Abstract
Thiamine (vitamin B1) is required in the diet of animals, and thiamine deficiency leads to diseases such as beri-beri and the Wernicke-Korsakoff syndrome. Dietary thiamine (vitamin B1) consists mainly of thiamine pyrophosphate (TPP), which is transformed into thiamine by gastrointestinal phosphatases before absorption. It is believed that TPP itself cannot be transported across plasma membranes in significant amounts. We have identified a partial loss-of-function mutation in the Caenorhabditis elegans gene (tpk-1) that encodes thiamine pyrophosphokinase, which forms TPP from thiamine at the expense of ATP inside cells. The mutation slows physiological rhythms and the phenotype it produces can be rescued by TPP but not thiamine supplementation. tpk-1 functions cell nonautonomously, as the expression of wild-type tpk-1 in one tissue can rescue the function of other tissues that express only mutant tpk-1. These observations indicate that, in contrast to expectation from previous evidence, TPP can be transported across cell membranes. We also find that thiamine supplementation partially rescues the phenotype of partial loss-of-function mutants of the Na/K ATPase, providing genetic evidence that thiamine absorption, and/or redistribution from the absorbing cells, requires the full activity of this enzyme.
VITAMIN B1 (thiamine), in the form of thiamine pyrophosphate (TPP), is necessary for oxidative phosphorylation and the pentose phosphate pathway by acting as a cofactor for α-ketoacid dehydrogenases such as pyruvate dehydrogenase (PDH), α-ketoglutarate dehydrogenase (KGDH), branched-chain α-ketoacid dehydrogenase, and transketolase (reviewed in Hohmann and Meacock 1998). Animals, including humans, cannot synthesize thiamine but extract it from a food source via intestinal absorption. Malnutrition or poor absorption of thiamine causes a deficiency that results in polyneuropathy, cardiomyopathy, disturbances of the blood-brain barrier, and metabolic acidosis. Well-known diseases caused by thiamine deficiency include beri-beri and the Wernicke-Korsakoff syndrome (reviewed in Singleton and Martin 2001).
Dietary thiamine consists mainly of TPP, but in the intestinal lumen it is converted to thiamine by gastrointestinal phosphatases (Rindi and Laforenza 2000). Previous studies in humans, rats, and other animal species have revealed a dual mechanism for thiamine absorption in intestinal tissue (Hoyumpa et al. 1982; Rindi and Laforenza 2000) as well as in other tissues such as liver (Moseley et al. 1992), kidney (Gastaldi et al. 2000), erythrocytes (Casirola et al. 1990), and neuroblastoma cells (Bettendorff 1995). At low physiological concentrations, thiamine is transported via a saturable, carrier-mediated process involving electroneutral thiamine/H+ antiport (reviewed in Rindi and Laforenza 2000). At high concentrations, some studies suggest that simple diffusion of thiamine prevails (Hoyumpa et al. 1982; Rindi and Laforenza 2000), while others point to carrier-mediated absorption (Moseley et al. 1992; Bettendorff and Wins 1994).
Recently, two closely related thiamine transporters have been identified, ThTr-1 (SLC19A2; Diaz et al. 1999; Fleming et al. 1999; Labay et al. 1999) and ThTr-2 (SLC19A3; Eudy et al. 2000; Rajgopal et al. 2001; Said et al. 2004). The high-affinity carrier ThTr-1 is widely expressed in human tissues but is most abundant in skeletal muscle, followed by heart, placenta, kidney, and liver. Patients lacking the ThTr-1 transporter suffer from thiamine-responsive megaloblastic anemia syndrome characterized by diabetes mellitus, megaloblastic anemia, and sensorineural deafness (Diaz et al. 1999; Fleming et al. 1999; Labay et al. 1999). Most of these symptoms can be reversed by administration of large amounts of thiamine.
The other high-affinity carrier, ThTr-2, is expressed in many human tissues as well. The highest expression is in placenta, followed by liver, kidney, and heart. The reduced folate carrier (RFC-1) shares ∼40% protein sequence identity with both thiamine transporters. Evidence obtained in murine leukemia cells suggests that RFC-1 might mediate influx of thiamine mono-phosphate (TMP) and efflux of TPP (Zhao et al. 2001, 2002).
Although thiamine uptake is not directly ATP dependent, it is driven by the rapid phosphorylation of thiamine by cytosolic thiamine pyrophosphokinase to form TPP at the expense of ATP (Lumeng et al. 1979; Yoshioka 1984; Bettendorff and Wins 1994; Bettendorff 1995). As a result, thiamine is found predominantly as TPP within cells, existing as a large, stable pool associated with enzymes such as transketolase, PDH, and α-KGDH. Since TPP is synthesized in the cytosol and PDH and α-KGDH are mitochondrial enzymes, the cofactor has to be transported across the mitochondrial membranes. Inside mitochondria, TPP is hydrolyzed to TMP (Barile et al. 1990). In Saccharomyces cerevisiae Tpc1p was shown to be involved in mitochondrial uptake of TPP in exchange for TMP (Marobbio et al. 2002).
It is generally assumed that TPP transport is of little, if any, importance in maintaining thiamine homeostasis, and it has been found that yeast (Nosaka et al. 1989) and rat hepatocytes (Yoshioka et al. 1983) are unable to take up TPP. As a consequence, thiamine pyrophosphokinase (TPK) is thought to be essential in every cell since it converts thiamine into TPP. Disruption of the gene coding for TPK in S. cerevisiae (THI80; Nosaka et al. 1993) or in Schizosaccharomyces pombe (TNR3; Fankhauser et al. 1995) is lethal. Although human TPK has been cloned (Nosaka et al. 2001), so far no multi-cellular organism that carries a mutation in the TPK gene has been described.
Here, we describe a partial loss-of-function mutation (qm162) in the nematode Caenorhabditis elegans gene that encodes thiamine pyrophosphokinase (tpk-1). tpk-1(qm162) was identified in a genetic screen for maternal-effect mutations resulting in slow development and behavior. Like other mutations found in this screen (Hekimi et al. 1995), tpk-1(qm162) also affects a number of biological rates and rhythms, including the rate of aging (Table 1). We take advantage of this mutation to show that, in C. elegans, TPP can be taken up directly from the environment and appears to be carried over plasma membranes without being converted into thiamine. We also demonstrate a role for thiamine deficiency in the phenotype of mutants of the sodium/potassium ATPase encoded by the eat-6 gene in C. elegans (Davis et al. 1995), which provides genetic evidence that thiamine absorption requires the activity of the Na+/K+ ATPase.
TABLE 1.
