Abstract
A total of 944 expressed sequence tags (ESTs) generated 2212 EST loci mapped to homoeologous group 1 chromosomes in hexaploid wheat (Triticum aestivum L.). EST deletion maps and the consensus map of group 1 chromosomes were constructed to show EST distribution. EST loci were unevenly distributed among chromosomes 1A, 1B, and 1D with 660, 826, and 726, respectively. The number of EST loci was greater on the long arms than on the short arms for all three chromosomes. The distribution of ESTs along chromosome arms was nonrandom with EST clusters occurring in the distal regions of short arms and middle regions of long arms. Duplications of group 1 ESTs in other homoeologous groups occurred at a rate of 35.5%. Seventy-five percent of wheat chromosome 1 ESTs had significant matches with rice sequences (E ≤ e−10), where large regions of conservation occurred between wheat consensus chromosome 1 and rice chromosome 5 and between the proximal portion of the long arm of wheat consensus chromosome 1 and rice chromosome 10. Only 9.5% of group 1 ESTs showed significant matches to Arabidopsis genome sequences. The results presented are useful for gene mapping and evolutionary and comparative genomics of grasses.
WHEATS are the universal cereals of Old World agriculture (Harlan 1992) and belong to the world's foremost crop plants (Feldman et al. 1995; Nevo et al. 2002). Wheat is one of the most widely cultivated food crops and is the staple food in >40 countries and for over 35% of the global population (Williams 1993). Modern wheat cultivars belong primarily to two species, i.e., hexaploid bread wheat (Triticum aestivum L., 2n = 6x = 42) and tetraploid durum-type wheat (T. turgidum L., 2n = 4x = 28). Bread wheat is most important and contains three genomes: A, B, and D (Sears 1969).
The genome size of hexaploid wheat is the largest (16,979 Mbp) among all cereal crops, including oat (Avena sativa L.; 12,961 Mbp), maize (Zea mays L.; 2671 Mbp), sorghum (Sorghum bicolor L.; 735–1642 Mbp), and rice (Oryza sativa L; 490 Mbp; Bennett and Leitch 2003). The hexaploid wheat genome is also much larger than any of the current plant model species, ∼35 times larger than rice and 99 times larger than Arabidopsis thaliana (172 Mbp) (Bennett and Leitch 2003).
In any genome-sequencing project, the central goal is the discovery of all the genes in the target organism and the establishment of their chromosomal location (Bennetzen 2002). The completion of genome sequencing in rice (Goff et al. 2002; Yu et al. 2002; http://rgp.dna.affrc.go.jp/cgi-bin/statusdb/irgsp-status.cgi, http://www.gramene.org/) and Arabidopsis (Arabidopsis Genome Initiative 2000) opened the door to comparative plant genomics or comparative plant biology. Because of the large size of the hexaploid wheat genome, complete sequencing has not been feasible. Large-scale discovery and isolation of genes and deciphering of gene function in wheat and its relatives must rely on other, less direct methods.
An EST is a segment of a sequence from a cDNA clone complementary to an mRNA sequence. Thus ESTs are segments of expressed genes (Adams et al. 1991). The ESTs (mRNAs → cDNAs) can be isolated from multiple tissues under various treatments and used to identify as many genes as possible in an organism. The EST approach facilitates the tagging of genes in a relatively short time at a fraction of the cost of complete genome sequencing, provides new genetic markers, and serves as a resource in diverse biological research fields (Adams et al. 1991). This approach has provided a new resource for the analysis of chromosome sequences and gene discovery in many organisms, such as Homo sapiens (Adams et al. 1991, 1995; Hillier et al. 1996), Mus musculus (Marra et al. 1999), Rattus norvegicus (Scheetz et al. 2001), Danio rerio (Clark et al. 2001), Medicago truncatula (Covitz et al. 1998), maize (Fernandes et al. 2002), and rice (Ewing et al. 1999). Developing ESTs has become a top priority for crop genomics worldwide (Briggs 1998). Development and deletion mapping analysis of ESTs from hexaploid wheat were conceived by a group of U. S. researchers as a cost-effective approach and a short cut to gene discovery, comparative genomics, and evolutionary genomics.
Hexaploid wheat has seven homoeologous groups of chromosomes, each containing one A, B, and D chromosome from each one of the donor genomes (Sears 1969). The group 1 homoeologous chromosomes are the most studied and understood of the wheat chromosome groups, primarily because they house major clusters of agronomically important genes. The group 1 and the group 2 chromosomes have ∼100 each of the 615 genes of known chromosomal location, more than any of the other groups (McIntosh et al. 2003). There are clusters of resistance genes in wheat chromosome 1B, including at least 22 genes and QTL conferring resistance (Peng 2000; Dilbirligi et al. 2004). Numerous genes and gene families expressed during seed development are located in group 1, such as high-molecular-weight glutenins, low-molecular-weight glutenins, γ- and ω-gliadins, the triplet protein, and several seed-specific globulins (Dubcovsky et al. 1997). Among seven domestication syndrome factors detected in wild emmer wheat [T. turgidum ssp. dicoccoides (Körn. ex Asch. & Graebn.) Thell.], two were located on chromosome 1B (Peng et al. 2003). In a single gene-rich region, the 1S0.8 region of the short arm of chromosome 1 in Triticeae, as many as 75 genes were identified (Sandhu et al. 2001).
The goal of the U.S. wheat EST project was to establish the chromosomal location of genes in the hexaploid wheat genomes. ESTs representing wheat unigenes were physically mapped to individual chromosomes/chromosomal intervals using wheat nullisomic-tetrasomic and ditelosomic lines (Sears 1966) and deletion stocks (Endo 1988, 1990; Endo and Gill 1996). This article summarizes the EST mapping results for group 1 chromosomes of hexaploid wheat. Patterns of distribution and duplication of ESTs within and among the group 1 chromosomes of wheat and comparisons with rice and Arabidopsis genome sequences are discussed.
MATERIALS AND METHODS
EST clones:
For the U.S. wheat EST project, 113,220 ESTs were produced from 41 libraries of wheat representing a wide range of tissues, developmental stages, and environmental stresses (Lazo et al. 2004; Zhang et al. 2004). The ESTs that mapped to wheat group 1 chromosomes were derived from 22 libraries. Amplified PCR products (inserts) were prepared and delivered by the U.S. Department of Agriculture Albany group to 10 mapping laboratories (http://wheat.pw.usda.gov/NSF) for Southern hybridization.
Plant materials:
Hexaploid wheat has an extensive collection of aneuploid and deletion stocks in the Chinese Spring background that are ideal for chromosome mapping, allowing for virtually every DNA marker to be assigned to a specific chromosome/arm/interval without requiring intragenomic polymorphism (Sears 1954, 1966; Sears and Sears 1978; Endo 1988, 1990; Endo and Gill 1996). A total of 146 cytogenetic stocks including 21 nullisomic-tetrasomic, 24 ditelosomic, and 101 deletion lines were used. The genetic stocks for EST mapping were provided by the Wheat Genetics Resource Center, Kansas State University, and cytologically and/or molecularly verified by all 10 mapping laboratories (http://wheat.pw.usda.gov/NSF; Qi et al. 2003, 2004). DNA samples were isolated following protocols established in the individual laboratories.
Southern hybridization:
The conventional Southern hybridization approach was adopted with some minor modifications (http://wheat.pw.usda.gov/NSF/project/mapping_data.html). About 20 μg of genomic DNA were digested with EcoRI enzyme. A mixture of digested products of λDNA using HindIII and BstEII was used as the size ladder. The digested DNA samples were separated on a 1.0% agarose gel and blotted onto a Hybond N+ membrane (Amersham Biosciences, Buckinghamshire, UK). A set consisting of five Southern blots, each with 30 lanes (a size ladder was included for the first four blots), was hybridized with a single EST clone in each hybridization reaction. More details about Southern hybridization were described by Lazo et al. (2004) and Qi et al. (2004).
Localization of ESTs:
Bread wheat is a hexaploid, and the allocation of homoeologous loci to specific chromosomes within a homoeologous group is based on interchromosomal polymorphism. According to presence or absence of the restriction fragments in a given set of DNA lanes of a Southern blot, EST loci were assigned to a specific chromosome, an arm, and/or a deletion bin (Sears 1954 1966; Endo 1988, 1990; Endo and Gill 1996). The EST loci were localized to individual bins in the homoeologous group 1 chromosomes as described by Akhunov et al. (2003) and Qi et al. (2003)(2004). Southern images were scored by at least two persons in each laboratory, and the edited images and mapping data were uploaded to the project website http://wheat.pw.usda.gov/NSF/project/. Each set of mapping data was further validated by corresponding coordinators of the seven homoeologous chromosome groups at the project website http://wheat.pw.usda.gov/cgi-bin/westsql/map_locus_rev.cgi.
Data analysis:
Only mapping data validated by three persons (http://wheat.pw.usda.gov/cgi-bin/westsql/map_locus_rev.cgi) were used for analyses. Mapping data were reverified by checking all the online images in the project database prior to the analyses. Any ambiguous data were excluded in the analyses. On the basis of the physical size (in micrometers) of chromosomes and chromosome arms (Gill et al. 1991) and the relative length of chromosome deletion bins (Endo and Gill 1996), the expected number of ESTs/EST loci was estimated under the assumption of random distribution among and along chromosomes. But the expected numbers of ESTs/EST loci in 1A, 1B, and 1D were estimated on the basis of a hypothetical 1:1:1 distribution. EST densities were calculated as the ratio of observed vs. expected ESTs/EST loci for the individual chromosomes, chromosome arms, and chromosome bins. The χ2 test was used to test for independence of distribution patterns of ESTs among and along chromosomes. Loci numbers and distributions of duplicated ESTs across the other six chromosome groups were also analyzed.
