Abstract
One of the most solid generalizations of transmission genetics is that the phenotypic variance of populations carrying a major mutation is increased relative to the wild type. At least some part of this higher variance is genetic and due to release of previously hidden variation. Similarly, stressful environments also lead to the expression of hidden variation. These two observations have been considered as evidence that the wild type has evolved robustness against genetic variation, i.e., genetic canalization. In this article we present a general model for the interaction of a major mutation or a novel environment with the additive genetic basis of a quantitative character under stabilizing selection. We introduce an approximation to the genetic variance in mutation-selection-drift balance that includes the previously used stochastic Gaussian and house-of-cards approximations as limiting cases. We then show that the release of hidden genetic variation is a generic property of models with epistasis or genotype-environment interaction, regardless of whether the wild-type genotype is canalized or not. As a consequence, the additive genetic variance increases upon a change in the environment or the genetic background even if the mutant character state is as robust as the wild-type character. Estimates show that this predicted increase can be considerable, in particular in large populations and if there are conditionally neutral alleles at the loci underlying the trait. A brief review of the relevant literature suggests that the assumptions of this model are likely to be generic for polygenic traits. We conclude that the release of hidden genetic variance due to a major mutation or environmental stress does not demonstrate canalization of the wild-type genotype.
THE idea that wild-type genotypes are mutationally robust, i.e., buffered against the effect of mutations, goes back to Waddington (1957), who originally introduced the concept as canalization. Waddington gave a simple, intuitive argument why wild types should evolve buffering: For a well-adapted trait almost all mutations with an effect on this trait are deleterious. For this reason, any modifier that reduces the effect of mutations and thus keeps the trait closer to its optimum should be selected. Waddington's intuition was backed by a series of impressive experiments, following his own work (cf. Scharloo 1991). The starting point is the common observation of an increased phenotypic variance in populations that carry a major mutation or are exposed to environmental stresses. The experiments show that much of the added variance is genetic (since it responds to artificial selection) and based on unexpressed (hidden) variation already present in the base population (since inbred lines show no selection response after a similar treatment). The increase in the genetic variance is then interpreted as evidence for reduced variability of the wild type with respect to mutations, hence genetic robustness or canalization. Subsequent theoretical work showed, however, that the evolution of genetic robustness, while possible, requires special assumptions and is often observed in population genetic models (Wagner et al. 1997; Hermisson et al. 2003). Considering the apparent empirical evidence, this has been perceived as a “discontinuity between theory and experiment” (Gibson and Wagner 2000).
Since genetic variance and the mutational variability of phenotypes underlie all Darwinian evolution, the finding that variability itself depends on the genotype and can evolve could have important implications for tempo and mode of the evolutionary process. In particular, if variability is itself adaptive, evolution actively forms and influences its own course. Evolution could modulate the adaptive process in two ways. The first possibility is the differential production of new variance. A well-known example is the accumulation of mutator strains in bacteria in times of environmental stress (Sniegowski et al. 2000). The second way is through buffering and the differential exposure of genetic variation to selection. In this case, evolvability is modulated by hide-and-release of genetic variation that is present in the population. As demonstarted most clearly by the above-mentioned experiments, there is indeed ample evidence for genetic variation that has no effect on the phenotype under normal conditions, but is expressed in mutants or in altered environments.
Given these observations, a lot of work has been devoted to the question of how a genetic system could accomplish the storing of variation, as well as its targeted exposure (Eshel and Matessi 1998; Rutherford and Lindquist 1998; Hansen et al. 2000; Masel and Bergman 2003; Rutherford 2003; see also Wagner and Altenberg 1996; Gerhart and Kirschner 1998; de Visser et al. 2003; Rutherford 2003 for reviews). Usually, the storing capacity is thought to be connected to a special mechanism that provides buffering of the wild population with respect to the effects of naturally occurring mutations. The release of variation then follows from the breakdown of this mechanism under certain circumstances.
In this note we point out that the evolution of such a buffering mechanism is not needed to obtain an increase of expressed genetic variation after an environmental or genetic change. The potential to release hidden variation is a generic property of a genetic system under two conditions: (1) a population in or near mutation-selection (and drift) balance and (2) epistasis or gene-environment interactions (G × E). The increase in variation can be considerable even if the character state under the changed conditions is no more—or even less—canalized than the wild type. There are two consequences of this finding. On the one hand, there is no need to demonstrate canalization of the wild type in arguments about a potential evolutionary role of hidden variation. On the other hand, the result shows that the observation of hidden variation is not sufficient to imply canalization of the wild type. As a consequence, we argue that the classic experiments demonstrating hidden variation do not provide convincing evidence for mutational robustness.
MODEL AND RESULTS
Consider a polygenic trait with genotypic value
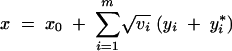 |
1 |
In this expression, x0 is the wild-type genotypic value and 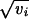 yi (respectively
yi (respectively 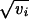 y*i) are the effects of single-locus substitutions of maternal (paternal) alleles. Both yi (y*i) and vi > 0 are taken from a continuum of values. Mutations at these loci occur at rate ui and add a random increment di to yi (y*i). The distribution of the di is normalized to unit variance and is assumed to have zero mean. This makes vi the variance of mutational effects on the ith locus, for brevity called the ith locus variance. The model assumes no sex differences, no dominance, and also neglects epistatic effects among segregating alleles.
y*i) are the effects of single-locus substitutions of maternal (paternal) alleles. Both yi (y*i) and vi > 0 are taken from a continuum of values. Mutations at these loci occur at rate ui and add a random increment di to yi (y*i). The distribution of the di is normalized to unit variance and is assumed to have zero mean. This makes vi the variance of mutational effects on the ith locus, for brevity called the ith locus variance. The model assumes no sex differences, no dominance, and also neglects epistatic effects among segregating alleles.
We are interested in the impact of a “major” change in the genetic background or in the ecological conditions on the statistical properties of the trait. Such a change can affect the trait in two ways. On the one hand, it can alter the trait mean and/or the trait optimum, thereby creating directional selection pressure. On the other hand, it can also lead to transient or permanent changes in the genetic variance and mutational variability properties of the trait, thereby affecting the ability of the population to respond to selection. The latter is a consequence of epistasis or G × E, which both lead to changes in the effects of new and segregating mutations. In our model this is included as a change of the locus variances vi. We refer to the conditions before and after the change as “old” and “new,” respectively, and use labels o and n (e.g., vo,i and vn,i) to distinguish both cases. For genetic changes, old represents the wild-type genetic background and new a mutant background. Some of the vo,i or vn,i may also be zero. The sum in Equation 1 runs over the variational basis of the trait, which we define as the collection of all polymorphic loci that affect the trait under either old or new conditions. Loci may be gene loci or adequately chosen smaller units. In averages over replicates, different loci are assumed to be statistically independent (i.e., we ignore linkage disequilibria due to selection).
In the following, we analyze the relation between two quantities before and directly after the environmental or genetic change: the (additive) genetic variance VG as a measure of the adaptive potential (evolvability) and the mutational variance VM as a measure of the sensitivity of the trait with respect to natural mutations (its variability, sensu Wagner and Altenberg 1996). For given locus variances, the mutational variance under old and new conditions is defined as
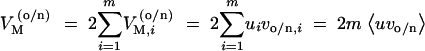 |
2 |
Here and in the following, the angle brackets denote the average over the m loci that contribute to the variational basis of the trait. For given mutation rates ui, low VM defines a state of relative mutational robustness or genetic canalization (Wagner et al. 1997; de Visser et al. 2003).
Assuming linkage equilibrium, the expected genetic variance (average over replicates or over time) under old conditions follows as
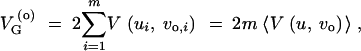 |
3 |
where V(ui, vo,i) is the expected genetic variance at a haploid locus (with mutation rate ui and variance vo,i). For the genetic variance directly after the change in the genetic background or the environmental conditions we assume that the variance of the standing genetic variation at each locus is changed by the same factor as the variance of new mutations. This is true, in particular, if all allelic effects at the same locus are rescaled by a single factor as in the multilinear model of Hansen and Wagner (2001). (While we need this assumption to keep the model tractable, note that reshuffling of allelic effects within loci would add to the effect of reshuffling effects among loci, which is what we focus on in this article. The assumption is therefore conservative with respect to our results.) We then obtain
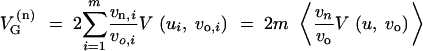 |
4 |
(the ratio of the locus variances, vn,i/vo,i, corresponds to the squared epistasis factor in Hansen and Wagner 2001).