Effect oftpk-1(qm162) on developmental, reproductive, and behavioral features in comparison to the effect ofclk-1(qm30)
| Phenotype | Wild type (N2) | clk-1(qm30) | tpk-1(qm162) |
tpk-1(qm162) maternally rescued |
|---|---|---|---|---|
| Embryonic development (hr) |
14.88 ± 0.97 (n = 297) | 23.6 ± 4.49 (n = 70) | 16.94 ± 1.51 (n = 116) | ND |
| Postembryonic development (hr) |
46.8 ± 1.4 (n = 200) | 99.2 ± 6.2a (n = 100) | 59.1 ± 5.2 (n = 30) | 51.6 ± 2.0 (n = 31) |
| Brood size | 261.8 ± 28.1 (n = 5) | 87.2 ± 27.2a (n = 10) | 78.8 ± 7.4 (n = 5) | ND |
| Life span (days) | 17.8 ± 4.5 (n = 150) max = 28 | 20.6 ± 7.1 (n = 150) max = 40 |
24.8 ± 6.6 (n = 50) max = 36 |
ND |
| Pharyngeal pumping (pumps/min) |
248 ± 6.9 (n = 20) | 170.3 ± 26.9a (n = 25) | 140 ± 23.5 (n = 25) | ND |
| Defecation cycle (sec) | 56 ± 3.1 (n = 5) | 92.4 ± 15.0a (n = 25) | 115.9 ± 19.7 (n = 20) | 65.5 ± 5.3 (n = 12) |
Data are from Wong et al. (1995).
MATERIALS AND METHODS
Materials and media:
Thiamine and thiamine pyrophosphate were purchased from Sigma. thiE− bacteria were cultured for 16 hr in M9-metal medium without thiamine, sufficiently depleting bacteria of thiamine. Control bacteria were cultured similarly, except that thiamine (0.5 μg/ml) was present in the medium. Subsequently, bacteria were plated on M9-metal plates containing 5 μg/ml cholesterol, with or without thiamine (0.5 μg/ml).
Cosmid ZK637 was kindly provided by Alan Coulson. The cDNA clone yk903f12 was kindly provided by Y. Kohara. The pPD95.77 vector was provided by A. Fire.
Nematode and bacterial strains:
C. elegans strains were cultured on OP50 bacteria at 20° as described by Brenner (1974). The N2 (Bristol) strain was used as wild type. Mutant strains analyzed include tpk-1(qm162), eat-4(ky5), and eat-6(ad601). thiE knock-out bacteria (genotype thiE::Tn5KAN-I-SceI at position 307 in minus orientation) were kindly provided by the Escherichia coli genome project at the University of Wisconsin-Madison.
Isolation and identification of tpk-1(qm162):
tpk-1(qm162) was isolated in a genetic screen for maternal-effect mutations affecting development and behavior (Wong et al. 1995) and was backcrossed four times with N2. To test if wild-type ZK637.9 could rescue the tpk-1(qm162) phenotypes, a 2.4-kb fragment including the ZK637.9 coding sequence and 425 bp upstream sequence was amplified from genomic DNA by nested PCR using outer forward primer CATTTTCGCCAATATTTTGTATTTC, inner forward primer ACCGTTGAATAAATAAGTTGATTGC, and reverse primer ACATTCCAATCATGTTTGATTTTCT. The PCR product was gel purified and co-injected into the germline of tpk-1(qm162) with the rol-6(su1006) marker plasmid. All tpk-1 phenotypes tested (growth rate, brood size, defecation cycle length) were rescued, demonstrating that the transgene complements the tpk-1(qm162) mutation. To identify the nature of the tpk-1(qm162) mutation, the above-mentioned primers were used to amplify ZK637.9 from tpk-1 mutant DNA and the resulting fragment was sequenced.
Construction of translational fusions:
The ZK637.9 coding sequence was amplified by PCR from genomic DNA with the forward primer ACG ACG TGC GAC GTC GAC GAT TCT CAT CAC AAG TGG containing the SalI restriction site (underlined) and the reverse primer AGC ACG ATC GCG GGT ACC GAA TCA AGT TTG TAG ACC containing the KpnI restriction site (underlined). After digestion, the 1.3-kb SalI/KpnI fragment was cloned into the corresponding sites in the pPD95.77 vector (obtained from A. Fire) under the control of the myo-3 and the myo-2 promoters.
Sequencing of cDNA:
Vector pME18S-FL containing cDNA clone yk903f12 (obtained from Y. Kohara) was sequenced using the forward primer 5′-GTA ATA CGA CTC ACT ATA GGG CGT CCA TCT GCC TGA TCA AGA C and the reverse primer 5′-GTA ATA CGA CTC ACT ATA GGG CTC CAA TTG AAC TGA CGA T (both contain the T7 promoter that is not used). The obtained sequence data covered the entire coding sequence of tpk-1.
Transgenic worms:
tpk-1 constructs were micro-injected in various concentrations into tpk-1 mutant worms with 150 ng/μl co-injection marker construct pRF4 [containing rol-6(su1006)] to create transgenic lines.
Scoring phenotypes:
Defecation and pharyngeal pumping cycle lengths of singled young adults were scored at 20° as described (Wong et al. 1995). Four successive defecation cycles were timed. Pharyngeal pumping rate was scored by measuring the number of pumps in 60 sec. To determine brood size, young adults were singled and transferred each day to a new plate until they stopped laying eggs. Progeny were counted on each of the plates.
O2 consumption rate:
Worms were cultured at 20° as described (Feng et al. 2001). Developmentally synchronized young adults were collected and washed with M9. Tubes were left on the table to allow the worms to sink for 5–10 min. The supernatant was removed as much as possible without removing worms. Oxygen consumption was analyzed using a Clark electrode. A total of 100 μl of the worms was put in the chamber and the volume was adjusted with M9 buffer. Measurements were taken for 6 min.
Electropharyngeograms:
Recording of electropharyngeograms (EPGs) was essentially as described in Raizen and Avery (1994) using a Warner Instruments (Hamden, CT), patch clamp PC-501A with a 1-GΩ headstage but without filtering in the amplifier. Borosilicate suction pipettes (1.2 OD) were pulled on a Sutter P-97 pipette puller (Sutter Instrument, Novato, CA). EPGs were digitized using a Digitdata1322A and recorded using Clampex 8.1 software (Axon Instruments, Union City, CA). Recordings were formatted and digitally filtered using a 1-kHz Gaussian filter with Clampfit 8.1 software (Axon Instruments).