The method of constructing the consensus map was as described by Gill et al. (1996a)(b). ESTs that were not mapped to specific bins or fell into two conflicting bins were assigned to consensus chromosome 1, to one chromosome arm, or to a larger combined bin encompassing the two conflicting bins.
To analyze the similarity of wheat consensus chromosome 1 with rice and Arabidopsis sequences, blastN searches of the ESTs—mapped to all three group 1 chromosomes and used to construct the consensus chromosome bin map against rice (http://rgp.dna.affrc.go.jp/cgi-bin/statusdb/irgsp-status.cgi) and Arabidopsis (http://www.arabidopsis.org/) sequence databases—were conducted. E ≤ e−10 was adopted as the standard to claim a significant match. BlastX was also used to retrieve the gene function of wheat chromosome 1 ESTs that matched to Arabidopsis. The binomial test was used to detect colinearity of consensus bins with rice chromosomes as described by Linkiewicz et al. (2004). A putative orthologous genetic map for each interval, significantly colinear with rice chromosomes 5 and/or 10, of wheat consensus chromosome 1 ESTs was constructed on the basis of the order and position of the corresponding P1-derived artificial chromosome (PAC)/bacterial artificial chromosome (BAC) clones in rice chromosomes 5 and 10. Details of wheat consensus chromosome 1 ESTs and the significantly matched PAC/BAC clones in rice chromosomes 5 and 10 can be retrieved as supplemental online material at http://wheat.pw.usda.gov/pubs/2004/Genetics/.
An anomaly was defined as an EST having loci mapped to nonoverlapping bins on homoeologous chromosomes (Munkvold et al. 2004). Mapping data and images of all the EST probes assigned to group 1 chromosomes were examined for such anomalies.
Types of EST duplications analyzed:
Two types of duplications were distinguished, intrachromosome and interchromosome duplications. An intrachromosome duplication is inferred to have occurred when an EST generates two or more loci in one chromosome. The number of intrachromosome duplications was estimated from the difference between the number of loci and the number of EST probes mapped in a particular chromosome. An interchromosome duplication refers to the situation in which an EST maps to chromosomes other than the three homoeologous group 1 chromosomes (1A, 1B, 1D). In testing the observed number vs. the expected number of duplications, we assumed that duplications in other locations occurred randomly, so that the greater the length of a chromosome or bin, the greater the chance of a duplication occurring in that region. The expected number of duplications was therefore determined on the basis of the size of the chromosome or chromosome bin.
RESULTS
ESTs mapped to homoeologous group 1 chromosomes and the consensus physical EST map:
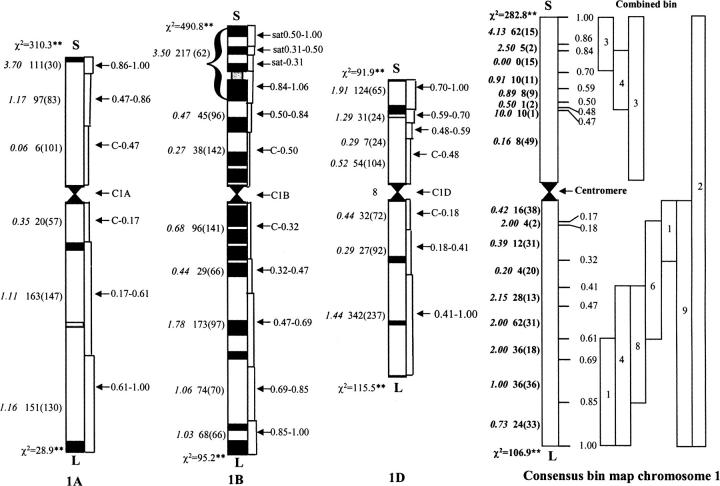
As of March 17, 2003, 944 ESTs were mapped to group 1 chromosomes. Of these, 597, 660, and 643 ESTs had loci in chromosomes 1A, 1B, and 1D, respectively (Table 1). A total of 367 (39%) of the 944 group 1 ESTs were mapped to each group 1 chromosome (1A, 1B, and 1D) and were used to construct the group 1 consensus deletion bin map. A list of these 367 ESTs has been deposited as supplemental online material at http://wheat.pw.usda.gov/pubs/2004/Genetics/. Of these 367 ESTs, 326 were allocated to specific bins and 41 were allocated to combined bins of the consensus chromosome 1. There were 251 and 114 ESTs located in the long and short arms, respectively, of the consensus chromosome bin map (Figure 1).
TABLE 1.
Distribution of EST probes and loci among the group 1 chromosomes in wheat
| All ESTs
|
ESTs with all bands mapped
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Chromosome | Probes | Loci | EST densitya |
Intrachromosome duplicationsb |
Probes | Loci | EST densitya |
Intrachromosome duplicationsb |
| 1A | 597 | 660 (29.8%) | 0.84 | 63 (20.2%) | 252 | 280 (31.3%) | 0.88 | 28 (24.6%) |
| 1B | 660 | 826 (37.3%) | 0.99 | 166 (53.2%) | 265 | 317 (35.5%) | 0.94 | 52 (45.6%) |
| 1D | 643 | 726 (32.8%) | 1.22 | 83 (26.6%) | 263 | 297 (33.2%) | 1.24 | 34 (29.8%) |
| Total | 944 | 2212 (100%) | 312 (100%) | 326 | 894 (100%) | 114 (100%) | ||
| χ2 | 3.355 | 16.344 | 57.365 | 0.377 | 2.302 | 8.211 | ||
| P | 0.187 | <0.001 | <0.001 | 0.828 | 0.316 | 0.016 | ||
EST density is the ratio of observed EST loci vs. expected EST loci based on chromosome size (Gill et al. 1991).
The difference between the number of loci and the number of probes mapped in a particular chromosome was used to estimate the level of intrachromosome duplications. The χ2 test was used to detect the probability of a departure from a hypothetically expected 1:1:1 ratio (even distribution) among the three chromosomes.
Figure 1.—
EST deletion bin maps of wheat chromosomes 1A, 1B, and 1D and the consensus group 1 chromosome. Numbers of EST loci mapped only to chromosomes and chromosome arms were not shown in the individual chromosome bin maps. The numbers of mapped EST loci (expected numbers are in parentheses) and the gene density (italic type) are shown on the left of each chromosome and the intervals are indicated on the right. The 1BS satellite region is incorporated into bin 1BS-0.84-1.06. On the left of the consensus chromosome bin map are the numbers of ESTs (expected numbers are in parentheses) and gene density (italic type) and on the right are the fraction lengths. In the consensus chromosome bin map, the boxes on the right represent the combined bins and the number of ESTs is shown inside the boxes. χ2 was used to test for random distribution of EST loci/probes on chromosome arms. * and **, significance of 0.05 and 0.01 probability, respectively.
Distribution of ESTs among homoeologous group 1 chromosomes:
The 944 EST probes generated 2212 loci mapped to the group 1 chromosomes with 660, 826, and 726 loci located on chromosomes 1A, 1B, and 1D, respectively (Table 1). Among the three chromosomes, 1B had the highest number and 1A the lowest. The χ2 test showed that deviation from the expected values based on a hypothetical ratio of 1:1:1 (even distribution) was highly significant (P < 0.01) for EST loci, but not significant for EST probes. Thus, it appeared that the mapped EST probes were randomly distributed among the three chromosomes, but the mapped EST loci were not. Chromosome 1B had a larger number and 1A had a smaller number than expected (Table 1).
As done by Linkiewicz et al. (2004) for homoeologous group 5, the previous analysis was repeated using the subset of EST probes for which all bands were mapped (326 ESTs). Again the 1B chromosome had the largest proportion of probes (265) and loci (317). However, the numbers of EST probes and loci did not significantly deviate from the expected values based on random distribution (Table 1). It seems that this subset of mapped EST probes was randomly distributed among the three group 1 chromosomes.
Distribution pattern of EST loci on the chromosome arms:
Of the 2212 EST loci (Table 1), 2076 were mapped to specific chromosome arms (Table 2) and the other 136 were mapped only to chromosomes or centromere bins. The numbers of EST loci mapped to the long arms of each of the three chromosomes were larger than those mapped to the short arms (Table 2). In comparison with the expected values based on arm length (Gill et al. 1991), the ESTs were randomly distributed between long and short arms for 1A (P = 0.27), nearly randomly for 1B (P = 0.06), and nonrandomly for 1D (P = 0.02) with more observed in the long arm and less than expected in the short arm (Table 2).
TABLE 2.
Distribution of EST loci between chromosome arms
| Chromosome arm
|
||||
|---|---|---|---|---|
| Chromosome | Item | Long | Short | Totala |
| 1A | Observed | 371 | 215 | 586 |
| Expectedb | 383.80 | 202.20 | 586 | |
| Deviation | −12.80 | 12.80 | 0 | |
| χ2 | 1.24 (P = 0.265) | |||
| Gene density | 0.97 | 1.06 | ||
| 1B | Observed | 483 | 326 | 809 |
| Expectedb | 509.40 | 299.60 | 809 | |
| Deviation | −26.40 | 26.40 | 0 | |
| χ2 | 3.69 (P = 0.054) | |||
| Gene density | 0.95 | 1.09 | ||
| 1D | Observed | 459 | 222 | 681 |
| Expectedb | 428.90 | 252.10 | 681 | |
| Deviation | 30.10 | −30.10 | 0 | |
| χ2 | 5.71 (P = 0.016) | |||
| Gene density | 1.07 | 0.88 | ||
EST loci mapped only to chromosomes or centromere bins were excluded.