We define the following dimensionless measures for the impact of the environmental or genetic change on VG and VM,
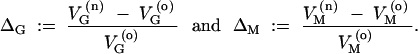 |
5 |
ΔG is the coefficient of hidden variation. It measures the amount of newly released genetic variation relative to the genetic variation that is expressed on the phenotype under the old conditions, i.e., the relative change in evolvability. ΔM is the canalization coefficient. It measures the relative change in the mutational variability. ΔM > 0 indicates that the population under the old conditions is mutationally robust (canalized). If the distributions of mutation rates and locus variances are independent (which we assume in the following), ΔG and ΔM are related according to
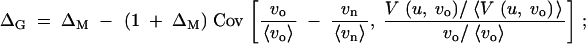 |
6 |
see the appendix for a derivation and discussion of general cases. All averages and the covariance are with respect to the distribution of the mutation rates ui and variances vo,i and vn,i across the loci.
We can distinguish three contributions to ΔG. The first is the change in the mutational variability ΔM. This term contributes to the hidden variation whenever the population under the old conditions is canalized. The second contribution is the (negative) covariance in Equation 6, −Cov[…], which we analyze in detail in the following section. Since this term does not vanish for ΔM = 0, it captures the change in expressed variation if the population is not particularly robust under the old conditions. Finally, there is an interaction term that is given by the product of the canalization coefficient and the covariance, −ΔMCov[…].
The release of hidden variation in epistatic systems:
Values of ΔG and ΔM that deviate from zero are the consequence of epistatis or G × E. To understand how different types of interactions influence these quantities, we introduce an explicit model for the effect of interactions on the locus variances. Assume the variances after the environmental or genetic change are related to the variances before the change as
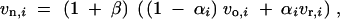 |
7 |
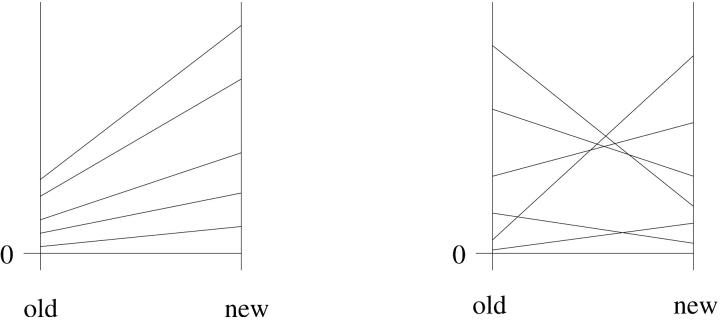
where β > −1 and αi ∈ [0, 1] are interaction parameters, αi = 0 = β corresponding to additivity. vr,i is a random variable that is independent of vo,i, normalized such that 〈vr〉 = 〈vo〉. Parameters αi are assumed independent of vo,i and vr,i. The different types of interactions that are defined by the parameters αi and β correspond to clearly distinguishable scenarios. (1 + β) acts as a uniform scaling factor of allelic effects across loci (and hence of the locus variances). In a reaction-norm picture of interactions, αi ≡ 0 and β ≠ 0 corresponds to a one-sided spread of lines. The αi collectively parameterize the randomization due to epistasis or G × E, i.e., all changes that lead to a reduced correlation of old and new locus variances. αi > 0 and β = 0 means that allelic effects do not change on average, but change relative to each other across loci. The typical pattern of this type of interaction in a reaction-norm picture are line crossings, see Figure 1 (cf. also Gibson and van Helden 1997, their Figure 1).
Figure 1.—
Different types of interactions in a schematic reaction-norm picture. Average (standard mean) allelic effects at various loci under “old” and “new” conditions (change of the environment or the genetic background) are shown. Left, αi ≡ 0 and β ≠ 0 leading to a one-sided spread of lines (canalization scenario). Right, αi > 0 and β = 0 leading to line crossing (variable interaction scenario).
Inserting (7) into (4) and (6), we find ΔM = β and
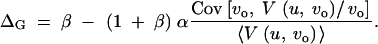 |
8 |
α := 〈α〉 is the mean (over loci) of the interaction parameters αi. We thus see that the canalization coefficient is affected only by β, but independent of the αi. Further, the covariance term in Equation 6 depends only on a single composite-interaction parameter 〈α〉, but is independent of β or further details of epistasis or G × E that are parameterized by the αi. In terms of the statistics of the old and new locus variances, this composite parameter can be expressed as
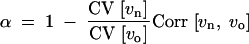 |
9 |
(This equation is proved by inserting Equation 7 for vn and using the independence of the vo,i and vr,i.) We see that two factors contribute α: The parameter increases if the correlation among old and new locus variances is small and if the genetic architecture of the trait is more inhomogeneous under old than under new conditions (i.e., the coefficient of variation, CV, decreases upon the change). In accordance with this interpretation of the interaction parameters, we call the case of α = 0, β ≠ 0 the canalization scenario (β > 0 and β < 0 corresponding to increased and reduced canalization of the wild type) and the case of α > 0, β = 0 the variable interaction scenario of the trait architecture.
We now proceed to analyze the covariance term in (8), which is the sole contribution to the hidden variation coefficient in the variable interaction scenario. The sign of this term depends on the functional dependence of V(u, vo) (the contribution of haploid loci to the genetic variance under the old conditions) on the locus variance vo. If the ratio V(u, vo)/vo decreases with vo (over the range of the distribution), the covariance term will be negative, and the contribution to ΔG positive, and vice versa. We investigate this functional dependence in the balance of mutation, drift, and stabilizing selection on the trait. We assume weak stabilizing viability selection with a quadratic fitness function,
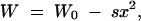 |
10 |
where s measures the strength of selection. Since we assume linkage equilibrium, the genetic variance of the trait VG is determined by the genetic variances of the haploid loci V(u, v) via Equation 3 (we drop the index o in this paragraph).
There are three standard approximations for V(u, v) under stabilizing selection, which are valid in different parameter regions (Bürger 2000). These are the neutral approximation
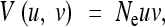 |
11 |
which holds whenever selection can be neglected relative to drift (svNe ≪ min{1, 1/(uNe)}); the Gaussian approximation,
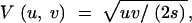 |
12 |
which is adequate if uNe ≫ svNe ≫ 1/(uNe); and the house-of-cards approximation,
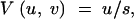 |
13 |
which is the leading-order term for strong selection, svNe ≫ max{1, uNe}. We see from these relations that V(u, v) increases less than linearly with v whenever selection plays a role. To cover the entire parameter range and for an estimate of ΔG in a trait with unequal locus effects, it is helpful to incorporate these approximations into a single analytical expression. This is accomplished by the following form, which is derived in the appendix:
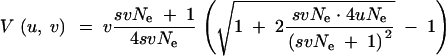 |
14 |
As discussed in the appendix, this expression interpolates between the three approximations above. It also reproduces the well-known stochastic house of cards and the stochastic Gaussian approximations in the respective limits (i.e., if u → 0, respectively svNe ≪ 1 ≪ uNe). We therefore call Equation 14 the stochastic house of Gauss (SHG) approximation. Taking the derivative with respect to v, it may readily be shown that, according to the SHG approximation, the ratio V(u, v)/v indeed strictly decreases with v for arbitrary values of s, u, and Ne.
We conclude that for a trait in the balance of mutation, stabilizing selection, and drift, hidden variation is released after an environmental or genetic change even if the population is originally not canalized (variable interaction scenario). This holds independently of the distributions of mutational effects or mutation rates, under the sole condition that (some of) the locus variances change due to epistasis or G × E (α ≠ 0). We can give an interpretation of this result by noting that v and V(u, v)/v in Equation 8 are measures for the mean-squared effect of a mutation at a given locus and its frequency in the equilibrium population. The expression of hidden variation is then the consequence of the following three-step argument: (1) For a well-adapted trait under stabilizing selection, almost all mutations are deleterious or neutral; (2) selection leads to a negative correlation of mutational effects on the phenotype and the frequency of the mutant allele in the equilibrium population (stronger deleterious mutants are kept at lower frequency); and (3) a change in the environmental conditions or in the genetic background partly removes this negative correlation, and hidden variation is revealed.
