Abstract
The transgenic insertional mouse mutation Odd Sex (Ods) represents a model for the long-range regulation of Sox9. The mutation causes complete female-to-male sex reversal by inducing a male-specific expression pattern of Sox9 in XX Ods/+ embryonic gonads. We previously described an A/J strain-specific suppressor of Ods termed Odsm1A. Here we show that phenotypic sex depends on a complex interaction between the suppressor and the transgene. Suppression can be achieved only if the transgene is transmitted paternally. In addition, the suppressor itself exhibits a maternal effect, suggesting that it may act on chromatin in the early embryo.
IN mice and humans, Sox9/SOX9 has been identified as a key regulator of the male sex determination pathway. In humans, loss of SOX9 function has been shown to lead to XY sex reversal (Foster et al. 1994; Wagner et al. 1994), while duplication of SOX9 leads to XX sex reversal (Huang et al. 1999). In mice carrying a Wt1:Sox9 transgene, or in the Odd Sex mutant, expression of Sox9 in the bipotential embryonic gonad leads to XX sex reversal (Bishop et al. 2000; Vidal et al. 2001).
In humans, SOX9 regulation is complex with critical elements scattered over a distance of ∼1 Mb (Pfeifer et al. 1999). We previously described a dominant insertional mouse mutation model for the long-range alteration of Sox9 expression, termed Odd Sex (Ods). In this model, two copies of a tyrosinase transgene (Yokoyama et al. 1990) integrated 980 kb upstream from Sox9 on chromosome 11 accompanied by a 134-kb deletion at the integration site (Bishop et al. 2000; Qin et al. 2004). Transgenic Ods/+ mice were born microphtalmic and all XX Ods/+ mice were sterile sex-reversed males. In wild-type embryonic genital ridges, Sox9 has a sexually dimorphic expression pattern being restricted to the Sertoli cell lineage in the male, beginning at 11.5 days post coitum (dpc; Kent et al. 1996). In 11.5-dpc XX Ods/+ fetal gonads the transgene, through an as yet unknown mechanism, is able to upregulate Sox9 expression, causing testes formation and sex reversal (Bishop et al. 2000; Qin et al. 2004). This mutation is dominant and fully penetrant in the original FVB/N inbred strain. In contrast, in F1 mice, hybrids between inbred A/J females and transgenic FVB/N males, the sex reversal is suppressed, resulting in XX Ods/+ fertile females. In 95% of mice the suppression is complete, leading to fertile females; however, a small number of mice (2.5%) are hermaphrodites and 2.5% remained male. We have shown, using genetic mapping in an A/J × FVB/N F2 intercross, that a single genetic locus, Odd Sex modifier 1 (Odsm1) on mouse chromosome 18, controlled the suppression (Qin et al. 2003). Here we report that the transgene is subject to imprinting and suppression can be achieved only if the transgene is transmitted paternally. In addition, the Odsm1 suppressor itself exhibits a maternal effect, suggesting that it may functionally modify chromatin structure in the very early embryo.
MATERIALS AND METHODS
The genotype at Odd Sex was scored, Ods/+ or +/+, by examination of the eyes (Bishop et al. 2000). Mice were genotyped for the Y chromosome as described previously (Bishop et al. 2000). Mice were recorded as male, hermaphrodite, or female after external and internal examination of the genitalia (Qin et al. 2003).
RESULTS
Parental effects at Odsm1 and Ods:
Surprisingly, among XX Ods/+ Odsm1A/Odsm1F mice, the deficit of males seen in the F1 generation (2.5% male) was not observed in the F2 generation, where 42% were male. One possibility was that this discrepancy between the F1 and F2 generations could be explained by a second locus controlling sex determination in mice heterozygous at Odsm1. We tested this hypothesis by reanalyzing the genome scan data for the mice heterozygous at Odsm1 in the original F2 intercross (Qin et al. 2003). We could not find any linkage disequilibrium with any marker, making the second-locus hypothesis unlikely. Unlike in the F1 progeny, the parental origin of the suppressor, Odsm1A, and the Ods transgene cannot be established in the F2 mice. This suggested the possibility that a parent of origin effect at Odsm1 and/or Ods could be responsible for the sex ratio discrepancy between the F1 and F2 groups.
