Abstract
The damaged DNA-binding protein (DDB) complex, thought to recognize (6-4) photoproducts and other lesions in DNA, has been implicated to have a role in global genomic nucleotide excision repair (NER) and E2F-1-mediated transcription. The complex consists of a heterodimer of p127 (DDB1) and p48 (DDB2), the latter also being known as XPE. We reported previously that in Drosophila expression of the DDB1 (D-DDB1) gene is controlled by the DRE/DREF system, and external injury to DNA is not essential for D-DDB1 function. In the present study of the function of D-DDB1 in a multicellular system, we prepared transgenic flies, which were knocked down for the D-DDB1 gene due to RNA interference (RNAi), and performed immunocytochemistry to ascertain the distribution of D-DDB1 in the eye imaginal disc. It was found to be abundant in the anterior of the morphogenetic furrow (MF). Whole-body overexpression of dsRNA of D-DDB1 in Drosophila using a GAL4-UAS targeted expression system induced melanotic tumors and caused complete lethality. When limited to the eye imaginal disc, a severe rough eye phenotype resulted. Correspondingly, all of the D-DDB1 gene knocked-out flies also died. D-DDB1 therefore appears to be an essential development-associated factor in a multicellular organism.
THE damaged DNA-binding protein (DDB) complex, which is a heterodimeric protein composed of 127-kD (DDB1) and 48-kD (DDB2) subunits, has been shown to recognize many types of DNA lesions (Feldberg 1980; Carew and Feldberg 1985; Hirschfeld et al. 1990; Keeney et al. 1993; Reardon et al. 1993; Payne and Chu 1994). DDB1 can interact with SPT3-TAFII31-GCN5L acetylase (STAGA) complex (Martinez et al. 2001) and p300 histone acetyltransferase (Rapic-Otrin et al. 2002), while DDB2 can interact with CREB-binding protein (Datta et al. 2001). DDB might function as a repair protein to alter chromatin structure and recruit nucleotide excision repair (NER) factors to DNA damage sites. DDB2 is also known as xeroderma pigmentosum complementation group E (XPE) and cells with mutations in this gene are mildly defective in NER of DNA damage (Shiyanov et al. 1999a; Liu et al. 2000). However, DDB was found not to be required in NER reconstitution studies in vitro (Aboussekhra and Wood 1995; Mu et al. 1995; Kazantsev et al. 1996) despite the fact that damaged DNA-binding activity of DDB is absent in cells of a subset of XPE patients (Chu and Chang 1988; Kataoka and Fujiwara 1991; Keeney et al. 1992, 1993). In vivo studies have shown that XPE cells (DDB2 mutants) are selectively defective in global genomic repair (GGR; Hwang et al. 1999). No mutations of mammalian DDB1 have been described although Zolezzi et al. (2002) discussed that although DDB1 is not necessarily a lethal factor in a unicellular system, the very potent defects that result as a consequence of the Schizosaccharomyces pombe DDB1 deletion would suggest that mutations in the mammalian DDB1 gene are likely to be lethal.
Because functions of DDB other than directly in DNA repair have recently been suggested (Nichols et al. 2000; Takata et al. 2002; Zolezzi et al. 2002), the present study was performed to investigate the properties of DDB1 in the whole body of Drosophila melanogaster. DDB2 binds to E2F-1, which is a cell cycle regulatory transcription factor (Hayes et al. 1998). DDB binds to cullin 4A, which is believed to be a ubiquitin-protein isopeptide ligase (type E3; Shiyanov et al. 1999b). The apolipoprotein B (apoB) gene regulatory factor-2 (BRF-2)/human hepatitis B virus X-associated protein-1 (XAP-1)/DDB1 may belong to a new family of transcription factors and control apoB gene transcription (Krishnamoorthy et al. 1997). DDB1 also binds to viral transcriptional transactivators, including the hepatitis B virus X protein (HBVx; Butel et al. 1995). DDB1 has roles in chromosome segregation and the aberrant nuclear structures observed in the ddb1Δ strain in S. pombe (Zolezzi et al. 2002). We found and reported previously that the expression of the Drosophila-DDB1 (D-DDB1) gene is controlled by the DNA replication-related element (DRE)/DRE-binding factor (DRE/DREF) system (Takata et al. 2002), which is generally responsible for activating the promoters of proliferation-related genes for proliferating cell nuclear antigen (PCNA) (Yamaguchi et al. 1995b), the 180-kD and 73-kD subunits of DNA polymerase α (Yamaguchi et al. 1995a; Takahashi et al. 1996), cyclin A (Ohno et al. 1996), ras (Lightfoot et al. 1994), raf (Ryu et al. 1997), and D-mtTFA (Takata et al. 2001, 2003). D-DDB1 is thus suggested to have roles in cell proliferation. We also indicated in a previous article that external injury to DNA is not essential to D-DDB1 function, and that as with UV-irradiation-induced transfer of D-DDB1 to the nucleus from the cytoplasm, during spermatogenesis the D-DDB1 protein transiently shifts from one cell compartment to another (Takata et al. 2002). The results indicated that D-DDB1 not only contributes to the DNA repair system, but also plays roles in cell proliferation and development.
In this report, we provide evidence from analyses of transgenic flies, knocked down for the D-DDB1 gene by RNAi, and knocked out for the D-DDB1 gene by P-element insertion, that D-DDB1 acts as a cell-proliferation and development-associated factor as well as in DNA repair. Interestingly, we found that a defect in D-DDB1 caused lethality in our multicellular system. Knock down of D-DDB1 in the entire eye imaginal disc, but not when posterior to the morphogenetic furrow (MF), further caused a severe rough eye phenotype.
MATERIALS AND METHODS
Plasmid construction:
The plasmid p5′-D-DDB1(650)-dsRNA contains the D-DDB1 open reading frame (1–750 bp) and the D-DDB1 ORF (101–750 bp) head to head (3′, 750–1 bp of D-DDB1 ORF and 5′, 101–750 bp of D-DDB1 ORF), in a P-element vector.
Establishment of transgenic flies:
P-element-mediated germ-line transformation was carried out as described earlier (Spradling and Rubin 1982). F1 transformants were selected on the basis of white eye color rescue (Robertson et al. 1988). Established transgenic strains carrying pUAS-D-DDB1(650)-dsRNA and their chromosomal linkages are listed in Table 1.
TABLE 1.
Transformants carrying the 650-bpD-DDB1double-strand RNA
| P-element plasmid | Strain | Chromosome linkage |
|---|---|---|
| pUAS-D-DDB1(650)-dsRNA | 10 | II |
| 23 | II | |
| 31 | III | |
| 41 | III |
Fly stocks:
Fly stocks were cultured at 25° on standard food. The Canton-S fly was used as the wild-type strain. The line expressed GAL4 under the control of the Act5C gene promoter, Act25 gene promoter, and eyeless gene promoter. Establishment of lines carrying GMR-GAL4 was described earlier (Robertson et al. 1988; Takahashi et al. 1999). Enhancer trap line carrying the lacZ marker X63 (inserted in rhomboid), D120 (inserted in scabrous), and BB02 (inserted in rhomboid) were obtained from Y. Hiromi. P-element insertion in the 5′ exon of D-DDB1, DDB1EY01408, and UAS-P35 were obtained from the Bloomington Indiana Stock Center. mei-9L1 (allele of the XPF gene) was obtained from the Drosophila Genetic Resource Center, Kyoto Institute of Technology. Establishment of lines carrying Collagen-GAL4 (CgGAL4) was kindly provided by Dr. C. R. Dearolf.
Ectopic expression of UAS-D-DDB1(650)-dsRNA:
Methods for ectopic expression of D-DDB1(650)-dsRNA were essentially as described by Brand and Perrimon (1993):
Expression in the whole body: A line carrying heterozygous Act5C-GAL4 on the third chromosome and Act25-GAL4 on the second chromosome was crossed with lines carrying the homozygous P[UAS-D-DDB1(650)-dsRNA] on the second chromosome.
Expression in the fat body: A line carrying homozygous CgGAL4 on the second chromosome was crossed with lines carrying the homozygous P[UAS-D-DDB1(650)-dsRNA] on the second chromosome.
Expression in the eye imaginal disc: Males carrying ey-GAL4 on the second chromosome and GMR-GAL4 on the X chromosome were crossed with females carrying homozygous P[UAS-D-DDB1(650)-dsRNA] on the second chromosome.
Northern hybridization:
Aliquots of 30 μg of total RNA extracted from living third-instar larvae carrying a single copy of Act5C-GAL4 (+/+;Act5C-GAL4/+) and single copies of Act5C-GAL4 and UAS-D-DDB1(650)-dsRNA (UAS-D-DDB1(650)-dsRNA/+;Act5C-GAL4/+) were resolved on 1.2% formaldehyde-containing agarose gels and transferred onto nylon membranes (Hybond-N+; Amersham, Arlington Heights, IL). After prehybridization, the filters were probed with a 32P-labeled D-DDB1 ORF (1774–3423 bp), with a 32P-labeled full-length PCNA ORF as a negative control or with a 32P-labeled full-length ribosomal protein 49 (RP-49) ORF as a control at 42° for 16 hr, followed by washing twice with 2× SSPE + 1% SDS at room temperature for 15 min and twice with 1× SSPE + 0.1% SDS at 60° for 20 min. Blots were exposed to Kodak X-Omat XAR films and quantified with a NIH imaging analyzer.
