Abstract
Zinc metalloproteases of the BMP-1/TOLLOID family (also known as astacins) are extracellular enzymes involved in important developmental processes in metazoans. We report the characterization of the Caenorhabditis elegans gene dpy-31, which encodes the first essential astacin metalloprotease identified in this organism. Loss-of-function mutations in dpy-31 result in cuticle defects, abnormal morphology, and embryonic lethality, indicating that dpy-31 is required for formation of the collagenous exoskeleton. DPY-31 is widely expressed in the hypodermal cells, which are responsible for cuticle secretion. We have investigated the dpy-31 function through reversion analysis. While complete reversion can be obtained only by intragenic suppressors, reversion of the Dpy-31 lethal phenotype also can be caused by dominant extragenic suppressors. Nine extragenic suppressors carry mutations in the uniquely essential collagen gene sqt-3, which we show is the same gene as rol-4. Most mutations exhibit the unusual property of exclusively dominant suppression and all affect the sequence of the SQT-3 collagen C terminus. This suggests that DPY-31 is responsible for C-terminal proteolytic processing of collagen trimers and is therefore a structural and functional homolog of vertebrate BMP-1. The results also demonstrate the critical importance of the collagen C-terminal sequence, which is highly conserved among all 49 members of the SQT-3 subfamily.
COLLAGENS constitute a major class of extracellular structural proteins in metazoans. Their importance is nowhere greater than in nematodes, in which the cuticle is largely constructed from an elaborate matrix of collagen molecules. In Caenorhabditis elegans, collagens account for ∼80% of the cuticle mass (Cox et al. 1981). The C. elegans cuticular collagen gene family includes >170 members, which are 1–2 kb long and encode proteins of ∼300 amino acids (aa). C. elegans collagen are structurally similar to vertebrate nonfibrillar FACIT collagens (Shaw and Olsen 1991), although considerably smaller in size. Like their vertebrate counterparts, nematode collagens possess Gly-X-Y repeats, which are required for formation of triple helical trimers. After assembly in the endoplasmic reticulum (ER), collagen trimers are secreted into the extracellular environment where they are highly crosslinked, thus forming a polymeric matrix (for a review on C. elegans cuticle collagens, see Johnstone 2000).
Genetic analysis has provided extensive insights into the process of cuticle formation in C. elegans. Many morphological mutants affecting the cuticle have been isolated in random and directed mutagenesis screens. These mutants are divided into distinct gene classes on the basis of visible phenotypes. Six types of viable morphological mutants have been described: dpy (dumpy), which is shorter than wild type (WT); bli, which has a blistered cuticle; rol (roller), which has a helically twisted body; sqt (squat), which has both a dominant Rol and a recessive Dpy phenotype; and lon, which is longer than WT (Brenner 1974). The molecular identity of most defined morphogenetic loci has now been determined; on one hand, 19 genes in these classes encode cuticle collagens, indicating that, despite the high level of redundancy, many collagens play distinct roles in cuticle structure. On the other hand, four morphogenetic loci, all of which were isolated in the course of the first C. elegans mutagenesis screens (Brenner 1974), encode core enzymatic components of the collagen assembly pathway, which is largely conserved between nematodes and vertebrates (for a recent review, see Myllyharju and Kivirikko 2004). The essential gene bli-4 encodes a furin proprotein convertase, which is thought to be involved in N-terminal proteolytic cleavage of collagen precursors (Thacker et al. 1995). Furin proteases have been implicated in collagen processing in mammals (Imamura et al. 1998). dpy-18 codes for one component of the prolyl 4-hydroxylase complex, which is required for collagen chain assembly in vertebrates (Winter and Page 2000). The dpy-11 gene encodes an ER-bound thioredoxin-like protein, which may also play a role in collagen assembly, presumably during trimer formation (Ko and Chow 2002). Finally, bli-3 codes for a dual oxidase, which is the putative catalyst of collagen tyrosine-derived crosslinking (Edens et al. 2001; Simmer et al. 2003). Herein, we examine a newly defined morphogenetic gene, which was named dpy-31 after its Dpy mutant phenotype. dpy-31 mutants also display a lethal phenotype, indicating that this gene is required for an essential aspect of cuticle formation. Upon cloning, dpy-31 was found to encode a zinc-metalloprotease of the BMP-1 (bone morphogenetic protein-1)/TOLLOID family. Proteases of this family are involved in important developmental processes, such as pattern formation, extracellular matrix (ECM) maturation, remodeling, and degradation (Bond and Beynon 1995). In particular, BMP-1 is responsible for proteolytic maturation of the C-propeptides of various collagen precursors. Reversion analysis of dpy-31 revealed unusual genetic interactions, which implicate the C-terminal region of a uniquely essential collagen, SQT-3, as a major target for processing by DPY-31. Involvement of zinc-metalloproteases in collagen maturation therefore appears to be crucial for collagen deposition and a conserved feature of the metazoan lineage.
MATERIALS AND METHODS
Strains:
Maintenance and handling of C. elegans were performed as described by Sulston and Hodgkin (1987). Worms were cultured at 20°, unless otherwise stated. Bristol N2 was used as WT.
The following mutant strains were used:
LG II: rrf-3(pk1426);
LG III: dpy-31(e2770, e2919, e2920, ju345); unc-32(e189); sma-3(e491); mab-5(e1239); unc-36(e251, e548); lon-1(e185); unc-119(e2498); nDf16;
LG IV: fem-1(hc17);
LG V: sqt-3(e24, e2117, sc63, sc8, sc42, e2639) sqt-3(e2809, e2888, e2889, e2890, e2896, e2901, e2906, e2911); ctDf1; arDf1;
LG X: xol-1(y9);
Arrays: kaIs(col-19::GFP).
Mapping and cloning of dpy-31:
After isolation in the mrt-2 mutant background, dpy-31(e2770) was mapped to the center of LG III and therefore tightly linked to the mrt-2 mutation. It was separated from mrt-2 by constructing a dpy-31 unc-32 double-mutant chromosome and then by removing the unc-32 marker by recombination against WT. Three-factor mapping was performed by using the triple-marked strain sma-3 mab-5 unc-36, whereby dpy-31 was placed between the two outside markers at position −0.80 (39 recombinants were examined). Snip-SNP mapping was performed as described by Wicks et al. (2001). Four double mutants containing dpy-31 and a flanking marker were constructed. These were the following: lon-1 dpy-31; sma-3 dpy-31; dpy-31 unc-36; and dpy-31 unc-32. We analyzed, respectively, 13 Lon-1, 1 Sma-3, 11 Unc-36, and 30 Unc-32 recombinants. Snip-SNP mapping defined a genomic region between cosmid clones F31E3 and T20B12. Clones from this interval were injected into the gonad of e2770 worms as described by Mello and Fire (1995) at an average concentration of 10 μg/ml with 20 μg/ml of pTG96 (a green fluorescent protein (GFP) reporter plasmid; Yochem et al. 1998). Following identification of R151 as a rescuing clone, the pD31FL construct including the genomic sequence of gene R151.5 was generated as below. PCR was performed on clone R151 with the following primers (bases added at the 5′ end are in lowercase; PstI sites are underlined): PstIPrF sense: 5′-aaactgcagTCCAACACCAGACTTTTCTCC-3′ and PstItoh2R antisense: 5′-tatttgacgTCCATCAGATCACCCAGC-3′. The 8.9-kb product was digested with PstI and ligated into vector pGEM-5Zf (Promega, Madison, WI) to generate pD31FL. PD31FL was injected into e2770 mutants at a concentration of 10 μg/ml with 20 μg/ml of pTG96. Gene R151.5 was amplified by PCR from e2770 genomic DNA and sequenced with gene-specific primers.
dpy-31 reporter gene:
pD31P3.4G was generated as described below. A 3.4-kb PCR product was amplified from cosmid R151 using the following primers: PstIPrF sense (primer used for construct pD31FL) and BaPrR antisense (BamHI site is underlined): 5′-cgggatcccgGCTGAAATTAAAGTTTAAAG-3′. The resulting PCR product was digested and cloned directionally into pPD96.04 (A. Fire, personal communication). pD31P3.4G was injected into unc-119 worms at a concentration of 20 μg/ml with 20 μg/ml of pDP#MM016b (an unc-119 rescuing construct; Maduro and Pilgrim 1995). To ascertain consistency in the expression pattern, we generated multiple transgenic unc-119 lines carrying pDP#MM016b and pD31P3.4G on an extrachromosomal array. Every line was found to display the same temporal and spatial pattern of expression.
pD31P3.4G-N, a version of pD31P3.4G devoid of nuclear localization signal (NLS), was generated by digestion of pD31P3.4G with KpnI followed by religation. PD31P3.4-N was directly injected into N2 worms.
RNAi of dpy-31:
For post-transcriptional silencing of the dpy-31 gene, RNA interference (RNAi) was performed by double-stranded RNA injection of N2 hermaphrodites (Fire et al. 1998). The double-stranded RNA injected corresponded to exons 2 and 3 of dpy-31 and the intervening sequence between the two exons. The following primers were used to amplify the fragment from N2 genomic DNA (T3 and T7 polymerase recognition sequences are in lowercase): T3toh23F sense 5′-attaaccctcactaaagGTTGGTAGCATGGGATCG-3′ and T7toh23R antisense 5′-aatacgactcactatagTGCGTTTAGACTTAATCC-3′.