Our analytical results use the assumption of linkage equilibrium among loci. However, negative covariances among loci that result from stabilizing selection should only make the effect stronger. It is well known that considerable amounts of genetic variation can be hidden in negative linkage disequilibria (Lynch and Gabriel 1983). If there is any reshuffling of the favorable and disfavorable effects of allele combinations after an environmental change, this will add to the released variation in a similar way to that described above for the reshuffling of locus effects.
Quantitative estimate of the released variation:
For a quantitative estimate of the hidden variation coefficient ΔG, we need to make specific assumptions on the distribution of locus variances. While no direct data are available, circumstantial evidence suggests a leptokurtic (L-shaped) distribution. This evidence comes from two directions. A strong leptokurcy is usually found for the distribution of the effects of new mutations on a quantitative trait (Garcia-Dorado et al. 1999). This trend could still be due to L-shaped distributions at single loci, rather then due to differences in the effects among loci. But at least for a special type of mutations—knock-outs of entire genes—huge differences in the effects among loci are well documented. Further evidence comes from quantitative trait loci (QTL) analyses, which generally find a leptokurtic distribution of effects (e.g., Dilda and Mackay 2002). Consequently, it has previously been suggested that a single-sided gamma distribution is an appropriate choice for a model distribution (Welch and Waxman 2002). Following this suggestion, we choose
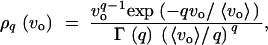 |
15 |
which is the single-sided Gamma distribution with mean 〈vo〉 and shape parameter q. Γ(q) denotes the Gamma function. The distribution has a maximum at vo = 0 for q ≤ 1 and is increasingly L-shaped with smaller q, 0 < q < 1. Different choices for the distribution of locus mutation rates ui (uniform, exponential, Gamma) had only very minor effects on our results. The numbers below are for uniform mutation rates, ui ≡ u. We concentrate on the transition between equivalent points in genotype space. This means that the variational properties of the genetic architecture before and after the environmental or genetic change are kept constant; in particular, there is no canalization, ΔM = 0. This can be seen as a null-model approach, assuming no special (evolved) properties of the genetic architecture in the ancestral population.
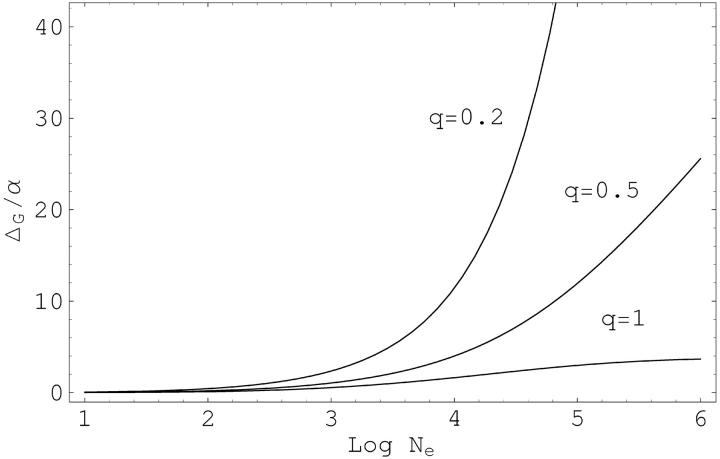
Figure 2 shows the dependence of ΔG on population size and the shape parameter q of the Gamma distribution. Both parameters have a strong effect. In small populations with Ne = 1000, and for commonly used values of the selection strength, s〈vo〉 = 1/800 and the mutation rate u = 10−5 (cf. Turelli 1984; Lynch and Walsh 1998; Welch and Waxman 2002), we obtain values from ΔG/α ≈ 1.6 for q = 1 to ΔG/α ≈ 11.4 for q = 0.2. In large populations, Ne = 106, these values increase to 3.7 for q = 1, and to 258.7 for a more strongly leptokurtic distribution with q = 0.2. ΔG increases with stronger selection s and lower mutation rates u, but the effect of both parameters is only moderate.
Figure 2.—
The hidden variation coefficient ΔG, in units of the epistasis parameter α, as a function of the effective population size. The three curves correspond to increasing leptokurcy of the distribution of the locus variances vo, with shape parameters q = 0.2, q = 0.5, and q = 1. The parameters for mutation rates and the average selection strength are u = 10−5 and s〈vo〉 = 1/800. For the strongly L-shaped distribution with q = 0.2, the values of 56.0α and 258.7α are reached for Ne = 105 and Ne = 106, respectively.
For ΔM = 0, hidden variation is directly proportional to the composite interaction parameter, ΔG ∼ α. For the general model, Equation 9 identifies two factors that determine α. If we assume equal variational properties before and after the change (and thus CV[vn] = CV[vo]), 1 − α is just the correlation among locus variances. As for the distribution of locus variances, there is again only indirect empirical evidence for this quantity. The correlation of the effects of new mutations on fitness across five different environments has been measured by Fry et al. (1996) using bottleneck lines of Drosophila melanogaster. Strong line crossing is observed, with cross-environmental correlations ranging from 0.5 to 0.93 with a mean of 0.75. Similarly, Gibson and van Helden (1997) measured the haltere size in 29 isogenic lines with and without a mutant Ubx allele. A very low correlation (0.41 in males and 0.18 in females) is found. From these estimates, a value of 1 − α ≤ 0.9 for the correlation among locus variances (i.e., α ≥ 0.1) appears realistic.
For a better qualitative understanding of these results, we consider a simplified distribution for vo that allows for a complete analytical treatment. This distribution consists of two parts, a uniform distribution (up to a maximum value depending on the choice of 〈vo〉) and a percentage p < 1 of loci that are neutral, vo = 0, under the old conditions. Derivations are given in the appendix. The results show that the variance increase can be estimated as
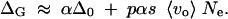 |
16 |
αΔ0 is the released variation for a uniform distribution (with p = 0). Δ0 takes values between 1 and 2 for population sizes from 104 to 106 and mutation and selection parameters as given above. For an epistasis parameter α = 0.1 this corresponds to an increase in VG by up to 20%.
The far bigger effect on ΔG, however, comes from the conditionally neutral loci that are not under selection under the old conditions, but are expressed after the change. Conditionally neutral loci typically do not refer to whole genes, but to a subset of alleles at a gene locus (see discussion). Note that these loci are in linkage equilibrium with all other loci (in averages over replicates or time) as assumed in our model. This holds true even if they are tightly linked to some other loci that contribute to the trait, since they are originally not under selection (we assume that the effective population size Ne accounts for the effects of background selection). The second term in Equation 16 is proportional to the percentage pα of loci that are added to the variational basis of the trait upon the change, i.e., that are neutral under the old conditions, but under selection afterward. For m loci in the variational basis, pα is a multiple of 1/m. It is also proportional to the effective population size Ne and to sv, which is the average effect on fitness of a mutation at a conditionally neutral locus under the new conditions. (In Equation 16 the latter is assumed to be equal to the average effect on fitness across all loci under the old conditions, s〈vo〉.) The result shows that for large populations even a single conditionally neutral locus can contribute a large amount of hidden variation. This can be seen from the following rule of thumb that directly follows from Equation 16: For a population with effective size Ne = 106, a single locus out of 100 that is shifted from (near) zero effect to a strong effect (sv = 1/100) leads to a >100-fold increase in VG.
This shows that in large populations a mere reshuffling of locus effects can explain virtually any increase of VG. A crucial condition for this result is that truly conditionally neutral alleles exist. These alleles must not have been under selection for a sufficiently long time for genetic variation to accumulate. In the following section, we discuss how these results change if alleles are not strictly neutral prior to the change of the environment or the genetic background, but are exposed to selection in rare environments or genetic backgrounds.
Rare environments:
So far, we have assumed that the changed environmental conditions are novel to the population, in that there is no previous adaptation to this environment. Under this assumption, large amounts of hidden variation are contributed by conditionally neutral loci that are expressed “for the first time” after the change. Examples for this scenario are the exploration of a novel niche by the population or an unusual experimental treatment such as ether shock in early development.