Western blotting analysis:
Western blotting analysis was carried out by the method of Towbin et al. (1979). A total of 60 μg of TCA-precipitated proteins of a homogenate of Drosophila bodies was separated on 10% SDS-PAGE. Polyclonal antibodies reacting with D-DDB1 were affinity purified by D-DDB1 protein-conjugated Sepharose column chromatography (Takata et al. 2002). DM1A mouse anti-α tubulin monoclonal antiboby (Accurate Chemical and Scientific, Westbury, NY) was used as a control. Anti-rat IgG and anti-mouse IgG conjugated with alkaline phosphatase (Promega, Madison, WI) were used as a secondary antibody. Color was developed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate as the substrates of alkaline phosphatase.
Immunohistochemistry:
The polyclonal anti-D-DDB1 antibody (Takata et al. 2002) used for the immunocytochemical study reacts specifically with the D-DDB1 protein in a crude extract of Drosophila embryos and with that produced in Escherichia coli carrying the expression plasmid for a recombinant D-DDB1 (Takata et al. 2002). Third instar larvae were dissected in Drosophila Ringer's solution and imaginal discs were fixed in 4% paraformaldehyde, PBS for 20 min at room temperature or at 4°. After washing with PBS, 0.3% Triton X-100 (PBS-T), the samples were blocked with PBS-T containing 10% normal goat serum for 30 min at room temperature and incubated with a rat anti-D-DDB1 polyclonal antibody at a 1:100 dilution, a rabbit anti-D-RPA70 polyclonal antibody at a 1:100 dilution, and mouse anti-β-galactosidase monoclonal antibody (Promega) at 1:500 dilution at 4° for 16 hr. After extensive washing with PBS-T, the imaginal discs were incubated with Alexa488-anti-rabbit IgG and Alexa594-anti-rat IgG (Sigma, St. Louis) at a 1:200 dilution or an alkaline phosphatase-conjugated goat anti-rabbit IgG (Promega) at 1:500 dilution for 2 hr at room temperature. After extensive washing with PBS-T, the tissues were also stained with 0.5 μm TOTO3 (Molecular Probes, Eugene, OR) for 30 min. The tissues were washed with PBS and mounted in 90% glycerol, PBS for confocal microscopic observation (Radiance 2100; Bio-Rad, Richmond, CA) or observation with an Olympus BX-50 microscope.
Scanning electron microscopy:
Adult flies were anesthetized, mounted on stages, and observed under a Hitachi S-3000N scanning electron microscope in the low vacuum mode.
Histology of adult eyes:
Adult eyes were fixed in Bouin's fixative, embedded in paraffin, sectioned, and stained with Giemsa solution.
Labeling with 5-bromo-2′-deoxyuridine:
Detection of cells in S-phase was performed by a 5-bromo-2′-deoxyuridine (BrdU) labeling method as described previously with minor modifications (Wilder and Perrimon 1995). Third instar larvae cultured at 28° were dissected in Grace's insect medium and then incubated in the presence of 20 μg/ml BrdU (Boehringer Mannheim, Indianapolis) for 30 min. The samples were fixed in Carnoy's fixative (ethanol:acetic acids:chloroform, 6:3:1) for 15 min at 25° and further fixed in 80% ethanol, 50 mm glycine buffer, pH 2.0 at −20° for 12 hr. Incorporated BrdU was visualized using an anti-BrdU antibody and an alkaline phosphatase detection kit (Boehringer).
Apoptosis assay:
Third instar larvae were dissected in Drosophila Ringer's solution and imaginal discs were fixed in 4.0% paraformaldehyde in PBS for 30 min at room temperature. After washing with PBS, endogenous peroxidase activity was blocked by treatment with methanol containing 0.3% H2O2 at room temperature for 30 min. The samples were then washed with PBS and permeabilized by incubation in a solution containing 0.1% sodium citrate and 0.1% Triton X-100 on ice for 2 min. After extensive washing, the apoptosis assay was carried out using an in situ cell death detection kit (POD, Boehringer) according to the manufacturer's recommendations.
RESULTS AND DISCUSSION
The DDB complex is thought to recognize (6-4) photoproducts and other lesions in DNA and consists of a heterodimer of p127 large subunit (DDB1) and p48 small subunit (DDB2) (Feldberg 1980; Carew and Feldberg 1985; Hirschfeld et al. 1990; Keeney et al. 1993; Reardon et al. 1993; Payne and Chu 1994). DDB2 has roles in GGR but not in transcriptional coupled repair (TCR). Although DDB1 is evolutionarily conserved from yeast to mammals, its functions remain unclear. To generate a deeper comprehension of its nature we here chose D. melanogaster as an experimental system. In Drosophila, DDB1 appears to act as a repair factor, because D-DDB1 binds to UV-irradiated DNA and a D-DDB1 knocked down Kc cultured cells generated with a RNAi method are sensitive to UV (Takata et al. 2002). We also found that the D-DDB1 gene is controlled by the DRE/DREF system, which is responsible for activating the promoters of proliferation-related genes (Takata et al. 2002), suggesting an importance for development.
Here we finally succeeded for the first time in knocking down D-DDB1 in the Drosophila whole body with the RNAi method. Mutations of DDB2, also termed XPE in human, are known to cause human disorders (Chu and Chang 1988; Kataoka and Fujiwara 1991; Keeney et al. 1992; Itoh et al. 1999; Nichols et al. 2000).
Expression of the 650-bp dsRNA fragment of D-DDB1 in transgenic flies and knock down of D-DDB1 in Drosophila whole body cause lethality:
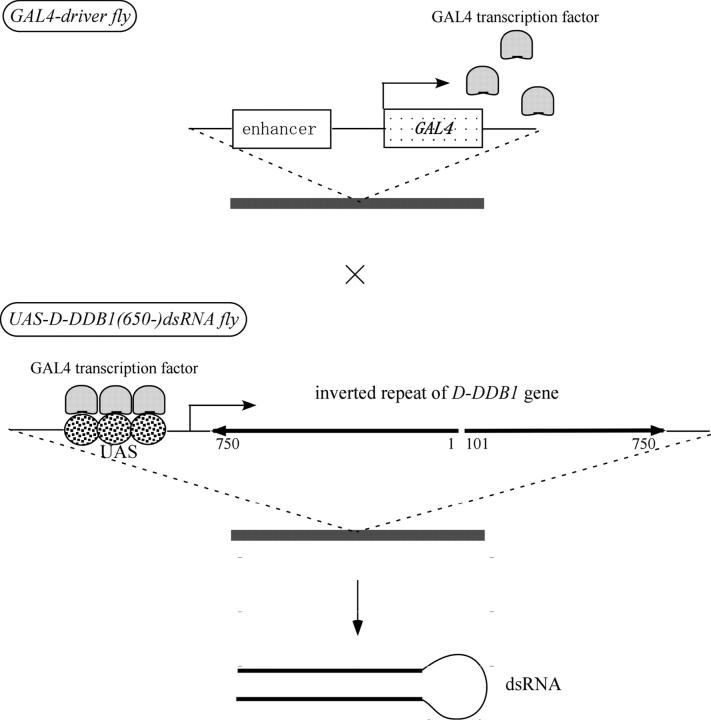
As shown in Figure 1, the 650-bp dsRNA fragment (from 101 to 750 bp of the ORF) of D-DDB1 was separated by using the D-DDB1 gene (from 1 to 100 bp of the ORF) sequence that acts as a spacer to give a hairpin loop-shaped RNA. Ectopic expression of the 650-bp dsRNA fragment of D-DDB1 (the D-DDB1 ORF is 3420 bp long) in living flies was performed using the GAL4-mediated expression system described in materials and methods. Lines with UAS-D-DDB1(650)-dsRNA transgenes were obtained with pUAST constructs according to standard procedures as described by Brand and Perrimon (1993). Four independent lines of germ-line transformants carrying UAS-D-DDB1(650)-dsRNA were established and used for the analysis. Established transgenic strains carrying UAS-D-DDB1(650)-dsRNA wild-type constructs and their chromosomal linkages are listed in materials and methods. Transgenic flies carrying UAS-D-DDB1(650)-dsRNA were then crossed with transgenic flies carrying GAL4 cDNA put under the control of the actin-specific enhancer-promoter (Act5C-GAL4 and Act25-GAL4), of the eye imaginal disc-specific promoter (ey-GAL4 and GMR-GAL4). A scheme of the heritable and inducible RNAi system is shown in Figure 1. There is no homology among the D-DDB1 ORFs (101–750 bp) used for RNAi and other Drosophila genes.