Screens for suppressors of dpy-31:
Nine suppressor screens were performed, three using ethyl methane sulfonate (EMS) and six using N-ethyl N-nitrosourea (ENU). In all screens, the e2770 allele of dpy-31 was used. The high level of lethality and infertility associated with e2770 precludes accurate measurement of the number of genomes tested in each suppressor screen. We estimate that at least 106 (EMS) and at least 2 × 106 genomes (ENU), in total, were tested.
EMS mutagenesis:
Mutagenesis of e2770 was performed as described by Brenner (1974). Following treatment with EMS, worms were plated and grown for 6–10 days before being shifted to restrictive temperature. Plates were then inspected for viable revertants.
ENU mutagenesis:
We used the protocol devised by De Stasio and Dorman (2001). Procedure was otherwise the same as the one followed in EMS screens.
Mutation analysis of WT revertants of e2770:
Mutations in WT revertants of e2770 were analyzed either by sequencing or by amplification refractory mutation system-polymerase chain reaction (ARMS-PCR; Newton et al. 1989). ARMS-PCR was performed by using a gene-specific reverse primer (LastR: antisense 5′-AATACCACCGCTGTCTGTCC-3′) paired in separate PCRs with three allele-specific forward primers. These were the following (the variable sequence is underlined): LeuF (sense 5′-GCAACTACTGATATGGTTGTACT-3′), which is specific for the Leu codon found in WT; SerF (sense 5′-GCAACTACTGATATGGTTGTATC-3′), which is specific for the Ser codon found in “Ser” revertants of e2770; and ProF (sense 5′-GCAACTACTGATATGGTTGTACC-3′), which is specific for the Pro codon found in e2770 mutants.
Mapping and cloning of rol-4:
Five cosmid clones contained within the interval covered by the deficiency ctDf1 were injected into rol-4(sc8) worms at a concentration of 10 μg/ml with 20 μg/ml of pTG96. Clone F23H12 rescued the left-handed Roller (Lrol) phenotype in transgenic sc8 worms. sqt-3 was amplified by single-worm PCR (Wicks et al. 2001) and sequenced with gene-specific primers. The procedure was similar for all the sqt-3 suppressors associated with a LRol phenotype, i.e., sc8, e2809, e2888, e2889, e2890, e2896, and e2901. The non-Rol sqt-3(sup) alleles e2906 and e2911 were identified by their failure to complement sqt-3 alleles. Other alleles assigned to sqt-3/rol-4 for which the sequence alteration had not been identified were b238 and sc42. No sqt-3 mutation was found in b238; this allele complements sqt-3/rol-4 alleles and probably defines a distinct locus. sc42 was sequenced with gene-specific primers and the sequence alteration was determined. Finally, the e2639 deletion allele of sqt-3, which was recovered in a mut-7 mutator background (M. Skipper and J. Hodgkin, unpublished results), was assigned to sqt-3 on the basis of the results of complementation tests. The e2639 allele of sqt-3 was then sequenced with gene-specific primers.
Isolation of new dpy-31 alleles:
Additional alleles of dpy-31 were obtained by crossing EMS-mutagenized N2 males with females of genotype dpy-31(e2770) unc-36(e548) III; fem-1(hc17) IV; sqt-3(sc8)/+ V; xol-1(y9) X at 22.5°. The unc-36 mutation is tightly linked to dpy-31 and thus acts as marker for the dpy-31 tester allele. The temperature-sensitive (ts) mutation fem-1(hc17) results in feminization of hermaphrodite worms at restrictive temperature. The sc8 dominant suppressor of the dpy-31 lethality allows viability of homozygotes for dpy-31. Finally, the xol-1 mutation (XO lethal) leads to the death of the male cross-progeny.
Approximately 5600 F1 progeny, of which half (2800) would have carried the sc8 suppressor, were scored for Dpy non-Unc animals. Candidates were cloned. ARMS-PCR performed using the primers described above was used to identify new alleles of dpy-31. Two Dpy lines, which appeared WT at the nucleotide position changed in e2770 mutants, were analyzed further. Sequencing of dpy-31 in these strains identified novel mutations in the coding region. The lines were retained and the new alleles of dpy-31 were named, respectively, e2919 and e2920. ju345 was assigned to dpy-31 on the basis of its failure to complement e2770.
Test for the effect of the sqt-3(sup) dosage on the viability of dpy-31 worms:
Worms of genotype dpy-31(e2770)/unc-32(189); sqt-3(sup)/+ were identified by inspection of the F1 progeny for the presence of viable Dpy and Unc segregants. Animals of correct genotype were grown at 22.5° and moved daily to a fresh plate. The F1 progeny were scored. We tested the sqt-3(sup) alleles sc8, e2889, e2901, e2906, and e2911. As unc-32 is tightly linked to dpy-31, recombination occurring between the two markers could be neglected. As a control, F1 progeny of dpy-31/unc-32 double heterozygotes were scored.
RESULTS
dpy-31: phenotypic characterization:
The dpy-31 locus of C. elegans was originally defined by a single mutation, e2770, which was recovered as a spontaneous event in a mrt-2(e2663) mutator background (Ahmed and Hodgkin 2000). In subsequent analysis, two further alleles of the gene, e2919 and e2920, were isolated in a directed EMS screen. A fourth allele of dpy-31, ju345, was a gift from Andrew Chisholm, who recovered it independently in an EMS screen.
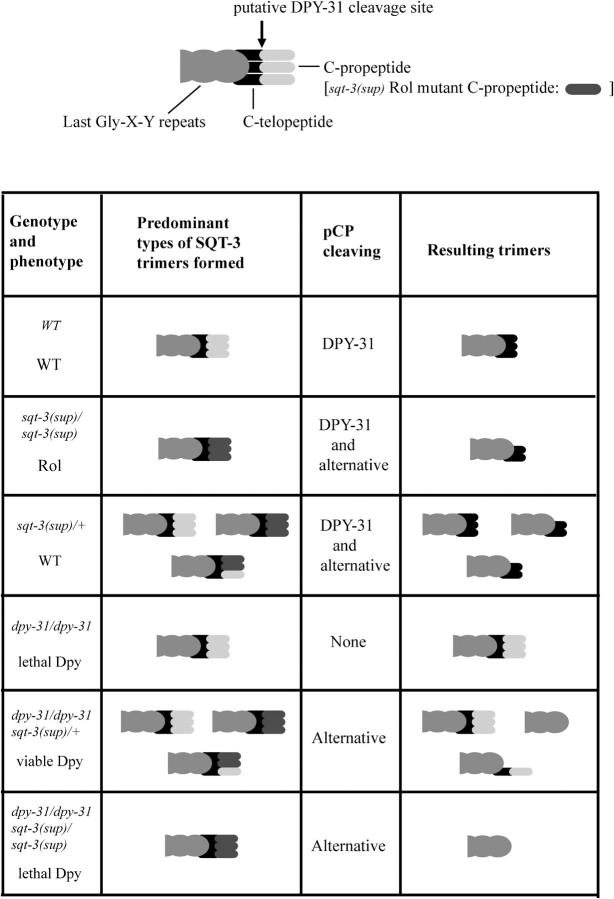
dpy-31(e2770, e2919, e2920) worms exhibit a strong recessive Dpy phenotype (Figure 1B) and are ts lethal. These dpy-31 mutants are barely viable at permissive temperature (15°), but totally inviable at restrictive temperature (20°–25°). ju345 animals display a milder Dpy phenotype and are ∼80% viable at 25°, indicating that ju345 is a hypomorphic allele of dpy-31. Embryos and first larval stages (L1) of dpy-31 are particularly sensitive to temperature upshifts. At 25°, dpy-31 embryos develop normally until the threefold stage; however, after completing elongation, mutant embryos retract in length and fail to hatch (Figure 1C). Rare hatchlings are arrested as L1 with a distinctive “retracted lumpy” appearance (Figure 1D). This phenotype has been observed in other mutants, such as sqt-3(e24, e2117) (Priess and Hirsh 1986; van der Keyl et al. 1994), bli-4(s90) (Thacker et al. 1995), and pdi-2(RNAi) (Winter and Page 2000). In all these cases, the lethal Dpy phenotype was found to be associated with defects in cuticle synthesis. Inspection of the mutant phenotype thus suggests that DPY-31 plays an essential role in formation of the collagenous exoskeleton.
Figure 1.—
Phenotype of dpy-31 mutants. (A) WT adult worm. (B) dpy-31(e2770) adult raised at 15°. (C) e2770 embryos raised at 25°: the cuticle surface in tail region of a dead embryo (solid arrow) appears abnormal. (D) e2770 arrested L1 larva. Note the severe internal disorganization resulting from hypercontraction. The solid arrow indicates the pharynx. (E) WT worm carrying the col-19::GFP reporter gene. The alae (open arrowhead) are visible. The circumferential rings perpendicular to the alae are the annuli. (F) col-19::GFP expression in dpy-31 mutants. Two worms are depicted. An area of concentrated fluorescence (open arrow) can be seen in the head region of a worm. The arrowheads mark the position of the alae. Bars: A and B, 0.1 mm; C–F, 10 μm.
To further characterize the defect in cuticle structure of dpy-31 mutants we made use of a strain carrying the transgenic array kaIs(col-19::GFP), which contains a fusion of the collagen gene col-19 and a GFP reporter (Thein et al. 2003). In adult WT worms, the COL-19::GFP protein localizes to the cortex of annuli, which are circumferential ridges covering the body of the worms, and to the alae, which are tripartite structures running perpendicular to the annuli (Figure 1E).
