Abstract
We selected on divergent photoperiodic response in three separate lines from a natural population of the pitcher-plant mosquito, Wyeomyia smithii. Line crosses reveal that there exists within a population, diverse epistatic variation for a fitness trait that could contribute to adaptive potential following founder events or rapid climate change.
A fundamental tenet of evolutionary genetics is that a trait's ability to respond to selection is directly related to the additive genetic variation (or evolvability, sensu Houle 1992) of that trait. In the absence of nonadditive genetic variation, response to stabilizing or directional selection or isolation and drift following a founder event should result in reduced additive genetic variation in the population. However, in the presence of nonadditive genetic variation, selection or isolation and drift can result in increased additive genetic variation, facilitating the evolution of populations having experienced a genetic bottleneck or confronting environmental change (Mayr 1954; Templeton 1980, 1996; Goodnight 1988, 2000; Carson 1990; Cheverud and Routman 1996; Slatkin 1996; Meffert 1999, 2000; Cheverud 2000; Naciri-Graven and Goudet 2003).
This generation of additive from epistatic variation has been invoked to explain the increase in additive genetic variation for photoperiodic response among populations of the pitcher-plant mosquito, Wyeomyia smithii, dispersing along a latitudinal gradient into postglacial northern North America (Hard et al. 1992, 1993). Hard et al.'s argument was based on three observations and two fundamental assumptions. The observations were, first, that contrary to the expectations of directional selection on a latitudinal scale and stabilizing selection on a local scale, the additive genetic variance for photoperiodic response increased with latitude. Second, genetic differences in photoperiodic response between southern and northern populations involved differences in epistasis. Third, the contribution of different forms of digenic epistasis (additive × additive, additive × dominance, dominance × dominance) was unique to each cross. The latter observation was important because it implied stochastic reordering of genic interactions as would be expected as a consequence of random drift following independent founder events. The two assumptions were that postglacial dispersal had taken place by sequential founder events along a latitudinal gradient and that there is actually epistatic variation for photoperiodic response within populations. The first of these assumptions was supported by Armbruster et al. (1998) who showed that average heterozygosity at 10 allozyme loci decreased with latitude from the approximate southern limit of the Laurentide Ice Sheet (∼40° N in New Jersey) northward. The second of these assumptions is the topic of the present article.
Hybridization experiments have revealed genetic differences attributable to epistasis among species (Doebley et al. 1995) and among populations within species (Hard et al. 1993; Lair et al. 1997; Fenster and Galloway 2000; Galloway and Fenster 2000; Carroll et al. 2001, 2003). In theory, one might expect little epistatic variation within populations as selection should favor an optimal combination of alleles (Whitlock et al. 1995), but crosses between selected lines within populations reveal that such epistatic variation can exist (Mather and Jinks 1982; Cheverud 2000). We use the latter approach to ask whether lines of W. smithii, selected for divergent photoperiodic response, differ in epistasis. W. smithii enters a larval dormancy that is initiated, maintained, and terminated by photoperiod (day length; Bradshaw and Lounibos 1977). The critical photoperiod is the length of day at which an individual switches between active development and dormancy and, in direct response to seasonal selection, increases with latitude and altitude of population origin (Bradshaw and Lounibos 1977; Bradshaw et al. 2003). The range of W. smithii extends from the Gulf of Mexico to northern Canada. We collected mosquitoes from a mid-latitude locality along a stream meandering through a cedar swamp in the New Jersey Pine Barrens (40° N, locality “PB” of earlier studies from this lab). To increase independence of the replicate lines, we collected from three subpopulations within a 200-m radius of each other (Bradshaw et al. 2003): “streamside” from along the stream itself; “backwater” from a backwater of the stream ∼100 m north of the first collection site; and “sandy bog” from a sandy bog ∼300 m to the west of the stream and separated from it by dry pine woodlands. We imposed divergent selection for long and short critical photoperiods and then crossed the selected lines from within each subpopulation (Table 1). We tested the specific prediction that, if there were genetic variation at epistatically acting loci in the original population, then genetic differences in photoperiodic response between selected lines derived from the same starting subpopulation should include epistasis.
TABLE 1.