An equally important scenario, however, is the case where the environment changes to conditions that are not altogether new, but previously have been rare. In this case, there will be some memory in the system of previous encounters. This means that relaxation to mutation-selection balance under the old conditions is not yet complete at the time of the change. In particular, conditionally neutral loci are not yet in equilibrium of mutation and drift, since variation at these loci is still reduced due to selection in previous generations. In the appendix we have calculated the expected contribution of conditionally neutral loci to the hidden variation in the SHG approximation. For a rare environment that occurs at a frequency f, we find that such loci can be treated, to a good approximation, as if in mutation-selection balance under effective selection of strength fs. Relative to the case of a novel environment (frequency f = 0), the variation that is released at the locus is reduced by a factor
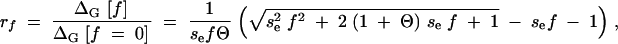 |
17 |
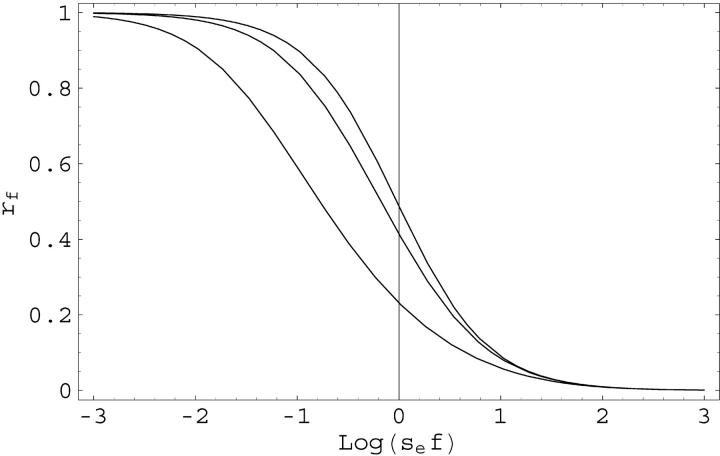
where se = Nesv and Θ = 4Neu are the effective selection strength and mutation rates. A graph of rf as a function of sef for different values of Θ is shown in Figure 3.
Figure 3.—
Reduction factor rf for the contribution of conditionally neutral loci to the hidden variation if the system changes to conditions that are not altogether new, but just rare with frequency f. rf is shown as a function of log10(sef) = log10(Nesvf) for three values of Θ = 4Neu, 0.2, 2, and 20 (top to bottom).
Qualitatively, we find that the contribution of conditionally neutral loci to ΔG is not large (as compared to other loci) as long as the frequency of the “rare” environment is f ≫ 10/se = 10/(Nesv). For f ≪ 1/(10Nesv), rf → 1 and we recover the results for truly novel conditions. The reduction is stronger in large populations, which have the larger release of variation at conditionally neutral loci, but also a longer memory of past environmental conditions (longer relaxation times). The same results hold if genetic instead of environmental changes are considered, e.g., if the alleles that define the new genetic background are already present at low frequency in the original wild population.
DISCUSSION
In this article a general model for gene-gene interaction (epistasis) and genotype-environment interaction (G × E) is presented and applied to a quantitative character in the balance of mutation and stabilizing selection. It is shown that the accumulation of hidden genetic variation is a generic property of these systems. This variation can be released if the genetic background or the environment changes, leading to an increase of the genetic variance directly after the change. The result does not require that the population has evolved genetic robustness (i.e., canalization) prior to the environmental change. Furthermore it is shown that this effect can be quantitatively important under plausible assumptions about the extent of epistasis and G × E.
As an intuitive picture for this effect, imagine that stabilizing selection tries to keep the house clean. Mutations, which are almost always deleterious for a well-adapted trait, correspond to the dirt. Not all dirt is equally visible, however: Some part is under the rug (loci that are weakly expressed or neutral under the old conditions). Since it is less visible to selection, it will accumulate. The size of the rug corresponds to the degree of canalization: With a large rug, new dirt at many places (new mutations at many loci) does not matter much. Now imagine that the room is rearranged (change of the environment or the genetic background). Clearly, some of the dirt will be exposed (genetic variation increased) if the size of the rug decreases (decanalization). But even without any change in size some of the previously hidden dirt will become visible if the rug is simply moved to a different location (loci with a large effect turn into loci with small effect and vice versa with equal probability). This moving-rug effect follows from two elementary principles: differential stabilizing selection that eliminates mutations at loci with a large effect more efficiently than at loci with a small effect (cleans the floor, but not under the rug) and epistasis or G × E interactions that reshuffle the locus effects (move the rug). Note that we do not predict an increase of the environmental variance under this scenario. The reason for this difference between VG and VE is that environmental “dirt” is not inherited and therefore cannot accumulate in a population.
There are thus two scenarios for the build-up and release of hidden variation. In the canalization scenario (the “shrinking rug”), the population prior to the change is mutationally robust and the average effect of a new mutation increases upon the change. This leads to a one-sided spread of lines in a reaction-norm picture of interactions. In the variable interaction scenario (the “moving rug”), neither state before or after the change is particularly robust, but there is extensive line crossing of mutational effects (cf. Figure 1). The amount of hidden variation released depends on a number of factors, which are the same in both scenarios. The most important ones are population size and the magnitude and kind of the interaction effects. Larger population size and larger interaction effects lead to more hidden genetic variation. For the latter, conditionally neutral alleles are particularly important, i.e., alleles that have no effect under the original conditions but are expressed in the new context. If these alleles have been not been under selection for a sufficiently long time, genetic variation can accumulate under the protection of neutrality. In contrast, the accumulation of hidden variation at these alleles is significantly reduced if there is a history of previous encounters with the new environment or genetic background prior to the change. Considering these factors, we find large amounts of released variation under plausible conditions. For a large population (Ne = 106), and no previous encounter with the novel environment or background, an increase of VG by more than a factor of 100 is possible if just 1% of the variational basis of the trait is expressed only under the new conditions.
To distinguish between the two scenarios, we need to take a closer look at how the interactions, and in particular conditionally neutral alleles, enter the genetic architecture of the trait. In the canalization scenario, conditional neutrality occurs predominantly in the wild type and under the prevailing ecological conditions. In the variable-interaction scenario, it is a generic phenomenon of gene networks that occurs independently of environmental conditions or of whether the genetic background is wild type or mutant. Below we first discuss the empirical evidence for conditional neutrality as such and then point out some evidence for the variable-interaction scenario, i.e., hidden variation without canalization. Finally, we discuss the implications of our result for the detection of genetic canalization and the potential evolutionary importance of hidden genetic variation.
Empirical evidence:
Interaction effects have been extensively documented and reviewed (Whitlock et al. 1995; Cheverud 2000; Templeton 2000; Mackay 2001). The amount and strength of interaction effects detectable has increased due to the application of molecular mapping techniques (Cheverud 2000; Mackay 2001) and there is no question that both G × E as well as epistasis are strong and ubiquitous.
There is also evidence for conditional neutrality, due to both epistasis and G × E. For example, in an analysis of the genetic differences between maize and its wild ancestor teosinte, Lauter and Doebley (2002) detected ample conditionally neutral genetic variation in teosinte. Alleles that exist in wild teosinte populations show effects in the hybrid background on traits that are phenotypically invariant in teosinte. Another striking example for conditional neutrality due to epistasis is provided by Polacysk et al. (1998). Although eye development in Drosophila is a highly stereotypic process, extensive genetic variation was revealed by introgression of mutant alleles of the epidermal growth factor receptor (Egfr). The phenotypes caused by the natural variation were in many cases more extreme than knockout phenotypes. Concerning the molecular nature of hidden variation, Dworkin et al. (2003) have shown that part of the genetic variation revealed by a mutation at the Egfr gene is due to alleles of the Egfr locus itself. This shows that conditionally neutral genetic variation is a fraction of the alleles at a gene locus and not the property of a gene locus per se. A gene that is maintained by stabilizing selection can nevertheless harbor conditionally neutral genetic variation.
In the case of G × E, a consensus that a considerable part takes the form of conditional neutrality seems to be emerging, which already found application in theories of ecological specialization (Fry 1996; Kawecki et al. 1997). Evidence comes from several directions. When testing mutation-accumulation lines of Drosophila for fitness in different environments, Kondrashov and Houle (1994) showed that a harsh environment reveals previously undetected differences between the lines. The authors estimated that a large fraction of mutations are conditionally neutral in benign lab environments but can have strong effect under less favorable conditions. In a study by Leips and Mackay (2000) on life span QTL effects of larval density, many QTL genotypes are neutral in low density but have detectable effects in high larval density. Similarly Vieira et al. (2000) found that life span QTL tested in various stress and temperature regimes show a high degree of conditional neutrality: Of the 17 QTL detected all were environment specific, i.e., were detectable only in a specific treatment. Similarly QTL for bristle number characters also exhibit strong environmental conditional neutrality (Dilda and Mackay 2002). Seventy-eight and 95% of the QTL affecting sternopleural and abdominal bristle number, respectively, are detected only in one temperature regime. These numbers are probably gross overestimates due to the detection thresholds of QTL measurements. However, our results show that as little as 1% of the genetic basis of a trait exhibiting conditional neutrality may contribute a 100-fold increase of the genetic variance if the genetic variation stored in these parts is released.