Construction of a line carrying the e2770 mutation together with the kaIs(col-19::GFP) array revealed that COL-19::GFP assembly is aberrant in dpy-31 mutants grown at permissive temperature (Figure 1F). In e2770 worms, COL-19::GFP fails to localize to the alae; instead, in this region an irregular mesh of fibers, extending into the annuli, can be seen. Furthermore, intensely bright patches of COL-19::GFP were observed in the animals' head region. These patches probably represent unsecreted COL-19::GFP accumulating intracellularly in the seam cells (A. Page, personal communication). The disruption of COL-19::GFP assembly in e2770 worms is similar to that observed by Thein et al. (2003) for dpy-5, which encodes a cuticle collagen. These observations support a role for dpy-31 in cuticle formation.
dpy-31: mapping and cloning:
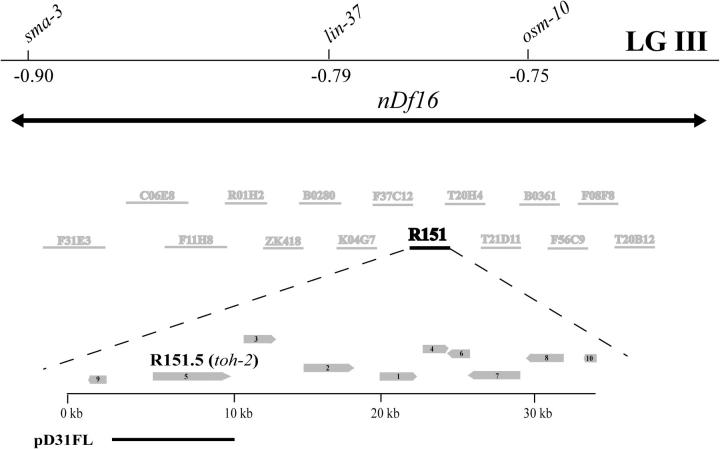
Visible marker mapping placed dpy-31 0.1 map unit to the right of sma-3 (−0.90) on LG III (Figure 2). We also found that dpy-31 is uncovered by the deficiency nDf16, extending between positions −1.05 and −0.23. Worms of genotype e2770/nDf16 were indistinguishable from e2770 homozygotes, showing that e2770 behaves as a classic loss of function and indicating that this allele represents the null state of dpy-31 at 25°.
Figure 2.—
Genetic and physical map of the dpy-31 locus. The deficiency nDf16 is depicted by a double arrow. The 15 cosmid clones defined as a physical interval by snip-SNP mapping are indicated by thin bars. The open reading frames in the rescuing cosmid R151 and the rescuing construct pD31FL are depicted by thick bars.
To refine the map position, we performed snip-SNP mapping, whereby we reduced the interval to 15 genomic clones. Within these we identified a single positive rescuing clone (R151; Figure 2). In further tests, we determined that a WT copy of gene R151.5 is sufficient to rescue the dpy-31 function. Gene R151.5 had already been named toh-2 (tollish-2) on the basis of the sequence similarity to Drosophila melanogaster tolloid (http://www.wormbase.org). In light of current data, the name toh-2 is misleading; we therefore renamed gene R151.5 after its mutant phenotype, i.e., dpy-31.
Structure of DPY-31 and identification of the DNA lesions in dpy-31 mutants:
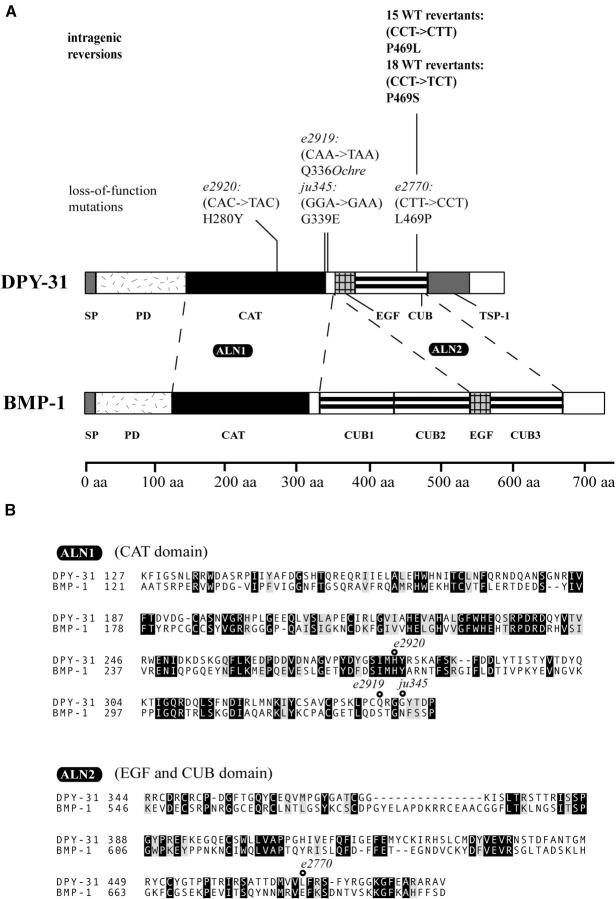
dpy-31 encodes a predicted zinc-metalloprotease of the BMP-1/TOLLOID family. Peptidases of this family are also known as astacins and are characterized by a zinc-binding catalytic domain, which is highly conserved across metazoans. In addition to the catalytic domain, astacin metalloproteases usually also contain a variable number of C-terminal extensions, which are thought to be required for regulation or modulation of activity. All astacin proteases identified so far are secreted or plasma membrane bound (Bond and Beynon 1995). TOLLOID is an essential component of pattern formation in Drosophila (Marqués et al. 1997). Mammalian BMP-1 is a major ECM regulator, which, in addition to its key role in the BMP-signaling pathway, is responsible for processing of ECM structural components (Pappano et al. 2003 and references cited therein). The deduced DPY-31 protein contains a signal peptide (SP: aa 1–16; Figure 3A), a prodomain (PD: aa 16–133), an astacin catalytic domain (CAT: aa 134–327), an epidermal growth factor motif (EGF: aa 344–370), a CUB (C1r/C1s, embryonic sea urchin protein Uegf, BMP-1) domain (CUB: aa 371–484), and a thrombospondin type I repeat (TSP-1: aa 494–539). DPY-31 shows 29% overall identity and 43% similarity to the shorter splicing variant of human BMP-1.
Figure 3.—
dpy-31 encodes a BMP-1 homolog. (A) Comparison of C. elegans DPY-31 (corresponding to gene R151.5) and human BMP-1 (isoform 1). BMP-1 has two additional CUB domains but lacks the TSP-1 motif. The molecular lesions identified in four dpy-31 alleles and the intragenic reversion events (boldface type) are shown above the protein structure. The corresponding codon changes are in parentheses. (B) ClustalW alignments of the CAT domain (ALN1) and EGF and CUB domains (ALN2) of DPY-31 and BMP-1. The CUB domain of DPY-31 is most homologous to the third CUB domain of BMP-1 (CUB3), which is shown in the alignment. The positions affected by the mutations identified in dpy-31 are shown.
Sequencing of the e2920, e2919, ju345, and e2770 alleles of dpy-31 identified mutations, respectively, in exons 4, 5, 5, and 6 of the gene. e2770, which arose spontaneously in the mrt-2 mutant background, is a T-to-C transition resulting in a Leu-to-Pro substitution at residue 469 of the encoded protein (L469P; Figure 3, A and B). This type of mutation would not be commonly induced by the standard chemical mutagen EMS, which causes chiefly GC-to-AT transitions (Sulston and Hodgkin 1987). At the protein level, the e2770 mutation maps to the CUB domain, which is a protein-protein interaction module composed of 10 β-strands arranged in a jellyroll-type topology (Romão et al. 1997). The Pro substitution affects the consensus sequence of β-strand 9, thus potentially leading to the disruption of the CUB domain fold.
In the three EMS-induced mutants, e2920, e2919, and ju345, GC-to-AT transitions were detected. e2920 maps to the conserved “Met-turn” region of the CAT domain, where it causes the substitution of an invariant His (H280Y; Figure 3, A and B), which is adjacent to a Tyr residue involved in zinc coordination (Bond and Beynon 1995). This conserved Tyr residue is essential for catalytic activity in BMP-1 (Yiallouros et al. 2000), indicating that DPY-31 is also likely to function as a protease. e2919 introduces a stop codon at position 336 of DPY-31 (Q336Ochre; Figure 3, A and B). The e2919 allele is predicted to encode a severely truncated protein lacking the EGF, CUB, and TSP-1 domain. Since the CUB domains are essential for catalytic activity and secretion of BMP-1 (Hartigan et al. 2003), it seems likely that this allele represents a dpy-31 null. Finally, the hypomorphic allele ju345 is a missense mutation causing a Gly-to-Glu substitution downstream of the CAT domain (G339E; Figure 3, A and B). e2770, e2920, and ju345 affect conserved motifs that are also present in BMP-1 (Figure 3).
Following identification of dpy-31, we attempted to phenocopy the Dpy-31 phenotype by RNAi. RNAi of dpy-31 occasionally resulted in an embryonic lethal phenotype in the progeny similar to that exhibited by dpy-31 mutants. However, we found that RNAi phenocopies the Dpy-31 lethality only at low penetrance [5% of the F1 progeny in WT; n = 5; 24.5% of the F1 progeny in rrf-3 mutants, which are hypersensitive to RNAi (n = 4; Simmer et al. 2002)]. Relative insensitivity to RNAi appears to be a common feature of astacin protease genes in C. elegans (Möhrlen et al. 2003).