Results of the joint-scaling test based on generation means and variances between lines ofW. smithii selected for long and short critical photoperiods
| Joint-scaling testa
|
||||
|---|---|---|---|---|
| Subpopulation | A 4 d.f. |
AD 3 d.f. |
ADM 4 d.f. |
ADME 1 d.f. |
| Streamside | ||||
| χ2 | 99.29 | 70.47 | 80.55 | 10.54b |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
| Backwater | ||||
| χ2 | 252.90 | 95.11 | 91.04 | 1.49 |
| P | <0.001 | <0.001 | <0.001 | <0.122 |
| Sandy bog | ||||
| χ2 | 298.66 | 58.21 | 86.25 | 0.04 |
| P | <0.001 | <0.001 | <0.001 | 0.844 |
Each subpopulation was transported to the lab, reared through three lab generations to minimize field effects, divergently selected for critical photoperiod for 13 generations with cumulative inbreeding <5% (Bradshaw et al. 2003), reared through 9 subsequent generations with N > 1000 to permit recombination, and then crossed to test for epistatic differences between lines within subpopulations. The two parent populations were hybridized to produce 14 “generations”: the two parents, F1, F2, both backcrosses, and all of their possible reciprocals. The experimental generations were reared on short days to induce dormancy and to synchronize development. Day lengths were then increased by 3 min·day−1, ultimately inducing development and pupation in each individual. The day length on the day of pupation was then scored as the critical photoperiod of the pupating individual. Since development under these conditions was log-normally distributed, individual critical photoperiods were log10 transformed prior to analyses. The number of parents of the experimental larvae averaged 85 females and 115 males; the number of experimental larvae per “generation” averaged 206.
The joint-scaling test (Lair et al. 1997) tests sequentially for goodness of fit to successively more inclusive models: A, additive; AD, additive-dominance; ADM, additive-dominance-maternal (including both additive-maternal and dominance-maternal effects); ADME, additive-dominance-maternal-digenic epistasis models. Mather and Jinks' (1982) F-∞ parameterization was used to calculate expected generation means. A high value of χ2 and a significant P-value indicate inadequacy (poor goodness of fit) of the model and that more inclusive genetic effects need to be considered. Note that in the presence of substantial epistasis, estimates of additive and dominance effects are difficult to interpret (Hayman 1958, 1960). A copy of our joint-scaling test written in Mathcad 4.0 is available on request.
Heterogeneity chi square (Zar 1996: χ2 = 11.95, d.f. = 2, P = 0.003) indicates significant differences among subpopulations in rejection of the ADME model.
Response to selection on critical photoperiod consistently revealed substantial epistatic differences between selected lines (Table 1; Figure 1). We interpret these results to mean that there was genetic variation among interacting loci in each of the respective subpopulations in nature. These results are consistent with Hard et al.'s (1992)(1993) assumption that within ancestral populations there existed the epistatic genetic variance from which additive genetic variance could have been released during successive, sequential founder events in the northward dispersal of W. smithii following recession of the Laurentide Ice Sheet.