RESULTS
Identification tpk-1(qm162):
The tpk-1 mutant was isolated in a genetic screen for maternal-effect mutations affecting development and behavior (clk mutants), which was similar to a previously described screen (Hekimi et al. 1995) and whose results will be described in detail elsewhere. The qm162 mutation was mapped to LG III between sma-2 and unc-69 and found to be genetically inseparable from unc-32 (mapping data have been deposited at http://www.wormbase.org). Cosmids from the corresponding genomic region were injected to assay for rescue of the mutant phenotypes. Injection of ZK637 allowed for phenotypic rescue and predicted genes on the rescuing cosmid were amplified by PCR and assayed by injection. Injection of a 2.4-kb PCR amplicon (coordinates: III:8911625 to 8914002) containing the predicted gene ZK637.9 was sufficient to rescue the developmental and behavioral phenotypes of the mutant strain (data not shown). To reveal the correct gene structure, an apparently full-length cDNA clone (yk903f12, kindly provided by Y. Kohara) that had sequences in common with ZK637.9 was sequenced. The sequence of yk903f12 (accession no. AY513235) indicated that it encodes a 228-amino-acid protein similar to TPK in vertebrates, plants, and yeast, which converts thiamine into the essential cofactor TPP. We identified the tpk-1(qm162) mutation (Cys67 → Tyr) by resequencing the gene from the mutant strain. Multiple alignment of C. elegans, human, mouse, fruit fly, and yeast TPK shows strong sequence conservation of the protein from yeast to humans (Figure 1).
Figure 1.—

Sequence conservation of thiamine pyrophosopkinase. Multiple alignment of thiamine pyrophosphokinase from human, mouse, Drosophila, C. elegans, and S. cerevisiae. Sequences were aligned using DNAman 4.1 and adjusted by hand. The tpk-1(qm162) mutation (Cys67 > Tyr) is shown in boldface type and indicated by an asterisk.
Phenotype of tpk-1(qm162) mutants:
clk mutants, including tpk-1(qm162), are characterized by a general slowdown and deregulation of physiological rhythms, including a lengthening of life span. Table 1 summarizes a variety of physiological traits that are affected in tpk-1(qm162) mutants. The average cycle lengths of adult rhythmic behaviors such as defecation and pharyngeal pumping are almost doubled. Other timed life-cycle phases, including embryonic and postembryonic development, are of increased duration. tpk-1 mutants have a substantially increased life span, which is due primarily to an increase in adult life span (Table 1). In addition, tpk-1 mutants can be maternally rescued. This means that tpk-1 homozygous mutant progeny from a heterozygous mother (tpk-1/+) are phenotypically wild type. Although all phenotypes appear rescued, only postembryonic development and the defecation cycle measured were exactly quantified (Table 1).
clk-1 mutants, which are the most extensively characterized animals with a Clk phenotype, look generally healthy and do not show any obvious morphological defects in spite of the slowdown and deregulation of physiological rhythms. However, tpk-1 mutants, although anatomically and behaviorally mostly normal, appear slimmer and more transparent than wild-type worms. Additionally, tpk-1 mutants tend to clump in groups and avoid the bacterial lawn. These behaviors are reminiscent of eat mutants, which have impaired pharyngeal pumping and consequently impaired food intake (Avery 1993). Since TPP is an essential cofactor for several key metabolic enzymes including mitochondrial pyruvate decarboxylase and α-ketoglutarate decarboxylase, reduced availability of TPP due to the tpk-1(qm162) mutation should affect mitochondrial metabolism. However, we did not observe a difference in oxygen consumption rates between wild-type (N2; 28.12 ± 4.09 nmol/min/mg protein) and tpk-1 mutant worms (29.36 ± 4.47 nmol/min/mg protein). The numbers represent the means ± standard error of two independent experiments with three independent samples measured per experiment. This suggests that the phenotype produced by the tpk-1(qm162) mutation is not mediated by a decrease in electron transport and ATP synthesis. This is consistent with previous studies of animals with reduced electron transport that are large and dark, rather than small and transparent like tpk-1 mutants (Feng et al. 2001). This means that the levels of TPP present in tpk-1 mutants are not limiting for mitochondrial function.
C. elegans requires thiamine from food:
Animals, unlike plants and micro-organisms, depend on external sources of thiamine to be able to produce TPP. In mammals, a thiamine-depleted diet eventually leads to severe neurological defects. To establish the effect of thiamine deficiency on C. elegans, wild-type worms at the L2, L3, or L4 larval stage were cultured on thiamine-free plates containing thiamine-depleted ThiE− bacteria, which cannot produce any thiamine and fail to grow without exogenous thiamine. To grow enough ThiE− bacteria to feed worms, we grew ThiE− bacteria in thiamine-deficient medium until they stopped growing. Control worms were cultured under the same conditions except that thiamine was added to the plates (see materials and methods). On the thiamine-deficient plates, the wild-type worms (N2) developed into normal-looking gravid adults that behave normally. After 2 days, the pharyngeal pumping rate, a behavior that is affected in tpk-1 mutants, was still similar to that of the controls (>230 pumps) as well as to that of N2 worms fed on OP50 bacteria (the standard bacterial strain). However, thiamine deficiency becomes apparent in the F1 progeny of animals grown continuously on deficient bacteria. Although F1 progeny develop into adults, these animals remain very small and slim and they fail to produce progeny. Thus wild-type animals can subsist on accumulated thiamine although the thiamine is eventually depleted in their progeny.
Unlike wild-type worms, young adult tpk-1 mutants that are challenged with a thiamine-depleted diet display symptoms of thiamine deficiency immediately: after 2 days the pharyngeal pumping rate is slowed down from 140 to 90 pumps/minute, and the worms start looking sick and die after ∼1 week. Although they produce F1 eggs, none of these hatch. The strong effect seen in tpk-1 worms suggests that these mutants are not able to subsist on previously accumulated thiamine. This is in line with the fact that most thiamine inside organisms is in the form of TPP. Thus, low activity of TPK might result in low levels of thiamine and TPP.
The tpk-1 mutant phenotypes are caused by thiamine pyrophosphate deficiency:
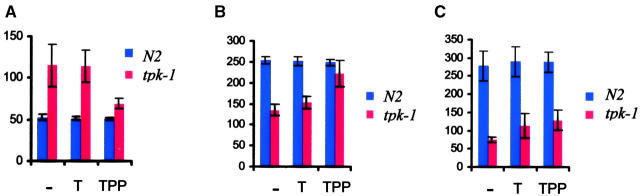
The strong phenotype of tpk-1 mutants implies that worms normally need to produce TPP endogenously to remain healthy. To determine directly the relationship between tpk-1 phenotypes and TPP, tpk-1 mutants were exposed to high concentrations of TPP by soaking a mix of L4 larvae and young adults in M9 buffer containing 2.5 mm TPP for 30 min. The pharyngeal pumping and defecation cycle lengths of the soaked worms were scored 24 hr later. Figure 2 illustrates the profound rescuing effect of TPP on rhythmic behaviors and brood size of tpk-1 mutant worms. In addition, the rescued worms look darker and bigger, suggesting that they are healthier. In principle, it is possible that TPP is first dephosphorylated inside the worm before cellular uptake, as is the case in the mammalian intestine. However, exposing worms to 2.5 mm thiamine has no effect on pharyngeal pumping and defecation cycle length and only a very slight effect on brood size, demonstrating that TPP is taken up and used directly. Neither thiamine nor TPP has any effect on wild-type worms (Figure 2).