To distinguish the variable interaction scenario from genetic canalization, we need to know whether conditional neutrality is particular to wild populations under prevailing ecological conditions or rather is a generic phenomenon. The characteristic difference of these two scenarios is the absence or presence of alleles that are neutral in the mutant background or new environment, but under selection under the old conditions. Since alleles of this type are usually kept at low frequency in a population, they are more difficult to detect than conditionally neutral alleles that are neutral under prevalent conditions. This results in a detection bias in favor of the latter in experimental settings that use genetic variation shaped and sorted by natural selection, such as QTL measurements. Nevertheless, some evidence points to variable interactions and a generic role of conditional neutrality.
One way to avoid the detection bias is to use genetic variation that is not sorted by selection. Fry et al. (1996) compared mutation-accumulation lines of D. melanogaster in five laboratory environments. They find strong line crossing with cross-environmental correlations ranging from 0.5 to 0.93 with a mean of 0.75. The authors assume that part of the line crossing is due to conditionally neutral alleles, although it is not possible to determine to what extent this is the case in this study. Similarly, Gibson and van Helden (1997) measured haltere size in isogenic lines of D. melanogaster with and without a mutant allele of the homeotic gene Ultrabithorax (Ubx). Although there is reason a priori to expect that Ubx function in haltere development may be canalized, the authors find no significant increase in variance between lines in the presence of the mutation. Instead, extensive line crossing is found, with a very low correlation between effects in the wild-type and Ubx backgrounds, as predicted in the variable-interaction scenario.
Among the best-documented cases of conditional neutrality is sex dependence of allelic effects. For example, Dilda and Mackay (2002) found that 57% of the QTL for bristle number are conditional on sex. Sex specificity was also noted in the study of eye development by Polacysk et al. (1998), discussed above. The authors remark that, since visual function is presumably equally necessary in both sexes, sex dependence would be unexpected if conditional neutrality was due to canalization, i.e., evolved robustness.
Evidence for the variable interaction scenario and conditionally neutral alleles in a mutant background also comes from some of the traits that are usually seen as classic examples for canalization. In an attempt to demonstrate canalization of the trait scutellar bristle number in D. melanogaster, Rendel (1959) and Fraser and Green (1964) used wild-type lines that were selected for higher bristle numbers and substituted the wild-type allele with the scute mutation. They find a large increase in the genetic variance, which is taken as evidence for canalization. Comparing bristle numbers of wild-type flies and those of their Scute sibs, however, Rendel finds only a small correlation, while Fraser and Green report no correlation at all. They conclude that there are two different sets of genes changing bristle number: one in the wild type, one in scute. Even more specifically, Fraser (1970) identifies a gene, x-vert, which increases bristle number in the wild-type background but had no effect in the presence of the scute mutation. These findings are corroborated by results of Sheldon and Milton (1972), who again find that genes that cause an increase in bristle number in wild-type flies have a much decreased effect in the presence of scute.
Similar findings are reported for vibrissa number in mice, another putatively canalized trait. Already Kindred (1967) noted that modifiers of the wild type are often not active in the presence of the tabby mutation. In a selection experiment for higher vibrissa number in a strain of Tabby mice, almost no selection response was found after 34 generations. Selection for increased number of vibrissae in the wild-type sibs, however, showed an immediate response, without affecting the Tabby sibs that still segregated in the line. While data are still too scarce to assess how general conditional neutrality is in mutant genotypes, the published literature suggests a generic role of conditional neutrality rather than the idea that conditional neutrality is limited to canalized states of the phenotype.
Implications for the study of genetic canalization/robustness:
The classical experimental paradigm for the study of genetic canalization consists in introducing a major mutation and then analyzing the genetic variation revealed in this new genetic background. Four model systems were studied most intensely: vibrissa number in mice (Dun and Fraser 1958, 1959) and scutellar bristles (Rendel 1959), ocelli and head bristles (Sondhi 1960, 1961), and wing vein interruption (Scharloo 1962, 1964), all in Drosophila. Release of hidden genetic variation was taken as prima facie evidence for the canalization of the wild type (for a review see Scharloo 1991).
Hidden genetic variation is valid evidence for wild-type canalization if all the genetic variation contributing to the focal character affects one and the same underlying physiological variable. This is assumed in the standard model for the quantitative analysis of these experiments by Dun and Fraser (1959) and Rendel (1959)(1967) that also found its way into textbooks (Lynch and Walsh 1998). Epistasis is included into this model as a change in the slope of the map that translates the underlying variable into the trait value. It thus affects all locus contributions in the same way (as the parameter β in our model, Equation 7) and predicts a large positive correlation of allele effects in both backgrounds. It does not allow for variable interaction effects, where some locus effects may increase and some decrease upon the change of the genetic background. This is the main difference from our model, which (in the language of Dun, Fraser, and Rendel) assumes that genes affect more than one underlying variable, maybe as many as or more than genes contributing to the character (modeled by the parameters αi in Equation 7). Under these conditions, however, as our results show, the release of cryptic genetic variation does not allow one to infer genetic canalization of the wild type.
The assumption of a single underlying character was soon challenged. In the case of scutellar bristle number, Robertson (1965) showed that scutellar bristle number is not a causally homogeneous character, but that anterior and posterior bristles are regulated differently. As briefly reviewed above, evidence for variable interactions (increases and decreases of allele effects) was subsequently found for scutellar bristles and wing vein interruptions in Drosophila and vibrissa number in mice (see above and Scharloo 1991). Sheldon and Milton (1972) explicitly state that “assumptions in the model about the similarity of effects in scute and wild-type flies were not met in the present material.” These results, however, were seen only as a challenge to the model of Dunn, Fraser, and Rendel. They were not seen as challenging the basic conclusion drawn from that model, namely that hidden variation demonstrates canalization of the wild type (see Scharloo 1991, as representing this classical era of canalization research).
Our results show that detecting differences in the degree of genetic canalization between two genotypes will require genetic variation that was not sorted and shaped by natural selection. One can estimate the mutational variance by mutation-accumulation experiments. To our knowledge, this approach has not been used on two different genotypic backgrounds because these experiments are very labor intensive. Stearns and Kawecki (1994) used P-element insertions to test the sensitivity of various life-history characters to mutations. They found a negative correlation among trait variability and the selection intensity on the traits and considered this as evidence for canalization. However, this conclusion was challenged by Houle (1998), who argued that the measurements are best explained by differences in the mutational target sizes of the traits. His arguments show that a mutational variance VM of a given trait that is small relative to the VM of other traits (instead of relative to the VM of the same trait in mutant backgrounds or across environments) does not provide sufficient evidence for evolved genetic robustness (canalization).
An alternative approach that avoids these problems was used by Gibson and van Helden (1997) with Drosophila haltere as phenotype. Gibson and van Helden introduced a mutant Ubx allele into an array of inbred lines derived from different populations. The idea here is that genetic variation across populations is not shaped by natural selection toward a common optimum. As reviewed above, these authors did not find evidence for canalization, but instead found evidence for line crossing and variable epistatic effects between wild-type and mutant backgrounds. An even more direct approach can be taken in Escherichia coli. Elena and Lenski (2001) genetically engineered the same mutations in two different genetic backgrounds, one adapted to the lab environment and one not. They tested a limited number of these mutations and did not find evidence for canalization; i.e., the adapted genotypes were not more robust with respect to mutations than the nonadapted genes. In yet another study using the same approach, Remold and Lenski (2001) tested the same set of mutations in two different environments, one to which the genotype was adapted (Glucose) and one that was new for the strain (Maltose). They found that the mutation effects in the new environment were much larger than those in the adapted environment, suggesting a higher degree of robustness in the adapted environment. Unfortunately, their result is confounded by the use of an inappropriate fitness scale (taking ratios of Malthusian parameters).