Figure 1.—
Schematic of the heritable and inducible RNAi system. Two transgenic fly stocks, GAL4-driver and UAS-D-DDB1(650)-dsRNA, are used in this system. The GAL4-driver fly used in this experiment has a transgene-conjugating yeast transcriptional factor GAL4. In this report, we used Act5C-GAL4 and Act25-GAL4 as a GAL4 driver to induce D-DDB1 gene silencing in all cells of the fly, ey-GAL4 and GMR-GAL4 to induce D-DDB1 gene silencing in the eye imaginal disc, and CgGAL4 to induce D-DDB1 gene silencing in the fat body. The UAS-D-DDB1(650)-dsRNA fly has a transgene containing the inverted repeat of the 650-bp dsRNA fragment (101–750 bp of the ORF) of D-DDB1 separated by the D-DDB1 gene (1–100 bp of the ORF) sequence ligated to the UAS promoter, a target of GAL4. In the F1 progeny of these flies, the 650-bp dsRNA of the target gene is expressed to induce the gene silencing.
Transgenic flies carrying single copies of Act5C-GAL4 and UAS-D-DDB1(650)-dsRNA [UAS-D-DDB1(650)-dsRNA/+;Act5C-GAL4/+] caused a striking phenotype with generation of melanotic tumors (Figure 2, A and B), which are thought to arise as a normal and heritable response to some form of abnormal development and form groups of cells that are recognized by the immune system and encapsulated in melanized cuticle (Watson et al. 1991). All transgenic flies died. Almost all transgenic flies died by the third instar larvae, after a 1- to 2-day delay in development. A few transgenic flies lived until early pupal stages (Figure 2B) but none grew to adults. Act25-GAL4/UAS-D-DDB1(650)-dsRNA showed the same phenotype. Note that no phenotypic differences were observed among four independent lines carrying UAS-D-DDB1(650)-dsRNA. No melanotic tumors or premature deaths were observed in control flies carrying a single copy of Act5C-GAL4 (+/+;Act5C-GAL4/+) (Figure 2, C and D). Biological activities of the D-DDB1 gene silencing during development were analyzed with transgenic flies of UAS-D-DDB1(650)-dsRNA/UAS-D-DDB1(650)-dsRNA and Act25-GAL4/Cyo,GFP. UAS-D-DDB1(650)-dsRNA/UAS-D-DDB1(650)-dsRNA transgenic flies were then crossed with Act25-GAL4/Cyo,GFP transgenic flies, and the numbers of F1 larvae of Act25-GAL4/UAS-D-DDB1(650)-dsRNA (RNAi active flies without GFP signal) and UAS-D-DDB1(650)-dsRNA/Cyo,GFP (control flies with GFP signal) were counted. About 70% of the RNAi active transgenic flies died by the third-instar larvae, compared with the control flies (Figure 2E). When they were crossed with the Act25-GAL4/Cyo,GFP transgenic flies and the wild type (+/+) flies, there was no significant difference between the number of Act25-GAL4/+ flies and Cyo,GFP/+ flies. The reason why the Act25-GAL4/UAS-D-DDB1(650)-dsRNA transgenic flies lived until the larval phase might be because of maternal loading of the factors (Takata et al. 2002). A line-inserted P element in the 5′ exon of D-DDB1, DDB1EY01408, is available (FlyBase ID: FBti0025194). Just the same, none of the DDB1EY01408/DDB1EY01408 flies grew to adults. Almost all of the DDB1EY01408/DDB1EY01408 flies died at the first instar larval stage, caused by melanotic tumors (Figure 2F). Reverting the lethality by excising the P element in the flies indicates that D-DDB1 must be an essential factor for development. The melanotic tumor develops not only in the RNAi knock-down flies (Figure 2A) but also in the P-element-insertion knock-out flies (Figure 2F).
Figure 2.—
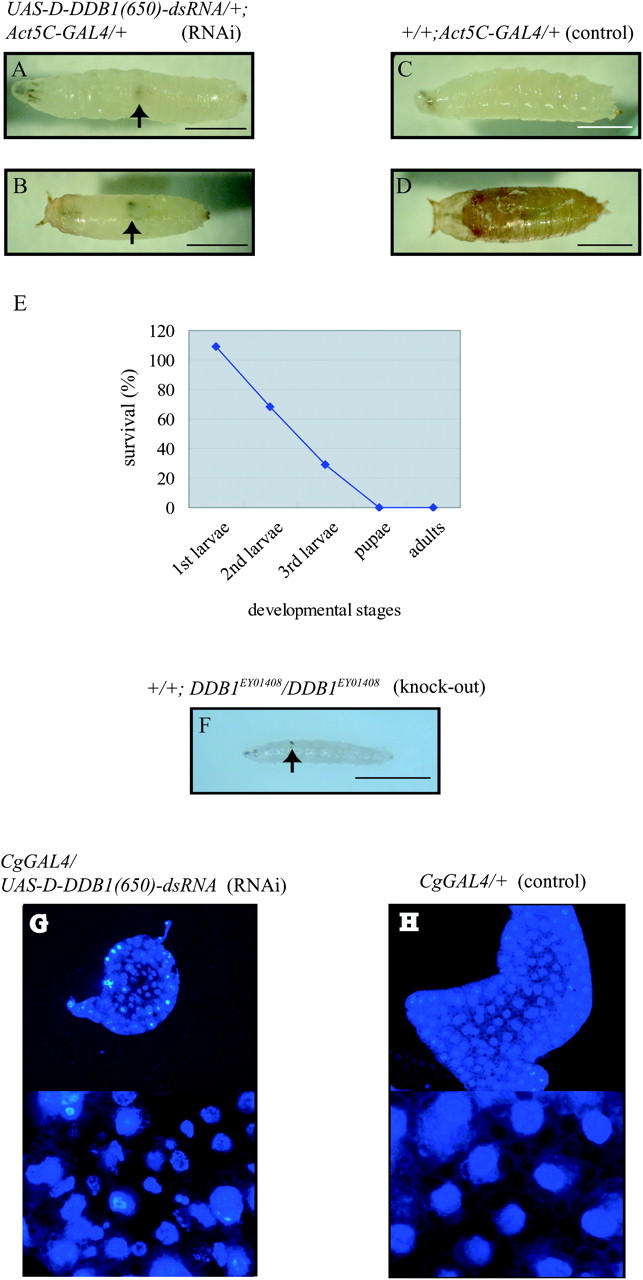
(A) Knock down of the D-DDB1 level by expression of the 650-bp D-DDB1 double-strand RNAs in living flies [UAS-D-DDB1(650)-dsRNA/+; Act5C-GAL4/+] caused melanotic tumors and almost all flies died in the larval stage. A few transgenic flies lived until early pupal stages (B) but none grew to adults. No melanotic tumors or early lethality was observed in control flies carrying a single copy of Act5C-GAL4 (+/+;Act5C/+) (C and D). (A and C) Third instar larvae; (B and D) pupae. (E) Lethality in the transgenic flies expressing D-DDB1(650)-dsRNA. UAS-D-DDB1(650)-dsRNA/UAS-D-DDB1(650)-dsRNA transgenic flies were crossed with Act25-GAL4/Cyo, GFP transgenic flies, and the numbers of F1 larvae of UAS-D-DDB1(650)-dsRNA/Act25-GAL4 (RNAi active flies without GFP signal) and UAS-D-DDB1(650)-dsRNA/Cyo,GFP (control flies with GFP signal) were counted. The values shown are the rate of the number of UAS-D-DDB1(650)-dsRNA/Act25-GAL4 flies divided by the number of UAS-D-DDB1(650)-dsRNA/Cyo,GFP flies. (F) The flies knocked out D-DDB1 showed melanotic tumors and almost all died at the first instar larval stage. (G and H) The DAPI staining images of the fat body from CgGAL4/UAS-D-DDB1(650)-dsRNA and CgGAL4/+. The bottoms of G and H show high-magnification views of the tops. The shape of nuclei in the CgGAL4/UAS-D-DDB1(650)-dsRNA fly (G) was abnormal as compared with control (H). Arrows indicate the position of the melanotic tumor. Bars, 0.5 mm.