Figure 2.—
Thiamine and thiamine pyrophosphate supplementation on tpk-1 mutants. Effect of soaking in M9 buffer (−), thiamine (T), or TPP on behavioral rhythms and brood size of wild-type animals (N2) and tpk-1 mutants. (A) Defecation cycle length in seconds (n = 10 animals). (B) Pharyngeal pumping rate in pumps per minute (n = 10 animals). (C) Brood size in number of offsprings (n = 5 animals). Treatments with either thiamine or TPP have no effect on the wild type. For tpk-1 mutants only TPP had a significant effect on the defecation rate. For pumping rate, both thiamine (P > 0.02) and TPP had a significant effect. However, the effect of thiamine is very small. For brood size, both thiamine and TPP appear to have an effect on mutant animals but we could detect only a statistically significant effect for TPP (P < 0.01).
tpk-1(qm162) results in a partial loss-of-function of TPK:
The finding that TPP but not thiamine supplementation rescues tpk-1 mutants indicates that qm162 is a loss-of-function mutation. However, this result also means that tpk-1(qm162) might be a null mutation and that the viability of mutants feeding on bacteria capable of synthesizing TPP is due to dietary TPP uptake. To establish whether tpk-1(qm162) is a full or partial loss-of-function mutation we used a particular phenotype associated with tpk-1(qm162). In the absence of food, newly hatched worms arrest as L1 larvae, which allows them to survive a prolonged period of starvation. When food becomes available, the wild-type arrested larvae resume normal development. However, tpk-1 mutant worms tend to die within a few days as arrested L1 larvae or fail to resume development when placed in the presence of food. To determine if this defect could be rescued exogenously, tpk-1 L1 larvae were arrested on plates containing thiamine or TPP but no bacteria. Each day, a sample of worms was transferred to a plate with bacteria and their development was monitored. As Table 2 documents, the presence of either thiamine or TPP in the plates on which the worms were arrested allows tpk-1 L1 larvae to resume development after transfer to a food plate. Thiamine appears to be as effective as TPP, strongly suggesting that TPK-1(QM162) has residual activity and converts exogenous thiamine to TPP. The inability of tpk-1 mutants to survive arrest and starvation without thiamine or TPP supplementation is likely due to a reduced ability to store thiamine as TPP. It also suggests that resuming growth after arrest requires less TPP than the other phenotypes we have described (e.g., pumping), which cannot be rescued by providing thiamine.
TABLE 2.
Effect of thiamine and TPP on recovery from larval arrest
| Duration of development to adulthood after L1 arrest (days)
|
||||||
|---|---|---|---|---|---|---|
| Wild type
|
tpk-1
|
|||||
| Duration of arrest (days)a |
T | TPP | M9 buffer | T | TPP | M9 buffer |
| 1 | 2 | 2 | 2 | 3 | 3 | 3 |
| 2 | 2 | 2 | 2 | 3 | 3 | 3–4 |
| 3 | 2 | 2 | 2 | 3 | 3 | 4 |
| 4 | 2–3 | 2–3 | 3 | 3–4 | 3–4 | 5b |
| 5 | 2–3 | 2–3 | 3 | 3–4 | 3–4 | 6–7b |
L1 larvae were arrested on empty plates supplemented with 200 μl thiamine (25 mm), TPP (25 mm), or M9 buffer. Worms were transferred to plates with bacteria at the indicated day and the duration of development to adulthood was determined.
Some worms had already died; others appeared developmentally arrested.
TPK-1 functions cell nonautonomously:
TPP is generally believed to be unable to cross cell membranes. Thus, given that TPP is essential for basic cellular metabolism, TPK is expected to be required in every cell. However, our observation that exogenous TPP can be taken up by C. elegans and appears to have global effects on the worm suggests that TPP can be transported without previous dephosphorylation from the absorbing cells to other cells in the body. Thus, tpk-1 might function cell nonautonomously. To explore this, a tpk-1::gfp translational fusion was expressed under the control of the myo-3 promoter (body-wall muscles) or the myo-2 promoter (pharyngeal muscles). In tpk-1 mutants that carry a [myo-3::tpk-1::gfp] or [myo-2::tpk-1::gfp] transgenic array, GFP fluorescence is detected as expected in the body-wall muscles or the pharyngeal muscles, respectively. However, in both cases the construct profoundly rescued all tested mutant phenotypes (Table 3). Thus, the rescue of phenotypes unrelated to the activity of a particular tissue (e.g., pharyngeal pumping should be independent of body-wall muscle function) suggests that tpk-1 functions cell nonautonomously. However, extrachromosomal arrays generally contain multiple copies of the gene, which can result in overexpression. To exclude the possibility that the expression of the transgene was not confined to a particular tissue because of overexpression, we used the myo-2::tpk-1::gfp construct in the following way. We produced transgenic arrays by injecting very low concentrations of rescuing DNA at a concentration so low (1 ng/μl) that two out of three independent transgenic lines were not rescued, presumably because they did not contain a full-length copy of the construct. The third line, however, was rescued for both the pharyngeal pumping rate and the defecation cycle length, which is not dependent on pharyngeal muscle function. Furthermore, the worms of this line have a general wild-type appearance. Together, these observations strongly suggest that tpk-1 functions cell nonautonomously, suggesting that TPP can be transported from one cell or tissue to others.
TABLE 3.
