterious synonymous mutations.
In conclusion, our findings show that Wolbachia, and probably other maternally transmitted endosymbionts, can severely depress levels of mtDNA diversity within an infected host species. In contrast, such infections may increase the rate of substitution in mtDNA. The significantly elevated dN/dS ratio in D. recens implicates the operation of Muller's ratchet and suggests that endosymbiont infection may contribute to mutational degradation of a host species' mtDNA. The greater rate of molecular evolution of mtDNA in endosymbiont-infected insect species, if general, has important consequences for the use of mtDNA as a molecular clock in insects. Finally, a positive correlation between polymorphism and divergence, as expected under the standard neutral theory, may not hold for vast numbers of endosymbiont-infected insects and other arthropods. Given these dramatic yet unappreciated effects and the widespread distribution of endosymbionts in arthropods, additional comparative studies similar to our study clearly are warranted to test the generality of these predictions. The discovery of appropriate species pairs for future comparisons rests on the reliable determination that a species in fact is not infected with Wolbachia or is infected with only a single strain, which in turn depends on more extensive within-species sampling than has been done in surveys to date (e.g., Werren et al. 1995; Jeyaprakash and Hoy 2000; Werren and Windsor 2000).
Acknowledgments
We thank L. Atkinson, C. Cornish, A. Cohn, D. Hinkle, R. Huey, J. Savage, and D. Shoemaker for helping to collect flies and A. Betancourt, J. Bollback, J. Crow, D. Goldfarb, D. Hinkle, L. Kaguni, P. Pamilo, J. Seger, N. Moran, D. Rand, E. Sia, C. Toomajian, and two anonymous reviewers for helpful discussions and/or comments on the manuscript. This research was supported by National Science Foundation grants DEB-0306554 to D.D.S. and DEB-0074141 to J.J.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession nos. AY154399, AY154400, AY154401, AY154402, AY154403, AY154404, AY154405, AY154406, AY154407, AY154408, AY154409, AY154410, AY154411, AY154412, AY154413, AY154414, AY154415, AY154416, AY154417, AY154418, AY154419, AY154420, AY154421, AY154422, AY154423, AY154424, AY154425, AY154426, AY154427, AY154428, AY154429, AY154430, AY154431, AY154432, AY154433, AY154434, AY154435, AY154436, AY154437, AY154438, AY154439, AY154440, AY154441, AY154442, AY154443, AY154444, AY154445, AY154446, AY154447, AY154448, AY154449, AY154450, AY154451, AY154452, AY154453, AY154454, AY154455, AY154456, AY154457.
References
- Abbot, P., and N. A. Moran, 2002. Extremely low levels of genetic polymorphism in endosymbionts (Buchnera) of aphids (Pemphigus). Mol. Ecol. 11: 2649–2660. [DOI] [PubMed] [Google Scholar]
- Andolfatto, P., and M. Przeworski, 2000. A genome-wide departure from the standard neutral model in natural populations of Drosophila. Genetics 156: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aris-Brosou, S., and L. Excoffier, 1996. The impact of population expansion and mutation rate heterogeneity on DNA sequence polymorphism. Mol. Biol. Evol. 13: 494–504. [DOI] [PubMed] [Google Scholar]
- Avise, J. C., 2000 Phylogeography: The History and Formation of Species. Harvard University Press, Cambridge, MA.