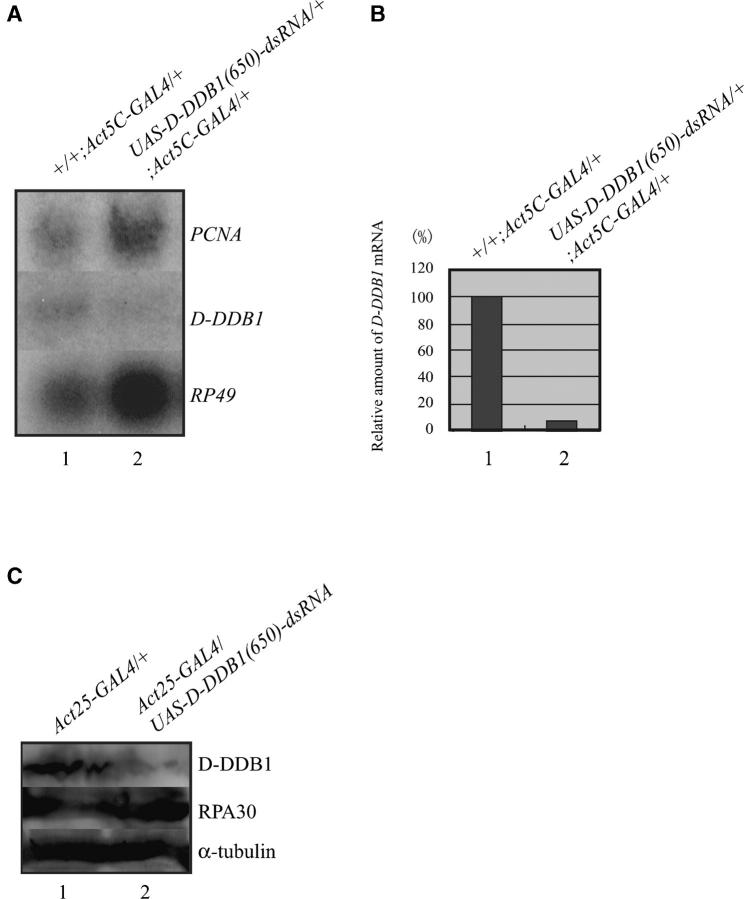
Next, expression of D-DDB1(650)-dsRNA in the transgenic animals was analyzed by Northern hybridization with tissue extracts. Living third instar larvae carrying a single copy of Act5C-GAL4 (+/+;Act5C-GAL4/+) and single copies of Act5C-GAL4 and UAS-D-DDB1(650)-dsRNA [UAS-D-DDB1(650)-dsRNA/+;Act5C-GAL4/+] were homogenized, and amounts of D-DDB1 transcripts in the total RNA extracts were determined with the D-DDB1 ORF (1774–3423 bp). As shown in Figure 3, the transcripts of D-DDB1 were reduced by 92.7% by RNAi compared with the amount of RP49 transcripts. As shown in Figure 3A, the amount of PCNA was not changed after inducing the D-DDB1 gene silencing. Like the D-DDB1 gene, PCNA gene is reportedly controlled by the DRE/DREF system (Yamaguchi et al. 1995a). The protein levels of D-DDB1 were also reduced and the amount of RPA30 was not changed by RNAi as compared with the amount of α-tubulin (Figure 3C). The results suggest that 650 bp of D-DDB1-dsRNA acts as a specific RNAi effector in vivo and that D-DDB1 is necessary for normal development. As shown in Figure 2, the knocked-down flies lived slightly longer than the knocked-out flies. The reason may be that the D-DDB1 gene is not completely degraded by the RNAi (Figure 3, A and B), and the D-DDB1 protein is tough to degrade. As described previously, Drosophila cultured Kc cells knocked down for D-DDB1 did not die completely, but became sensitive to UV (Takata et al. 2002). There is also a report that a DDB1 knocked-out strain (FZ150) of S. pombe does not always suffer mortality, although aberrant chromosome segregation is caused (Zolezzi et al. 2002). The nuclei of the fat body cells in transgenic flies carrying single copies of CgGAL4 and UAS-D-DDB1(650)-dsRNA [CgGAL4/UAS-D-DDB1(650)-dsRNA] were abnormal in shape as well as the phenotype of the S. pombe strain lacking DDB1 (Figure 2, G and H). The CgGAL4 transgenic fly can express GAL4 in the fat body cells and the circulating hemocytes (Asha et al. 2003). Aberrant chromosome segregation in the flies lacking D-DDB1 may result in reduced cell numbers and thus cause lethality in Drosophila. DDB1 is not necessarily an essential factor in a unicellular system, but has an essential role in the development of multicellular systems.
Figure 3.—
Effects of RNA interference (RNAi) in living flies. (A) The 650-bp D-DDB1 double-strand RNAs were expressed using the Act5C-GAL4 driver and Northern hybridization analysis was performed for total RNAs from third instar larvae carrying a single copy of Act5C-GAL4 (lane 1, +/+;Act5C-GAL4/+) or single copies of Act5C-GAL4 and UAS-D-DDB1(650)-dsRNA (lane 2, UAS-D-DDB1(650)-dsRNA/+;Act5C-GAL4/+). Total RNAs were separated on a 1.2% agarose-formaldehyde gel, transferred onto a nylon membrane, and probed with a random-primed 32P-labeled D-DDB1 ORF (1774–3420 bp). The blot was reprobed for the RP49 message as a loading control. (B) The transcripts of D-DDB1 were reduced to 7.3% by RNAi compared with the amount of RP49 transcripts. The amount of PCNA was not changed after inducing the D-DDB1 gene silencing. (C) D-DDB1, RPA30, and α-tubulin in an Act25-GAL4/+ fly (lane 1) and an Act25-GAL4/UAS-D-DDB1(650)-dsRNA fly (lane 2) were determined by immunoblotting. The protein levels of D-DDB1 were also reduced by RNAi.
Distribution and knock down of D-DDB1 in Drosophila eye imaginal discs:
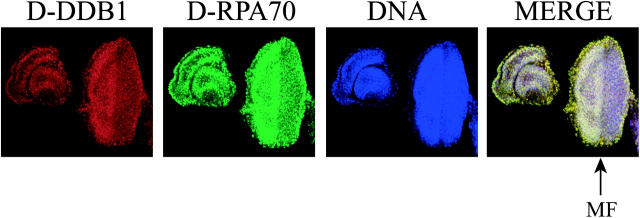
Melanotic masses in D. melanogaster are generally accompanied by overproliferation, premature differentiation, and aggregation of hemocytes (Dearolf 1998). Drosophila Act5C and Act25 promotion leads to death in the developmental stage and generation of melanotic tumors (Figure 2, A and B). Either the overproliferation or the premature differentiation could induce melanotic masses. In a wild-type eye disc, cells divide asynchronously, which occurs anterior to the MF (Figure 4). As they enter the furrow, they are arrested in G0/G1 phase and synchronously enter the last round of the mitotic cell cycle. Cell differentiation then occurs posterior to the MF (Figure 4). We here applied double labeling with anti-D-DDB1 and anti-D-RPA70 (70-kD subunit of Drosophila-replication protein A) antibodies, because there is a report that DDB mediates NER activity (Wakasugi et al. 2001) and because replication protein A (RPA) is known to be an indispensable factor for replication. Triple staining (D-DDB1 in red, DNA in blue, and D-RPA70 in green) showed D-DDB1 was abundant anterior to the MF (Figure 4), suggesting roles in cell proliferation.
Figure 4.—
Distribution of D-DDB1 in the eye imaginal disc. The anterior of the discs is on the left and the posterior is on the right. Triple labeling (D-DDB1 in red, DNA in blue, and D-RPA70 in green) shows, in merged views: red, D-DDB1; blue, DNA; green, D-RPA70; and yellow, D-DDB1 and D-RPA70. Arrows indicate the position of the morphogenetic furrow (MF). D-DDB1 is especially abundant anterior to the MF. Essentially, similar patterns were found for both D-DDB1 and D-RPA70.