DPY-31 is expressed in the hypodermis:
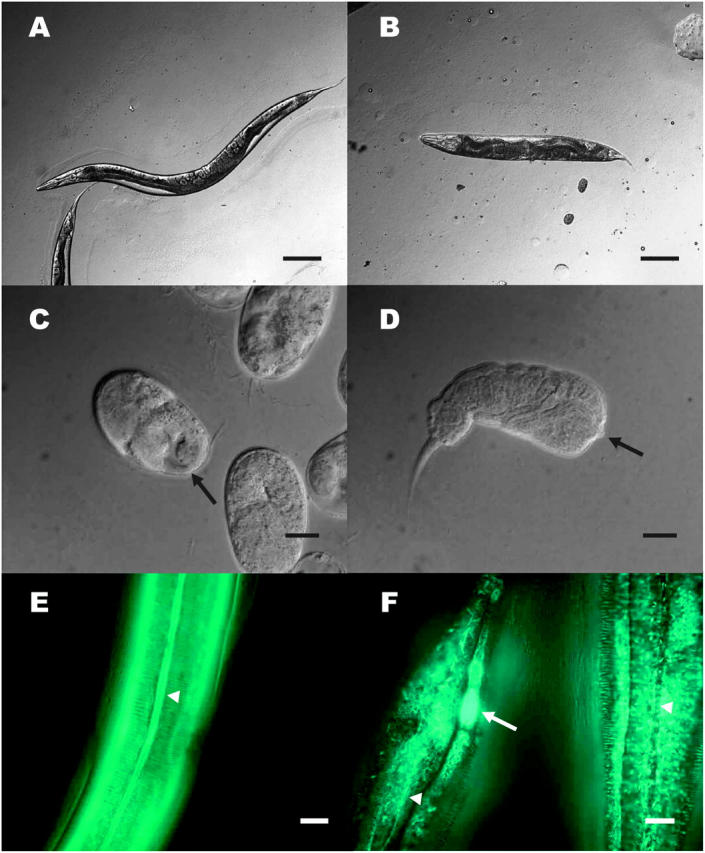
To investigate the expression pattern of dpy-31, we generated a reporter gene (pD31P3.4G) containing the dpy-31 5′ flanking region fused to the GFP sequence with a NLS. Transgenic worms carrying pD31P3.4G express GFP in most hypodermal nuclei throughout the life cycle (Figure 4). Expression was first detected in embryos at the twofold stage. The GFP signal was particularly intense in embryos (Figure 4A) and early larvae (Figure 4B).
Figure 4.—
Tissue-specific localization of dpy-31. Expression of GFP reporter driven by the dpy-31 promoter is detected in the hypodermal cells. (A) Fully elongated embryo and (B) L2 larva carrying a multicopy array of pD31P3.4G, which contains the dpy-31 promoter sequence fused to GFP with a NLS. (C and C′) L4 larva carrying a multicopy array of pD31P3.4G-N, which is a NLS negative version of pD31P3.4G. No expression of DPY-31 is detected in the seam cells (open arrowheads), which have not yet undergone fusion in this animal. The rectal epithelial cells expressing DPY-31 are indicated by an open arrow. Bars: A, 10 μm; B, 20 μm; C and C′, 50 μm.
A version of the pD31P3.4G construct devoid of NLS (pD31P3.4G-N) confirmed this expression pattern. In transgenic animals carrying pD31P3.4G-N, GFP was detected in most hypodermal cells, except in the seam cells of larval stages (Figure 4, C and C′). Expression of DPY-31 is particularly pronounced at all life stages in the amphid socket cells at the anterior end of the animal and in the rectal epithelial cells at the posterior end (Figure 4, C and C′). In L4 and adults, DPY-31 expression was also seen in the vulval epithelial cells. Fluorescence was also observed in some head neurons, although the identity of such neurons has not been determined.
Investigation of the dpy-31 function through reversion analysis:
In vertebrates, the astacin metalloprotease BMP-1 acts as a procollagen C-proteinase (pCP): BMP-1 converts collagen trimers into a mature form by cleaving their C-terminal propeptides (Kessler et al. 1996). Taken together, our results suggested that DPY-31 could be involved in processing of cuticle collagens. However, the extreme redundancy of the collagen gene family made it arduous to identify potential targets of DPY-31 by biochemical means. We therefore sought to characterize the dpy-31 gene function by genetic means. The fully penetrant ts lethality of dpy-31 mutants lent itself to analysis of genetic interaction via isolation of extragenic suppressors. We first carried out an EMS pilot screen in the course of which five independent revertant strains were isolated. To obtain a wide range of suppressing mutations, most subsequent screens were performed using the mutagen ENU, which generates a wider spectrum of mutations than EMS (De Stasio et al. 1997).
Nine mutagenesis screens yielded >50 independent revertants of e2770. Such revertants have different properties but can be grouped into two main classes: WT revertants, i.e., phenotypically WT worms, and non-ts Dpy revertants, which are Dpy but no longer lethal at restrictive temperatures.
The WT revertants carry intragenic mutations restoring the dpy-31 function:
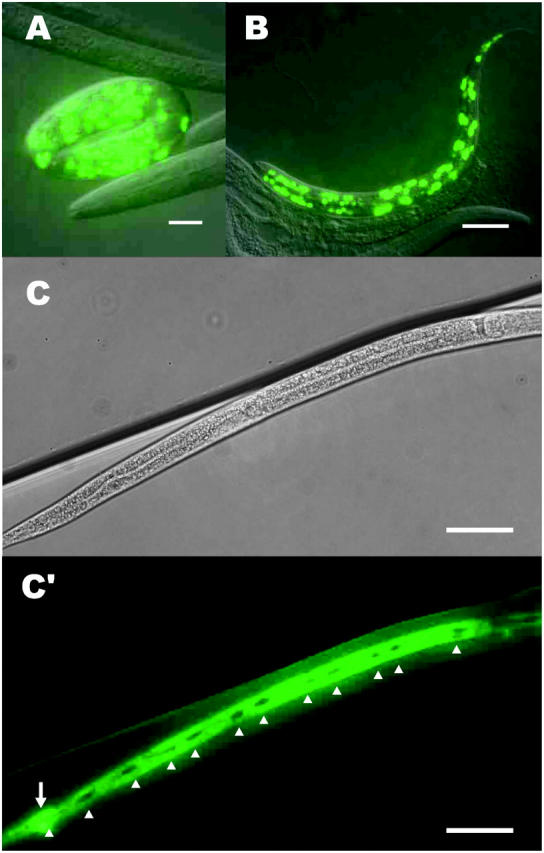
A total of 33 WT revertants were isolated: 19 strains were recovered in EMS screens and 14 in ENU screens. The WT revertants had almost identical properties, resembling normal worms with respect to body size (Figure 5A). All revertants complemented the e2770 allele and the deficiency nDf16, indicating that the suppressor mutations were cis dominant and tightly linked to e2770 and therefore presumably intragenic.
Figure 5.—
Revertants of e2770. (A) WT revertant animals. This strain is a Ser revertant: the worms appear perfectly WT as far as body size and shape is concerned. (B) Non-ts Dpy revertants (e2770/e2770; sc8/+) grown at 25°. The strain is viable at restrictive temperature. Bars: A, 0.1 mm; B, 1 mm.
Intragenic reversion events were confirmed by sequence analysis of these alleles. Five WT revertants carried an exact reversion at the codon changed by e2770, restoring the WT sequence of DPY-31 (P469L; Figure 3). Five other WT revertants carried a second-site missense mutation on the same codon. This mutation would cause a Pro-to-Ser substitution in the encoded protein (P469S; Figure 3), suggesting that amino acids other than Leu can restore the function of the CUB domain in dpy-31. While the “Leu” revertants were indistinguishable from WT, the “Ser” revertants had a residual lethality (9% of the F1 progeny at 20°; n = 5).
To rapidly analyze the remaining 23 WT revertants, we used ARMS-PCR (Newton et al. 1989). This revealed that all the WT revertants carried either a codon for Leu (8/19 EMS; 10/14 ENU) or a codon for Ser (11/19 EMS; 4/14 ENU) at the codon changed by e2770. ENU might be expected to generate additional single-base changes to codons for Ala, Thr, Arg, or His at position 469, but these were not recovered as revertants nor were any compensatory mutations located elsewhere in the coding sequence.
The non-ts Dpy revertants carry extragenic mutations that suppress dpy-31 lethality:
Twenty-three non-ts Dpy revertants of e2770 were isolated, 6 from EMS screens and 17 from ENU screens. All non-ts Dpy revertants are Dpy at all temperatures and viable at 25° (Figure 5B). Adults of these strains are Dpy but often larger than e2770 viable worms (Figure 5B) while early larvae are markedly less Dpy than e2770 and sometimes close to WT. Genetic analysis showed that all the non-ts Dpy revertants contain the original e2770 mutation as well as a dominant extragenic suppressor of the lethality.
When separated from dpy-31, six of the dominant suppressors (e2809, e2888, e2889, e2890, e2896, and e2901) were found to be associated with a recessive LRol phenotype. Further analysis showed that the six mutations were allelic and linked to LGV. In complementation tests against known genes mapping to LGV, the alleles failed to complement rol-4, which also displays a LRol mutant phenotype. Finally, we found that the standard rol-4 allele sc8 could also act as a dominant suppressor of the dpy-31 lethality. This showed that these suppressors are allelic to rol-4.