The expected number is based on the arm ratio value (Gill et al. 1991).
Chromosome bin mapping of 2212 EST loci (Table 1) yielded 1913 that were mapped to specific chromosome intervals/bins (Figure 1); the remaining 299 were mapped only to chromosomes or chromosome arms. Most of the EST loci were located in the distal regions of both long and short arms for all three chromosomes (Figure 1). This distribution of loci deviated significantly (P = 0.01) from the expected numbers based on interval lengths in each arm (Figure 1). The consensus chromosome deletion bin map had a higher resolution (the chromosome arms were divided into more and smaller intervals than any individual chromosome) and it also revealed a majority of the ESTs in the distal intervals of the chromosome arms (Figure 1). The number of ESTs located in distal regions (0.84–0.86 and 0.86–1.00) of the short arm was significantly larger than expected, but in the long arm this was true only for the middle intervals (0.41–0.47, 0.47–0.61, and 0.61–0.69); the reverse was found for the long-arm distal bin (0.85–1.00; Figure 1). In the 1A, 1B, and 1D long arms, ESTs were allocated to the most distal bins, but they appear to be located in the proximal regions of those bins and not at the ends of the chromosomes. The consensus map with a greater number of long-arm bins clearly points this out (Figure 1). Therefore, distribution patterns of mapped ESTs were different for the two arms of all group 1 chromosomes.
EST density:
EST density is the ratio of the number of mapped ESTs to the expected value based on the length of chromosome/arm/interval. Chromosome 1D had relatively high EST density values, both when all ESTs were used (1.22) and when only the subset for which all loci were mapped was used (1.24); chromosome 1A had the lowest EST density with values 0.84 and 0.88; and chromosome 1B was in between with values of 0.94 and 0.99 (Table 1). The short arms had slightly higher EST densities (>1) than the long arms (<1) for 1A and 1B, but the reverse was true for 1D where the long arm had a higher EST density (>1) than did the short arm (<1; Table 2).
Most of the intervals with EST density <1.0 were in proximal regions near the centromeres. Five intervals were EST rich, having high-EST density: 1AS-0.86-1.00 (3.70), 1BL-0.47-0.69 (1.78), 1DS-0.70-1.00 (1.91), 1DL-0.41-1.00 (1.44), and the 1BS satellite region (3.50; Figure 1). In the consensus chromosome bin map, the EST-rich regions in the short arm were located in the intervals 1S-0.47-0.48 (10.00), 1S-0.84-0.86 (2.50), and 1S-0.86-1.00, including the satellite region (4.13). The EST-rich regions in the long arm were all located in the middle intervals 1L-0.41-0.47 (2.15), 1L-0.47-0.61 (2.00), and 1L-0.61-0.69 (2.00; Figure 1).
Duplications of group 1 ESTs:
EST intrachromosome duplications were not randomly distributed among the three chromosomes with ∼50% of the duplications occurring in 1B (Table 1). Approximately 13% of the mapped EST unigenes contained at least one EcoRI restriction site, not including sites within introns (Munkvold et al. 2004). Because internal EcoRI sites within the region of hybridization can create duplicated fragments within a bin, the rate of intrachromosome duplications might be overestimated in the present study. However, the multiple EcoRI restriction sites within ESTs would not affect the rates of interchromosome and intergenome duplications.
About 35% (335) of the ESTs mapped to group 1 chromosomes also had loci in one or more of the six other homoeologous groups (Table 3). The χ2 test showed that deviation of the observed number of ESTs with duplications in other homoeologous groups from the expected number based on chromosome size was not significant, although group 2 had a relatively large number. It seems that none of the six homoeologous chromosome groups had a significantly closer homoeology with group 1 chromosomes.
TABLE 3.
EST probes mapped to group 1 chromosomes and to other homoeologous groups in wheat
| No. of duplicated ESTs
|
|||
|---|---|---|---|
| Combination | Observed | Expecteda | Percentage of duplicated ESTsb |
| Group 1 + group 2 | 158 | 135.0 | 16.74 |
| Group 1 + group 3 | 132 | 136.9 | 13.98 |
| Group 1 + group 4 | 113 | 122.8 | 11.97 |
| Group 1 + group 5 | 126 | 129.3 | 13.35 |
| Group 1 + group 6 | 118 | 123.2 | 12.50 |
| Group 1 + group 7 | 129 | 128.9 | 13.67 |
| χ2 | 5.18 (P = 0.39) | ||
The expected number is based on the chromosome length (Gill et al. 1991).
The percentage is based on 944 mapped ESTs.
The numbers of duplicated group 1 EST loci in the other homoeologous groups were evaluated for each of the group 1 chromosome bins (Table 4). The total numbers of duplications in the other six homoeologous groups were 430, 551, and 411 for 1A, 1B, and 1D, respectively. Therefore, the group 1 EST duplications in other homoeologous group chromosomes were not evenly distributed among the three group 1 chromosomes (χ2 = 24.9, P < 0.01), with a larger number than expected in 1B and a smaller number than expected in 1A and 1D. A similar result was observed for distribution of EST duplications along chromosome arms with clustering in the distal bins. In comparison with the expected number derived from chromosome size, deviations of EST duplication in the six other homoeologous groups for all three group 1 chromosomes were not significant.
TABLE 4.
Distribution of group 1 ESTs having duplicated loci in other chromosome groups
| Homoeologous group
|
Total
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2
|
3
|
4
|
5
|
6
|
7
|
||||||||||
| Chromosome interval | E | O | E | O | E | O | E | O | E | O | E | O | E | O | χ2 |
| 1AS3-0.86-1.00 | 3 | 10 | 3 | 6 | 2 | 6 | 3 | 8 | 4 | 17 | 5 | 16 | 19 | 63 | |
| 1AS1-0.47-0.86 | 7 | 8 | 7 | 13 | 4 | 5 | 9 | 8 | 12 | 14 | 13 | 17 | 53 | 65 | |
| C-1AS1-0.47 | 8 | 0 | 9 | 0 | 5 | 0 | 11 | 7 | 15 | 0 | 15 | 0 | 63 | 7 | |
| χ2 | 24.5** | 17.1** | 13.3** | 9.9** | 57.6** | 40.4** | 154.4** | ||||||||
| C1A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| C-1AL1-0.17 | 6 | 0 | 9 | 0 | 7 | 2 | 6 | 1 | 5 | 0 | 4 | 0 | 38 | 3 | |
| 1AL1-0.17-0.61 | 17 | 30 | 24 | 22 | 19 | 35 | 14 | 17 | 11 | 15 | 9 | 18 | 99 | 137 | |
| 1AL3-0.61-1.00 | 15 | 8 | 21 | 32 | 17 | 6 | 13 | 15 | 10 | 21 | 8 | 3 | 88 | 85 | |
| χ2 | 19.2** | 14.9** | 24.2** | 5.1 | 18.6** | 16.1** | 46.9** | ||||||||
| Sum of 1A | 75 | 69 | 76 | 88 | 68 | 63 | 72 | 65 | 68 | 80 | 71 | 65 | 464 | 430 | 6.05 |
| 1BS9-0.84-1.06 | 10 | 27 | 6 | 17 | 4 | 8 | 10 | 29 | 6 | 19 | 8 | 21 | 46 | 121 | |
| 1BS10-0.50-0.84 | 16 | 14 | 9 | 4 | 7 | 9 | 14 | 3 | 7 | 1 | 13 | 13 | 71 | 44 | |
| C-1BS10-0.50 | 23 | 8 | 13 | 7 | 10 | 4 | 21 | 13 | 13 | 7 | 19 | 6 | 104 | 45 | |
| χ2 | 38.9** | 25.7** | 8.17* | 47.8** | 36.1** | 30.0** | 166.0** | ||||||||
| C1B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| C-1BL6-0.32 | 18 | 18 | 20 | 9 | 18 | 8 | 11 | 6 | 11 | 10 | 14 | 5 | 91 | 56 | |
| 1BL6-0.32-0.47 | 9 | 4 | 9 | 2 | 8 | 8 | 5 | 2 | 5 | 0 | 6 | 0 | 42 | 16 | |
| 1BL1-0.47-0.69 | 12 | 16 | 14 | 24 | 12 | 22 | 7 | 10 | 7 | 12 | 10 | 24 | 63 | 108 | |
| 1BL2-0.69-0.85 | 9 | 13 | 10 | 17 | 9 | 13 | 5 | 10 | 5 | 10 | 7 | 10 | 45 | 73 | |
| 1BL3-0.85-1.00 | 9 | 6 | 9 | 10 | 8 | 4 | 5 | 5 | 5 | 1 | 6 | 4 | 42 | 30 | |
| χ2 | 7.9 | 23.7** | 17.7** | 10.4* | 16.9** | 33.3** | 82.6** | ||||||||
| Sum of 1B | 96 | 118 | 97 | 99 | 87 | 84 | 92 | 82 | 87 | 73 | 92 | 95 | 464 | 551 | 8.62 |
| 1DS5-0.70-1.00 | 12 | 26 | 4 | 5 | 7 | 18 | 4 | 7 | 8 | 20 | 9 | 16 | 42 | 92 | |
| 1DS1-0.59-0.70 | 4 | 2 | 1.5 | 3 | 2 | 1 | 1.5 | 0 | 3 | 0 | 3 | 4 | 16 | 10 | |
| 1DS3-0.48-0.59 | 4 | 9 | 1.5 | 1 | 2 | 1 | 1.5 | 1 | 3 | 0 | 3 | 0 | 16 | 12 | |
| C-1DS3-0.48 | 19 | 2 | 6 | 4 | 10 | 1 | 6 | 5 | 12 | 6 | 14 | 9 | 67 | 27 | |
| χ2 | 38.8** | 2.6 | 26.4** | 4.1 | 27.0** | 10.6* | 86.2** | ||||||||
| C1D | 4 | 0 | 3 | 0 | 0 | 0 | 7 | ||||||||
| C-1DL4-0.18 | 6 | 1 | 8 | 3 | 7 | 6 | 7 | 5 | 5 | 3 | 5 | 2 | 38 | 20 | |
| 1DL4-0.18-0.41 | 8 | 3 | 10 | 4 | 9 | 2 | 9 | 5 | 6 | 0 | 7 | 4 | 49 | 18 | |
| 1DL2-0.41-1.00 | 20 | 30 | 27 | 38 | 24 | 32 | 23 | 29 | 14 | 22 | 18 | 24 | 126 | 175 | |
| χ2 | 12.3** | 11.2** | 8.3* | 3.9 | 11.4** | 5.1 | 47.2** | ||||||||
| Sum of 1D | 71 | 91 | 72 | 63 | 65 | 72 | 68 | 57 | 65 | 59 | 68 | 69 | 464 | 411 | 9.86 |
| Overall χ2 for the three chromosomes |
24.9** | ||||||||||||||
E, expected values based on length of chromosome or interval except for the overall χ2; O, observed value. * and **, significant at P < 0.05 and 0.01, respectively. Sum of each chromosome includes those group 1 ESTs not mapped to specific chromosome intervals. 1BS satellite region was combined into 1BS9-0.84-1.06.