Hence, while the release of hidden variation due to the introduction of a mutation is a general observation, the evidence for canalization is ambiguous. Recent theoretical models predict the evolution of genetic canalization on the level of a trait only for high mutation rates and under restrictive conditions on the genetic architecture (Wagner et al. 1997; Hermisson et al. 2003). The empirical evidence accumulated to date shows variable genetic interactions with conditionally neutral alleles in wild-type and mutant backgrounds and across environments. We thus think that generic interaction effects are the most plausible explanation for the release of hidden genetic variation under changes of the environment or the genetic background.
Implications for the evolutionary role of hidden variation:
Major changes in the ecological conditions or in the genetic background (through gene flow or rapid fixation of new alleles) provide new challenges for a population, to which it is, initially, not optimally adapted. It is intriguing to think that the expressed genetic variation may be increased in just these situations. Several recent studies have therefore suggested an important evolutionary role of hidden variation (Rutherford and Lindquist 1998; Hansen et al. 2000; see also de Visser et al. 2003 for review). Two questions need to be answered in addressing this issue: What are the circumstances and mechanisms that allow for the build-up and release of hidden variation? And whether or how does any newly released variation provide a benefit for the further course of evolution, e.g., by facilitating the crossing of fitness valleys? This article is concerned only with the first question.
Since mutational buffering of the wild type obviously leads to the accumulation of genetic variation in a population, the evolutionary role of hidden variation has usually been discussed in the context of genetic canalization (e.g., Eshel and Matessi 1998). This makes it necessary to explain how canalization evolves, breaks down upon the change, and is subsequently restored. As detailed above, however, the evolution of canalization faces empirical and theoretical problems. Our results show that canalization is not necessary for hidden variation to accumulate. There is no need to explain the evolution of canalization in a theory that uses hidden variation. The only assumption needed is sufficient time for mutations to accumulate at conditionally neutral loci.
In recent years, several studies proposed the molecular chaperone Hsp90 as a specific mechanism for the release of hidden variation (Rutherford and Lindquist 1998; Queitsch et al. 2002; Rutherford 2003). These studies show that mutations at the gene encoding Hsp90 as well as a chemical knock down of the protein reveal hidden genetic variation. It was therefore suggested that Hsp90 may “act as a capacitor of morphological evolution” by allowing the accumulation of hidden genetic variation, which may be released and used in times of stress. Using group selection arguments, it has even been speculated that this molecular mechanism may have evolved because of the putative benefits of hidden variation (Rutherford 2003).
The assignment of a privileged role to Hsp90 in the maintenance and release of hidden variation was recently challenged by a computer simulation study (Bergman and Siegal 2003). The authors showed that in a model of a complex gene network, any knock-out mutation of a gene is likely to reveal hidden phenotypic variation. Our result explains and generalizes their conclusion in an analytical framework. An increase of the genetic variance after an environmental or genetic change is a generic phenomenon of traits with variable G × E or epistasis. Any genetic element that interacts with other genes or the environment will facilitate the accumulation of hidden genetic variation. Thus no specific molecular mechanisms are needed for the existence and putative evolutionary role of hidden variation. The phenomenon is most likely entirely generic and due to a large set of mechanistically heterogeneous proximate causes. These findings corroborate arguments by Phillips et al. (2000) in favor of a potentially important role of variable epistatic effects, even if the average epistatic effect is small or zero. Our results further predict that hidden variation should be most prevalent in large populations.
Whether there is any important evolutionary role of hidden variation remains an open question. The main unresolved issue is the benefit of this type of variation for the adaptive process. Our results show that these questions can and should be addressed separately from problems concerning the evolution of canalization mechanisms.
Acknowledgments
We thank T. Hansen, P. Pennings, N. Stoletzki, and two anonymous referees for helpful comments on the manuscript. J.H. acknowledges financial support by an Emmy-Noether fellowship of the German Science Foundation (Deutsche Forschungsgemeinschaft).
APPENDIX
The stochastic house-of-Gauss approximation:
We derive and briefly discuss the approximation for the single-locus genetic variance in mutation-selection-drift balance that is used above.
Consider a single haploid locus with a continuum of alleles y ∈ R. We assume weak stabilizing selection toward an optimum at y = 0; i.e., W = W0 − sy2. Mutations add random increments d to y, with a distribution of d that is symmetric around zero and has variance v.
For an infinite population, the change of the genetic variance V under the combined action of mutation and selection reads
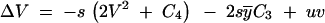 |
A1 |
(e.g., Bürger 2000), where C3 and C4 denote the third and forth cumulant of the distribution of y. In equilibrium, C3 vanishes due to symmetry. For the fourth-order cumulant, two approximations are widely used. In the Gaussian allelic approximation (Lande 1976), which holds for sufficiently large mutation rates or small mutational effects (v ≪ u/s), C4 is neglected relative to 2V2. In the house-of-cards (HC) approximation (Turelli 1984) on the other hand, the fourth-order cumulant is replaced by its asymptotic estimate for u → 0, where C4 relates to the variance as C4 = Vv (Bürger 2000). Since v ≫ V under the assumptions of the HC approximation, the term 2V2 in Equation A1 is neglected in this approximation.
To obtain an analytic approximation for the whole parameter range we replace C4 by Vv as in the HC case, but retain the term 2V2 in Equation A1. Solving for V at equilibrium, we obtain
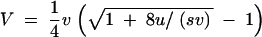 |
A2 |
This expression for the equilibrium variance was previously derived by Waxman (2003) as the exact solution of the mutation-selection balance model with a special distribution of mutational effects d. This distribution is given by 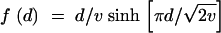 . It is symmetric with variance v and kurtosis 1 and does not deviate much from a Gaussian distribution in its appearance. One readily checks from the expressions in Waxman (2003) that C4 = vV (and in general C2n+2 = c2nV with ck the kth cumulant of f) exactly in this case. As an approximate solution for a general mutation distribution, Equation A2 may be called the house-of-Gauss (HG) approximation since it reduces to the house-of-cards and Gaussian approximations in the limits u → 0 and v → 0, respectively. Expanding to second order for small v, Equation A2 also reproduces Fleming's second-order approximation (cf. Bürger 2000) for mutation distributions with a kurtosis of 1 (as to be expected from an exact solution).
. It is symmetric with variance v and kurtosis 1 and does not deviate much from a Gaussian distribution in its appearance. One readily checks from the expressions in Waxman (2003) that C4 = vV (and in general C2n+2 = c2nV with ck the kth cumulant of f) exactly in this case. As an approximate solution for a general mutation distribution, Equation A2 may be called the house-of-Gauss (HG) approximation since it reduces to the house-of-cards and Gaussian approximations in the limits u → 0 and v → 0, respectively. Expanding to second order for small v, Equation A2 also reproduces Fleming's second-order approximation (cf. Bürger 2000) for mutation distributions with a kurtosis of 1 (as to be expected from an exact solution).
We include drift into the model in the same way as is usually done in the Gaussian or HC approximations (Bürger 2000) by assuming a constant effective population size Ne and an average reduction of the genetic variance due to drift of V/Ne per generation, i.e.,
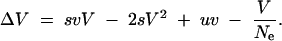 |
A3 |
Solving for V we arrive at the expression in Equation 14. Alternatively, we can write V as a function of the effective selection strength se = Nesv and Θ = 4Neu as
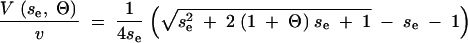 |
A4 |
In analogy to the above, we call this the SHG approximation. The SHG approximation fully interpolates between the stochastic versions of the Gaussian and the HC approximation and reduces to the HG approximation in the deterministic limit.