The genetic interaction between rol-4 and dpy-31 suggested that this gene was involved at some level in dpy-31 function. rol-4 was defined >20 years ago (Cox et al. 1980), but its molecular identity has not been hitherto determined. Since seven of eight genes associated with a LRol mutant phenotype have been found to encode collagens, it seemed probable that rol-4 would encode a collagen target of dpy-31 or, alternatively, a collagen-modifying enzyme functionally related to dpy-31.
The extragenic suppressor rol-4 defines a group of mutations affecting the C-terminal region of the SQT-3 collagen:
rol-4 had been previously mapped to position 4.30 on LGV, but contradictory data have been reported as to whether rol-4 is uncovered by the deficiency ctDf1, spanning map positions 3.4–4.1 (http://www.wormbase.org). We determined that rol-4 is contained within ctDf1 but we found that sc8/ctDf1 worms roll less vigorously than sc8 homozygotes, suggesting that ctDf1 contains a hypomorphic allele of rol-4.
To clone rol-4, a candidate gene approach was taken. We searched the genomic sequences covered by or in proximity to ctDf1 for genes encoding collagen or modiying enzymes and selected candidates on five cosmid clones. Clone F23H12 rescued the Rol phenotype of rol-4 worms when carried on an extrachromosomal array (data not shown). F23H12 contains the genomic sequence of the collagen gene sqt-3, which has been previously characterized and defined mutationally (Kramer et al. 1985; van der Keyl et al. 1994). Two lines of evidence indicated that sqt-3 and rol-4 are the same gene: (i) the mutant phenotypes of the two genes are similar, as sqt-3 mutants display a LRol phenotype and tail abnormalities and both defects are also found in rol-4 worms and (ii) sqt-3 alleles exhibit aberrant complementation patterns with rol-4(sc8), which have been interpreted as cases of intergenic noncomplementation (Kusch and Edgar 1986). Our results instead suggest that the complex complementation arises from intragenic interactions.
We then tested the remaining extragenic suppressors for allelism to sqt-3. Two other dominant suppressors of dpy-31 lethality, e2906 and e2911, failed to complement sqt-3/rol-4 alleles. e2911 worms display a weak Dpy phenotype but seem otherwise WT; e2906 homozygotes are also weakly Dpy. In addition, this strain exhibits a strong cold sensitive (cs) lethality; i.e., e2906 worms are viable at 25°, but embryonic or early larval lethal at 15°.
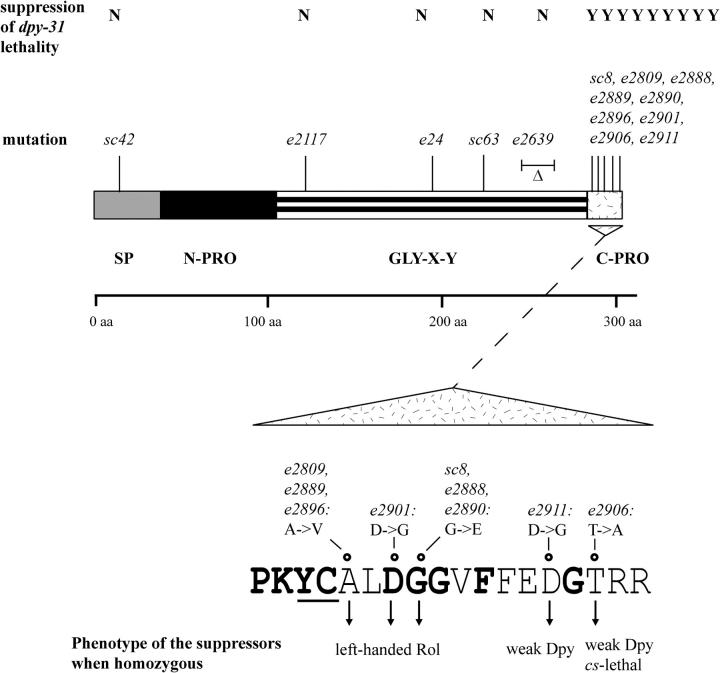
Sequence analysis of the sqt-3 gene isolated from the nine allelic suppressor strains (sc8, e2809, e2888, e2889, e2890, e2896, e2901, e2906, and e2911) revealed that each of them carried a missense mutation in the last exon of sqt-3. In all, five different mutations were obtained, because sc8, e2888, and e2890 as well as e2809, e2889, and e2896 were found to represent independent isolations of the same events (Figure 6). It should be noted that the use of ENU as a mutagen was instrumental in generating such a diverse range of mutational events. All alleles generated with EMS carry, as expected, GC-to-AT transitions (sc8, e2888: G to A; e2809: C to T). In contrast, three ENU-induced mutations are GC-to-AT transitions (e2889, e2890, and e2896), while e2901, e2906, and e2911 are A-to-G transitions.
Figure 6.—
Structure of SQT-3. For clarity, interruptions in the Gly-X-Y repeats are not shown in the diagram. Locations of the molecular lesions corresponding to 14 sqt-3 alleles are indicated along with their ability to suppress the dpy-31 lethality. The sequence of the SQT-3 C terminus, the molecular lesions identified in the sqt-3(sup) alleles, and the phenotype associated with each suppressor are shown below the protein structure. Residues in boldface type are conserved in all collagens of the SQT-3 subfamily. The YC motif (underlined) may be required for crosslinking of SQT-3.
The five suppressor mutations cause five different amino acid substitutions in the encoded protein. The SQT-3 predicted protein contains a signal peptide (Figure 6; SP: aa 1–37), a N-terminal propeptide (N-PRO: aa 37–105), Gly-X-Y interrupted repeats (GLY-X-Y: aa 105–284), and a C-terminal domain (C-PRO: aa 285–301). The five substitutions map between amino acids 288 and 299 of the SQT-3 C terminus. Significantly, the Tyr and Cys residues occupying positions 286–287 (Figure 6) are likely to be required for tyrosine crosslinking of SQT-3 (Yang and Kramer 1994, 1999).
The nature of the suppressor mutations suggested that structural alterations in the SQT-3 C terminus could cause a partial restoration or replacement of the dpy-31 function. The most plausible scenario is that DPY-31 is responsible for cleavage of the SQT-3 C terminus. However, an alternative explanation is that the dominant suppression of the dpy-31 lethality could be a consequence of decreased SQT-3 levels. To discriminate between these two possibilities, we tested mutant alleles of sqt-3 for suppression of the dpy-31 lethality. All four mutations defining sqt-3 are loss of function, but no true genetic null has been hitherto isolated. sc63, e24, and e2117 have missense mutations in the triple helical domain (van der Keyl et al. 1994). In the sqt-3 hypomorphic allele sc42 (Cox et al. 1980), we identified a missense mutation causing a Gly-to-Asp substitution in the signal peptide of SQT-3 (Figure 6). Moreover, we have recovered a deletion allele of sqt-3, e2639. This allele contains a 60-bp deletion mapping to the last block of Gly-X-Y repeats (Figure 6). In 2639, the correct frame is retained and an “in-frame” Gly-X-Y repeat is recreated by rejoining. e2639 worms display a pronounced Sqt phenotype [i.e., dominant Rol, recessive Dpy, like the canonical sqt-3 allele sc63 (Cox et al. 1980)]. In addition, e2639 homozygotes are strongly ts-lethal. The dominant negative phenotype and the nature of the mutation suggest that e2639 is not a null allele of sqt-3, which would be expected to behave recessively. None of the 5 sqt-3 alleles was found to alleviate the dpy-31 lethality in either heterozygous or homozygous combination (Figure 6). No dominant suppression of dpy-31 lethality was conferred by the deficiency arDf1, which entirely deletes sqt-3 (http://www.wormbase.org). These results demonstrate that partial suppression of the Dpy-31 phenotype by sqt-3 is specifically associated with this newly identified subset of C-terminal missense mutations. For clarity, in the rest of the article this class of sqt-3 mutations will be collectively referred to as sqt-3(sup).
Most sqt-3 suppressors are exclusively dominant:
In subsequent tests, we found that all four mutant alleles of dpy-31 are equally suppressible by sqt-3(sup). Since the e2770, e2920, and ju345 mutations affect different domains of DPY-31, and e2919 is a probable null allele, it seems unlikely that the suppressors could be acting by restoring function to DPY-31 by protein-protein interaction. Rather, the sqt-3(sup) mutations might permit action by an alternative processing enzyme, which can substitute for the defective or missing DPY-31. Alternatively, a less likely possibility is that the sqt-3(sup) mutations might allow SQT-3 to function in the absence of C-terminal cleavage.
Further analysis revealed that the mechanism of suppression has unusual properties. As stated above, all the sqt-3(sup) are dominant; therefore a worm of genotype dpy-31/dpy-31; sqt-3(sup)/+ is viable at 25°. One might expect that such animals would segregate self-progeny according to the ratio of three viable Dpy to one lethal Dpy. However, for most of the suppressors a 1:1 ratio was observed. These observations suggested that double homozygous worms for dpy-31 and sqt-3(sup) were not viable.
To test the effect of heterozygosity vs. homozygosity of the suppressors, we scored the self-progeny of unc-32/dpy-31; sqt-3(sup)/+ worms raised at restrictive temperature. We found that all but one of the suppressors of the dpy-31 lethality act as such only in heterozygous combination with a WT copy of sqt-3 (Table 1). Therefore, worms of genotype dpy-31/dpy-31; sqt-3(sup)/+ are fully viable at 25°, whereas double homozygotes for dpy-31 and sqt-3(sup) are invariably lethal at restrictive temperature. The only exception is e2906. Double homozygotes for this suppressor and dpy-31 do not display any sign of lethality at 25° and produce fully viable progeny.