Figure 1.—
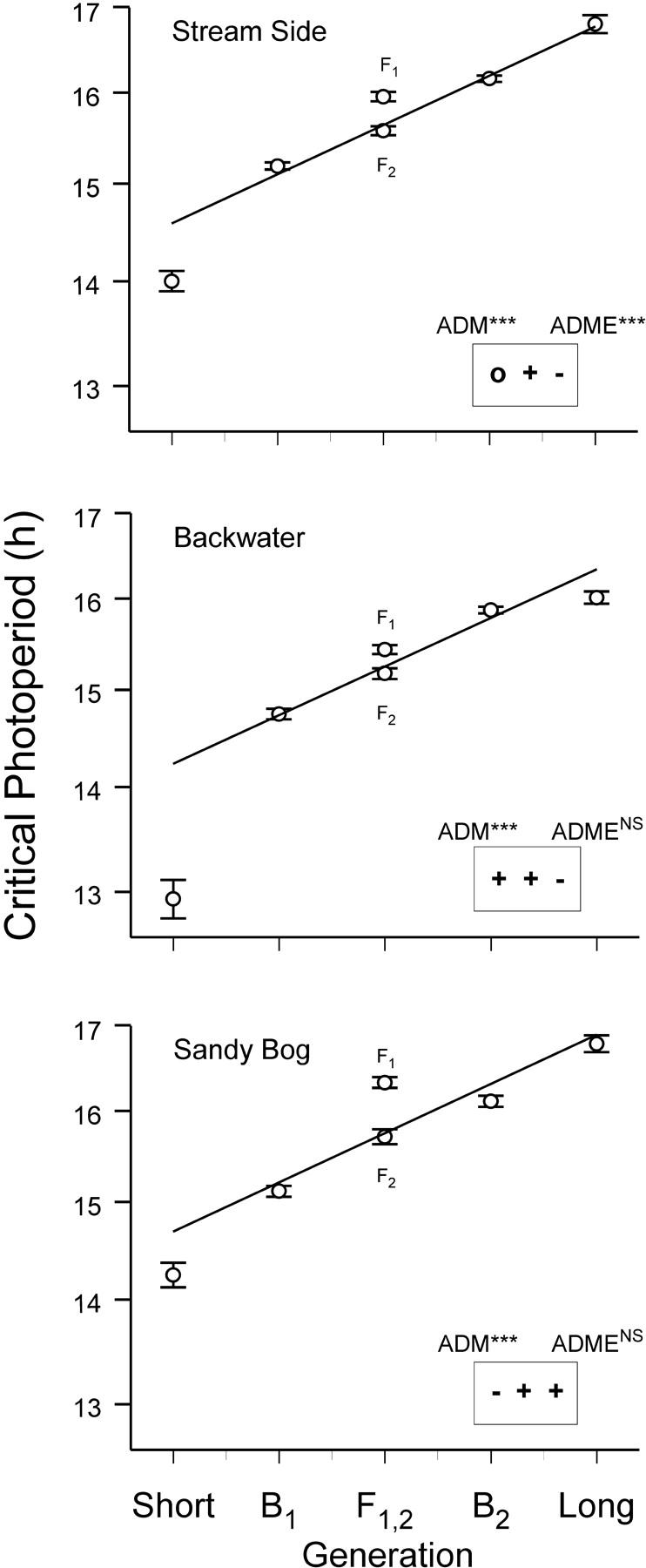
Critical photoperiod of long and short selected lines and their F1 and F2 hybrids and backcrosses (B1, B2). The plots show mean log10(critical photoperiod, hr ± 2SE). The solid line shows the weighted least-squares expectation of an additive model. The results of the joint-scaling test (see also Table 1) are shown for the additive-dominance-maternal (ADM) and the ADME models; ***P < 0.001; NSP > 0.05. The symbols in the boxes refer to the sign of digenic epistasis (+, significant and positive; −, significant and negative; 0, not significant) in the order A × A, A × D, D × D from the coefficients in Table 2.
The genetic “fingerprint” of epistasis was distinct for each subpopulation (Figure 1). In two of the subpopulations, additive, dominance, maternal, and digenic epistatic effects were sufficient to account for genetic differences in critical photoperiod between the selected lines; but, in one of the subpopulations (streamside), higher-order epistasis or linkage was implicated. Since the selected lines had been maintained for nine potentially recombining generations without selection prior to testing for epistasis, higher-order epistasis was more likely than linkage to be responsible for rejection of the additive-dominance-maternal-digenic epistasis (ADME) model in this cross. In addition, the pattern of coefficients of digenic epistasis was distinct for each subpopulation (Figure 1; Table 2). First, the contribution of additive-by-additive epistasis was negative in the sandy bog subpopulation and positive in the other two. Second, the contribution of additive by dominance epistasis was less in the sandy bog than in the backwater subpopulation. Third, the contribution of dominance-by-dominance epistasis differed among all three subpopulations and was positive in the sandy bog but negative in the other two subpopulations.
TABLE 2.