Homoeology of wheat consensus chromosome 1 with the Arabidopsis genome:
BlastN searches of the 367 ESTs involved in the consensus chromosome deletion bin map against sequence databases of the Arabidopsis genome (http://www.arabidopsis.org/) revealed that only 35 (9.5%) had significant (E ≤ e−10) matches with Arabidopsis sequences (Table 5). Of these, 27 (77%) were located on the long arm of wheat consensus chromosome 1 and the other 8 (23%) were on the short arm. These 35 that significantly matched wheat chromosome 1 ESTs were not randomly distributed (P < 0.05) among the five Arabidopsis chromosomes, with an apparently larger number located to chromosome 3 (37%) and fewer to chromosome 1 (11%). Therefore, homoeology of wheat consensus chromosome 1 (W1) ESTs with Arabidopsis was not high (<10%), and the relatively greatest homoeology exists with Arabidopsis chromosome 3.
TABLE 5.
Wheat consensus chromosome 1 ESTs having significant matches with Arabidopsis sequences
| GenBank accession no. |
E-value | Arabidopsis chromosome |
Gene functiona | Wheat consensus chromosome bin |
|---|---|---|---|---|
| BE404660 | 6.00E-17 | 3 | Ubiquitin-conjugating enzyme | 1L-0.47-0.61 |
| BE405167 | 8.00E-13 | 3 | Ubiquitin-conjugating enzyme | 1L-0.32-0.41 |
| BE423193 | 7.00E-32 | 2 | 60S ribosomal protein | 1L-0.47-0.61 |
| BE426097 | 7.00E-54 | 5 | Dim1 homolog, putative thioredoxin-like U5 small ribonucleoprotein particle protein |
1L-0.41-0.85 |
| BE426257 | 9.00E-16 | 3 | Tetrahydrofolate dehydrogenase/cyclohydrolase, Methyltetrahydrofolate cyclohydrolase |
1S-0.47-0.84 |
| BE442716 | 7.00E-14 | 1 | T-complex protein 1, ε-subunit/chaperonin | 1L-0.41-0.47 |
| BE442818 | 1.00E-11 | 3 | Expressed protein | 1L-0.47-0.61 |
| BE443332 | 2.00E-11 | 4 | Kelch repeat containing F-box protein family | 1L-0.47-0.61 |
| BE443378 | 2.00E-14 | 5 | Microtubule binding protein D-CLIP-190 (GTP-binding protein) |
1L-0.18-0.32 |
| BE443531 | 2.00E-14 | 2 | Neutral leucine aminopeptidase preprotein; metallo-exopeptidase; leucyl aminopeptidase |
1S-0.47-0.48 |
| BE444620 | 8.00E-16 | 3 | Expressed protein | 1L-0.61-0.69 |
| BE446010 | 1.00E-11 | 3 | Cyanate hydratase (cyanase), cyanate lyase | C-1L-0.32 |
| BE446240 | 2.00E-13 | 2 | Thiol methyltransferase, GDP dissociation inhibitor protein |
1L-0.61-0.69 |
| BE490592 | 2.00E-32 | 3 | Mitochondrial NAD-dependent malate dehydrogenase |
1L-0.85-1.00 |
| BE490596 | 3.00E-37 | 5 | H+-transporting ATP synthase β-chain (mitochondrial) |
1L-0.41-0.85 |
| BE494850 | 2.00E-20 | 3 | 20S proteasome β-subunit B (PBB1) | C-1S-0.48 |
| BE495028 | 3.00E-55 | 3 | Leucine-rich repeat transmembrane protein kinase, calmodulin |
1L-0.47-0.61 |
| BE497107 | 7.00E-14 | 3 | Pseudogene, similar to En/Spm transposon protein; Ras family GTP-binding protein |
C-1L-0.17 |
| BE497584 | 6.00E-26 | 2 | Mitochondrial F1-ATPase, mitochondrial precursor | C-1L-0.17 |
| BE497808 | 7.00E-29 | 5 | Glycosyl hydrolase family 17, histone H4 (TH091) | 1L-0.61-0.69 |
| BE500310 | 3.00E-15 | 4 | Reverse transcriptase (RNA-dependent DNA polymerase) kinase-like protein |
1L-0.17-0.61 |
| BE500541 | 1.00E-14 | 1 | GPI-anchor transamidase | 1S-0.70-1.00 |
| BE518048 | 5.00E-27 | 3 | Mitogen-activated protein kinase | 1L-0.85-1.00 |
| BE518393 | 2.00E-34 | 1 | 60S ribosomal protein L23 (RPL23A) | C-1L-0.17 |
| BE590822 | 1.00E-15 | 2 | Glycosyltransferase family, UDP-glucoronosyl and UDP-glucosyl transferase, cyclophilin |
1L-0.69-0.85 |
| BE637867 | 6.00E-14 | 3 | Crooked neck-related protein, receptor-protein kinase | 1L-0.47-0.61 |
| BF145399 | 1.00E-14 | 2 | Homeodomain protein, vacuolar ATP synthase 16-kD proteolipid subunit |
1S-0.86-1.00 |
| BF200980 | 4.00E-15 | 5 | Protein translation factor SUI1 homolog | 1L-0.61-0.69 |
| BF473056 | 3.00E-16 | 5 | Phosphoinositide-specific phospholipase, actin-depolymerizing factor 1 |
1S-0.86-1.00 |
| BF474139 | 1.00E-10 | 4 | Fructose-6-phosphate-1 phosphotransferase | 1L-0.61-0.69 |
| BF483378 | 2.00E-14 | 2 | DnaJ domain-containing protein, proteasome subunit α-type 3 |
1L-0.47-0.61 |
| BF483456 | 6.00E-14 | 3 | Nodulin/glutamate-ammonia ligase-like protein; pseudogene-gag-pol polyprotein; UDP-galactose transporter related protein 1; dTDP-glucose 4-6-dehydratase |
1L-0.47-0.61 |
| BF485305 | 2.00E-16 | 1 | Unknown protein | 1L-0.47-0.61 |
| BG262410 | 7.00E-17 | 4 | Transcriptional adaptor; Shaggy related protein kinase | 1S-0.47-0.48 |
| BM134392 | 1.00E-13 | 4 | Cellulose synthase-1 | 1S-0.47-0.48 |
Gene function was retrieved via blastX.
Homoeology of wheat consensus chromosome 1 with rice genome:
BlastN searches of 367 ESTs against the rice sequence database (http://rgp.dna.affrc.go.jp/cgi-bin/statusdb/irgsp-status.cgi) indicated that 274 (75%) had significant (E ≤ e−10) matches to rice sequences (Table 6). All but two of these were associated with specific rice chromosomes. Wheat group 1 ESTs that significantly matched to rice sequences were unevenly distributed (P < 0.01) among the 12 rice chromosomes with 49, 20, and 1–5% located on chromosome 5, chromosome 10, and the other 10 chromosomes, respectively. Therefore, the wheat group 1 ESTs analyzed have high homoeology (∼75%) with rice, especially rice chromosomes 5 and 10.
TABLE 6.