TABLE 1.
Effect of thesqt-3(sup) gene dosage on the viability ofdpy-31 mutants
| sqt-3(sup) allele | Genotype of the parental and broods counted |
Viable phenotypes |
Viable Dpy expected if the sqt-3(sup) acts exclusively when heterozygous |
Viable Dpy expected if the sqt-3(sup) acts both when heterozygous and when homozygous |
|---|---|---|---|---|
| None | e2770/e189 | 586 WT | ||
| +/+ | 283 Unc | |||
| (5) | 0 Dpy | 0 | 0 | |
| sc8 | e2770/e189; | 841 WT | ||
| sc8/+ | 494 Unc | |||
| (7) | 264 Dpy | 280 | 420 | |
| 319 Rol | ||||
| 109 Rol Unc | ||||
| e2889 | e2770/e189; | 553 WT | ||
| e2889/+ | 301 Unc | |||
| (5) | 181 Dpy | 184 | 265 | |
| 166 Rol | ||||
| 54 Rol Unc | ||||
| e2901 | e2770/e189; | 465 WT | ||
| e2901/+ | 247 Unc | |||
| (5) | 100 Dpy | 155 | 232 | |
| 140 Rol | ||||
| 63 Rol Unc | ||||
| e2906 | e2770/e189; | 1183 WT | ||
| e2906/+ | 608 Unc | |||
| (8) | 433 Dpy | 295 | 443 | |
| e2911 | e2770/e189; | 812 WT | ||
| e2911/+ | 406 Unc | |||
| (5) | 136 Dpy | 203 | 304 |
Note that sc8, e2889, and e2901 are associated with a recessive Rol phenotype; therefore, worms homozygous for these suppressors could be scored. e2906 and e2911 mutants cannot be easily distinguished from WT; therefore, in this case worms homozygous for the suppressors were scored as WT. For suppression in both heterozygous and homozygous combinations we expected a viable Dpy frequency in the F1 of ∼3/16. For suppression exclusively in heterozygous combination, we expected a viable Dpy frequency of ∼1/8. The expected figure of viable Dpy was calculated on the basis of the WT scores. The number of observed and expected viable Dpy is underlined. The number of broods counted is in parentheses.
Isolation of double homozygotes for the sc8 suppressor and dpy-31 confirmed that e2770/e2770; sc8/sc8 worms are not suppressed. Moreover, we found that this strain is even less viable than dpy-31 mutants devoid of suppressor. At permissive temperature, e2770 homozygotes produce an average of 16 viable self-progeny (n = 7) and e2770/e2770; sc8/+ worms produce an average of 80 (n = 7). In contrast, worms of genotype e2770/e2770; sc8/sc8 produce on average only 3 viable self-progeny (n = 7). Enhancement is also seen in homozygotes with the hypomorphic dpy-31 allele ju345. While ju345 mutants are usually viable at 25°, ju345 worms carrying the sc8 suppressor in homozygous combination are inviable at 25° and display very low viability at 15°. Thus, while presence of a sqt-3(sup) in trans to a WT copy of the gene leads to a significant alleviation of the dpy-31 lethality, homozygosity of the suppressors has deleterious effects on the viability of dpy-31 mutants.
Other extragenic suppressors of the dpy-31 lethality:
Overall, about half of the extragenic suppressors of dpy-31 were found to be allelic to sqt-3. Preliminary mapping of the remaining suppressors demonstrates that they define several distinct loci. We expect that cloning of the other extragenic suppressors will further our understanding of the collagen-processing pathway.
DISCUSSION
We have shown that the dpy-31 locus of C. elegans, which encodes a predicted astacin zinc-metalloprotease, is required for viability and normal morphogenesis. dpy-31 plays a crucial role in the late stages of embryogenesis following elongation. Loss of DPY-31 causes the embryonic exoskeleton to collapse to a length comparable to that of the twofold stage. The marginal viability of dpy-31 mutants observed at 15° may result either from the activity of a redundant astacin protease or from residual DPY-31 activity, which is sufficient to permit formation of a functional, albeit highly defective, cuticle. Alternatively, the targets of dpy-31 may be capable of limited function in the absence of cleavage. While the lethality of dpy-31 mutants indicates that the gene is essential at all temperatures above 15°, the recovery of a ts allele has allowed a reversion analysis of the locus. The mutant phenotype and sequence similarities of DPY-31, together with the reversion analysis discussed below, indicate that it acts as an essential pCP for one or more cuticle collagens. Consistent with this proposed role, our expression analysis shows that DPY-31 is widely expressed in the hypodermal cells, which are responsible for cuticle secretion. Our results do not exclude the formal possibility that DPY-31 acts only as a maturase for another protease, which in turn acts as the direct collagen-processing enzyme. However, the structure of DPY-31 and its similarity to a known pCP enzyme favor the simpler model.
In a recent survey, it was determined that the C. elegans genome contains as many as 39 actively transcribed astacin protease genes. This is in striking contrast with what has been observed for other metazoan genomes, in which only three to four astacin paralogs can be generally found (Möhrlen et al. 2003). The functional significance of the 10-fold expansion undergone by astacins in nematodes is unclear. Since RNAi screens in C. elegans have failed to highlight astacin protease genes essential for viability, this class of genes has been hitherto overlooked. Only one astacin gene so far has been defined mutationally. The locus was named hch-1 (for hatch defective). Loss of function of hch-1 results in a delayed hatching phenotype, indicating that HCH-1 is responsible for degradation of eggshell proteins (Hishida et al. 1996). The inefficient and poorly penetrant phenocopy of the dpy-31 mutant phenotype obtained by RNAi suggests that no conclusion can be drawn from genome-wide RNAi screens on the actual number of astacin protease genes required for viability, and therefore no inference can be made about their actual level of redundancy. In the light of our findings, it seems possible that the expansion of astacin protease genes in the nematode C. elegans partially reflects subfunctionalization or specialization in cuticle collagen processing and degradation.
We have investigated the gene function by generating revertants of dpy-31(e2770). Analysis of WT revertants of the mutation showed that full reversion can be obtained only through direct restoration of the dpy-31 function. In our screens, we have isolated exclusively Leu and Ser WT revertants of the e2770 mutation in the CUB domain. Failure to recover any other ENU-induced mutations at the codon changed by e2770 suggests that tight molecular constraints exist on position 469 of DPY-31. Indeed, Ser revertants, albeit WT in size, are partially lethal, showing that even this substitution causes some reduction of function of dpy-31. The CUB domain may be essential for catalytic activity or for secretion of the protein, as shown for BMP-1 (Hartigan et al. 2003).
Analysis of extragenic suppressors of dpy-31 has revealed a peculiar genetic interaction between dpy-31 and the collagen gene sqt-3. Five clustered missense mutations, which are associated with recessive hypomorphic phenotypes and alter the structure of the SQT-3 C terminus, possess the property of acting as dominant suppressors of dpy-31 lethality.
sqt-3 is a unique cuticle collagen in that it is the only member of this gene family that is indispensable for viability. sqt-3 is most abundantly expressed in embryos (Kramer et al. 1985), where it exerts a crucial structural function in maintenance of the body shape achieved through elongation (Priess and Hirsh 1986). Strong loss-of-function mutations in sqt-3 result in embryonic lethality and in early larval arrest characterized by the same retracted Dpy phenotype as dpy-31 mutants (van der Keyl et al. 1994), supporting the hypothesis that DPY-31 may be required for assembly of SQT-3. SQT-3-like collagens are evolutionarily conserved, as they have been detected in a vast array of distantly related nematode species (Cox et al. 1990) and are almost certainly ubiquitous in the phylum. The fundamental role played by SQT-3 in cuticle structure is also probably conserved: in the parasitic nematode species Strongyloides stercoralis, the transcript of the sqt-3 ortholog gene is found among the 12 most abundant in L1 larvae (Mitreva et al. 2004).
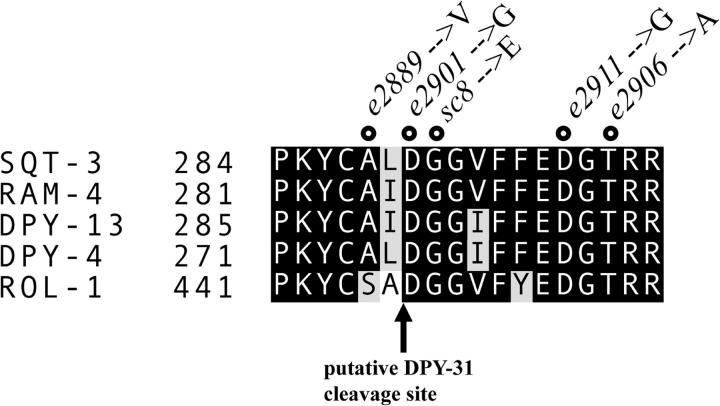
In the C. elegans genome, we find 49 SQT-3 paralog genes, according to the classification based on the structural homology proposed by Johnstone (2000). In Figure 7, we show an amino acid alignment of the C-terminal regions of the five collagens of the SQT-3 subfamily that are associated with mutant phenotypes, in addition to SQT-3: DPY-13 (von Mende et al. 1988), ROL-1 (L. Molin, personal communication), RAM-4 (R. Y. Yu and K. L. Chow, personal communication), and DPY-4 (Simmer et al. 2003). While the average sequence identity of these proteins is ∼60%, the C-terminal regions are almost identical, with only a few conservative substitutions. Also, in the remaining members of the SQT-3 subfamily, the C-terminal sequence appears invariably conserved (minimal amino acid identity to SQT-3: 78%; absolutely conserved residues are highlighted by boldface type in Figure 6).