Next, we analyze the case of a conditionally neutral locus that is under selection under rare environmental conditions, but not under the prevalent conditions. We want to calculate the variation that is released if the environment changes to these rare conditions. Consider an environmental cycle of length k, with a change to the rare selective environment k generations after the previous occurrence. The change of the genetic variance in the ith generation, V(i), is given by
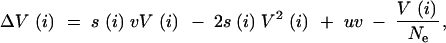 |
A5 |
with s0 = sk = s in generations 0 and k, and si = 0, 0 < i < k. Summing up the mutation and drift terms from generation 0 to k − 1, we obtain the following recursion for V over the environmental cycle:
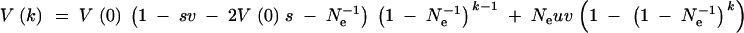 |
A6 |
V(k) is the genetic variance that is expressed after the change to the rare environment in generation k. To obtain the average variance that is expressed in a rare environment that occurs with a frequency f = 〈k〉, we need to average this expression over many environmental cycles (we denote this average as 〈·〉). For simplicity, we assume that there is no autocorrelation in the lengths k of these cycles and therefore no correlation among k and V (a strictly periodic environment is a simple case that fits into this scheme). In this case,
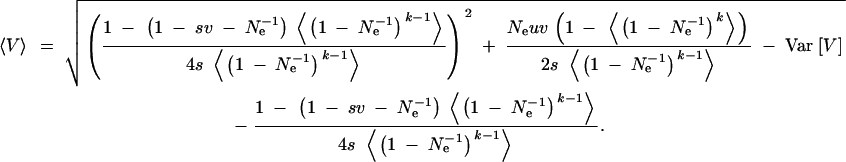 |
A7 |
For k ≡ 1, this expression reduces to the usual SHG approximation (14). For the general case, we assume that the variance and higher-order cumulants in k can be ignored. Note that this is always the case for small k ≪ Ne, or more generally if Vark ≪ N2e. In this case, also the variance in the released variance is Var[V] ≪ 〈V〉2 and can be neglected. We then find that Equation A7 approximately reduces to the standard SHG expression (14), with the selection strength s replaced by its average over the generations, s/〈k〉 = sf. It is easily seen that this approximation is exact for both very small and very large f. For intermediate frequencies, numerical results (not shown) demonstrate its accuracy almost to the precision of drawing in plots over biologically relevant parameter regions.
Further derivations:
The larger derivations that were left out in the main text are presented in this section. For a derivation of Equation 6, express ΔG as
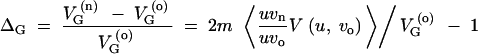 |
A8 |
 |
A9 |
Using
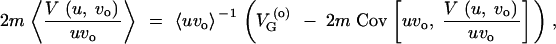 |
A10 |
this may be written as
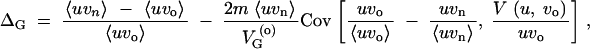 |
A11 |
which gives Equation 6. An alternative way to write this expression is
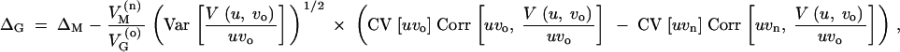 |
A12 |
where CV is the coefficient of variation. Release of hidden variation is obtained if the population under the old conditions is more robust, VoM < VnM, if the genetic basis of the trait is more inhomogeneous, CV[uvo] > CV[uvn], or if uvo is more strongly anticorrelated with V(u, vo)/vo than is uvn. In the above model, this is analyzed for independent mutation rates and locus variances. If there is a tendency for populations to evolve a negative correlation among mutation rates and locus variances (as predicted by Hermisson et al. 2003), this decreases 〈uvo〉 relative to 〈u〉〈vo〉 and leads to a further increase of ΔG.
We now derive an exact result for the hidden variation coefficient ΔG in the limit of many loci. The probability density for the locus variances is best expressed in terms of the effective selection strength se = Nesvo and is given by
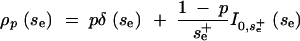 |
A13 |
δ is the delta function and Ia,b(x) an index function, which is 1 for a ≤ x ≤ b and 0 else. This density consists of two parts. A proportion p < 1 of all loci is unexpressed under the old conditions and therefore not under selection. A proportion 1 − p of loci is expressed, with selection on these following a uniform distribution up to a maximum 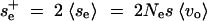 , where the averages are taken with respect to the expressed loci only.
, where the averages are taken with respect to the expressed loci only.
The probability density for se after the genetic or environmental change can be constructed according to Equation 7 with an arbitrary distribution of epistasis parameters αi and β = 1 without any change of the result. For concreteness, assume that se remains constant with probability (1 − α) and changes to an uncorrelated value taken from the same distribution with probability α. In this case, the proportion of conditional neutral loci that are under selection only after the change, but not before, of the entire variational basis of the trait (loci that are expressed before or after the change) is αp/(1 + αp). Integrating the genetic variance of a single locus, (A4), with (A13), we obtain
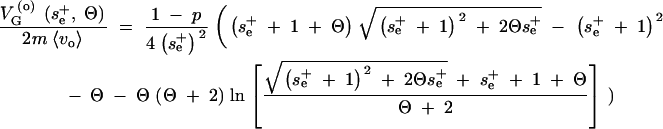 |
A14 |
Similarly, the genetic variance under the new conditions follows from the integral of V(se, Θ)〈se〉/se, with the same density for all loci that change their variance as
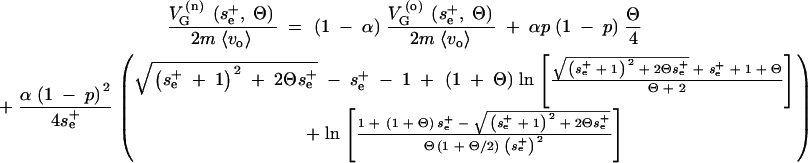 |
A15 |
By combining these expressions, we obtain the hidden variation coefficient ΔG. For small p this may be approximated by
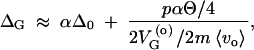 |
A16 |
where αΔ0 is the hidden variation coefficient in the absence of conditional neutral loci (p = 0). For 〈se〉 ≫ Θ, the first term, Δ0, dominates in the drift regime, 〈se〉 ≪ 1. In the selection regime, 〈se〉 ≫ 1, we can further approximate VoG/2mvo ≈ Θ/4se (HC approximation), which results in Equation 16. Numerical evaluation of the exact expression shows that this approximation is reasonable for the entire parameter range of biological interest.
References
- Bergman, A., and M. L. Siegal, 2003. Evolutionary capacitance as a general feature of complex gene networks. Nature 424: 549–552. [DOI] [PubMed] [Google Scholar]
- Bürger, R., 2000 The Mathematical Theory of Selection, Recombination, and Mutation. Wiley, Chichester, UK.
- Cheverud, J., 2000 Detecting epistasis among quantitative trait loci, pp. 82–98 in Epistasis and the Evolutionary Process, edited by J. B. Wolf, E. D. Brodie III and M. J. Wade. Oxford University Press, Oxford.