Components of digenic epistasis contributing to genetic differences among subpopulations ofW. smithiiselected for long and short critical photoperiods
| Digenic epistatic effectsa
|
|||
|---|---|---|---|
| Subpopulation | A × A | A × D | D × D |
| Streamside | |||
| Coefficients | 0.00733A | 0.253MN | −0.0254X |
| t-testb | 1.71NS | 7.90*** | 4.08*** |
| Backwater | |||
| Coefficients | 0.0163A | 0.0289M | −0.0609Y |
| t-test | 3.43*** | 6.68*** | 8.61*** |
| Sandy bog | |||
| Coefficients | −0.0187B | 0.0158N | 0.0431Z |
| t-test | 3.09*** | 4.24*** | 5.54*** |
Coefficients for the components of digenic epistasis: A × A, additive by additive; A × D, additive by dominance; D × D, dominance by dominance; within each column, coefficients followed by the same letter do not differ by Wald χ2 (Fox et al. 2004); significant differences: M vs. N, P < 0.05; A vs. B and X vs. Y vs. Z, P < 0.001.
t-test for significant effect of the components of digenic epistasis: NSP = 0.089, ***P < 0.001.
These distinct genetic fingerprints indicate that either population subdivision is occurring over a very fine microscale (e.g., Fenster and Galloway 2000) or unique genetic trajectories underlie similar phenotypic trajectories in response to a uniform selection gradient (e.g., Travisano and Lenski 1996), or a combination of these processes. Regardless of which of these processes is operating in W. smithii, our results show that diverse genetic trajectories of genic interactions are available to respond to short-term selection within a natural population of W. smithii. This availability may have contributed not only to the generation of additive genetic variation over millennial time scales since the recession of the Laurentide Ice Sheet, but also to the rapid genetic response of W. smithii to recent climate change (Bradshaw and Holzapfel 2001).
Acknowledgments
We are grateful to P. Zani for useful discussion, Thomas Hansen and Tadeusz Kawecki for helpful comments on earlier versions of the manuscript, Charles Fox and Derek Roff for statistical advice, and the National Science Foundation for support through grants DEB-9806278 and IBN-9814438.
References
- Armbruster, P. A., W. E. Bradshaw and C. M. Holzapfel, 1998. Effects of postglacial range expansion on allozyme and quantitative genetic variation in the pitcher-plant mosquito, Wyeomyia smithii. Evolution 52: 1697–1704. [DOI] [PubMed] [Google Scholar]
- Bradshaw, W. E., and C. M. Holzapfel, 2001. Genetic shift in photoperiodic response correlated with global warming. Proc. Natl. Acad. Sci. USA 98: 14509–14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw, W. E., and L. P. Lounibos, 1977. Evolution of dormancy and its photoperiodic control in pitcher-plant mosquitoes. Evolution 31: 546–567. [DOI] [PubMed] [Google Scholar]
- Bradshaw, W. E., M. C. Quebodeaux and C. M. Holzapfel, 2003. Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: Adaptive response to the photic environment or correlated response to the seasonal environment? Am. Nat. 161: 735–748. [DOI] [PubMed] [Google Scholar]
- Carroll, S. P., H. Dingle, T. R. Famula and C. W. Fox, 2001. Genetic architecture of adaptive differentiation in evolving host races of the soapberry bug, Jadera haematoloma. Genetica 112/113: 257–272. [PubMed] [Google Scholar]
- Carroll, S. P., H. Dingle and T. R. Famula, 2003. Rapid appearance of epistasis during adaptive divergence following colonization. Proc. R. Soc. Lond. Ser. B 270(Suppl. 1): S80–S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson, H. L., 1990. Increased genetic variation after a bottleneck. Trends Ecol. Evol. 5: 228–230. [DOI] [PubMed] [Google Scholar]
- Cheverud, J. M., 2000 Detecting epistasis among quantitative trait loci, pp. 58–81 in Epistasis and the Evolutionary Process, edited by J. B. Wolf, E. D. Brodie, III and M. J. Wade. Oxford University Press, Oxford.
- Cheverud, J. M., and E. J. Routman, 1996. Epistasis as a source of increased additive genetic variance at population bottlenecks. Evolution 50: 1042–1051. [DOI] [PubMed] [Google Scholar]
- Doebley, J., A. Stec and C. Gustus, 1995. teosinte branched1 and the origin of maize: evidence for epistasis and the evolution of dominance. Genetics 141: 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster, C. B., and L. F. Galloway, 2000 The contribution of epistasis to the evolution of natural populations: a case study of an annual plant, pp. 232–244 in Epistasis and the Evolutionary Process, edited by J. B. Wolf, E. D. Brodie, III and M. J. Wade. Oxford University Press, Oxford.