Tissue-specific extrachromosomal expression of the wild-typetpk-1 gene fused togfpand expressed in atpk-1 mutant background
| Genotype (extrachromosomal arrays) | Brood size (progeny) (n = 5) | Defecation cycle length (sec) (n = 20) |
Pharyngeal pumping (pumps/min) (n = 20) |
Postembryonic development (hr) (n = 50) |
|---|---|---|---|---|
| Wild type (N2) | 305 ± 16 | 55.6 ± 3.8 | 248 ± 16.8 | 47.7 ± 3.5 |
| tpk-1 | 76 ± 7 | 115 ± 23.6 | 140 ± 23.8 | 60.5 ± 4.5 |
| [myo-3::tpk-1:gfp] 25 ng/μl, line 1a | 253 ± 11 | 59.3 ± 4.1 | 243 ± 14.2 | 49.2 ± 3.2 |
| [myo-2::tpk-1:gfp] 25 ng/μl, line 1a | 167 ± 21 | 65 ± 5.9 | 245 ± 12 | 49.1 ± 3.4 |
| [myo-2::tpk-1:gfp] 5 ng/μl, line 1a | 176 ± 10 | 77 ± 6.2 | 207 ± 11.2 | 56.1 ± 3.5 |
| [myo-2::tpk-1:gfp] 5 ng/μl, line 2a | 191 ± 40 | 64.9 ± 5.9 | 215 ± 12 | 52.4 ± 3.3 |
| [myo-2::tpk-1:gfp] 5 ng/μl, line 3a | 167 ± 46 | 83.5 ± 20.3 | 217 ± 21.7 | 51.4 ± 3.4 |
| [myo-2::tpk-1:gfp] 1 ng/μl, line 1 | 84 ± 10 | 101 ± 20 | 141 ± 19.7 | 58.2 ± 4.7 |
| [myo-2::tpk-1:gfp] 1 ng/μl, line 2a | ND | 73.4 ± 3.8 | 208 ± 12.6 | ND |
| [myo-2::tpk-1:gfp] 1 ng/μl, line 3 | 80 ± 16 | 114.3 ± 18.5 | 142 ± 18.2 | 59.9 ± 4.9 |
Lines whose individuals have a wild-type appearance.
Slow pumping in tpk-1 mutants is due to failure to trigger muscle contraction:
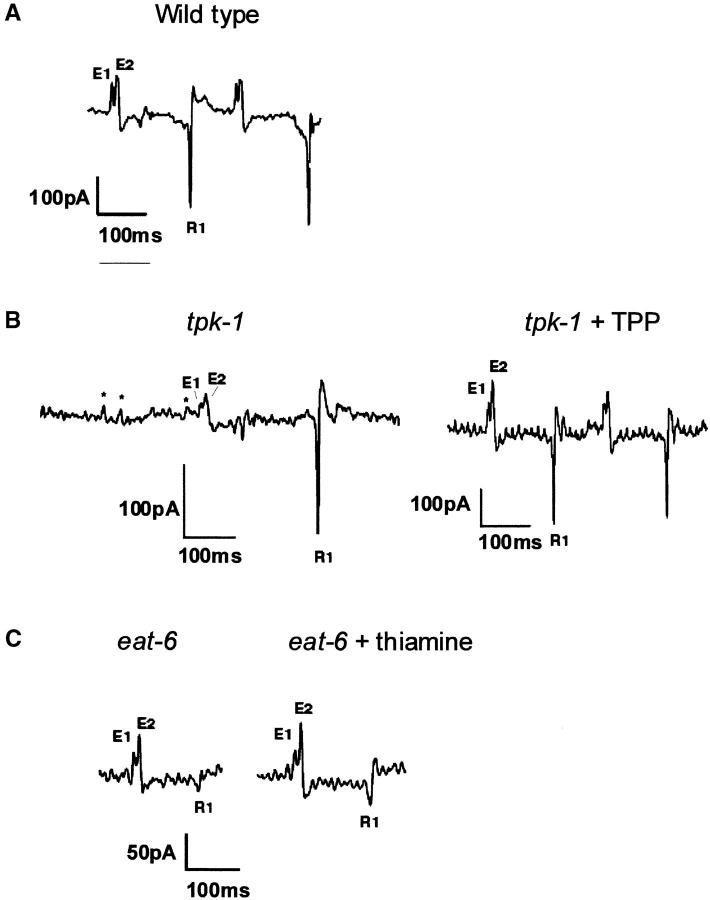
To better understand the slow-pumping phenotype of tpk-1 mutants, we examined pumping by electrophysiological methods. We recorded EPGs, extracellular recordings that measure changes in the membrane potential of pharyngeal muscles during rhythmic pumping of the pharynx (Raizen and Avery 1994; Davis et al. 1995). Normally, the rate of pumping in the presence of food is determined by the activity of the marginal cells (MC) pacemaker motor neurons. We found that in tpk-1 mutants, in contrast to what is observed in the wild type, the excitatory postsynaptic potentials (EPSPs) produced by the MC motor neurons do not always trigger muscle contraction (Figure 3, A and B), which can explain the slow-pumping rate of the mutants. Indeed, treatment of mutants with TPP rescues the coupling between EPSP and contraction so that virtually every EPSP triggers a muscle contraction (Figure 3B). Inefficient triggering of muscle action potentials is characteristic of mutants, such as snt-1, that have compromised neurotransmitter release (Raizen et al. 1995). However, reduced muscle excitability could also explain this phenotype.
Figure 3.—
EPGs. E1 is the EPSPs produced by the action of the two MC motor neurons on the muscle. E2 reflects the depolarization of the muscle and the initiation of the action potential in response to E1. R1 indicates the repolarization of the muscle that terminates the muscle action potential. (A) Typical recording from wild-type muscle. (B) Typical recording from a tpk-1 mutant and a tpk-1 mutant treated with TPP. The smaller amplitude of the spikes reflects the smaller size of the tpk-1 mutant animals. Many EPSPs (marked by an asterisk) failed to elicite a muscle action potential. This phenotype is fully rescued by treatment with TPP. (C) Typical recording from an eat-6(ad601) mutant without and with treatment with thiamine. In the untreated mutant the R1 spike is very small compared to those in the wild type, and this phenotype is not rescued by treatment with thiamine, although thiamine rescues the pumping rate.
All currents in the EPG traces of the tpk-1 worms are smaller because tpk-1 worms are starved and small worms have smaller amplitude EPG currents. This is because the EPG measures capacitative currents and the capacitance of the cell decreases with size. However, this does not affect our conclusions. Even in normal small worms (i.e., larvae) a normal small EPSP triggers a pump almost every time.