- de Visser, J. A. G. M., J. Hermisson, G. P. Wagner, L. Ancel Meyers, H. Bagheri-Chaichian et al., 2003. Perspective: evolution and detection of genetic robustness. Evolution 57: 1959–1972. [DOI] [PubMed] [Google Scholar]
- Dilda, C. L., and T. F. C. Mackay, 2002. The genetic architecture of Drosophila sensory bristle number. Genetics 162: 1655–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun, R. B., and A. S. Fraser, 1958. Selection for an invariant character—‘vibrissae number’—in the house mouse. Nature 181: 1018–1019. [DOI] [PubMed] [Google Scholar]
- Dun, R. B., and A. S. Fraser, 1959. Selection for an invariant character, vibrissae number in the house mouse. Aust. J. Biol. Sci. 12: 506–523. [DOI] [PubMed] [Google Scholar]
- Dworkin, I., A. Palsson, K. Birdsall and G. Gibson, 2003. Evidence that Egfr contributes to cryptic genetic variation for photoreceptor determination in natural populations of Drosophila melanogaster. Curr. Biol. 13: 1888–1893. [DOI] [PubMed] [Google Scholar]
- Elena, S. F., and R. E. Lenski, 2001. Epistasis between new mutations and genetic background and a test of genetic canalization. Evolution 55: 1746–1752. [DOI] [PubMed] [Google Scholar]
- Eshel, I., and C. Matessi, 1998. Canalization, genetic assimilation and preadaptation: a quantitative genetic model. Genetics 149: 2119–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, A. S., 1970. Variation of scutellar bristles in Drosophila XVI: major and minor genes. Genetics 65: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, A. S., and M. M. Green, 1964. Variation of scutellar bristles in Drosophila III: sex-dimorphism. Genetics 50: 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry, J. D., 1996. The evolution of host specialisation: Are trade-offs overrated? Am. Nat. 148: S84–S107. [Google Scholar]
- Fry, J. D., S. L. Heinsohn and T. F. C. Mackay, 1996. The contribution of new mutations to genotype-environment interaction for fitness in Drosophila melanogaster. Evolution 50: 2316–2327. [DOI] [PubMed] [Google Scholar]
- Garcia-Dorado, A., C. Lopez-Fanjul and A. Caballero, 1999. Properties of spontaneous mutations affecting quantitative traits. Genet. Res. 74: 341–350. [DOI] [PubMed] [Google Scholar]
- Gerhart, J., and M. Kirschner, 1998. Evolvability. Proc. Natl. Acad. Sci. USA 95: 8420–8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, G., and S. van Helden, 1997. Is the function of the Drosophila homeotic gene Ultrabithorax canalized? Genetics 147: 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, G., and G. P. Wagner, 2000. Canalization in evolutionary genetics: A stabilizing theory? BioEssays 22: 372–380. [DOI] [PubMed] [Google Scholar]
- Hansen, T. F., and G. P. Wagner, 2001. Modeling genetic architecture: a multilinear theory of gene interaction. Theor. Popul. Biol. 59: 61–86. [DOI] [PubMed] [Google Scholar]
- Hansen, T. F., A. J. R. Carter and C.-H. Chiu, 2000. Gene conversion may aid adaptive peak shifts. J. Theor. Biol. 207: 495–511. [DOI] [PubMed] [Google Scholar]
- Hermisson, J., T. F. Hansen and G. P. Wagner, 2003. Epistasis in polygenic traits and the evolution of genetic architecture under stabilizing selection. Am. Nat. 161: 708–734. [DOI] [PubMed] [Google Scholar]
- Houle, D., 1998. How should we explain variation in the genetic variance of traits? Genetica 102/103: 241–253. [PubMed] [Google Scholar]
- Kawecki, T. J., N. H. Barton and J. D. Fry, 1997. Mutational collapse of fitness in marginal habitats and the evolution of ecological specialisation. J. Evol. Biol. 10: 407–429. [Google Scholar]
- Kindred, B., 1967. Selection for an invariant character, vibrissae in house mouse. V. Selection on non-Tabby segregants from Tabby selection lines. Genetics 55: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov, A. S., and D. Houle, 1994. Genotype-environment interactions and the estimation of genomic mutation rate in Drosophila melanogaster. Proc. R. Soc. Lond. Ser. B 258: 221–227. [DOI] [PubMed] [Google Scholar]
- Lande, R., 1976. The maintenance of genetic variability by mutation in a polygenic character with linked loci. Genet. Res. 26: 221–235. [DOI] [PubMed] [Google Scholar]
- Lauter, N., and J. Doebley, 2002. Genetic variation for phenotypically invariant traits detected in teosinte: implications for the evolution of novel forms. Genetics 160: 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leips, J., and T. F. C. Mackay, 2000. Quantitative trait loci for life span in Drosophila melanogaster: interactions with genetic background and larval density. Genetics 155: 1773–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, M., and W. Gabriel, 1983. Mutation load and survival of small populations. Am. Nat. 122: 745–764. [DOI] [PubMed] [Google Scholar]
- Lynch, M., and J. B. Walsh, 1998 Genetics and Analysis of Quantitative Traits. Sinauer, Sunderland, MA.
- Mackay, T. F. C., 2001. The genetic architecture of quantitative traits. Annu. Rev. Genet. 35: 303–339. [DOI] [PubMed] [Google Scholar]
- Masel, J., and A. Bergman, 2003. The evolution of the evolvability properties of the yeast prion. Evolution 57: 1498–1512. [DOI] [PubMed] [Google Scholar]
- Phillips, P., S. Otto and M. Whitlock, 2000 Beyond the average: the evolutionary importance of gene interactions and variability of epistatic effects, pp. 20–38 in Epistasis and the Evolutionary Process, edited by J. Wolf, E. Brodie and M. Wade. Oxford University Press, Oxford.
- Polacysk, P. J., R. Gasperini and G. Gibson, 1998. Naturally occurring genetic variation affects Drosophila photoreceptor determination. Dev. Genes Evol. 207: 462–470. [DOI] [PubMed] [Google Scholar]
- Queitsch, C., T. A. Sangster and S. Lindquist, 2002. Hsp90 as a capacitor of phenotypic variation. Nature 417: 618–624. [DOI] [PubMed] [Google Scholar]
- Remold, S. K., and R. E. Lenski, 2001. Contribution of individual random mutations to genotype-by-environment interactions in Escherichia coli. Proc. Natl. Acad. Sci. USA 98: 11388–11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendel, J. M., 1959. Canalization of the scute phenotype of Drosophila. Evolution 13: 425–439. [Google Scholar]
- Rendel, J. M., 1967 Canalization and Gene Control. Logos Press, New York.
- Robertson, A., 1965. Variation in scutellar bristle number—an alternative hypothesis. Am. Nat. 99: 19–24. [Google Scholar]
- Rutherford, S. L., 2003. Between genotype and phenotype: protein chaperones and evolvability. Nat. Rev. Genet. 4: 263–274. [DOI] [PubMed] [Google Scholar]
- Rutherford, S. L., and S. Lindquist, 1998. Hsp90 as a capacitor for morphological evolution. Nature 396: 336–342. [DOI] [PubMed] [Google Scholar]
- Scharloo, W., 1962. The influence of selection and temperature on a mutant character (ciD) in Drosophila melanogaster. Arch. Neerl. Zool. 14: 431–512. [Google Scholar]
- Scharloo, W., 1964. Mutant expression and canalization. Nature 203: 1095–1096. [DOI] [PubMed] [Google Scholar]
- Scharloo, W., 1991. Canalization: genetic and developmental aspects. Annu. Rev. Ecol. Syst. 22: 65–93. [Google Scholar]
- Sheldon, B. L., and M. K. Milton, 1972. Studies on the scutellar bristles of Drosophila melanogaster. II. Long-term selection for high bristle number in the Oregon RC strain and correlated responses in abdominal chaetae. Genetics 71: 567–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniegowski, P., P. Gerrish, T. Johnson and A. Shaver, 2000. The evolution of mutation rates: separating causes from consequences. BioEssays 22: 1057–1066. [DOI] [PubMed] [Google Scholar]
- Sondhi, K. C., 1960. Selection for a character with a bounded distribution of phenotypes in Drosophila subobscura. J. Genet. 57: 193–221. [Google Scholar]
- Sondhi, K. C., 1961. Developmental barriers in a selection experiment. Nature 189: 249–250.13733634 [Google Scholar]
- Stearns, S. C., and T. J. Kawecki, 1994. Fitness sensitivity and the canalization of life-history traits. Evolution 48: 1438–1450. [DOI] [PubMed] [Google Scholar]
- Templeton, A. R., 2000 Epistasis and complex traits, pp. 82–98 in Epistasis and the Evolutionary Process, edited by J. B. Wolf, E. D. Brodie III and M. J. Wade. Oxford University Press, Oxford.
- Turelli, M., 1984. Heritable genetic variation via mutation-selection balance: Lerch's zeta meets the abdominal bristle. Theor. Popul. Biol. 25: 138–193. [DOI] [PubMed] [Google Scholar]
- Vieira, C., E. G. Pasyukova, Z-B. Zeng, J. B. Hackett, R. F. Lyman et al., 2000. Genotype-environment interaction for quantitative trait loci affecting life span in Drosophila melanogaster. Genetics 154: 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington, C. H., 1957 The Strategy of the Genes. MacMillan, New York.
- Wagner, G. P., and L. Altenberg, 1996. Complex adaptations and the evolution of evolvability. Evolution 50: 967–976. [DOI] [PubMed] [Google Scholar]
- Wagner, G. P., G. Booth and H. Bagheri-Chaichian, 1997. A population genetic theory of canalization. Evolution 51: 329–347. [DOI] [PubMed] [Google Scholar]
- Waxman, D., 2003. Numerical and exact solutions for continuum of alleles models. J. Math. Biol. 46: 225–240. [DOI] [PubMed] [Google Scholar]
- Welch, J., and D. Waxman, 2002. Nonequivalent loci and the distribution of mutant effects. Genetics 161: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock, M. C., P. C. Philips, F. B.-G. Moore and S. J. Tonsor, 1995. Multiple fitness peaks and epistasis. Annu. Rev. Ecol. Syst. 26: 601–629. [Google Scholar]