Imprinting at Ods:
To test this hypothesis, three backcrosses to FVB/N were set up. In the first two crosses, Ods was transmitted through the paternal germline while the suppressor (Odsm1A) was transmitted through either the paternal (Table 1A, cross 1) or the maternal germline (Table 1A, cross 2). In the third cross (Table 1A, cross 3), both the suppressor and the transgene were transmitted through the maternal germline. Among the N2 XX Ods/+ progeny, three types of mice were recorded: males, females, and hermaphrodites. Analysis of the data showed that suppression of sex reversal (12 females out of 63 progeny—19%) occurred only when the suppressor was transmitted maternally and the transgene was transmitted paternally (Table 1A, cross 2). Sex determination in XX Ods/+ mice was therefore controlled by loci with a parental effect. Suppression could be achieved only through paternal transmission of the transgene, demonstrating imprinting at the Ods locus.
TABLE 1.
Evidence for parental effects in backcross mice
| N2 XX Ods/+ progeny
|
Transmission
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cross | Mother | Father | Males | Female+hermaphrodite | Total | Ods | Odsm1A | ||
| A. Backcrosses to FVB/Na | |||||||||
| 1 | FVB+/+ | F1 (AxFVB)Ods/+ | 114 | 0 | 114 | Paternal | Paternal | ||
| 2 | F1 (AxFVB)+/+ | FVBOds/+ | 51 | 12 | 63 | Paternal | Maternal | ||
| 3 | F1 (AxFVB)Ods/+ | FVB+/+ | 60 | 0 | 60 | Maternal | Maternal | ||
| 4 | F1 (FVBxA)+/+ | FVBOds/+ | 21 | 2 | 23 | Paternal | Maternal | ||
| Females
|
Hermaphrodites
|
Males
|
|||||||
| B. Genotype from cross 2 progenyb | |||||||||
| D18Mit184 | FF | AF | FF | FF | AF | FF | AF | FF | AF |
| D18Mit210 | FF | AF | FF | FF | AF | FF | AF | FF | FF |
| D18Mit25 | FF | AF | AF | FF | AF | FF | AF | AF | FF |
| No. of mice | 2* | 1 | 1 | 5* | 3 | 24 | 23 | 3 | 1 |
The numbers of males, females, and hermaphrodites, among XX Ods/+ progeny, are reported following paternal or maternal transmission of the transgene (Ods) and the suppressor (Odsm1A). At a 5% risk, the female and hermaphrodite frequencies are <2.5% (cross 1), between 9.4 and 28.7% (cross 2), and <4.8% (cross 3).
For XX Ods/+ mice from cross 2, the number of mice and their genotype around Odsm1 as a function of their phenotypic sex. A, A/J-derived allele; F, FVB/N-derived allele.
Maternal effect at Odsm1:
To check for imprinting at Odsm1, all the XX Ods/+ backcross progeny were genotyped for markers spanning the Odsm1 critical region: D18Mit184, D18Mit210, and D18Mit25 (Qin et al. 2003). Among females and hermaphrodites, 7 out of 12 (Table 1B, asterisks) were homozygous for the FVB/N allele (FF) at each of the three microsatellites, implying they were homozygous FF at Odsm1 (Table 1B). In these 7 mice, suppression of the sex reversal was achieved in the absence of the Odsm1A suppressor. This indicates that the maternal Odsm1 genotype itself, rather than the genotype of the offspring, controls the sex determination. To exclude a role for the mitochondrial genome, we set up an additional backcross to FVB (Table 1A, cross 4); the only difference between cross 2 and cross 4 is the mitochondrial genome from A/J (cross 2) and FVB/N (cross 4). In both crosses, suppression was observed, ruling out any role of mitochondrial DNA.
Thus sex reversal in Odd Sex mice can be suppressed through the action of a maternal-effect locus. Such loci are usually expressed during oogenesis, and their products play an essential role in the early stages of embryogenesis. In XX Ods/+ mice, male sex determination is a cell-autonomous process initiated at ∼11.5 dpc by the expression of Sox9 in the presumptive Sertoli cells. In the 2 XX Ods/+ females and the 5 XX Ods/+ hermaphrodites homozygous for Odsm1F (Table 1B), any maternal Odsm1A product is presumably long gone by the time sex determination is initiated. This urged us to reconfirm the association of the suppression with the A/J chromosome 18 found in the original F2 data (Qin et al. 2003). To do this, we backcrossed the Odd Sex mutation to either C57BL/6J females (Table 2A, cross 5) or consomic females (Table 2A, cross 6) carrying chromosome 18 from the A/J strain in a C57BL/6J genetic background (B6-18A; Nadeau et al. 2000; Singer et al. 2004). Surprisingly, complete sex reversal was observed in the F1 progeny of FVB/N Ods/+ males and either C57BL/6J, 20 males out of 20 F1 XX Ods/+, or consomic females, 25 males out of 25 F1 XX Ods/+. In the pure C57BL/6J backcross, however, (Table 2A, cross 5) we observed 3 females/hermaphrodites out of 26 XX Ods/+ offspring. This could be due to the existence of additional C57BL/6J recessive suppressor loci or a penetrance reduction associated with the genetic background shift. In the consomic backcross (Table 2A, cross 6), we observed 17 females/hermaphrodites out of 43 XX Ods/+, representing a significant increase in the suppression (P < 1.3%) and confirming linkage to chromosome 18.