Figure 7.—
ClustalW alignment of the C-terminal domains of five C. elegans collagens belonging to the SQT-3 subfamily. The protein sequences correspond, respectively, to genes F23H12.4 (sqt-3), F36A4.10 (ram-4), F30B5.1 (dpy-13), Y41E3.2 (dpy-4), and Y57A10A.11 (rol-1). Circles indicate the positions affected by the sqt-3(sup) mutations.
Amino acid conservation implies an important role for the C-terminal regions of these collagens. In vertebrates, the C-propeptides of fibrillar procollagens have been shown to perform three distinct functions. First, during assembly, a conserved motif of the collagen C-propeptide is required to bring the three monomers together (Bachinger et al. 1980). Second, the C-propeptides of vertebrate procollagen also contain variable sequences, which promote selective oligomerization of structurally compatible monomers (Lees et al. 1997). Third, the C-propeptide harbors a consensus sequence for the C-terminal proteinase: upon secretion, cleavage of the C-propeptide occurs at this site, leaving only a short nonhelical region, termed telopeptide, C-terminal to the triple helical domain (Leung et al. 1979).
By analogy, the C terminus of collagens of the SQT-3 subfamily could have similar functions. However, given the absence of variable sequences, it seems unlikely that this motif would be able to direct type-specific oligomerization of collagen chains. Presumably, in nematode collagens determinants of chain assembly reside elsewhere. Analysis of the molecular lesions found in the 5 sqt-3(sup) alleles suggests that the two other functions of the C-propeptide are probably conserved in members of the SQT-3 subfamily.
The sqt-3(sup) mutations can be further divided into two groups. The first includes e2889, e2901, and sc8, which display very similar LRol mutant phenotypes and affect adjacent amino acids in the encoded protein. The second group is represented by e2911 and e2906, which are associated with non-Rol mutant phenotypes and cause the substitution of two close residues at the C-terminal end of SQT-3 (Figures 6 and 7).
The e2889, e2901, and sc8 mutations affect, respectively, residues 288, 290, and 291 of SQT-3. Positions 290 and 291 are strictly conserved in collagens of the SQT-3 subfamily whereas position 288 is conserved in most of the cases (Figures 6 and 7). Two lines of evidence support the hypothesis that the YCALD motif between aa 286 and 290 may represent a site for cleavage by DPY-31. First, this motif closely resembles the Y(X)XXD degenerate consensus site for BMP-1 found in various collagen precursors and in chordin (Scott et al. 2000 and references cited therein). Second, use of this site for cleavage is consistent with the fact that SQT-3 C-terminal processing should occur downstream of the YC motif (aa 286–287), which is known to be required for tyrosine crosslinking and therefore likely to be retained in the mature trimers. The Asp residue occupying position 290 may be the actual site of cleavage (YCAL/D). Almost all proven targets of BMP-1 are processed at an Asp residue, which was shown to be indispensable for BMP-1 action (Lee et al. 1990).
Likewise, the e2911 and e2906 mutations could act to cause indirect alteration or distortion of the DPY-31 cleavage site. However, the nature of the e2906 mutation suggests that it may have a pleiotropic effect. e2906 homozygotes are cs lethal: the strain is inviable at 15°, while WT-like, or weakly Dpy, at higher temperatures. At 15°, e2906 arrested embryos display the retracted Dpy phenotype typical of sqt-3 loss of function. This phenotype is particularly puzzling because it represents the mirror image of what is observed in standard loss-of-function sqt-3 mutants, such as e24, e2117, and e2639, which are viable only at low temperatures. At the protein level, e2906 causes a Thr-to-Ala substitution at position 299 of SQT-3. Given that this is the C-terminal-most nucleophilic residue, it seems possible that it may be involved in the initial steps of collagen oligomerization. According to this model, the amino acid substitution could decrease the rate of chain assembly, which would not occur efficiently at low temperatures.
The recovery of five distinct but clustered missense suppressor mutations in sqt-3 might suggest a direct compensatory interaction between SQT-3 and DPY-31. However, since the sqt-3(sup) mutations suppress equally the four dpy-31 mutant alleles, including e2919, which represents a putative molecular null, it is more likely that suppression involves an alternative pCP that acquires the ability to process SQT-3 as a consequence of conformational changes in the collagen C-propeptide. Possibly, this alternative pathway will be defined by one or more of the remaining extragenic suppressors of dpy-31.
Finally, the behavior of the sqt-3(sup) alleles requires explanation. Most sqt-3(sup) alleles were found to act as suppressors of the dpy-31 lethality only in trans to a WT copy of sqt-3, while the homozygous combination of the suppressors worsens the Dpy-31 mutant phenotype. This unusual genetic interaction may result from the trimeric nature of functional sqt-3 gene products. We propose a model to explain suppression by the sqt-3(sup) Rol alleles, i.e., e2889, sc8, and e2901. Our hypothesis is based on three assumptions: (i) SQT-3 forms exclusively homotrimers; (ii) the sqt-3(sup) mutations allow processing of SQT-3 by the alternative pCP, but DPY-31 retains the ability of cleaving the mutant collagen chains; (iii) cleavage by the alternative pCP interferes with crosslinking of SQT-3 trimers. This could happen in various ways; a reasonable interpretation (illustrated in Figure 8) could be that cleavage of SQT-3 by the alternative protease occurs upstream of the DPY-31 processing site, thus causing loss or inactivation of the YC motif required for crosslinking of SQT-3. A similar type of unspecific processing of the C-telopeptide occurs in vertebrate collagens in the absence of cleavage by BMP-1 (Bateman et al. 1987; Lee et al. 1990). This model has the advantage of explaining the recessiveness of the suppressors' LRol phenotype as well as the exclusively dominant suppression of dpy-31 lethality. On one hand, in a dpy-31(+) background, the Rol phenotype of the sqt-3(sup) may be recessive because in a heterozygous combination, SQT-3 heterotrimers (composed of a mixture of mutant and WT chains) and homotrimers (composed of purely mutant or purely WT chains) would form. Cleavage by DPY-31 of WT homotrimers and WT chains in the heterotrimers could then be sufficient to ensure normal levels of SQT-3 crosslinking, as a large fraction of collagen trimers would possess C-telopeptides. In sqt-3(sup) homozygotes, the alternative protease would compete with DPY-31 for the cleavage of the SQT-3 mutant homotrimers, leading to the partial loss of telopeptide sequences and, consequently, to a reduction in crosslinking of SQT-3. This hypothesis is consistent with findings by Yang and Kramer (1994)(1999), who demonstrated that mutations affecting the C-terminal YC crosslinking motif of the collagen SQT-1 and ROL-6 are associated with a recessive LRol phenotype identical to that displayed by the sqt-3(sup) Rol alleles. On the other hand, in a dpy-31 mutant background, the LRol sqt-3(sup) alleles may suppress the dpy-31 lethality because, in the absence of DPY-31, imprecise cleavage of the SQT-3 C terminus by the alternative pCP could allow more efficient packing of the collagen trimers. However, a WT copy of sqt-3 would be required to ensure formation of heterotrimers containing at least one YC crosslinking motif. The deleterious effects observed when the suppressors are homozygous would then be a consequence of the total loss of SQT-3 C-terminal crosslinking sites, which would preclude SQT-3 deposition. This interpretation provides a plausible explanation of the suppression by sqt-3(sup) alleles. However, the existence of many additional suppressor mutations suggests that there are other ways to ameliorate the lethal effects of the dpy-31 mutation. Future analysis of these other suppressors, which define at least two distinct loci unlinked to sqt-3, will shed further light on collagen processing and astacin proteases in C. elegans.
Figure 8.—
A model proposing an explanation for the exclusively dominant rescue of the dpy-31 lethality conferred by the sqt-3(sup) Rol alleles. The shaded segmented bar represents a SQT-3 trimer. For clarity, only part of the Gly-X-Y repeats, the C-propeptides and C-telopeptides, are shown in the diagram. The C-telopeptides, which contain the sequence required for crosslinking of trimers, are represented as solid. The C-propeptides, which are predicted to be removed by proteolytic cleavage, are shown with light shading in the case of WT monomers and with dark shading in the case of sqt-3 Rol suppressor mutant monomers.
Acknowledgments
We thank Debbie Whittington for technical assistance, members of the Hodgkin lab, Petros Ligoxygakis for critical reading of the manuscript, and Iain Johnstone and members of the Woollard lab for helpful discussions. We are grateful to the Caenorhabditis Genetics Centre for providing nematode strains, to Alan Coulson for providing cosmid clones, to Anthony Page for the col-19::GFP marker, and to Andrew Chisholm for ju345. This work is supported by the UK Medical Research Council.