- Ballard, J. W. O., 2000. Comparative genomics of mitochondrial DNA in Drosophila simulans. J. Mol. Evol. 51: 64–75. [DOI] [PubMed] [Google Scholar]
- Ballard, J. W. O., 2004. Sequential evolution of a symbiont inferred from the host: Wolbachia and Drosophila simulans. Mol. Biol. Evol. 21: 428–442. [DOI] [PubMed] [Google Scholar]
- Ballard, J. W. O., and M. C. Whitlock, 2004. The incomplete natural history of mitochondria. Mol. Ecol. 13: 729–744. [DOI] [PubMed] [Google Scholar]
- Ballard, J. W. O., O. J. Hatzidakis, T. L. Karr and M. Kreitman, 1996. Reduced variation in Drosophila simulans mitochondrial DNA. Genetics 144: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard, J. W. O., B. Chernoff and A. C. James, 2002. Divergence of mitochondrial DNA is not corroborated by nuclear DNA, morphology, or behavior in Drosophila simulans. Evolution 56: 527–545. [DOI] [PubMed] [Google Scholar]
- Begun, D. J., 2001. The frequency distribution of nucleotide variation in Drosophila simulans. Mol. Biol. Evol. 18: 1343–1352. [DOI] [PubMed] [Google Scholar]
- Betran, E., and M. Ashburner, 2000. Duplication, dicistronic transcription, and subsequent evolution of the alcohol dehydrogenase and alcohol dehydrogenase-related genes in Drosophila. Mol. Biol. Evol. 17: 1344–1352. [DOI] [PubMed] [Google Scholar]
- Brynnel, E. U., C. G. Kurland, N. A. Moran and S. G. E. Andersson, 1998. Evolutionary rates for tuf genes in endosymbionts of aphids. Mol. Biol. Evol. 15: 574–582. [DOI] [PubMed] [Google Scholar]
- Caspari, E., and G. S. Watson, 1959. On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution 13: 568–570. [Google Scholar]
- Clark, M. A., N. A. Moran and P. Baumann, 1999. Sequence evolution in bacterial endosymbionts having extreme base compositions. Mol. Biol. Evol. 16: 1586–1598. [DOI] [PubMed] [Google Scholar]
- Clary, D., and D. Wolstenholme, 1985. The mitochondrial DNA molecule of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J. Mol. Evol. 22: 252–271. [DOI] [PubMed] [Google Scholar]
- Dean, M. D., K. J. Ballard, A. Glass and J. W. O. Ballard, 2003. Influence of two Wolbachia strains on population structure of East African Drosophila simulans. Genetics 165: 1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver, D. R., K. Morris, M. Lynch, L. L. Vassilieva and W. K. Thomas, 2000. High direct estimate of the mutation rate in the mitochondrial genome of Caenorhabditis elegans. Science 289: 2342–2344. [DOI] [PubMed] [Google Scholar]
- Dyer, K., and J. Jaenike, 2004. Evolutionarily stable infection by a male-killing endosymbiont in Drosophila melanogaster: molecular evidence from the host and parasite genomes. Genetics 168: 1443–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay, J., and C.-I Wu, 2000. Hitchhiking under positive Darwinian selection. Genetics 155: 1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine, P. E. M., 1978. On the dynamics of symbiote-dependent cytoplasmic incompatibility in culicine mosquitoes. J. Invertebr. Pathol. 30: 10–18. [DOI] [PubMed] [Google Scholar]
- Gentile, K. L., W. D. Burke and T. H. Eickbush, 2001. Multiple lineages of R1 retrotransposable elements can coexist in the rDNA loci of Drosophila. Mol. Biol. Evol. 18: 235–245. [DOI] [PubMed] [Google Scholar]
- Gleason, J. M., and J. R. Powell, 1997. Interspecific and intraspecific comparisons of the period locus in the Drosophila willistoni sibling species. Mol. Biol. Evol. 14: 741–753. [DOI] [PubMed] [Google Scholar]
- Guillemaud, T., N. Pasteur and F. Rousset, 1997. Contrasting levels of variability between cytoplasmic genomes and incompatibility types in the mosquito Culex pipiens. Proc. R. Soc. Lond. Ser. B Biol. Sci. 264: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A., D. J. Clancy and E. Merton, 1994. Cytoplasmic incompatibility in Australian populations of Drosophila melanogaster. Genetics 136: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A., M. Hercus and H. Dagher, 1998. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, N., C. B. Smejkal, D. A. Mackey, P. F. Chinnery, D. M. Turnbull et al., 2003. The pedigree rate of sequence divergence in the human mitochondrial genome: there is a difference between phylogenetic and pedigree rates. Am. J. Hum. Genet. 72: 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. R., M. Kreitman and M. Aguadé, 1987. A test of neutral molecular evolution based on nucleotide data. Genetics 116: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, L. D., and G. T. McVean, 1996. Clade selection, reversible evolution, and the persistence of selfish elements: the evolutionary dynamics of cytoplasmic incompatibility. Proc. R. Soc. Lond. Ser. B Biol. Sci. 262: 97–104. [Google Scholar]