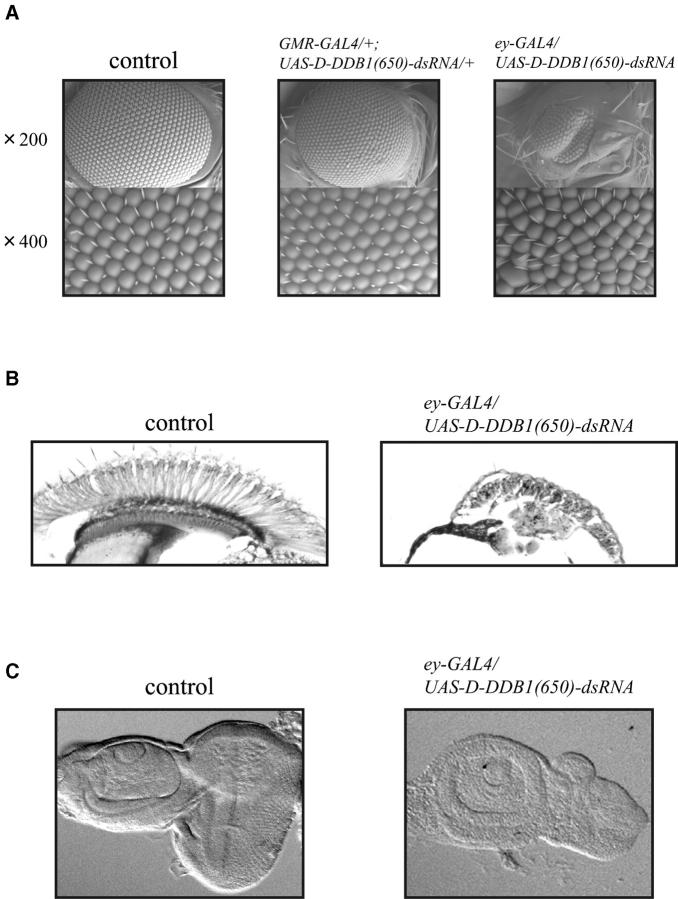
We next investigated effects on the eye imaginal disc using our transgenic flies. Knock down of D-DDB1 in the whole eye disc caused a severe rough eye phenotype but when posterior to the MF of eye disc caused no phenotype. In discs of larvae bearing one copy of ey-GAL4 and one copy of UAS-D-DDB1(650)-dsRNA [ey-GAL4/UAS-D-DDB1(650)-dsRNA] a severe rough eye phenotype, with irregular compound eyes and bristles (Figure 5A, right), was noted. Control flies bearing one copy of ey-GAL4 (ey-GAL4/+) exhibited a normal eye morphology. In discs of larvae bearing one copy of GMR-GAL4 and one copy of UAS-D-DDB1(650)-dsRNA [GMR-GAL4/+; UAS-D-DDB1(650)-dsRNA/+], no rough eye phenotype was observed (Figure 5A, middle). Flies carrying two copies of GMR-GAL4 and two copies of UAS-D-DDB1(650)-dsRNA [GMR-GAL4/GMR-GAL4; UAS-D-DDB1(650)-dsRNA/UAS-D-DDB1(650)-dsRNA] also exhibited normal eye morphology (data not shown). The normal compound eye has regular ommatidia and bristles (Figure 5A, left). Horizontal sections of eyes of adult flies also showed the D-DDB1 gene silencing-induced eye degeneration (Figure 5B). We next observed effects on the eye imaginal disc of transgenic flies. The eye imaginal disc of ey-GAL4/UAS-D-DDB1(650)-dsRNA was smaller than that of wild type (Figure 5C). The results also indicate that D-DDB1 has a function in cell proliferation so the D-DDB1 gene silencing caused small eye imaginal disc morphology.
Figure 5.—
(A) Scanning electron micrographs of adult compound eyes. (Control) Compound eye of Canton-S. (GMR-GAL4/+;UAS-D-DDB1(650)-dsRNA/+) Knock down of D-DDB1 posterior to the morphogenetic furrow (MF) of the eye disc resulted in a normal eye morphology. (ey-GAL4/UAS-D-DDB1(650)-dsRNA) In contrast, knock down of D-DDB1 in the whole eye disc caused a severe rough eye phenotype. Negative control flies of ey-GAL4/+and GMR-GAL4/+;+/+ have no difference in phenotype compared with the wild-type (Canton-S) compound eye. Top parts are at 200× and bottom parts are at 800× magnification. (B) Horizontal sections of adult Drosophila eyes. Canton-S (left) and ey-GAL4/UAS-D-DDB1(650)-dsRNA transgenic flies (right) are shown. (C) The D-DDB1 gene silencing in the whole eye disc [ey-GAL4/UAS-D-DDB1(650)-dsRNA] caused a shrunken eye disc phenotype.
D-DDB1 gene silencing induces an ectopic S phase posterior to the MF, causes apoptosis, and interferes with photoreceptor differentiation:
Imaginal discs were pulse labeled with BrdU for 30 min in vitro and stained with an anti-BrdU antibody (Figure 6A). No significant difference between control and D-DDB1 gene-silencing discs was observed in the region anterior to the MF. D-DDB1 gene silencing induced an extra S phase in some cells that are normally postmitotic. In addition, some cells in the region more posterior to the MF, where commitment to a neuronal fate occurs and is normally followed by differentiation into specific cells such as photoreceptors, were labeled with BrdU (Figure 6A, right).
Figure 6.—
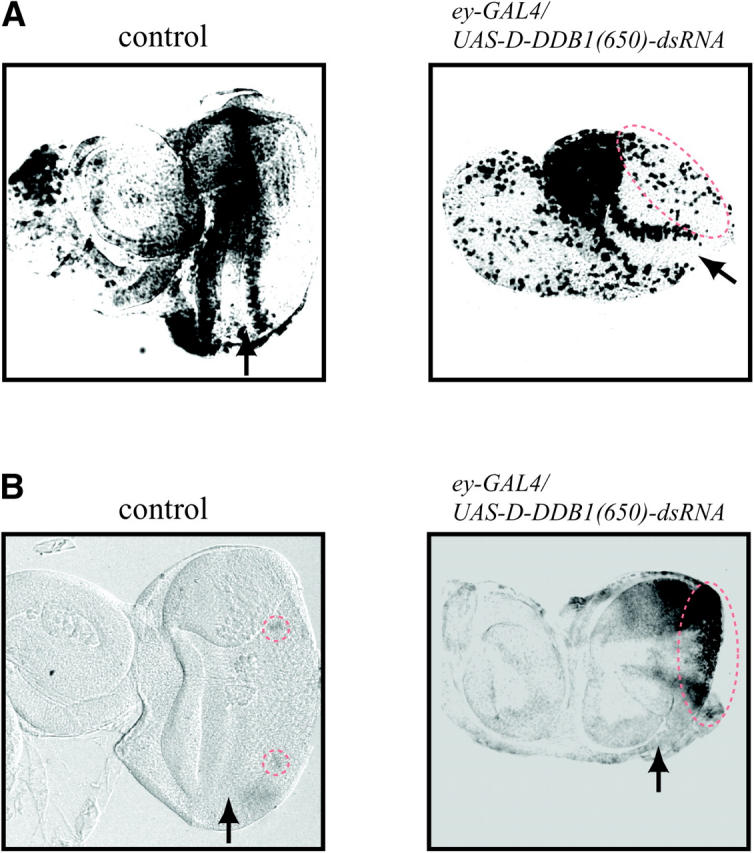
(A) DNA synthesis. Patterns of BrdU incorporation in eye imaginal discs. Left, ey-GAL4/+; right, ey-GAL4/UAS-D-DDB1(650)-dsRNA. The eye disc was stained with an anti-BrdU antibody. Arrows indicate the position of the MF. The anterior of the discs is on the left. The region of an extra S phase in some cells that are normally postmitotic is indicated by a dotted line. (B) Apoptosis. Detection of apoptotic cells in eye imaginal discs. Left, ey-GAL4/+; right, ey-GAL4/UAS-D-DDB1(650)-dsRNA. The apoptosis assay was carried out with terminal deoxynucleotidyl transferase. The anterior of the discs is on the left. Arrows indicate the position of the MF. The apoptotic cells are indicated by a dotted line.
Failure of normal cell cycle progression and disturbance of differentiation processes are known to cause apoptosis (Harrington et al. 1994). For example, it has been reported that overexpression of dE2F and dDP in eye imaginal discs using a GMR promoter induces apoptosis and that this counterbalances cells that enter an abnormal S phase (Du et al. 1996). We investigated whether D-DDB1 gene silencing can induce apoptosis in eye imaginal disc cells or not. In wild-type discs of third instar larvae, there were very few apoptotic cells (Figure 6B, left). In contrast, staining of eye imaginal discs from transgenic flies expressing 650 bp dsRNA of D-DDB1 revealed apoptotic cells to be significantly increased in the region posterior to the MF (Figure 6B, right). The apoptosis by D-DDB1 gene silencing could also produce the morphologically small eye in Figure 5, A and C. Apoptosis seemed to begin in the imaginal disc cells in the region where late commitment to photoreceptor cells takes place, suggesting that failure of differentiation might induce apoptosis. Therefore, we analyzed the effect of D-DDB1 gene silencing on photoreceptor specification. In wild-type discs, developmentally uncommitted cells are sequentially recruited into clusters that comprise ommatidial precursors. Cluster formation is first observed within the MF, where cells are in G1. Cells either leave the cell cycle and differentiate or undergo a final synchronous round of cell division. Overt ommatidial organization starts in the MF when cells are grouped into equally spaced concentric aggregates, which convert into preclusters. Photoreceptor cells have been found to develop in stereotyped order: R8 is generated first, with movement posterior from the MF, then cells are added pairwise (R2 and R5, R3 and R4, and R1 and R6), and R7 is the last photoreceptor to be added to each cluster. We used three enhancer trap lines, X63 (inserted in rhomboid; Figure 7, top), D120 (inserted in scabrous; Figure 7, middle) and BB02 (inserted in rhomboid; Figure 7, bottom), specifically expressing the nucleus-localized form of E. coli β-galactosidase marker in photoreceptor cells (R) of R2/R5/R8, early R8, and late R8, respectively. The imaginal discs from F1 larvae generated by mating of enhancer trap lines and 650 bp dsRNA of D-DDB1-expressing transgenic flies [ey-GAL4,UAS-D-DDB1(650)-dsRNA/+;rhomboid-lacZ/+] were immunohistochemically stained with anti-β-galactosidase antibody. In the ommatidia of 650 bp dsRNA of D-DDB1-expressing animals, positive nuclei of late R2/R5/R8, early R8, and late R8 were fewer than in the control case (Figure 7, a and c, top, middle, and bottom), and an abnormal staining pattern was apparent (Figure 7, b and d, top, middle, and bottom). The results indicate that D-DDB1 gene silencing inhibits the differentiation of photoreceptor cells.