TABLE 2.
The maternal effect is linked to chromosome 18
| N2 XX Ods/+ progeny
|
||||||
|---|---|---|---|---|---|---|
| Cross | Mother | Father | Males | Females+hermaphrodites | Total | |
| A. Outcrosses to C57BL/6Ja | ||||||
| 5 | B6 | F1 (B6xFVB)Ods/+ | 23 | 3 | 26 | |
| 6 | B6-18A | F1 (B6-18A × FVB)Ods/+ | 26 | 17 | 43 | |
| 7 | B6 | F1 (B6-18A × FVB)Ods/+ | 26 | 3 | 29 | |
| B. XX Ods/+ progenies from the consomic backcross (no. 6)b | ||||||
| D18Mit184 | AF | AA | AF | AA | AF | AA |
| D18Mit210 | AF | AA | AF | AA | AA | AF |
| D18Mit25 | AF | AA | AA | AF | AA | AF |
| Females + hermaphrodites | 7 | 7 | 1 | 1 | 1 | 0 |
| Males | 13 | 8 | 2 | 2 | 0 | 1 |
The numbers of males, females, and hermaphrodites, among XX Ods/+ progeny, are reported in backrosses to C57BL/6J (cross 5), to the consomic strain (cross 6), and in an outcross (cross 7).
Genotype around Odsm1 for the females/hermaphrodites and males. At each microsatellite the genotype distribution between the two groups, females/hermaphrodites and males, was compared with a chi-square analysis. No difference between the two groups was detected. P = 75.9% at D18Mit184; P = 35% at D18Mit210 and D18Mit25.
We next confirmed the maternal effect by two independent methods. First, we genotyped the XX Ods/+ progeny from the consomic backcross (Table 2A, cross 6) around the Odsm1 locus (Table 2B). As expected for a maternal effect, no transmission distortion was detected. Second, we crossed C57BL/6J females with F1 (B6-18A × FVB) Ods/+ males (Table 2A, cross 7). Here we observed only three females/hermaphrodites out of 29 XX Ods/+ mice. As predicted for a maternal effect, this shows a significant decrease in suppression compared to the consomic backcross, cross 6 (P < 0.6%), which carries Odsm1A maternally. Taken together the data obtained from these crosses confirmed the importance of the A/J-derived chromosome 18 in the suppression and the maternal effect itself.
Background effect:
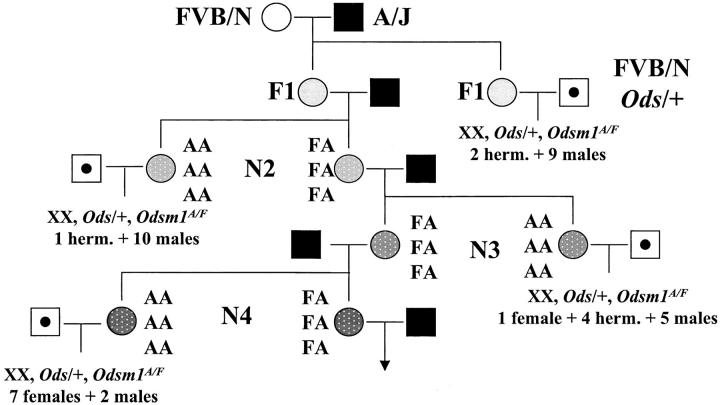
The suppression of sex reversal seen in the C57BL/6J backcross suggests the presence of other minor loci that contribute to the sex determination, with the suppressive C57BL/6J alleles recessive to the FVB/N alleles. To check this hypothesis, we started to generate a congenic line carrying the D18Mit184-D18Mit25 genomic segment from FVB/N in the A/J strain (Figure 1). At each generation, we crossed FVB/N Ods/+ males with females homozygous for the A/J alleles at the three microsatellites, D18Mit184, D18Mit210, and D18Mit25. The external and internal genitalia of all XX Ods/+ offspring were observed and we scored them as male, female, or hermaphrodite. XX Ods/+, Odsm1A/Odsm1F offspring were merged in two groups; the first group consisted of mice born from F1 and N2 females, the second group consisted of mice born from N3 and N4 females. In the first group, we observed 19 sex-reversed males and 3 hermaphrodites; in the second group, we observed only 7 sex-reversed males and 12 females/hermaphrodites. The sex distribution between these two groups, i.e., the effect of suppression, was significantly different, P = 1.7 × 10−4 risk (from a chi-square test). From generation N2 to N4, we observed a reduction in males and concomitantly an increase in females, suggesting both a quantitative and a qualitative effect of the genetic background.