Thiamine redistribution after uptake requires the activity of Na+/K+-ATPase:
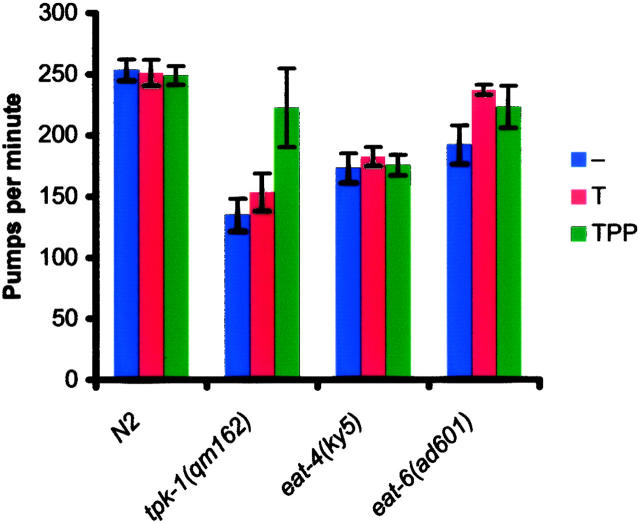
Although uptake of thiamine appears to be driven by TPK-dependent phosphorylation, export of thiamine from cells is believed to require the activity of Na+/K+-ATPase (Laforenza et al. 1993). In C. elegans, the α-subunit of this enzyme is encoded by the eat-6 gene (Davis et al. 1995), and partial loss-of-function mutants in this gene have decreased Na+/K+-ATPase activity (Shima et al. 1998) and display slow, abnormal pumping (Avery 1993). We treated eat-6 mutants by soaking them in thiamine or TPP and found that both compounds dramatically improved the pharyngeal pumping rate of the mutants (Figure 4). In contrast, no effect was seen on mutants (ky5) of the eat-4 gene, which encodes a vesicular glutamate transporter (Bellocchio et al. 2000). This suggests that at least part of the phenotype of eat-6 mutants is due to a deficiency in thiamine. A likely explanation is that, after thiamine is taken up by competent cells, for example, in the gut, it cannot be reexported to the rest of the body because of the defect in Na+/K+-ATPase.
Figure 4.—
Effect of thiamine and thiamine pyrophosphate supplementation on pumping mutants. Effect of soaking in M9 buffer (−), thiamine (T), or TPP on pharyngeal pumping rate of wild-type, tpk-1, eat-4(ky5), and eat-6(ad601) worms (n = 10 animals for each strain). The effect of thiamine on tpk-1 mutants is small but significant (P > 0.02). The effects of both thiamine (P < 0.001) and TPP (P < 0.001) on eat-6 mutants are highly significant, but there is no significant effect of either compound on eat-4 (P = 0.18 and P = 0.72, for thiamine and TPP, respectively).
eat-6 mutants display an electrophysiological phenotype in which the R spike, which corresponds to the repolarization phase of the action potential just before the muscles relax, is dramatically reduced (Davis et al. 1995; Figure 4). However, treatment with thiamine, which rescues the pumping-rate phenotype (Figure 3), does not rescue the electrophysiological phenotype. Thus the muscle defect that results in the reduced R spike is not a result of thiamine deficiency and does not explain the reduced pumping rate. Decreased levels of TPP in the nervous system, specifically in the pacemaker neurons, might explain the decreased pumping rate in eat-6 mutants. This would be consistent with findings in vertebrates that the nervous system is the first tissue to be affected by thiamine deficiency.
DISCUSSION
Thiamine and tpk-1:
Thiamine plays an essential role in energy metabolism in the form of TPP in all organisms. To synthesize TPP, animals have to extract thiamine from food, which is converted into the active coenzyme by TPK. This enzyme is essential for viability in yeast and probably in other species as well, since no salvation pathway for TPP is known to exist. We identified tpk-1(qm162) in a screen for C. elegans mutants with a slow life cycle and behavioral rhythms and used positional cloning to discover that the gene encodes C. elegans TPK.
Function of dietary thiamine:
Symptoms associated with thiamine deficiency are being studied in organisms such as rats and mice. Rats that are fed a thiamine-deficient diet for 30 days start to show the first signs of thiamine deficiency, that is, anorexia, after 3 weeks (Pires et al. 2001). In mice, anorexia sets in after 10–15 days of thiamine depletion (Nakagawasai et al. 2001). By feeding wild-type worms a thiamine-depleted diet and monitoring growth and reproduction, we show that C. elegans, like other animal species, needs dietary thiamine for survival. Moreover, tpk-1(qm162) mutants are hypersensitive to thiamine depletion. When tpk-1 adults are fed a thiamine-depleted diet they immediately display a significantly reduced feeding rate and start dying after only a few days. On the other hand, although tpk-1 mutants presumably have reduced levels of TPP (see results), their behavioral and developmental phenotypes are not improved by excess thiamine (Figure 2). Together, these observations suggest that cellular uptake of thiamine in worms is coupled to TPK activity as in other organisms (Lumeng et al. 1979; Yoshioka 1984; Bettendorff and Wins 1994; Bettendorff 1995), which is why tpk-1 mutants are hypersensitive to thiamine depletion. However, the amount of thiamine that tpk-1 mutants can extract from normal food is not limiting and allows for maximal activity of the mutant TPK in most tissues, which is why the tpk-1 mutant phenotype cannot be improved by pharmacological amounts of thiamine, except when endogenous thiamine is depleted by developmental arrest (Table 2).
Uptake and transport of TPP in C. elegans:
Dietary thiamine, which consists mostly of TPP, is converted into thiamine in the mammalian intestine before absorption. In fact, TPP is thought to be produced only where it is used, and its transport is thought to be of minor, if any, importance in thiamine metabolism. Dephosphorylation of dietary TPP is likely to occur as well in the C. elegans intestine, given that worms need TPK activity to maintain a wild-type physiology. This would suggest that direct uptake and utilization of dietary TPP is not possible, is insufficient, or is prevented by rapid dephosphorylation. Yet, we show that tpk-1 mutants are rescued by soaking in pharmacological amounts of TPP but not of thiamine. This indicates that TPP can be taken up directly without being dephosphorylated.
All aspects of the phenotype of tpk-1 mutants improve upon TPP treatment, which indicates that it is not only taken up in the intestine, but also efficiently distributed through the worms. This finding is surprising, given that it is believed that only thiamine but not TPP can be exported from cells (Yoshioka et al. 1983; Nosaka et al. 1989). To rule out the possibility that the effect of soaking is merely an artifact induced by the extremely high amounts of TPP, we tested whether expression of wild-type tpk-1 in a single tissue could rescue the entire animal. As shown in Table 3, extrachromosomal expression of tpk-1::gfp restricted to the pharyngeal muscles rescues not only pharyngeal pumping, but also unrelated phenotypes such as the defecation cycle, as well as the overall aspect of the animal. This strongly indicates that TPP produced in the pharyngeal muscles is efficiently distributed throughout the worm by endogenous physiological mechanisms. Taken together, these findings show that TPP can be effectively transported across plasma membranes in C. elegans.
The tpk-1 gene lies in an operon whose first gene is unc-32 (Blumenthal et al. 2002), a gene whose pattern of expression has been studied in detail (Oka et al. 2001; Pujol et al. 2001). The promoter of this operon directs expression to many tissues, including to the entire embryo, the intestine, the excretory cell, the pharynx, the somatic gonad, the nervous system, and various hypodermal cells. However, the operon appears not to be expressed in the body-wall muscle cells and in the main hypodermal multinucleated hypodermal cell. In addition, tpk-1 might be expressed in fewer tissues because of post-transcriptional controls. Thus, the TPP transport system might be essential to provide TPP that has been synthesized elsewhere to muscle cells, the hypodermis, and, possibly, other tissues.