Colinearity between wheat consensus chromosome 1 and rice chromosomes
| Rice chromosomeb
|
Probability
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat consensus chromosome 1 bin |
1c | 2 | 3 | 4 | 5c | 6 | 7 | 8 | 9 | 10c | 11 | 12 | 0 | No. with no match |
Total matches |
1st | 2nd |
| Chromosome 1a | 1 | 1 | 1 | 0.0833 | — | ||||||||||||
| 1Sa | 1 | 1 | 1 | 2 | 0.1597 | — | |||||||||||
| 1S-0.86-1.00 | 1 | 3 | 3 | 1 | 16 | 2 | 4 | 3 | 0 | 1 | 1 | 1 | 1 | 25 | 37 | <0.0001 | 0.1022 |
| 1S-0.84-0.86 | 3 | 2 | 3 | 0.0006 | — | ||||||||||||
| 1S-0.70-1.00a | 1 | 1 | 1 | 2 | 0.1597 | — | |||||||||||
| 1S-0.70-0.84 | 0 | 0 | — | — | |||||||||||||
| 1S-0.59-0.70 | 1 | 1 | 1 | 1 | 6 | 4 | 0.2939 | — | |||||||||
| 1S-0.50-0.59 | 7 | 1 | 0 | 8 | <0.0001 | 0.0909 | |||||||||||
| 1S-0.48-0.50 | 1 | 0 | — | — | |||||||||||||
| 1S-0.47-0.84a | 1 | 1 | 2 | 2 | 0.1597 | — | |||||||||||
| 1S-0.47-0.48 | 1 | 1 | 8 | 0 | 10 | <0.0001 | 0.1736 | ||||||||||
| C-1S-0.47 | 2 | 1 | 1 | 2 | 2 | 6 | 0.0831 | 0.0438 | |||||||||
| C-1L-0.17 | 1 | 2 | 1 | 1 | 9 | 2 | 14 | <0.0001 | 0.0686 | ||||||||
| C-1L-0.32a | 1 | 0 | 1 | 0.0833 | — | ||||||||||||
| 1L-0.17-0.18 | 1 | 1 | 2 | 2 | 0.1597 | — | |||||||||||
| 1L-0.17-0.61a | 1 | 2 | 1 | 2 | 4 | 0.0372 | 0.1736 | ||||||||||
| 1L-0.18-0.32 | 1 | 1 | 8 | 2 | 10 | <0.0001 | 0.1736 | ||||||||||
| 1L-0.32-0.41 | 1 | 3 | 0 | 4 | 0.0022 | 0.0909 | |||||||||||
| 1L-0.41-0.47 | 1 | 5 | 1 | 14 | 1 | 2 | 4 | 24 | <0.0001 | 0.0011 | |||||||
| 1L-0.41-0.85a | 1 | 2 | 1 | 1 | 3 | 5 | 0.0586 | 0.2487 | |||||||||
| 1L-0.41-1.00a | 1 | 1 | 1 | 1 | 3 | 0.2297 | — | ||||||||||
| 1L-0.47-0.61 | 4 | 2 | 3 | 29 | 1 | 4 | 1 | 3 | 1 | 14 | 48 | <0.0001 | 0.0744 | ||||
| 1L-0.61-0.69 | 4 | 2 | 1 | 22 | 1 | 6 | 30 | <0.0001 | 0.0035 | ||||||||
| 1L-0.61-1.00a | 1 | 0 | — | — | |||||||||||||
| 1L-0.69-0.85 | 1 | 19 | 3 | 1 | 1 | 3 | 1 | 7 | 29 | <0.0001 | 0.0554 | ||||||
| 1L-0.85-1.00 | 1 | 1 | 1 | 13 | 1 | 2 | 5 | 19 | <0.0001 | 0.0968 | |||||||
| 1La | 1 | 1 | 1 | 3 | 3 | 6 | 0.0095 | 0.2487 | |||||||||
| Total | 13 | 10 | 13 | 7 | 133 | 12 | 11 | 8 | 3 | 53 | 6 | 3 | 2 | 93 | 274 | <0.0001 | <0.0001 |
Combined bins.
A figure in the table with P < 0.01 is claimed as significant and is in boldface type for rice chromosome 5 (R5), in boldface and italic for R10, and in italic type for R1.
Underlining denotes W1 bins with significant colinearity with rice chromosomes.
Colinearity of wheat consensus chromosome 1 and rice chromosomes:
The results shown in Table 6 indicate that bins in the regions of 0.47–1.00 on the short arm and of 0.41–1.00 on the long arm were significantly colinear with rice chromosome 5 (R5) and that bins in the region of C-0.47 on the long arm were significantly colinear with rice chromosome 10 (R10). The 1L-0.41-0.47 bin was significantly colinear with both R5 and R10. The 1L-0.61-0.69 bin was significantly colinear with both rice chromosomes 5 and 1. A large portion of the W1 short arm, especially the C-0.47 and 0.59-0.84 regions, was not significantly colinear with any of the 12 rice chromosomes.
Putative EST order in wheat consensus chromosome 1 based on rice sequences:
Due to the high homoeology with wheat group 1 chromosomes, sequence orders of rice chromosomes R5 and R10 (http://rgp.dna.affrc.go.jp/cgi-bin/statusdb/irgsp-status.cgi) were used to construct an orthologous genetic consensus EST bin map of W1 as shown in Figure 2. Details of this orthologous genetic map are also presented as supplemental online material at http://wheat.pw.usda.gov/pubs/2004/Genetics/.
Figure 2.—
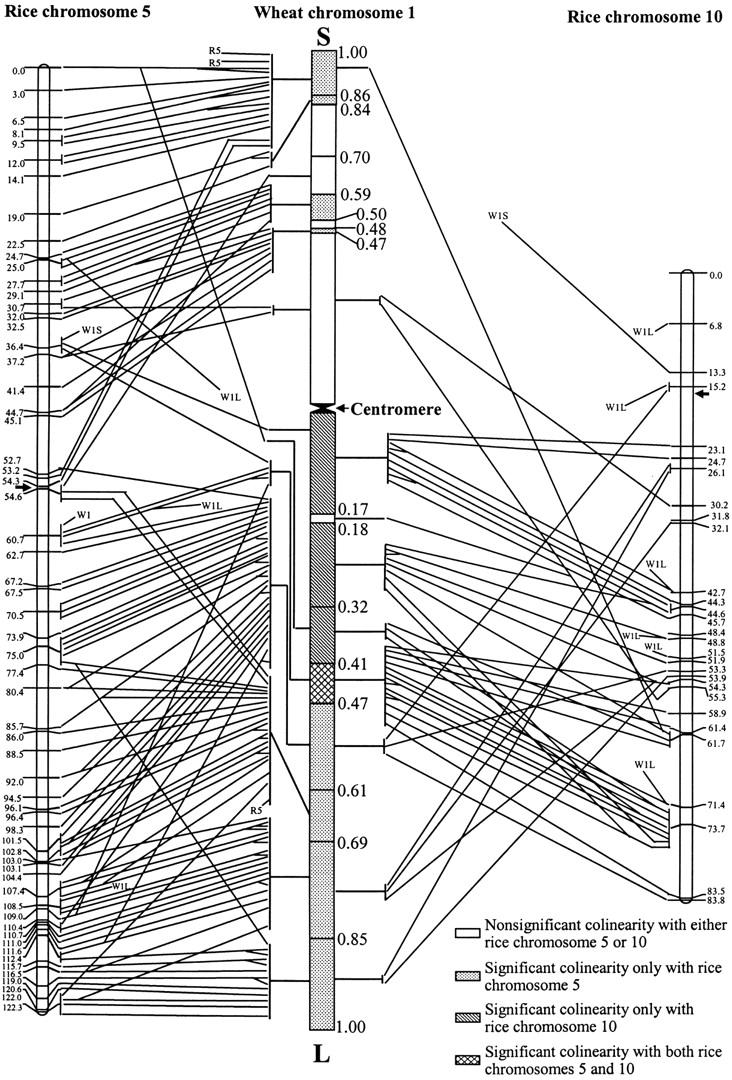
Schematic orthologous map of wheat consensus chromosome 1 ESTs with rice chromosomes 5 and 10. The wheat consensus chromosome 1 intervals are linked using lines with rice genetic maps in the locations where wheat ESTs are sequence matched with rice BAC/PAC clones. Order of the lines (ESTs) in wheat consensus chromosome 1 intervals was based on the order of homoeologous rice sequences (BAC/PAC clones). In the consensus bin map of wheat chromosome 1, the numbers and located intervals of wheat group 1 ESTs (lines) and the distance from the centromere (fraction length) are indicated. In the rice maps, the locations and numbers of BAC/PAC clones (lines linked with wheat map) and interval lengths (in centimorgans) are indicated. The arrows in rice chromosomes 5 and 10 indicate the approximate regions of centromere cited from http://www.gramene.org/. For those wheat ESTs not mapped to specific intervals, the chromosome (W1) or chromosome arms (W1S for the short and W1L for the long arm) are indicated. The specific map locations of three rice BAC/PAC clones on chromosome 5 are not available, and the corresponding wheat ESTs are thus linked with a code R5 in the figure. The details including accession numbers of wheat ESTs and rice BAC/PAC clones and the orthologous map of wheat group 1 ESTs are also presented as supplemental online materials at http://wheat.pw.usda.gov/pubs/2004/Genetics/.
A total of 133 W1 ESTs corresponded to 93 R5 BAC/PAC clones spanning a genetic length of 122.3 cM. More than one-third (33) of the R5 sequences matched to two or more W1 ESTs in the same bins or different bins in both short and long arms. There was arm correspondence between R5 and W1, with a small number (six) of exceptions where the linear order was not conserved (Figure 2).
A total of 53 W1 ESTs significantly matched to 37 R10 BAC/PAC clones spanning a genetic distance of 77.0 cM. About one-third (12) of the R10 sequences matched to two or more W1 ESTs in the proximal regions of the long arm of W1. Of the 37 R10 sequences, 33 matched to W1 ESTs on the long arm. Of the four exceptions, two were duplicated and two were specific to the short arm of W1 (Figure 2).