Figure 7.—
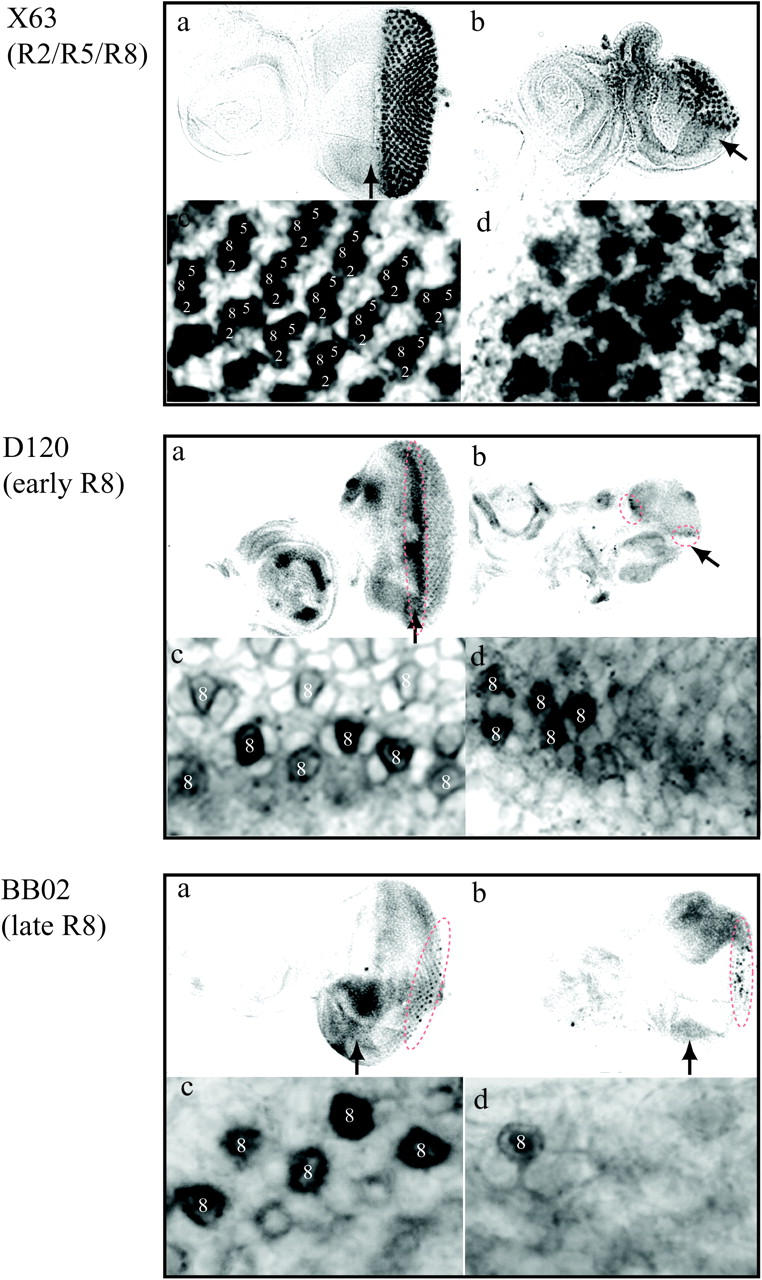
Immunostaining of eye imaginal discs with anti-β-galactosidase antibody. The anterior of the discs is on the left. Arrows indicate the position of the MF. Wild-type (a and c) or ey-GAL4,UAS-D-DDB1(650)-dsRNA/+ transgenic (b and d) flies were crossed with an enhancer trap line carrying X63 (inserted in rhomboid), D120 (inserted in scabrous), and BB02 (inserted in rhomboid), specifically expressing the β-galactosidase marker in photoreceptor cells of R2/R5/R8, early R8, and late R8, respectively, and F1 larvae were immunohistochemically stained with the anti-β-galactosidase antibody. c and d show high-magnification basal views of the same focal planes as a and b, respectively. The differentiated cells are indicated by a dotted line (a and b in D120 and a and b in BB02).
The S. pombe strain lacking ddb1 has slow growth due to delayed replication progression. Flow-cytometric analysis shows an extensive heterogeneity in DNA content. A large number of cells specifically displayed DNA content intermediate between 1N and 2N, which may represent slow replication (Bondar et al. 2003). While it may be due in part to defective chromosomal segregation in the Δddb1 strain, as reported (Zolezzi et al. 2002), aberrant S-phase progression also could explain the heterogeneous DNA distribution. Thus DDB1 appears to have roles in cell proliferation, especially relevant to replication progression and mitosis. Because differentiation is correlated with modification of cell cycle processes, D-DDB1 gene silencing may cause inhibition of differentiation due to accumulation of ectopic S-phase cells posterior to the MF. Although GMR-GAL4/+;UAS-D-DDB1(650)-dsRNA/+ transgenic flies showed normal phenotype, the results suggest that the D-DDB1 protein may be stable and left, that abnormal cells in mitosis were more accumulated in the ey-GAL4/UAS-D-DDB1(650)-dsRNA transgenic fly than in the GMR-GAL4/+;UAS-D-DDB1(650)-dsRNA/+ transgenic fly, and that the D-DDB1 gene silencing caused inhibition of cell proliferation and differentiation by accumulating abnormal mitotic cells. In the eye disc of the ey-GAL4/UAS-D-DDB1(650)-dsRNA transgenic fly, the cell cycle synchronization for the cluster formation in the MF may be disturbed. If synchronized, the cells are arrested at G1 in the MF. We used a D120 enhancer trap line (inserted in scabrous) to confirm the possibility. The number of the early photoreceptor cells (early R8) immediately after MF was decreased in the ey-GAL4,UAS-D-DDB1(650)-dsRNA/scabrous-lacZ transgenic fly (Figure 7, middle). The other photoreceptor cell (R1–R7) differentiation begins after the R8 cell differentiation. Shortage of the early R8 cells can lead to abnormal differentiation of the cells from R1 to R7 (Figure 7). Failure of all differentiation might induce apoptosis (Figure 6B, right). This might be one of the reasons that the ey-GAL4/UAS-D-DDB1(650)-dsRNA transgenic fly shows a more severe phenotype such as rough eye than the GMR-GAL4/+;UAS-D-DDB1(650)-dsRNA/+ transgenic fly does. According to the studies using yeast (Zolezzi et al. 2002; Bondar et al. 2003), the shortage of DDB1 causes abnormal cell division (Figure 2G). The results showed that cell proliferation is required before differentiation occurs, and aberrant cell proliferation must cause abnormal differentiation. D-DDB1 must be required for normal cell differentiation.
The rough eye phenotype was more strongly expressed in the line [ey-GAL4,UAS-D-DDB1(650)-dsRNA/UAS-P35] that was crossed between the transgenic fly expressing 650 bp dsRNA of D-DDB1 and the fly expressing the baculovirus caspase inhibitor P35 protein, which is an inhibitor of cell death (Figure 8H). Figure 8, A–D, showed the normal eyes of the wild-type (+/+), UAS-D-DDB1(650)-dsRNA/+, ey-GAL4/UAS-P35, and DDB1EY01408/+ flies, respectively. As compared with the eyes in Figure 8, A–D, the eyes of ey-GAL4,UAS-D-DDB1(650)-dsRNA/+ (Figure 8E) looked more severely abnormal. ey-GAL4,UAS-D-DDB1(650)-dsRNA/+;DDB1EY01408/+ (Figure 8F) showed a more severe rough eye phenotype than the eyes in Figure 8E, meaning that D-DDB1 gene silencing certainly occurs in ey-GAL4/UAS-D-DDB1(650)-dsRNA. On the other hand, the eyes of mei-9L1/+;ey-GAL4,UAS-D-DDB1(650)-dsRNA/+ looked same as the eyes of ey-GAL4,UAS-D-DDB1(650)-dsRNA/+ (data not shown). The XPF homolog mei-9 is implicated in nucleotide excision repair. Reduction of the XPF protein cannot induce the abnormality on the phenotype by D-DDB1 gene silencing. The results also indicate that DDB1 has more important roles in the events other than XPF-related repair. As shown in Figure 8G, when the fly carried two copies of ey-GAL4 and two copies of UAS-D-DDB1(650)-dsRNA [ey-GAL4,UAS-D-DDB1(650)-dsRNA/ey-GAL4,UAS-D-DDB1(650)-dsRNA], the fly lacked the head and died at the pupa stage. Since Eyeless protein is known to have roles in Drosophila head development (Benassayag et al. 2003), D-DDB1 must function in brain development. Therefore, we speculate that mouse knocked-out DDB1 may lack the brain and subsequently die at the early embryo stage. In a mouse knocked out the genes of XPA and CSB (XPA−/−CSB−/−), the cerebellum was remarkably smaller but did not lack the brain (Murai et al. 2001). These results suggested that the function of DDB1 was different from the other XP-related proteins. A few reports showed that the abnormal phenotype occurred when baculovirus P35 protein and dE2F/dDP were simultaneously expressed (Du et al. 1996; Staehling-Hampton et al. 1999). This indicated that the majority of cells ectopically entering S phase as a result of dE2F/dDP expression are eliminated by apoptosis; this phenotype resembles those in Figure 8H. The abnormal mitotic cells caused by D-DDB1 gene silencing interfere with normal differentiation, and consequently these abnormal cells are eliminated by apoptosis. Although coexpression of P35 appears to severely decrease the number of cells, the normal cells are few, and the unusual cells remain without being removed.