Redistribution of dietary thiamine:
While uptake of thiamine into cells is energized by the activity of TPK, export of thiamine requires the activity of Na+/K+-ATPase (Laforenza et al. 1993). We found that this also appears to be the case in C. elegans, as the phenotype of eat-6 mutants, which have partially impaired Na+/K+-ATPase function, is rescued by thiamine. It suggests that thiamine redistribution to other tissues after uptake from dietary sources by the intestine might be one of the cellular processes that is most sensitive to a partial reduction of the function of Na+/K+-ATPase. It also indicates that part of the eat-6 phenotype is due to thiamine (and therefore TPP) deficiency.
TPP transport may also be important in mammals:
TPP appears to be transported rather efficiently between cells in C. elegans. Does similar transport occur in mammals and, if so, how important is it for thiamine homeostasis? Our observations suggest that all the basic aspects of thiamine metabolism previously described in mammalian systems exist in C. elegans as well. Furthermore, mammalian genes known to be directly involved in thiamine metabolism, including the two thiamine transporters and TPK, have close homologs in C. elegans. An inability to transport TPP across membranes was directly demonstrated only in yeast and liver cells (Yoshioka et al. 1983; Nosaka et al. 1989). More circumstantial evidence that TPP does not cross membranes includes the observations that TPP can be detected in erythrocytes but not in blood serum (Tallaksen et al. 1997) and that dietary TPP is taken up in the form of thiamine. However, what we observe in worms might nonetheless be a phylogenetically common mechanism. Indeed, there have been early observations that Ehrlich ascites carcinoma cells rapidly take up labeled thiamine and that after 2 hr practically all the labeled thiamine is in the phosphorylated form, including that in the medium (Menon and Quastel 1966). More recently, mouse leukemia cells were also shown to transport TPP through plasma membranes. Thiamine uptake appears to be impaired in thiamine-responsive megaloblastic anemia syndrome patients. Yet the symptoms of these patients appear to be distinct from those induced by dietary thiamine depletion (Oishi et al. 2002). Possibly, the basis of this disease is impaired TPP transport.
Acknowledgments
We thank Robyn Branicky for insightful comments on the manuscript; A. Coulson, A. Fire, and Y. Kohara for providing clones; and the Caenorhabditis Genetics Centre, which is funded by the National Institute of Health National Center for Research Resources, for providing strains. S.H. is a Canadian Institutes of Health Research Scientist.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession no. AY513235.
References
- Avery, L., 1993. The genetics of feeding in Caenorhabditis elegans. Genetics 133: 897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile, M., S. Passarella and E. Quagliariello, 1990. Thiamine pyrophosphate uptake into isolated rat liver mitochondria. Arch. Biochem. Biophys. 280: 352–357. [DOI] [PubMed] [Google Scholar]
- Bellocchio, E. E., R. J. Reimer, R. T. Fremeau, Jr. and R. H. Edwards, 2000. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 289: 957–960. [DOI] [PubMed] [Google Scholar]
- Bettendorff, L., 1995. Thiamine homeostasis in neuroblastoma cells. Neurochem. Int. 26: 295–302. [DOI] [PubMed] [Google Scholar]
- Bettendorff, L., and P. Wins, 1994. Mechanism of thiamine transport in neuroblastoma cells. Inhibition of a high affinity carrier by sodium channel activators and dependence of thiamine uptake on membrane potential and intracellular ATP. J. Biol. Chem. 269: 14379–14385. [PubMed] [Google Scholar]
- Blumenthal, T., D. Evans, C. D. Link, A. Guffanti, D. Lawson et al., 2002. A global analysis of Caenorhabditis elegans operons. Nature 417: 851–854. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casirola, D., C. Patrini, G. Ferrari and G. Rindi, 1990. Thiamin transport by human erythrocytes and ghosts. J. Membr. Biol. 118: 11–18. [DOI] [PubMed] [Google Scholar]
- Davis, M. W., D. Somerville, R. Y. Lee, S. Lockery, L. Avery et al., 1995. Mutations in the Caenorhabditis elegans Na,K-ATPase alpha-subunit gene, eat-6, disrupt excitable cell function. J. Neurosci. 15: 8408–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, G. A., M. Banikazemi, K. Oishi, R. J. Desnick and B. D. Gelb, 1999. Mutations in a new gene encoding a thiamine transporter cause thiamine-responsive megaloblastic anaemia syndrome. Nat. Genet. 22: 309–312. [DOI] [PubMed] [Google Scholar]
- Eudy, J. D., O. Spiegelstein, R. C. Barber, B. J. Wlodarczyk, J. Talbot et al., 2000. Identification and characterization of the human and mouse SLC19A3 gene: a novel member of the reduced folate family of micronutrient transporter genes. Mol. Genet. Metab. 71: 581–590. [DOI] [PubMed] [Google Scholar]
- Fankhauser, H., A. Zurlinden, A. M. Schweingruber, E. Edenharter and M. E. Schweingruber, 1995. Schizosaccharomyces pombe thiamine pyrophosphokinase is encoded by gene tnr3 and is a regulator of thiamine metabolism, phosphate metabolism, mating, and growth. J. Biol. Chem. 270: 28457–28462. [DOI] [PubMed] [Google Scholar]
- Feng, J., F. Bussiere and S. Hekimi, 2001. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell 1: 633–644. [DOI] [PubMed] [Google Scholar]
- Fleming, J. C., E. Tartaglini, M. P. Steinkamp, D. F. Schorderet, N. Cohen et al., 1999. The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat. Genet. 22: 305–308. [DOI] [PubMed] [Google Scholar]
- Gastaldi, G., E. Cova, A. Verri, U. Laforenza, A. Faelli et al., 2000. Transport of thiamin in rat renal brush border membrane vesicles. Kidney Int. 57: 2043–2054. [DOI] [PubMed] [Google Scholar]
- Hekimi, S., P. Boutis and B. Lakowski, 1995. Viable maternal-effect mutations that affect the development of the nematode Caenorhabditis elegans. Genetics 141: 1351–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann, S., and P. A. Meacock, 1998. Thiamin metabolism and thiamin diphosphate-dependent enzymes in the yeast Saccharomyces cerevisiae: genetic regulation. Biochim. Biophys. Acta 1385: 201–219. [DOI] [PubMed] [Google Scholar]