- Itoh, T., W. Martin and M. Nei, 2002. Acceleration of genomic evolution caused by enhanced mutation rate in endocellular symbionts. Proc. Natl. Acad. Sci. USA 99: 12944–12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, A. C., and J. W. O. Ballard, 2000. Expression of cytoplasmic incompatibility in Drosophila simulans and its impact on infection frequencies and distribution of Wolbachia pipientis. Evolution 54: 1661–1672. [DOI] [PubMed] [Google Scholar]
- James, A. C., M. D. Dean, M. E. McMahon and J. W. O. Ballard, 2002. Dynamics of double and single Wolbachia infections in Drosophila simulans from New Caledonia. Heredity 88: 182–189. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash, A., and M. A. Hoy, 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropods. Insect Mol. Biol. 9: 393–405. [DOI] [PubMed] [Google Scholar]
- Jiggins, F. M., 2003. Male-killing Wolbachia and mitochondrial DNA: selective sweeps, hybrid introgression and parasite population dynamics. Genetics 164: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone, R., and G. Hurst, 1996. Maternally inherited male-killing microorganisms may confound interpretation of mitochondrial DNA variability. Biol. J. Linn. Soc. 58: 453–470. [Google Scholar]
- Kambhampati, S., K. S. Rai and D. M. Verleye, 1992. Frequencies of mitochondrial DNA haplotypes in laboratory cage populations of the mosquito Aedes albopictus. Genetics 132: 20–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, M., 1968. Evolutionary rate at the molecular level. Nature 217: 624–626. [DOI] [PubMed] [Google Scholar]
- Kimura, M., 1987. Molecular evolutionary clock and the neutral theory. J. Mol. Evol. 26: 24–33. [DOI] [PubMed] [Google Scholar]
- Lacy, R. C., 1982. Niche breadth and abundance as determinants of genetic variation in populations of mycophagous Drosophilid flies (Diptera: Drosophilidae). Evolution 36: 1265–1275. [DOI] [PubMed] [Google Scholar]
- Machado, C. A., R. M. Kliman, J. A. Markert and J. Hey, 2002. Inferring the history of speciation from multilocus DNA sequence data: the case of Drosophila pseudoobscura and close relatives. Mol. Biol. Evol. 19: 472–488. [DOI] [PubMed] [Google Scholar]
- Marcade, I., C. Souty-Grosset, D. Bouchon, T. Rigaud and R. Raimond, 1999. Mitochondrial DNA variability and Wolbachia infection in two sibling woodlice species. Heredity 83: 71–78. [DOI] [PubMed] [Google Scholar]
- Moran, N., 1996. Accelerated evolution and Muller's ratchet in endosymbiotic bacteria. Proc. Natl. Acad. Sci. USA 93: 2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muse, S. V., and B. S. Weir, 1992. Testing for equality of evolutionary rates. Genetics 132: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei, M., 1987 Molecular Evolutionary Genetics. Columbia University Press, New York.
- Patterson, J. T., and W. S. Stone, 1952 Evolution in the Genus Drosophila. Macmillan, New York.
- Peek, A. S., R. C. Vrijenhoek and B. S. Gaut, 1998. Accelerated evolutionary rate in sulfur-oxidizing bacteria associated with the mode of symbiont transmission. Mol. Biol. Evol. 15: 1514–1523. [DOI] [PubMed] [Google Scholar]
- Perlman, S. J., G. S. Spicer, D. D. Shoemaker and J. Jaenike, 2003. Associations between mycophagous Drosophila and their Howardula nematode parasites: a worldwide phylogenetic shuffle. Mol. Ecol. 12: 237–249. [DOI] [PubMed] [Google Scholar]
- Przeworski, M., J. D. Wall and P. Andolfatto, 2001. Recombination and the frequency spectrum in Drosophila melanogaster and Drosophila simulans. Mol. Biol. Evol. 18: 291–298. [DOI] [PubMed] [Google Scholar]
- Rand, D. M., D. M. Weinrich and B. O. Cezairliyan, 2000. Neutrality tests of conservative-radical amino acid changes in nuclear- and mitochondrially-encoded proteins. Gene 291: 115–125. [DOI] [PubMed] [Google Scholar]
- Rigaud, T., D. Bouchon, C. Souty-Grosset and R. Raimond, 1999. Mitochondrial DNA polymorphism, sex ratio distorters, and population genetics in the isopod Armadillidium vulgare. Genetics 152: 1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Rechavi, M., and D. Huchon, 2000. RRTree: relative-rate tests between groups of sequences on a phylogenetic tree. Bioinformatics 16: 296–297. [DOI] [PubMed] [Google Scholar]
- Rokas, A., R. J. Atkinson, G. S. Brown, S. A. West and G. N. Stone, 2001. Understanding patterns of genetic diversity in the oak gallwasp Biorhiza pallida: Demographic history or a Wolbachia selective sweep? Heredity 87: 294–304. [DOI] [PubMed] [Google Scholar]