R5 sequence-matched ESTs apparently predominated over R10 sequence-matched ESTs for the bins with fraction length (FL) > 0.47 in both short and long arms of W1, the reverse was true for the bins with FL < 0.47 in the long arm of W1. There were five intervals without significant colinearity with R5 or R10, and four of them were in the short arm (C-1S-0.47, 1S-0.48-0.50, 1S-0.59-0.70, and 1S-0.70-0.84) of W1. Thus, either these five intervals have no similarity to R5 and R10 sequences or the similarity could not be detected with the existing data.
Anomaly:
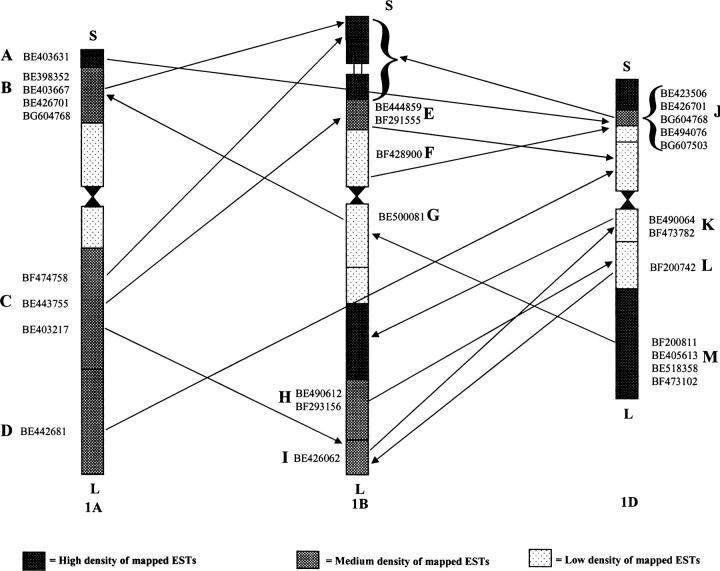
An anomaly refers to nonoverlapping map positions for an EST in the chromosome bin maps of the homoeologues. Of the 944 EST probes mapped to group 1 chromosomes, 26 (2.8%) detected anomalies, as defined by Munkvold et al. (2004), involving all three group 1 chromosomes and arms. The distribution of these anomalies among the three group 1 chromosomes is shown in Figure 3. For example, EST BE500081 in the bin C-1BL-0.32 was not mapped to the near-centromere bin on the long arm of either 1A or 1D, but it did map to bin 1AS-0.47-0.86 on 1AS. A total of 13 anomalies were present among 18 of 21 noncentromere bins, and 4, 5, and 4 anomalies were present for 1A, 1B, and 1D chromosomes, respectively. But only 7 of the 13 anomalies were detected by two or more ESTs.
Figure 3.—
Chromosome locations of mapped ESTs detecting anomalies. The GenBank accession numbers for the ESTs contained in the anomalies are included next to the appropriate chromosome intervals. A letter code was given to each anomaly for reference.
DISCUSSION
Large number of ESTs mapped to homoeologous group 1 chromosomes:
In the catalog of wheat gene symbols (McIntosh et al. 2003), >1500 DNA marker loci including RFLPs and SSRs were documented for wheat group 1 chromosomes from ∼100 publications. With the results reported here, 2212 loci, and the previous 1500, >3700 loci have been identified for this group, making it particularly rich in DNA marker loci.
Gene-rich regions in wheat group 1 chromosomes:
It is well established from this study and others that polymorphic DNA markers are not evenly distributed along the chromosomes in both genetic (Devey and Hart 1993; Dubcovsky et al. 1995; Gale et al. 1995; Van Deynze et al. 1995; Cadalen et al. 1997; Peng 2000; Peng et al. 2000) and physical maps (Delaney et al. 1995a,b; Gill et al. 1996a,b; Faris et al. 2000; Ma et al. 2001). Gill et al. (1996b) identified the 1S0.8 consensus region as gene rich. Sandhu et al. (2001) localized 75 useful genes into this region. We mapped >100 EST loci in regions of similar size in this study on each short arm of the group 1 chromosomes, accounting for >50% of those on the short arm for each chromosome. The short arm region of similar size in the wheat consensus chromosome 1 (16% of the arm) contained 67 ESTs (64.4% of the total in the consensus short-arm map; Figure 1; supplemental online material at http://wheat.pw.usda.gov/pubs/2004/Genetics/).
The distribution of ESTs can reveal the approximate expressed gene distribution patterns. We found that ESTs clustered in a few regions in the three group 1 chromosomes (Figure 1). It has been shown that centromeric/proximal regions are relatively gene poor and distal/telomeric regions on the short arms are gene rich (Gill et al. 1996a,b; Faris et al. 2000). Our results further showed that the telomeric regions on the long arms had a lower gene density than the middle portion of the arms, which contained EST-rich regions/clusters. There are ESTs in the distal bins, but they are located in the proximal regions of those bins and are not at the ends of the chromosomes. The consensus map with a higher number of long-arm bins than the individual chromosomes clearly points this out (Figure 1).
We found that the number of ESTs mapped to the proximal parts of chromosome arms was significantly lower than that mapped to the distal parts (Figure 1). Comparisons of genetic linkage maps and physical maps have indicated that recombination is dramatically reduced in the centromeric regions of grass chromosomes. This recombination reduction is explained as the result of suppression around the centromere or by the centromere itself (Dvořák and Chen 1984; Lukaszewski and Curtis 1993; Van Deynze et al. 1995). As shown by Akhunov et al. (2003), EST density was positively related with recombination rate (i.e., EST-rich regions have high recombination rates). But the reason for recombination reduction near the centromere may be the low EST (gene) density, rather than centromere suppression.
Duplication of wheat group 1 ESTs:
If an EST probe had loci mapping to more than one chromosome in a genome, then those loci were considered duplicated (Akhunov et al. 2003). Anderson et al. (1992) reported that 40 of 210 DNA probes hybridized to fragments in more than one homoeologous group and that group 1 chromosomes were involved in the majority of these duplications. Van Deynze et al. (1995) found that many loci were duplicated between group 1 and group 7 chromosomes. Dubcovsky et al. (1996) pointed out that 30% of the loci mapped in T. monococcum were duplicated in other chromosomes. Akhunov et al. (2003) found that 21% of 730 ESTs had duplicated loci. In the present study, 335 (35%) of 944 ESTs analyzed were mapped to group 1 and at least one of the other six homoeologous groups. The number of group 1 duplications shared by each of the other six homoeologous groups ranged from 113 to 158 ESTs (Table 3). The EST duplication rate in this article seemed much higher than that found by Akhunov et al. (2003), and the difference may be derived from the data source (group 1, studied here, vs. all groups studied by Akhunov et al. 2003). Duplications of group 1 ESTs were randomly distributed among the other six homoeologous groups, but unevenly distributed among the three group 1 chromosomes with a larger number in 1B and a smaller number in 1A and 1D than expected and, along the chromosome arms, with a larger number in the distal and a smaller number in the proximal regions except 1AL and 1BL (Table 4). These results confirm the conclusion of Akhunov et al. (2003) who studied a smaller sample of the ESTs mapped in this project. The high rate of interchromosome duplication of ESTs also confirms that homoology exists among the seven groups of homoeologous chromosomes of wheat.
Anomalies in group 1 chromosomes:
The rate of anomalies was much lower in group 1 than in group 3 (Munkvold et al. 2004). Anomalies may result from biological events such as chromosomal rearrangements, transposition, and gene duplication or be an artifact of technical errors. An anomaly evidenced by a single EST (6 of 13 for group 1 chromosomes) is questionable and likely due to a technical error. However, anomalies involving two or more mapped ESTs with the same location pattern (7 of 13) are more likely to result from a biological event. Of the 7 multi-EST anomalies, three (B, J, and M, Figure 3) were supported by four to five ESTs. These three striking anomalies belong to an intra-arm anomaly, i.e., long (or short) arm to long (or short) arm of two homoeoleogous chromosomes. The chromosome bins involved had different EST densities (Figure 3). These anomalies could have resulted from chromosomal rearrangements, possibly resulting from transposition and gene duplication mainly between chromosome intervals with different gene density.
Homoeologous relationship between wheat consensus chromosome 1 and the rice genome:
Homoeology between wheat and rice genomes was first studied by Ahn et al. (1993) and followed by Kurata et al. (1994) and Van Deynze et al. (1995) at the macro level. Recently, Sorrells et al. (2003), studying a subset of project EST loci involving all homoeologous groups, compared rice and wheat genomes at the DNA sequence level. All studies indicated that rice chromosomes 5 and 10 were homoeologous with group 1 chromosomes of wheat. Sorrells et al. (2003) also showed that 81% of the rice BAC/PAC clones were matched by wheat ESTs. Our results (Figure 2; Table 6; supplemental online material at http://wheat.pw.usda.gov/pubs/2004/Genetics/) further corroborated the close syntenic or homoeologous relationship of wheat consensus chromosome 1 with rice chromosomes 5 and 10.
Comparative mapping based on cDNA clones indicated that rice chromosome 5 was largely conserved with wheat consensus chromosome 1 and that rice chromosome 10 was conserved with a portion of the long arm of wheat consensus chromosome 1. Our results, based on sequence comparison between wheat consensus chromosome 1 ESTs and rice BAC/PAC clones, further identified specific wheat consensus chromosome 1 bins with significant colinearity to rice chromosomes 5 and 10 (Figure 2; Table 6). Since distal segments of wheat chromosomes are gene-rich and recombination-rich regions (Delaney 1995a,b; Gill et al. 1996a,b; Faris et al. 2000), we may expect rice chromosome 5 to have close relationships to the gene clusters in the distal regions of wheat consensus chromosome 1. Rice chromosomes 5 and 10 and wheat chromosome 1 were probably differentiated by a chromosome fission/fusion after evolutionary divergence (Ahn et al. 1993). As shown in Figure 1, both the EST number and EST density in the C-1L-0.47 region significantly (P < 0.01) exceeded those in the C-1S-0.47 region of wheat consensus chromosome 1. Therefore, we also speculate that, in the process of divergence, the DNA segments corresponding to the current rice chromosome 10 might have been translocated to the proximal region of the long arm of rice chromosome 5. Because of this possible “translocation,” the proximal part of the long arm of wheat chromosome 1 is not as gene poor as the counterpart of the short arm appears (Figures 1 and 2).