Figure 1.—
Qualitative and quantitative effect of the genetic background. Odsm1F was backcrossed from FVB/N female, open circle, to A/J males, solid square, until generation N4. Its transmission was followed by three microsatellites, D18Mit184, D18Mit210, and D18Mit25, from top to bottom, AA homozygous for the A/J allele, FA heterozygous. At each generation, females homozygous for Odsm1A were mated with FVB/N Ods/+ males, open square with solid dot; the number of XX Ods/+ Odsm1A/F offspring and their sex are reported, female, hermaphrodites (herm.), and males.
DISCUSSION
In mice, male sex determination is a cell-autonomous process initiated by the Y-located testes-determining gene, Sry (Koopman et al. 1991). In XX Ods/+ embryos, however, Sox9 triggers this process (Bishop et al. 2000). Here, we show that the Ods suppressor, Odsm1, displays a maternal effect in addition to the zygotic effect detected in the F2 mapping (Qin et al. 2003). The suppression is also dependent on the parental origin of the tyrosinase transgene, i.e., imprinting, suggesting an interaction between Odsm1 and Ods in the suppression.
In mice, mutations with true maternal effects are very rare and are usually associated with death of the embryos conceived by females homozygous for the mutation. Death can occur before the morula stage (Christians et al. 2000; Tong et al. 2000; Burns et al. 2003; Wu et al. 2003), during blastocyst formation (Renard et al. 1994), around 9.5 dpc (Bourc'his et al. 2001), or even later after 14 dpc (Howell et al. 2001). In the cases of DNA methyltransferases, Dnmt3L and Dnmt1o, death occurred around mid-gestation and was associated with the partial loss of methylation at maternally imprinted loci, leading to an inappropriate expression of the corresponding genes (Bourc'his et al. 2001; Howell et al. 2001). Another maternal effect, without any visible consequence, was described in the transgenic mouse line TKZ751. Here, the transgene was methylated when transmitted through the paternal germline, but the methylation was controlled by a locus with maternal effect mapped to chromosome 17 (Pickard et al. 2001).
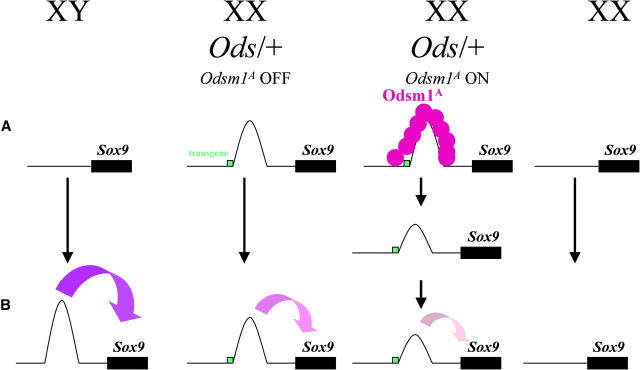
As for Dnmt3L (Bourc'his et al. 2001) and Dnmt1o (Howell et al. 2001), the consequence of Odsm1A maternal effect is seen relatively late in development, beginning at ∼11.5 dpc. As for TKZ751 (Pickard et al. 2001), the maternal effect was detected only through paternal transmission of the transgene. The suppression of the sex reversal in the Odd Sex model bears striking similarities with three reported cases involving methylation: Dnmt3L (Bourc'his et al. 2001), Dnmt1o (Howell et al. 2001), and the transgenic line TKZ751 (Pickard et al. 2001). Methylation is known to regulate the architecture of chromatin and, once modified, the new structure can be faithfully transmitted through somatic divisions reviewed in Li (2002). Our working hypothesis is that the insertion of the transgene somehow modified the surrounding chromatin structure, thereby allowing expression of Sox9 in XX Ods/+ fetal gonads through a long-distance effect (Ptashne 1986). We postulate that the product of Odsm1A modifies the chromatin structure around Ods/Sox9 on the paternal chromosome in the early embryo. The new structure of the chromatin would then be transmitted through somatic divisions during embryogenesis. This new chromatin conformation would be less permissive to the expression of Sox9 in the XX Ods/+ fetal gonad, reducing its level below a critical threshold leading to the suppression of the sex reversal in some cases (Figure 2).