References
- Ahmed, S., and J. Hodgkin, 2000. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature 403: 159–164. [DOI] [PubMed] [Google Scholar]
- Bachinger, H. P., P. Bruckner, R. Timpl, D. J. Prockop and J. Engel, 1980. Folding mechanism of the triple helix in type-III collagen and type-III pN-collagen. Role of disulfide bridges and peptide bond isomerization. Eur. J. Biochem. 106: 619–632. [DOI] [PubMed] [Google Scholar]
- Bateman, J. F., J. J. Pillow, T. Mascara, S. Medvedec, J. Ramshaw et al., 1987. Cell-layer-associated proteolytic cleavage of the telopeptides of type I collagen in fibroblast culture. Biochem. J. 245: 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond, J. S., and R. J. Beynon, 1995. The astacin family of metalloendopeptidases. Protein Sci. 4: 1247–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, G. N., J. S. Laufer, M. Kusch and R. S. Edgar, 1980. Genetic and phenotypic characterization of roller mutants of Caenorhabditis elegans. Genetics 95: 317–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, G. N., M. Kusch and R. S. Edgar, 1981. Cuticle of Caenorhabditis elegans: its isolation and partial characterization. J. Cell Biol. 90: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, G. N., L. M. Shamansky and R. J. Boisvenue, 1990. Evidence that the 3A3 collagen gene is a member of an evolutionarily conserved family of nematode cuticle collagens. Exp. Parasitol. 70: 175–185. [DOI] [PubMed] [Google Scholar]
- De Stasio, E. A., and S. Dorman, 2001. Optimization of ENU mutagenesis of Caenorhabditis elegans. Mutat. Res. 495: 81–88. [DOI] [PubMed] [Google Scholar]
- De Stasio, E. A., C. Lephoto, L. Azuma, C. Holst, D. Stanislaus et al., 1997. Characterization of revertants of unc-93(e1500) in Caenorhabditis elegans induced by N-ethyl-N-nitrosourea. Genetics 147: 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens, W. A., L. Sharling, G. Cheng, R. Shapira, J. M. Kinkade et al., 2001. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 154: 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver et al., 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 744–745. [DOI] [PubMed] [Google Scholar]
- Hartigan, N., L. Garrigue-Antar and K. E. Kadler, 2003. Bone morphogenetic protein-1 (BMP-1). Identification of the minimal domain structure for procollagen C-proteinase activity. J. Biol. Chem. 278: 18045–18049. [DOI] [PubMed] [Google Scholar]
- Hishida, R., T. Ishihara, K. Kondo and I. Katsura, 1996. hch-1, a gene required for normal hatching and normal migration of a neuroblast in C. elegans, encodes a protein related to TOLLOID and BMP-1. EMBO J. 15: 4111–4122. [PMC free article] [PubMed] [Google Scholar]
- Imamura, Y., B. M. Steiglitz and D. S. Greenspan, 1998. Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-α1(V) collagen. J. Biol. Chem. 273: 27511–27517. [DOI] [PubMed] [Google Scholar]
- Johnstone, I. L., 2000. Cuticle collagen genes. Expression in Caenorhabditis elegans. Trends Genet. 16: 21–27. [DOI] [PubMed] [Google Scholar]
- Kessler, E., K. Takahara, L. Biniaminov, M. Brusel and D. S. Greenspan, 1996. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science 271: 360–362. [DOI] [PubMed] [Google Scholar]
- Ko, F. C., and K. L. Chow, 2002. A novel thioredoxin-like protein encoded by the C. elegans dpy-11 gene is required for body and sensory organ morphogenesis. Development 129: 1185–1194. [DOI] [PubMed] [Google Scholar]
- Kramer, J. M., G. N. Cox and D. Hirsh, 1985. Expression of the Caenorhabditis elegans collagen genes col-1 and col-2 is developmentally regulated. J. Biol. Chem. 260: 1945–1951. [PubMed] [Google Scholar]
- Kusch, M., and R. S. Edgar, 1986. Genetic studies of unusual loci that affect body shape of the nematode Caenorhabditis elegans and may code for cuticle structural proteins. Genetics 113: 621–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. T., E. Kessler and D. S. Greenspan, 1990. Analysis of site-directed mutations in human pro-α2(I) collagen which block cleavage by the C-proteinase. J. Biol. Chem. 265: 21992–21996. [PubMed] [Google Scholar]
- Lees, J. F., M. Tasab and N. J. Bulleid, 1997. Identification of the molecular recognition sequence which determines the type-specific assembly of procollagen. EMBO J. 16: 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, M. K., L. I. Fessler, D. B. Greenberg and J. H. Fessler, 1979. Separate amino and carboxyl procollagen peptidases in chick embryo tendon. J. Biol. Chem. 254: 224–232. [PubMed] [Google Scholar]
- Maduro, M., and D. Pilgrim, 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141: 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués, G., M. Musacchio, M. J. Shimell, K. Wunnenberg-Stapleton, K. W. Cho et al., 1997. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 91: 417–426. [DOI] [PubMed] [Google Scholar]
- Mello, C., and A. Fire, 1995. DNA transformation. Methods Cell Biol. 48: 451–482. [PubMed] [Google Scholar]
- Mitreva, M., J. P. McCarter, J. Martin, M. Dante, T. Wylie et al., 2004. Comparative genomics of gene expression in the parasitic and free-living nematodes Strongyloides stercoralis and Caenorhabditis elegans. Genome Res. 14: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhrlen, F., H. Hutter and R. Zwilling, 2003. The astacin protein family in Caenorhabditis elegans. Eur. J. Biochem. 270: 4909–4920. [DOI] [PubMed] [Google Scholar]
- Myllyharju, J., and K. I. Kivirikko, 2004. Collagen, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 20: 33–43. [DOI] [PubMed] [Google Scholar]
- Newton, C. R., A. Graham, L. E. Heptinstall, S. J. Powell, C. Summers et al., 1989. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 17: 2503–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappano, W. N., B. M. Steiglitz, I. C. Scott, D. R. Keene and D. S. Greenspan, 2003. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol. Cell. Biol. 23: 4428–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess, J. R., and D. I. Hirsh, 1986. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev. Biol. 117: 156–173. [DOI] [PubMed] [Google Scholar]
- Romão, M. J., I. Kolln, J. M. Dias, A. L. Carvalho, A. Romero et al., 1997. Crystal structure of acidic seminal fluid protein (aSFP) at 1.9 Å resolution: a bovine polypeptide of the spermadhesin family. J. Mol. Biol. 274: 650–660. [DOI] [PubMed] [Google Scholar]
- Scott, I. C., Y. Imamura, W. N. Pappano, J. M. Troedel, A. D. Recklies et al., 2000. Bone morphogenetic protein-1 processes probiglycan. J. Biol. Chem. 275: 30504–30511. [DOI] [PubMed] [Google Scholar]
- Shaw, L. M., and B. R. Olsen, 1991. FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem. Sci. 16: 191–194. [DOI] [PubMed] [Google Scholar]
- Simmer, F., M. Tijsterman, S. Parrish, S. Koushika, M. Nonet et al., 2002. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 12: 1317–1319. [DOI] [PubMed] [Google Scholar]
- Simmer, F., C. Moorman, A. M. van der Linden, E. Kuijk, P. V. van den Berghe et al., 2003. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 1: E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J., and J. Hodgkin, 1987 Methods, pp. 587–606 in The Nematode Caenorhabditis elegans, edited by W. B. Wood. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Thacker, C., K. Peters, M. Srayko and A. M. Rose, 1995. The bli-4 locus of Caenorhabditis elegans encodes structurally distinct kex2/subtilisin-like endoproteases essential for early development and adult morphology. Genes Dev. 9: 956–971. [DOI] [PubMed] [Google Scholar]
- Thein, M. C., G. McCormack, A. D. Winter, I. L. Johnstone, C. B. Shoemaker et al., 2003. Caenorhabditis elegans exoskeleton collagen COL-19: an adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology. Dev. Dyn. 226: 523–539. [DOI] [PubMed] [Google Scholar]
- van der Keyl, H., H. Kim, R. Espey, C. V. Oke and M. K. Edwards, 1994. Caenorhabditis elegans sqt-3 mutants have mutations in the col-1 collagen gene. Dev. Dyn. 201: 86–94. [DOI] [PubMed] [Google Scholar]
- von Mende, N., D. M. Bird, P. S. Albert and D. L. Riddle, 1988. dpy-13: a nematode collagen gene that affects body shape. Cell 55: 567–576. [DOI] [PubMed] [Google Scholar]
- Wicks, S. R., R. T. Yeh, W. R. Gish, R. H. Waterston and R. H. Plasterk, 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28: 160–164. [DOI] [PubMed] [Google Scholar]
- Winter, A. D., and A. P. Page, 2000. Prolyl 4-hydroxylase is an essential procollagen-modifying enzyme required for exoskeleton formation and the maintenance of body shape in the nematode Caenorhabditis elegans. Mol. Cell. Biol. 20: 4084–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., and J. M. Kramer, 1994. In vitro mutagenesis of Caenorhabditis elegans cuticle collagens identifies a potential subtilisin-like protease cleavage site and demonstrates that carboxyl domain disulfide bonding is required for normal function but not assembly. Mol. Cell. Biol. 14: 2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., and J. M. Kramer, 1999. Proteolytic processing of Caenorhabditis elegans SQT-1 cuticle collagen is inhibited in right roller mutants whereas cross-linking is inhibited in left roller mutants. J. Biol. Chem. 274: 32744–32749. [DOI] [PubMed] [Google Scholar]
- Yiallouros, I., E. Grosse Berkhoff and W. Stocker, 2000. The roles of Glu93 and Tyr149 in astacin-like zinc peptidases. FEBS Lett. 484: 224–228. [DOI] [PubMed] [Google Scholar]
- Yochem, J., T. Gu and M. Han, 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149: 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]