- Hoyumpa, Jr., A. M., R. Strickland, J. J. Sheehan, G. Yarborough and S. Nichols, 1982. Dual system of intestinal thiamine transport in humans. J. Lab. Clin. Med. 99: 701–708. [PubMed] [Google Scholar]
- Labay, V., T. Raz, D. Baron, H. Mandel, H. Williams et al., 1999. Mutations in SLC19A2 cause thiamine-responsive megaloblastic anaemia associated with diabetes mellitus and deafness. Nat. Genet. 22: 300–304. [DOI] [PubMed] [Google Scholar]
- Laforenza, U., G. Gastaldi and G. Rindi, 1993. Thiamine outflow from the enterocyte: a study using basolateral membrane vesicles from rat small intestine. J. Physiol. 468: 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng, L., J. W. Edmondson, S. Schenker and T. K. Li, 1979. Transport and metabolism of thiamin in isolated rat hepatocytes. J. Biol. Chem. 254: 7265–7268. [PubMed] [Google Scholar]
- Marobbio, C. M., A. Vozza, M. Harding, F. Bisaccia, F. Palmieri et al., 2002. Identification and reconstitution of the yeast mitochondrial transporter for thiamine pyrophosphate. EMBO J. 21: 5653–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, I. A., and J. H. Quastel, 1966. Transport and metabolism of thiamine in Ehrlich ascites-carcinoma cells. Biochem. J. 99: 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley, R. H., P. G. Vashi, S. M. Jarose, C. J. Dickinson and P. A. Permoad, 1992. Thiamine transport by basolateral rat liver plasma membrane vesicles. Gastroenterology 103: 1056–1065. [DOI] [PubMed] [Google Scholar]
- Nakagawasai, O., T. Tadano, S. Hozumi, R. Taniguchi, K. Tan-No et al., 2001. Characteristics of depressive behavior induced by feeding thiamine-deficient diet in mice. Life Sci. 69: 1181–1191. [DOI] [PubMed] [Google Scholar]
- Nosaka, K., H. Nishimura and A. Iwashima, 1989. Identity of soluble thiamine-binding protein with thiamine repressible acid phosphatase in Saccharomyces cerevisiae. Yeast 5(Spec no.): S447–S451. [PubMed] [Google Scholar]
- Nosaka, K., Y. Kaneko, H. Nishimura and A. Iwashima, 1993. Isolation and characterization of a thiamin pyrophosphokinase gene, THI80, from Saccharomyces cerevisiae. J. Biol. Chem. 268: 17440–17447. [PubMed] [Google Scholar]
- Nosaka, K., M. Onozuka, N. Kakazu, S. Hibi, H. Nishimura et al., 2001. Isolation and characterization of a human thiamine pyrophosphokinase cDNA. Biochim. Biophys. Acta 1517: 293–297. [DOI] [PubMed] [Google Scholar]
- Oishi, K., S. Hofmann, G. A. Diaz, T. Brown, D. Manwani et al., 2002. Targeted disruption of Slc19a2, the gene encoding the high-affinity thiamin transporter Thtr-1, causes diabetes mellitus, sensorineural deafness and megaloblastosis in mice. Hum. Mol. Genet. 11: 2951–2960. [DOI] [PubMed] [Google Scholar]
- Oka, T., T. Toyomura, K. Honjo, Y. Wada and M. Futai, 2001. Four subunit a isoforms of Caenorhabditis elegans vacuolar H+-ATPase. Cell-specific expression during development. J. Biol. Chem. 276: 33079–33085. [DOI] [PubMed] [Google Scholar]
- Pires, R. G., S. R. Pereira, J. E. Pittella, G. C. Franco, C. L. Ferreira et al., 2001. The contribution of mild thiamine deficiency and ethanol consumption to central cholinergic parameter dysfunction and rats' open-field performance impairment. Pharmacol. Biochem. Behav. 70: 227–235. [DOI] [PubMed] [Google Scholar]
- Pujol, N., C. Bonnerot, J. J. Ewbank, Y. Kohara and D. Thierry-Mieg, 2001. The Caenorhabditis elegans unc-32 gene encodes alternative forms of a vacuolar ATPase a subunit. J. Biol. Chem. 276: 11913–11921. [DOI] [PubMed] [Google Scholar]
- Raizen, D. M., and L. Avery, 1994. Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron 12: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen, D. M., R. Y. Lee and L. Avery, 1995. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics 141: 1365–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajgopal, A., A. Edmondnson, I. D. Goldman and R. Zhao, 2001. SLC19A3 encodes a second thiamine transporter ThTr2. Biochim. Biophys. Acta 1537: 175–178. [DOI] [PubMed] [Google Scholar]
- Rindi, G., and U. Laforenza, 2000. Thiamine intestinal transport and related issues: recent aspects. Proc. Soc. Exp. Biol. Med. 224: 246–255. [DOI] [PubMed] [Google Scholar]
- Said, H. M., K. Balamurugan, V. S. Subramanian and J. S. Marchant, 2004. Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 286: G491–G498. [DOI] [PubMed] [Google Scholar]
- Shima, Y., Y. Tada, M. Furuki, Y. Hara and H. Ohta, 1998. A missense mutation of the gene for Na+,K(+)-ATPase alpha-subunit causes abnormal feeding behavior in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 248: 778–782. [DOI] [PubMed] [Google Scholar]
- Singleton, C. K., and P. R. Martin, 2001. Molecular mechanisms of thiamine utilization. Curr. Mol. Med. 1: 197–207. [DOI] [PubMed] [Google Scholar]
- Tallaksen, C. M., T. Bohmer, J. Karlsen and H. Bell, 1997. Determination of thiamin and its phosphate esters in human blood, plasma, and urine. Methods Enzymol. 279: 67–74. [DOI] [PubMed] [Google Scholar]
- Wong, A., P. Boutis and S. Hekimi, 1995. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics 139: 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka, K., 1984. Some properties of the thiamine uptake system in isolated rat hepatocytes. Biochim. Biophys. Acta 778: 201–209. [DOI] [PubMed] [Google Scholar]
- Yoshioka, K., H. Nishimura, K. Sempuku and A. Iwashima, 1983. Inability of thiamine phosphates transport in isolated rat hepatocyte. Experientia 39: 505–507. [DOI] [PubMed] [Google Scholar]
- Zhao, R., F. Gao, Y. Wang, G. A. Diaz, B. D. Gelb et al., 2001. Impact of the reduced folate carrier on the accumulation of active thiamin metabolites in murine leukemia cells. J. Biol. Chem. 276: 1114–1118. [DOI] [PubMed] [Google Scholar]
- Zhao, R., F. Gao and I. D. Goldman, 2002. Reduced folate carrier transports thiamine monophosphate: an alternative route for thiamine delivery into mammalian cells. Am. J. Physiol. Cell Physiol. 282: C1512–C1517. [DOI] [PubMed] [Google Scholar]