Figure 2.—
Hypothetical model for the suppression of sex reversal. In XY and XX embryos at 0.5 dpc (A) the chromatin, around paternal Sox9, is in an inactive state (line). At 11.5 dpc in XY embryonic gonads, Sry, directly or indirectly, induces a dramatic conformation change (parabola), allowing upregulation of Sox9 (curved arrow). In XX bipotential gonads at ∼11.5 dpc in the absence of Sry, no change in chromatin conformation is detected leading to Sox9 downregulation and female sex determination. The insertion/deletion created a change in conformation around Sox9 in the XX Ods/+ fertilized egg (A); this new conformation mimicks the XY conformation at 11.5 dpc, inducing Sox9 upregulation and consequently sex reversal. Suppressor product, Odsm1A, is able to alter the conformation induced by the transgene in the XX Ods/+ fertilized egg (A); this altered conformation has a weaker effect on Sox9 upregulation, allowing sex-reversal suppression. Green box, tyrosinase transgene; shaded circle, Odsm1A, the suppressor product.
Interestingly, two genes, Mbd1 and Mbd2, encoding proteins that bind to methylated DNA, have been mapped to the Odsm1 critical region (Hendrich et al. 1999). Mice homozygous for null alleles at Mbd1 and Mbd2 (Hendrich et al. 2001; Zhao et al. 2003) are viable and fertile but have defects in overall methylation. Moreover, cells lacking Mbd2 were unable to silence methylated transgene (Hendrich et al. 2001). Those genes were therefore attractive candidates for Odsm1 but no sequence polymorphism, between A/J and FVB/N, was found in their coding sequence (data not shown).
Acknowledgments
This work was supported by grants from the National Institute of Health and the March of Dimes births defects foundation (to C.E.B.).
References
- Bishop, C. E., D. J. Whitworth, Y. Qin, A. I. Agoulnik, I. U. Agoulnik et al., 2000. A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat. Genet. 26: 490–494. [DOI] [PubMed] [Google Scholar]
- Bourc'his, D., G. L. Xu, C. S. Lin, B. Bollman and T. H. Bestor, 2001. Dnmt3L and the establishment of maternal genomic imprints. Science 294: 2536–2539. [DOI] [PubMed] [Google Scholar]
- Burns, K. H., M. M. Viveiros, Y. Ren, P. Wang, F. J. DeMayo et al., 2003. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science 300: 633–636. [DOI] [PubMed] [Google Scholar]
- Christians, E., A. A. Davis, S. D. Thomas and I. J. Benjamin, 2000. Maternal effect of Hsf1 on reproductive success. Nature 407: 693–694. [DOI] [PubMed] [Google Scholar]
- Foster, J. W., M. A. Dominguez-Steglich, S. Guioli, G. Kowk, P. A. Weller et al., 1994. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372: 525–530. [DOI] [PubMed] [Google Scholar]
- Hendrich, B., C. Abbott, H. McQueen, D. Chambers, S. Cross et al., 1999. Genomic structure and chromosomal mapping of the murine and human Mbd1, Mbd2, Mbd3, and Mbd4 genes. Mamm. Genome 10: 906–912. [DOI] [PubMed] [Google Scholar]
- Hendrich, B., J. Guy, B. Ramsahoye, V. A. Wilson and A. Bird, 2001. Closely related proteins MBD2 and MBD3 play distinctive but interacting roles in mouse development. Genes Dev. 15: 710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, C. Y., T. H. Bestor, F. Ding, K. E. Latham, C. Mertineit et al., 2001. Genomic imprinting disrupted by a maternal effect mutation in the Dnmt1 gene. Cell 104: 829–838. [DOI] [PubMed] [Google Scholar]
- Huang, B., S. Wang, Y. Ning, A. N. Lamb and J. Bartley, 1999. Autosomal XX sex reversal caused by duplication of SOX9. Am. J. Med. Genet. 87: 349–353. [DOI] [PubMed] [Google Scholar]