Kurata et al. (1994) provided evidence for the conservation of gene order between rice and wheat; i.e., many wheat chromosomes contain genes and genomic DNA fragments in a similar order to that found on rice chromosomes. Van Deynze et al. (1995) pointed out that although wheat orthologous loci span all of rice chromosome 5 and 10 linkage maps, loci from the distal portion of the linkage maps for the short arms of the Triticeae chromosomes are not represented in these rice chromosomes. The only two loci from the distal short arm of the linkage maps of the Triticeae that could be detected in rice did not map to homoeologous positions in rice chromosome 5. The linear organizations of genes in nine different genomes of grasses, including wheat, can be described in terms of only 25 “rice linkage blocks” based on genetic mapping using common DNA probes (Gale and Devos 1998). Our results, based on DNA sequence comparison and chromosome deletion bin mapping, corroborate, to some extent, the conservation of gene orders between rice and wheat. However, a number of genes not showing any colinearity were also identified. This noncolinearity may be due to chromosome structural changes, gene inversions, transposon-like movements, and inclusion of multi-copy probes. The ESTs in the orthologous regions of wheat consensus chromosome 1 may be ordered following the sequence (BAC/PAC clone) order in rice chromosome 5 or 10, as shown in Figure 2 and supplemental online material at http://wheat.pw.usda.gov/pubs/2004/Genetics/. The order of orthologous EST loci in the individual wheat consensus chromosome 1 deletion bins needs to be verified by genetic mapping or complete sequencing of the bins.
It is clear that the three chromosomes of homoeologous group 1 are not perfectly conserved. They are different in physical size and structure (Gill et al. 1991), the extent of polymorphism detected by both molecular markers (Peng et al. 2000) and QTL analysis (Peng et al. 2003), and gene content, density, and duplication (Table 1). Therefore, even though the consensus chromosome 1 is of considerable value in obtaining a general profile of EST distribution patterns of wheat group 1 chromosomes and their colinearity with rice chromosomes 5 and 10, there are limitations to its resolution and validity.
Homoeologous relationship of wheat consensus chromosome 1 with the Arabidopsis genome:
Arabidopsis is the first plant with a completely sequenced genome (Arabidopsis Genome Initiative 2000). A. thaliana was estimated to contain 25,000 genes, but its genome is 99 times smaller than that of hexaploid wheat (Bennett and Leitch 2003). It would be greatly helpful for wheat genomics studies if Arabidopsis information could be transferable to wheat. Initial observations indicated that synteny might be extrapolated to monocot and dicot species (Paterson et al. 1996). Attempts to establish colinearity between the rice and Arabidopsis genomes suggest that colinearity cannot be detected in comparative genetic mapping studies (Devos et al. 1999). In the present study, only 35 (9.5%) of wheat homoeologous group 1 ESTs showed significant matches to the Arabidopsis genome sequences. No significant conservation of gene order was detected between wheat consensus chromosome 1 and Arabidopsis chromosomes (data not shown) with this small number of ESTs. These 35 ESTs represent <30 types of gene function (Table 5). The number of conserved genes shared by monocotyledonous and dicotyledonous plant species seems small. Therefore the DNA sequence data of Arabidopsis do not appear to be very useful in the study of genome organization of wheat or other grass species.
Arabidopsis chromosome 3 accounted for 13 (37.4%) of the DNA segments with significant matches to wheat group 1 ESTs and seems to have the closest relationship, among the five Arabidopsis chromosomes, with wheat consensus chromosome 1 (Table 5). Five (38.5%) of these 13 segments spanning 20.5 Mbp (2.6–23.1 Mbp) of Arabidopsis chromosome 3 corresponded to the ESTs located in the 1L-0.47-0.61 region of wheat consensus chromosome 1 (data not shown). However, as observed between rice and Arabidopsis (Devos et al. 1999), there is poor colinearity between wheat consensus chromosome 1 and Arabidopsis chromosome 3.
Interestingly, only 5 (14.3%) of the 35 group 1 ESTs with matches to Arabidopsis were located in the terminal regions of wheat consensus chromosome 1. The common genes shared by wheat consensus chromosome 1 and Arabidopsis appear located in the middle parts of the chromosome arms or the proximal region of the long arm (Table 5).
Acknowledgments
We thank Fang Shi for her participation during the early period of this project. We appreciate the technical support at Colorado State University of Hong Wang, Jason Hunt, John Gajewski, and the following students: Dustin Arellano, Huixia Wang, Leah Roberts, Kellen Nelson, Jan Williams, Elise Woodruff, Ashley Lock, and Jason Haybarker. This material is based upon work supported by the National Science Foundation under Cooperative Agreement no. DBI-9975989.
References
- Adams, M. D., J. M. Kelley, J. D. Gocayne, M. Dubnick, M. H. Polymeropoulos et al., 1991. Complementary DNA sequencing: expressed sequence tags and human genome project. Science 252: 1651–1656. [DOI] [PubMed] [Google Scholar]
- Adams, M. D., A. R. Kerlavage, R. D. Fleischmann, R. A. Fuldner, C. J. Bult et al., 1995. Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence. Nature 377(Suppl.): 3–17. [PubMed] [Google Scholar]
- Ahn, S. N., J. A. Anderson, M. E. Sorrells and S. D. Tanksley, 1993. Homoeologous relationships of rice, wheat and maize chromosomes. Mol. Gen. Genet. 241: 483–490. [DOI] [PubMed] [Google Scholar]
- Akhunov, E. D., A. W. Goodyear, S. Geng, L. L. Qi, B. Echalier et al., 2003. The organization and rate of evolution of wheat genomes are correlated with recombination rates along chromosome arms. Genome Res. 13: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. A., Y. Ogihara, M. E. Sorrells and S. D. Tanksley, 1992. Development of a chromosomal arm map for wheat based on RFLP markers. Theor. Appl. Genet. 83: 1035–1043. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative, 2000. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815. [DOI] [PubMed] [Google Scholar]
- Bennett, M. D., and I. J. Leitch, 2003 Plant DNA C-values database (release 2.0, Jan. 2003; http://www.rbgkew.org.uk/cval/homepage.html).
- Bennetzen, J., 2002. Opening the door to comparative plant biology. Science 296: 60–63. [DOI] [PubMed] [Google Scholar]
- Briggs, S. P., 1998. Plant genomics: more than food for thought. Proc. Natl. Acad. Sci. USA 95: 1986–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadalen, T., C. Boeuf, S. Bernard and M. Bernard, 1997. An intervarietal molecular marker map in Triticum aestivum L. em. Thell. and comparison with a map from a wide cross. Theor. Appl. Genet. 94: 367–377. [Google Scholar]
- Clark, M. D., S. Hennig, R. Herwig, S. W. Clifton, M. A. Marra et al., 2001. An oligonucleotide fingerprint normalized and expressed sequence tag characterized zebrafish cDNA library. Genome Res. 11: 1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covitz, P. A., L. S. Smith and S. R. Long, 1998. Expressed sequence tags from a root-hair-enriched Medicago truncatula cDNA library. Plant Physiol. 117: 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, D. E., S. Nasuda, T. R. Endo, B. S. Gill and S. H. Hulbert, 1995. a Cytologically based physical maps of the group-2 chromosomes of wheat. Theor. Appl. Genet. 91: 568–573. [DOI] [PubMed] [Google Scholar]
- Delaney, D. E., S. Nasuda, T. R. Endo, B. S. Gill and S. H. Hulbert, 1995. b Cytologically based physical maps of the group 3 chromosomes of wheat. Theor. Appl. Genet. 91: 780–782. [DOI] [PubMed] [Google Scholar]
- Devey, M. E., and G. E. Hart, 1993. Chromosomal localization of intergenomic RFLP loci in hexaploid wheat. Genome 36: 913–918. [DOI] [PubMed] [Google Scholar]
- Devos, K. M., J. Beales, Y. Nagamura and T. Sasaki, 1999. Arabidopsis-rice: Will colinearity allow gene prediction across the eudicot-monocot divide? Genome Res. 9: 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilbirligi, M., M. Erayman, D. Sandhu, D. Sidhu and K. S. Gill, 2004. Identification of wheat chromosomal regions containing expressed resistance genes. Genetics 166: 461–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky, J., M. C. Luo and J. Dvořák, 1995. Differentiation between homoeologous chromosomes 1A of wheat and 1Am of Triticum monococcum and its recognition by the wheat Ph1 locus. Proc. Natl. Acad. Sci. USA 92: 6645–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky, J., M. C. Luo, G. Y. Zhong, R. Bransteitter, A. Desai et al., 1996. Genetic map of diploid wheat, Triticum monococcum L., and its comparison with maps of Hordeum vulgare L. Genetics 143: 983–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky, J., M. Echeide, F. Giancola, M. Rousset, M. C. Luo et al., 1997. Seed storage protein loci and RFLP maps of diploid, tetraploid, and hexaploid wheat. Theor. Appl. Genet. 95: 1169–1180. [Google Scholar]
- Dvořák, J., and K. C. Chen, 1984. Distribution of nonstructural variation between wheat cultivars along chromosome arm 6Bp: evidence from the linkage map and physical map of the arm. Genetics 106: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo, T. R., 1988. Induction of chromosomal structural changes by a chromosome of Aegilops cylindrica L. in common wheat. J. Hered. 79: 366–370. [DOI] [PubMed] [Google Scholar]
- Endo, T. R., 1990. Gametocidal chromosomes and their induction of chromosome mutations in wheat. Jpn. J. Genet. 65: 135–152. [Google Scholar]
- Endo, T. R., and B. S. Gill, 1996. The deletion stocks of common wheat. J. Hered. 87: 295–307. [Google Scholar]
- Ewing, R. M., A. B. Kahla, O. Poirot, F. Lopez, S. Audic et al., 1999. Large-scale statistical analyses of rice ESTs reveal correlated patterns of gene expression. Genome Res. 9: 950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris, J. D., K. M. Haen and B. S. Gill, 2000. Saturation mapping of a gene-rich recombination hot spot region in wheat. Genetics 154: 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, M., F. G. H. Lipton and T. E. Miller, 1995 Wheats, Triticum spp. (Gramineae-Triticinae), pp. 184–192 in Evolution of Crop Plants, edited by J. Smart and N. W. Simmonds. Longman Scientific & Technical Press, London.