Figure 8.—
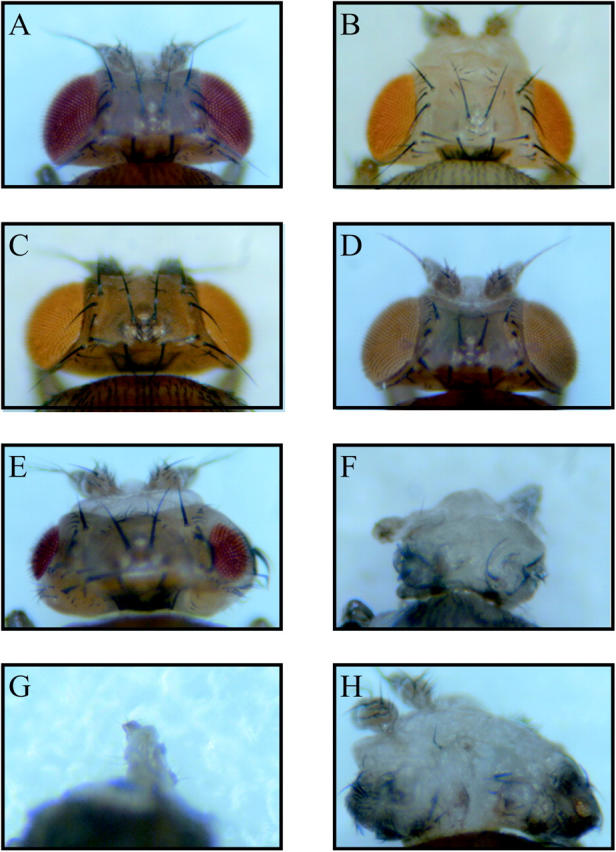
The sensitivity of the ey-GAL4/UAS-D-DDB1(650)-dsRNA transgenic flies phenotype was enhanced by UAS-P35. (A) wild-type fly (+/+); (B) UAS-D-DDB1(650)-dsRNA/+; (C) ey-GAL4/UAS-P35; (D) DDB1EY01408/+; (E) ey-GAL4,UAS-D-DDB1(650)-dsRNA/+; (F) ey-GAL4,UAS-D-DDB1(650)-dsRNA/+;DDB1EY01408/+; (G) ey-GAL4,UAS-D-DDB1(650)-dsRNA/ey-GAL4,UAS-D-DDB1(650)-dsRNA; (H) ey-GAL4,UAS-D-DDB1(650)-dsRNA/UAS-P35. The P35, anti-apoptotic protein strongly enhances the the D-DDB1 gene silencing phenotype. Note that the fly carrying two copies of ey-GAL4 and two copies of UAS-D-DDB1(650)-dsRNA showed no head and died at the pupa stage. (G) The content of the dissected pupa.
One of the functions of DDB1 might be not only in DNA repair but also in development. S. pombe DDB1 is reportedly linked to the replication checkpoint control gene cds1, and the S. pombe strain lacking DDB1 grows slowly because of prolongation of S phase (Bondar et al. 2003). Aberrant chromosome segregation also occurs in the S. pombe strain lacking DDB1 (Zolezzi et al. 2002). DDB1 possibly has a role in chromosome segregation. As in S. pombe DDB1, we showed that D-DDB1 has an important role in cell proliferation. Since cell proliferation is required before cell differentiation occurs, Drosophila eye differentiation must be closely linked to the cell cycle machinery using D-DDB1.
Recently, the evidence that not only DDB2 but also CSA can bind directly to DDB1 was reported. Furthermore, like SCF complex and other cullin-based ubiquitin ligases, the DDB1 complex containing DDB2 or CSA has Cul4A and Roc1. DDB1 is part of a cullin-containing E3 ligase complex (Groisman et al. 2003). Since both DDB2 and CSA contain WD40 domains, the other proteins with the WD40 domain may also be able to bind to DDB1. On the other hand, the D-DDB1 gene was highly conserved in yeast to mammals, and the genes of DDB2 and CSA were still not found in the genome of D. melanogaster.
To conclude, this report documents the first observation of an altered phenotype in a multicellular organism knocked down or knocked out for DDB1. The results indicate that, different from the unicellular system case, DDB1 is an essential gene in multicellular organisms, playing important roles in development.
Acknowledgments
We are grateful to Fumiko Hirose of Himeji Institute of Technology, Yoshihiro H. Inoue and Kyoko Otsuki of the University of Kyoto Institute of Technology, Richard D. Wood of University of Pittsburgh Cancer Institute, Yoko Ogasawara of Tokyo University of Science, Kaori Shimanouchi and Shizuka Murakami of our laboratory, and Malcolm Moore for technical assistance, valuable comments, and helpful discussion. We are also grateful to Yasushi Hiromi of the National Institute of Genetics for fly stocks.
References
- Aboussekhra, A., and R. D. Wood, 1995. Detection of nucleotide excision repair incisions in human fibroblasts by immunostaining for PCNA. Exp. Cell Res. 221: 326–332. [DOI] [PubMed] [Google Scholar]
- Asha, H., I. Nagy, G. Kovacs, D. Stetson, I. Ando et al., 2003. Analysis of ras-induced overproliferation in Drosophila hemocytes. Genetics 163: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benassayag, C., S. Plaza, P. Callaerts, J. Clements, Y. Romeo et al., 2003. Evidence for a direct functional antagonism of the selector genes proboscipedia and eyeless in Drosophila head development. Development 130: 575–586. [DOI] [PubMed] [Google Scholar]
- Bondar, T., E. V. Mirkin, D. S. Ucker, W. E. Walden, S. M. Mirkin et al., 2003. S. pombe Ddb1 is functionally linked to the replication checkpoint pathway. J. Biol. Chem. 278: 37006–37014. [DOI] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Butel, J. S., T. H. Lee and B. L. Slagle, 1995. Viral co-factors in liver cancer: lessons from hepatitis B virus. Princess Takamatsu Symp. 25: 185–198. [PubMed] [Google Scholar]
- Carew, J. A., and R. S. Feldberg, 1985. Recognition of a cytosine base lesion by a human damage-specific DNA binding protein. Nucleic Acids Res. 13: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, G., and E. Chang, 1988. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science 242: 564–567. [DOI] [PubMed] [Google Scholar]
- Datta, A., S. Bagchi, A. Nag, P. Shiyanov, G. R. Adami et al., 2001. The p48 subunit of the damaged-DNA binding protein DDB associates with the CBP/p300 family of histone acetyltransferase. Mutat. Res. 486: 89–97. [DOI] [PubMed] [Google Scholar]
- Dearolf, C. R., 1998. Fruit fly “leukemia”. Biochim. Biophys. Acta 1377: M13–M23. [DOI] [PubMed] [Google Scholar]
- Du, W., J. E. Xie and N. Dyson, 1996. Ectopic expression of dE2F and dDP induces cell proliferation and death in the Drosophila eye. EMBO J. 15: 3684–3692. [PMC free article] [PubMed] [Google Scholar]
- Feldberg, R. S., 1980. On the substrate specificity of a damage-specific DNA binding protein from human cells. Nucleic Acids Res. 8: 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman, R., J. Polanowska, I. Kuraoka, J. Sawada, M. Saijo et al., 2003. The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell 113: 357–367. [DOI] [PubMed] [Google Scholar]
- Harrington, E. A., M. R. Bennett, A. Fanidi and G. I. Evan, 1994. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 13: 3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, S., P. Shiyanov, X. Chen and P. Raychaudhuri, 1998. DDB, a putative DNA repair protein, can function as a transcriptional partner of E2F1. Mol. Cell. Biol. 18: 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld, S., A. S. Levine, K. Ozato and M. Protic, 1990. A constitutive damage-specific DNA-binding protein is synthesized at higher levels in UV-irradiated primate cells. Mol. Cell. Biol. 10: 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, B. J., J. M. Ford, P. C. Hanawalt and G. Chu, 1999. Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc. Natl. Acad. Sci. USA 96: 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, T., T. Mori, H. Ohkubo and M. Yamaizumi, 1999. A newly identified patient with clinical xeroderma pigmentosum phenotype has a non-sense mutation in the DDB2 gene and incomplete repair in (6–4) photoproducts. J. Invest. Dermatol. 113: 251–257. [DOI] [PubMed] [Google Scholar]
- Kataoka, H., and Y. Fujiwara, 1991. UV damage-specific DNA-binding protein in xeroderma pigmentosum complementation group E. Biochem. Biophys. Res. Commun. 175: 1139–1143. [DOI] [PubMed] [Google Scholar]
- Kazantsev, A., D. Mu, A. F. Nichols, X. Zhao, S. Linn et al., 1996. Functional complementation of xeroderma pigmentosum complementation group E by replication protein A in an in vitro system. Proc. Natl. Acad. Sci. USA 93: 5014–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney, S., H. Wein and S. Linn, 1992. Biochemical heterogeneity in xeroderma pigmentosum complementation group E. Mutat. Res. 273: 49–56. [DOI] [PubMed] [Google Scholar]
- Keeney, S., G. J. Chang and S. Linn, 1993. Characterization of a human DNA damage binding protein implicated in xeroderma pigmentosum E. J. Biol. Chem. 268: 21293–21300. [PubMed] [Google Scholar]