- Rozas, J., and R. Rozas, 1999. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15: 174–175. [DOI] [PubMed] [Google Scholar]
- Shoemaker, D. D., and J. Jaenike, 1997. Habitat continuity and the genetic structure of Drosophila populations. Evolution 51: 1326–1332. [DOI] [PubMed] [Google Scholar]
- Shoemaker, D. D., V. Katju and J. Jaenike, 1999. Wolbachia and the evolution of reproductive isolation between Drosophila recens and Drosophila subquinaria. Evolution 53: 1157–1164. [DOI] [PubMed] [Google Scholar]
- Shoemaker, D. D., K. G. Ross, L. Keller, E. L. Vargo and J. H. Werren, 2000. Wolbachia infections in native and introduced populations of fire ants (Solenopsis spp.). Insect Mol. Biol. 9: 661–673. [DOI] [PubMed] [Google Scholar]
- Shoemaker, D. D., G. Keller and K. G. Ross, 2003. Effects of Wolbachia on mtDNA variation in two fire ant species. Mol. Ecol. 12: 1757–1771. [DOI] [PubMed] [Google Scholar]
- Simon, C., F. Frati, A. Beckenbach, B. Crespi, H. Liu et al., 1994. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 81: 651–701. [Google Scholar]
- Solignac, M., D. Vautrin and F. Rousset, 1994. Widespread occurrence of the proteobacteria Wolbachia and partial cytoplasmic incompatibility in Drosophila melanogaster. C. R. Acad. Sci. III 317: 461–470. [Google Scholar]
- Stouthamer, R., J. A. J. Breeuwer and G. D. D. Hurst, 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53: 71–102. [DOI] [PubMed] [Google Scholar]
- Tajima, F., 1983. Evolutionary relationship of DNA sequences in finite populations. Genetics 105: 437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima, F., 1989. The effect of change in population size on DNA polymorphism. Genetics 123: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thao, M. L., N. A. Moran, P. Abbot, E. B. Brennan, D. H. Burckhardt et al., 2000. Cospeciation of psyllids and their primary prokaryotic endosymbionts. Appl. Environ. Microbiol. 66: 2898–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M., 1994. Evolution of incompatibility-inducing microbes and their hosts. Evolution 48: 1500–1513. [DOI] [PubMed] [Google Scholar]
- Turelli, M., and A. A. Hoffmann, 1991. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353: 440–442. [DOI] [PubMed] [Google Scholar]
- Turelli, M., and A. A. Hoffmann, 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140: 1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M., A. A. Hoffmann and S. W. McKechnie, 1992. Dynamics of cytoplasmic incompatibility and mtDNA variation in natural Drosophila simulans populations. Genetics 132: 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R.-L., and J. Hey, 1996. The speciation history of Drosophila pseudoobscura and close relatives: inferences from DNA sequence variation at the period locus. Genetics 144: 1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson, G. A., 1975. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 7: 256–276. [DOI] [PubMed] [Google Scholar]
- Weinrich, D. M., and D. M. Rand, 2000. Contrasting patterns of nonneutral evolution in proteins encoded in nuclear and mitochondrial genomes. Genetics 156: 385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernegreen, J. J., and N. A. Moran, 1999. Evidence for genetic drift in endosymbionts (Buchnera): analyses of protein-coding genes. Mol. Biol. Evol. 16: 83–97. [DOI] [PubMed] [Google Scholar]
- Werren, J. H., and J. Jaenike, 1995. Wolbachia and cytoplasmic incompatibility in mycophagous Drosophila and their relatives. Heredity 74: 320–326. [DOI] [PubMed] [Google Scholar]
- Werren, J. H., and D. M. Windsor, 2000. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. R. Soc. Lond. Ser. B Biol. Sci. 267: 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, J. H., L. Guo and D. W. Windsor, 1995. Distribution of Wolbachia in neotropical arthropods. Proc. R. Soc. Lond. Ser. B Biol. Sci. 262: 197–204. [Google Scholar]
- Woolfit, M., and L. Bromham, 2003. Increased rates of sequence evolution in endosymbiotic bacteria and fungi with small effective population sizes. Mol. Biol. Evol. 20: 1545–1555. [DOI] [PubMed] [Google Scholar]
- Yang, Z. H., 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13: 555–556. [DOI] [PubMed] [Google Scholar]
- Yang, Z. H., 2000 Phylogenetic Analysis by Maximum Likelihood (PAML), Version 3.0. University College, London.
- Zhou, W., F. Rousset and S. O'Neill, 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. Ser. B Biol. Sci. 265: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]