- Fox, C. W., M. E. Czesak and W. G. Wallin, 2004. Complex genetic architecture of population differences in adult lifespan of a beetle: nonadditive inheritance, gender differences, body size, and a large maternal effect. J. Evol. Biol. 17: 1007–1017. [DOI] [PubMed] [Google Scholar]
- Galloway, L. F., and C. B. Fenster, 2000. Population differentiation in an annual legume: local adaptation. Evolution 54: 1173–1181. [DOI] [PubMed] [Google Scholar]
- Goodnight, C. J., 1988. Epistasis and the effect of founder events on the additive genetic variance. Evolution 42: 441–454. [DOI] [PubMed] [Google Scholar]
- Goodnight, C. J., 2000 Modeling gene interaction in structured populations, pp. 129–145 in Epistasis and the Evolutionary Process, edited by J. B. Wolf, E. D. Brodie, III and M. J. Wade. Oxford University Press, Oxford.
- Hard, J. J., W. E. Bradshaw and C. M. Holzapfel, 1992. Epistasis and the genetic divergence of photoperiodism between populations of the pitcher-plant mosquito, Wyeomyia smithii. Genetics 131: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard, J. J., W. E. Bradshaw and C. M. Holzapfel, 1993. The genetic basis of photoperiodism and evolutionary divergence among populations of the pitcher-plant mosquito, Wyeomyia smithii. Am. Nat. 142: 457–473. [DOI] [PubMed] [Google Scholar]
- Hayman, B. I., 1958. The separation of epistatic from additive and dominance variation in generation means. Heredity 12: 371–390. [DOI] [PubMed] [Google Scholar]
- Hayman, B. I., 1960. The separation of epistatic from additive and dominance variation in generation means II. Genetica 31: 133–146. [DOI] [PubMed] [Google Scholar]
- Houle, D., 1992. Comparing evolvability of quantitative traits. Genetics 143: 1467–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lair, K. P., W. E. Bradshaw and C. M. Holzapfel, 1997. Evolutionary divergence of the genetic architecture underlying photoperiodism in the pitcher-plant mosquito, Wyeomyia smithii. Genetics 147: 1873–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather, K., and J. L. Jinks, 1982 Biometrical Genetics. Chapman & Hall, London.
- Mayr, E., 1954 Change of genetic environment and evolution, pp. 157–180 in Evolution as a Process, edited by J. S. Huxley, A. C. Hardy and E. B. Ford. Allen & Unwin, London.
- Meffert, L. M., 1999. How speciation experiments relate to conservation biology. Bioscience 139: 365–374. [Google Scholar]
- Meffert, L. M., 2000 The evolutionary potential of morphology and mating behavior: the role of epistasis in bottlenecked populations, pp. 177–193 in Epistasis and the Evolutionary Process, edited by J. B. Wolf, E. D. Brodie, III and M. J. Wade. Oxford University Press, Oxford.
- Naciri-Graven, Y., and J. Goudet, 2003. The additive genetic variance after bottlenecks is affected by the number of loci involved in epistatic interactions. Evolution 57: 706–716. [DOI] [PubMed] [Google Scholar]
- Slatkin, M., 1996. In defense of founder-flush theories of speciation. Am. Nat. 147: 493–505. [Google Scholar]
- Templeton, A. R., 1980. The theory of speciation via the founder principle. Genetics 94: 1011–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton, A. R., 1996. Experimental evidence for the genetic-transilence model of speciation. Evolution 50: 909–915. [DOI] [PubMed] [Google Scholar]
- Travisano, M., and R. E. Lenski, 1996. Long-term experimental evolution in Escherichia coli. IV. Targets of selection and the specificity of adaptation. Genetics 143: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock, M. C., P. C. Phillips, F. B.-G. Moore and S. J. Tonsor, 1995. Multiple fitness peaks and epistasis. Annu. Rev. Ecol. Syst. 26: 601–629. [Google Scholar]
- Zar, J. H., 1996 Biostatistical Analysis. Prentice-Hall, Upper Saddle River, NJ.