- Krishnamoorthy, R. R., T. H. Lee, J. S. Butel and H. K. Das, 1997. Apolipoprotein B gene regulatory factor-2 (BRF-2) is structurally and immunologically highly related to hepatitis B virus X associated protein-1 (XAP-1). Biochemistry 36: 960–969. [DOI] [PubMed] [Google Scholar]
- Lightfoot, K., L. Maltby, R. Duarte, R. Veale and O. Segev, 1994. Conserved cis-elements bind a protein complex that regulates Drosophila ras2/rop bidirectional expression. Br. J. Cancer 69: 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W., A. F. Nichols, J. A. Graham, R. Dualan, A. Abbas et al., 2000. Nuclear transport of human DDB protein induced by ultraviolet light. J. Biol. Chem. 275: 21429–21434. [DOI] [PubMed] [Google Scholar]
- Martinez, E., V. B. Palhan, A. Tjernberg, E. S. Lymar, A. M. Gamper et al., 2001. Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo. Mol. Cell. Biol. 21: 6782–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, D., C. H. Park, T. Matsunaga, D. S. Hsu, J. T. Reardon et al., 1995. Reconstitution of human DNA repair excision nuclease in a highly defined system. J. Biol. Chem. 270: 2415–2418. [DOI] [PubMed] [Google Scholar]
- Murai, M., Y. Enokido, N. Inamura, M. Yoshino, Y. Nakatsu et al., 2001. Early postnatal ataxia and abnormal cerebellar development in mice lacking Xeroderma pigmentosum group A and Cockayne syndrome group B DNA repair genes. Proc. Natl. Acad. Sci. USA 98: 13379–13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, A. F., T. Itoh, J. A. Graham, W. Liu, M. Yamaizumi et al., 2000. Human damage-specific DNA-binding protein p48. Characterization of XPE mutations and regulation following UV irradiation. J. Biol. Chem. 275: 21422–21428. [DOI] [PubMed] [Google Scholar]
- Ohno, K., F. Hirose, K. Sakaguchi, Y. Nishida and A. Matsukage, 1996. Transcriptional regulation of the Drosophila CycA gene by the DNA replication-related element (DRE) and DRE binding factor (DREF). Nucleic Acids Res. 24: 3942–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, A., and G. Chu, 1994. Xeroderma pigmentosum group E binding factor recognizes a broad spectrum of DNA damage. Mutat. Res. 310: 89–102. [DOI] [PubMed] [Google Scholar]
- Rapic-Otrin, V., M. P. Mclenigan, D. C. Bisi, M. Gonzalez and A. S. Levine, 2002. Sequential binding of UV DNA damage binding factor and degradation of the p48 subunit as early events after UV irradiation. Nucleic Acids Res. 30: 2588–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon, J. T., A. F. Nichols, S. Keeney, C. A. Smith, J. S. Taylor et al., 1993. Comparative analysis of binding of human damaged DNA-binding protein (XPE) and Escherichia coli damage recognition protein (UvrA) to the major ultraviolet photoproducts: T[c,s]T, T[t,s]T, T[6–4]T, and T[Dewar]T. J. Biol. Chem. 268: 21301–21308. [PubMed] [Google Scholar]
- Robertson, H. M., C. R. Preston, R. W. Phillis, D. M. Johnson-Schlitz, W. K. Benz et al., 1988. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, J. R., T. Y. Choi, E. J. Kwon, W. H. Lee, Y. Nishida et al., 1997. Transcriptional regulation of the Drosophila-raf proto-oncogene by the DNA replication-related element (DRE)/DRE-binding factor (DREF) system. Nucleic Acids Res. 25: 794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiyanov, P., S. A. Hayes, M. Donepudi, A. F. Nichols, S. Linn et al., 1999. a The naturally occurring mutants of DDB are impaired in stimulating nuclear import of the p125 subunit and E2F1-activated transcription. Mol. Cell. Biol. 19: 4935–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiyanov, P., A. Nag and P. Raychaudhuri, 1999. b Cullin 4A associates with the UV-damaged DNA-binding protein DDB. J. Biol. Chem. 274: 35309–35312. [DOI] [PubMed] [Google Scholar]
- Spradling, A. C., and G. M. Rubin, 1982. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218: 341–347. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton, K., P. J. Ciampa, A. Brook and N. Dyson, 1999. A genetic screen for modifiers of E2F in Drosophila melanogaster. Genetics 153: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y., M. Yamaguchi, F. Hirose, S. Cotterill, J. Kobayashi et al., 1996. DNA replication-related elements cooperate to enhance promoter activity of the drosophila DNA polymerase alpha 73-kDa subunit gene. J. Biol. Chem. 271: 14541–14547. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y., F. Hirose, A. Matsukage and M. Yamaguchi, 1999. Identification of three conserved regions in the DREF transcription factors from Drosophila melanogaster and Drosophila virilis. Nucleic Acids Res. 27: 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata, K., H. Yoshida, F. Hirose, M. Yamaguchi, M. Kai et al., 2001. Drosophila mitochondrial transcription factor A: characterization of its cDNA and expression pattern during development. Biochem. Biophys. Res. Commun. 287: 474–483. [DOI] [PubMed] [Google Scholar]
- Takata, K., G. Ishikawa, F. Hirose and K. Sakaguchi, 2002. Drosophila damage-specific DNA-binding protein 1 (D-DDB1) is controlled by the DRE/DREF system. Nucleic Acids Res. 30: 3795–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata, K., Y. H. Inoue, F. Hirose, S. Murakami, K. Shimanouchi et al., 2003. Spatio-temporal expression of Drosophila mitochondrial transcription factor A during development. Cell Biol. Int. 27: 361–374. [DOI] [PubMed] [Google Scholar]
- Towbin, H., T. Staehelin and J. Gordon, 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76: 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi, M., M. Shimizu, H. Morioka, S. Linn, O. Nikaido et al., 2001. Damaged DNA-binding protein DDB stimulates the excision of cyclobutane pyrimidine dimers in vitro in concert with XPA and replication protein A. J. Biol. Chem. 276: 15434–15440. [DOI] [PubMed] [Google Scholar]
- Watson, K. L., T. K. Johnson and R. E. Denell, 1991. Lethal(1) aberrant immune response mutations leading to melanotic tumor formation in Drosophila melanogaster. Dev. Genet. 12: 173–187. [DOI] [PubMed] [Google Scholar]
- Wilder, E. L., and N. Perrimon, 1995. Dual functions of wingless in the Drosophila leg imaginal disc. Development 121: 477–488. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M., Y. Hayashi, Y. Nishimoto, F. Hirose and A. Matsukage, 1995. a A nucleotide sequence essential for the function of DRE, a common promoter element for Drosophila DNa replication-related genes. J. Biol. Chem. 270: 15808–15814. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M., F. Hirose, Y. Nishimoto, T. Naruge, M. Ikeda et al., 1995. b Expression patterns of DNA replication enzymes and the regulatory factor DREF during Drosophila development analyzed with specific antibodies. Biol. Cell 85: 147–155. [DOI] [PubMed] [Google Scholar]
- Zolezzi, F., J. Fuss, S. Uzawa and S. Linn, 2002. Characterization of a Schizosaccharomyces pombe strain deleted for a sequence homologue of the human damaged DNA binding 1 (DDB1) gene. J. Biol. Chem. 277: 41183–41191. [DOI] [PubMed] [Google Scholar]