- Fernandes, J., V. Brendel, X. Gai, S. Lal and V. L. Chandler, 2002. Comparison of RNA expression profile based on maize expressed sequence tag frequency analysis and micro-array hybridization. Plant Physiol. 128: 896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, M. D., and K. M. Devos, 1998. Comparative genetics in the grasses. Proc. Natl. Acad. Sci. USA 95: 1971–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, M. D., M. D. Atkinson, C. N. Chinoy, R. L. Harcourt, J. Jia et al., 1995 Genetic maps of hexaploid wheat, pp. 29–40 in Proceedings of the 8th International Wheat Genetics Symposium, edited by Z. S. Li and Z. Y. Xin. China Agricultural Scientech Press, Beijing.
- Gill, B. S., B. Friebe and T. R. Endo, 1991. Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome 34: 830–839. [Google Scholar]
- Gill, K. S., B. S. Gill, T. R. Endo and E. V. Boyko, 1996. a Identification and high-density mapping of gene-rich regions in chromosome group 5 of wheat. Genetics 143: 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, K. S., B. S. Gill, T. R. Endo and T. Taylor, 1996. b Identification and high-density mapping of gene-rich regions in chromosome 1 of wheat. Genetics 144: 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff, S. A., D. Ricke, T. H. Lan, G. Presting, R. Wang et al., 2002. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296: 92–100. [DOI] [PubMed] [Google Scholar]
- Harlan, J. R., 1992 Crops and Man, Ed 2. American Society of Agronomy/Crop Science Society of America. Madison, WI.
- Hillier, L., G. Lennon, M. Becker, M. F. Bonaldo, B. Chiapelli et al., 1996. Generation and analysis of 280,000 human expressed sequence tags. Genome Res. 6: 807–828. [DOI] [PubMed] [Google Scholar]
- Kurata, N., G. Moore, Y. Nagamura, T. Foote, M. Yano et al., 1994. Conservation of genome structure between rice and wheat. Biotechnology 12: 276–278. [Google Scholar]
- Lazo, G. R., S. Chao, D. D. Hummel, H. Edwards, C. C. Crossman et al., 2004. Development of an expressed sequence tag (EST) resource for wheat (Triticum aestivum L.): EST generation, unigene analysis, probe selection, and bioinformatics for a 16,000-locus bin-delineated map. Genetics 168: 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkiewicz, A. M., L. L. Qi, B. S. Gill, B. Echalier, S. Chao et al., 2004. A 2500-locus bin map of wheat homoeologous group 5 provides new insights on gene distribution and colinearity with rice. Genetics 168: 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewski, A. J., and C. A. Curtis, 1993. Physical distribution of recombination in B-genome chromosomes of tetraploid wheat. Theor. Appl. Genet. 84: 121–127. [DOI] [PubMed] [Google Scholar]
- Ma, X.-F., K. Ross and J. P. Gustafson, 2001. Physical mapping of restriction fragment length polymorphism (RFLP) markers in homeologous groups 1 and 3 chromosomes of wheat by in situ hybridization. Genome 44: 401–412. [PubMed] [Google Scholar]
- Marra, M., L. Hillier, T. Kucaba, M. M. Allen, R. Barstead et al., 1999. An encyclopedia of mouse genes. Nat. Genet. 21: 191–194. [DOI] [PubMed] [Google Scholar]
- McIntosh, R. A., Y. Yamazaki, K. M. Devos, J. Dubcovsky, W. J. Rogers et al., 2003 Catalogue of gene symbols for wheat, pp. 1–34 in Proceedings of the 10th International Wheat Genetics Symposium, Vol. 4, edited by N. E. Pogna, M. Romano, E. Pogna and G. Galterio. Instituto Sperimentale per la Cerealicotura, Rome.
- Munkvold, J. D., R. A. Greene, C. E. Bermudez-Kandianis, C. M. La Rota, H. Edwards et al., 2004. Group 3 chromosome bin maps of wheat and their relationship to rice chromosome 1. Genetics 168: 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo, E., A. B. Korol, A. Beiles and T. Fahima, 2002 Evolution of Wild Emmer and Wheat Improvement—Population Genetics, Genetic Resources, Genome Organization of Wheat's Progenitor, Triticum dicoccoides. Springer-Verlag, Berlin/Heidelberg, Germany/New York.
- Paterson, A. H., T. H. Lan, K. P. Reischmann, C. Chang, Y. R. Lin et al., 1996. Toward a unified genetic map of higher plants, transcending the monocot-dicot divergence. Nat. Genet. 14: 380–382. [DOI] [PubMed] [Google Scholar]
- Peng, J. H., 2000 Genomics of wild emmer wheat, Triticum dicoccoides: genetic maps, mapping of stripe rust resistance genes and QTLs for agronomic traits. Ph.D. Thesis, University of Haifa, Haifa, Israel.
- Peng, J. H., A. B. Korol, T. Fahima, M. S. Röder, Y. I. Ronin et al., 2000. Molecular genetic maps in wild emmer wheat, Triticum dicoccoides: genome-wide coverage, massive negative interference, and putative quasi-linkage. Genome Res. 10: 1509–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J. H., Y. I. Ronin, T. Fahima, M. S. Röder, Y. C. Li et al., 2003. Domestication quantitative trait loci in Triticum dicoccoides, the progenitor of wheat. Proc. Natl. Acad. Sci. USA 100: 2489–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, L. L., B. Echalier, B. Friebe and B. S. Gill, 2003. Molecular characterization of a set of wheat deletion stocks for using in chromosome bin mapping of ESTs. Funct. Integr. Genomics 3: 39–55. [DOI] [PubMed] [Google Scholar]
- Qi, L. L., B. Echalier, S. Chao, G. R. Lazo, G. E. Butler et al. 2004. A chromosome bin map of 16,000 expressed sequence tag loci and distribution of genes among the three genomes of polyploid wheat. Genetics 168: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu, D., J. A Champoux, S. N. Bondareva and K. S. Gill, 2001. Identification and physical location of useful genes and markers to a major gene-rich region on wheat group 1S chromosomes. Genetics 157: 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz, T. E., M. R. Raymon, D. Y. Nishimura, A. McClain, C. W. Roberts et al., 2001. Generation of a high-density rat EST map. Genome Res. 11: 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears, E. R., 1954. The aneuploids of common wheat. Univ. Mo. Agric. Exp. Stn. Bull. 572: 1–58. [Google Scholar]
- Sears, E. R., 1966 Nullisomic-tetrasomic combinations in hexaploid wheat, pp. 29–45 in Chromosome Manipulations and Plant Genetics, edited by R. Riley and K. R. Lewis. Oliver & Boyd, Edinburgh.
- Sears, E. R., 1969. Wheat cytogenetics. Annu. Rev. Genet. 3: 451–468. [Google Scholar]
- Sears, E. R., and L. M. S. Sears, 1978 The telocentric chromosomes of common wheat, pp. 389–407 in Proceedings of the 5th International Wheat Genetics Symposium, edited by S. Ramanujam. Indian Society of Genetics and Plant Breeding, New Delhi.
- Sorrells, M. E., M. La Rota, C. E. Bermudez-Kandianis, R. A. Greene, R. Kantety et al., 2003. Comparative DNA sequence analysis of wheat and rice genomes. Genome Res. 13: 1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deynze, A. E., J. Dubcovsky, K. S. Gill, J. C. Nelson, M. E. Sorrells et al., 1995. Molecular-genetic maps for group 1 chromosomes of Triticeae species and their relation to chromosomes in rice and oat. Genome 38: 45–59. [DOI] [PubMed] [Google Scholar]
- Williams, P. C., 1993 The world of wheat, pp. 557–602 in Grains and Oilseeds: Handling, Marketing, Processing. Canadian International Grains Institute, Winnipeg, Manitoba, Canada.
- Yu, J., S. Hu, J. Wang, G. K. S. Wong, S. Li et al., 2002. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296: 79–92. [DOI] [PubMed] [Google Scholar]
- Zhang, D., D. W. Choi, S. Wanamaker, R. D. Fenton, A. Chin et al., 2004. Construction and evaluation of cDNA libraries for large-scale expressed sequence tag sequencing in wheat (Triticum aestivum L.). Genetics 168: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]