- Kent, J., S. C. Wheatley, J. E. Andrews, A. H. Sinclair and P. Koopman, 1996. A male-specific role for SOX9 in vertebrate sex determination. Development 122: 2813–2822. [DOI] [PubMed] [Google Scholar]
- Koopman, P., J. Gubbay, N. Vivian, P. Goodfellow and R. Lovell-Badge, 1991. Male development of chromosomally female mice transgenic for Sry. Nature 351: 117–121. [DOI] [PubMed] [Google Scholar]
- Li, E., 2002. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 3: 662–673. [DOI] [PubMed] [Google Scholar]
- Nadeau, J. H., J. B. Singer, A. Matin and E. S. Lander, 2000. Analysing complex genetic traits with chromosome substitution strains. Nat. Genet. 24: 221–225. [DOI] [PubMed] [Google Scholar]
- Pfeifer, D., R. Kist, K. Dewar, K. Devon, E. S. Lander et al., 1999. Campomelic dysplasia translocation breakpoints are scattered over 1 Mb proximal to SOX9: evidence for an extended control region. Am. J. Hum. Genet. 65: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard, B., W. Dean, S. Engemann, K. Bergmann, M. Fuermann et al., 2001. Epigenetic targeting in the mouse zygote marks DNA for later methylation: a mechanism for maternal effects in development. Mech. Dev. 103: 35–47. [DOI] [PubMed] [Google Scholar]
- Ptashne, M., 1986. Gene regulation by proteins acting nearby and at a distance. Nature 322: 697–701. [DOI] [PubMed] [Google Scholar]
- Qin, Y., C. Poirier, C. Truong, A. Schumacher, A. I. Agoulnik et al., 2003. A major locus on mouse chromosome 18 controls XX sex reversal in Odd Sex (Ods) mice. Hum. Mol. Genet. 12: 509–515. [DOI] [PubMed] [Google Scholar]
- Qin, Y., L. K. Kong, C. Poirier, C. Truong, P. A. Overbeek et al., 2004. Long-range activation of Sox9 in Odd Sex (Ods) mice. Hum. Mol. Genet. 13: 1213–1218. [DOI] [PubMed] [Google Scholar]
- Renard, J. P., P. Baldacci, V. Richoux-Duranthon, S. Pournin and C. Babinet, 1994. A maternal factor affecting mouse blastocyst formation. Development 120: 797–802. [DOI] [PubMed] [Google Scholar]
- Singer, J. B., A. E. Hill, L. C. Burrage, K. R. Olszens, J. Song et al., 2004. Genetic dissection of complex traits with chromosome substitution strains of mice. Science 304: 445–448. [DOI] [PubMed] [Google Scholar]
- Tong, Z. B., L. Gold, K. E. Pfeifer, H. Dorward, E. Lee et al., 2000. Mater, a maternal effect gene required for early embryonic development in mice. Nat. Genet. 26: 267–268. [DOI] [PubMed] [Google Scholar]
- Vidal, V. P., M. C. Chaboissier, D. G. de Rooij and A. Schedl, 2001. Sox9 induces testis development in XX transgenic mice. Nat. Genet. 28: 216–217. [DOI] [PubMed] [Google Scholar]
- Wagner, T., J. Wirth, J. Meyer, B. Zabel, M. Held et al., 1994. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79: 1111–1120. [DOI] [PubMed] [Google Scholar]
- Wu, X., M. M. Viveiros, J. J. Eppig, Y. Bai, S. L. Fitzpatrick et al., 2003. Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat. Genet. 33: 187–191. [DOI] [PubMed] [Google Scholar]
- Yokoyama, T., D. W. Silversides, K. G. Waymire, B. S. Kwon, T. Takeuchi et al., 1990. Conserved cysteine to serine mutation in tyrosinase is responsible for the classical albino mutation in laboratory mice. Nucleic Acids Res. 18: 7293–7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., T. Ueba, B. R. Christie, B. Barkho, M. J. McConnell et al., 2003. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc. Natl. Acad. Sci. USA 100: 6777–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]