Abstract
Objective:
To analyze long-term weight loss, changes in comorbidities and quality of life, and late complications after laparoscopic and open gastric bypass.
Summary Background Data:
Early results from our prospective randomized trial comparing the outcome of laparoscopic versus open gastric bypass demonstrated less postoperative pain, shorter length of hospital stay, fewer wound-related complications, and faster convalescence for patients who underwent laparoscopic gastric bypass.
Methods:
Between May 1999 and March 2001, 155 morbidly obese patients were enrolled in this prospective trial, in which 79 patients were randomized to laparoscopic gastric bypass and 76 to open gastric bypass. Two patients in the laparoscopic group required conversion to open surgery; their data were analyzed within the laparoscopic group on an intention-to-treat basis. The 2 groups were well matched for body mass index, age, and gender. Outcome evaluation included weight loss, changes in comorbidities and quality of life, and late complications.
Results:
The mean follow-up was 39 ± 8 months. There were no significant differences in the percent of excess body weight loss between the 2 groups at the 3-year follow-up (77% for laparoscopic versus 67% for open). The rate of improvement or resolution of comorbidities was similar between groups. Improvement in quality of life, measured by the Moorehead-Ardelt Quality of Life Questionnaire, was observed in both groups without significant differences between groups. Late complications were similar between groups except for the rate of incisional hernia, which was significantly greater after open gastric bypass (39% versus 5%, P < 0.01), and the rate of cholecystectomy, which was greater after laparoscopic gastric bypass (28% versus 5%, P = 0.03).
Conclusions:
In this randomized trial with a 3-year follow-up, we found that laparoscopic gastric bypass was equally effective as open gastric bypass with respect to weight loss and improvement in comorbidities and quality of life. A major advantage at long-term follow-up for patients who underwent laparoscopic gastric bypass was the reduction in the rate of incisional hernia.
In this randomized trial of laparoscopic versus open gastric bypass with a 3-year follow-up, we found that laparoscopic gastric bypass was equally effective as open gastric bypass with regard to weight loss, improvement in comorbidities, and quality of life. A major advantage of the laparoscopic approach on long-term follow-up is the reduction in incisional hernias.
Roux-en-Y gastric bypass is an effective surgical treatment of morbid obesity. The mortality and development of health-related conditions have been shown to decrease in patients who underwent Roux-en-Y gastric bypass compared with severely obese patients who had not undergone weight reduction surgery.1 Roux-en-Y gastric bypass is conventionally performed through an upper midline incision. One of the disadvantages of the open technique is the high incidence of wound-related complications, primarily infection and incisional hernia. The incidence of wound infection after open gastric bypass has been reported to be as high as 25%, and the incidence of incisional hernia has been reported to be as high as 26%.2,3 In an attempt to improve the outcome of conventional Roux-en-Y gastric bypass, Wittgrove et al,4 in 1994, reported the first clinical series of gastric bypass performed via laparoscopy. The primary differences between open and laparoscopic gastric bypass are the method of access (upper midline abdominal incision versus 5 trocar incisions) and method of exposure (abdominal wall retractors versus pneumoperitoneum). The clinical benefits of laparoscopic gastric bypass have been reported in 2 randomized controlled trials.5,6 In 2001, we reported our initial results of a randomized trial of laparoscopic versus open gastric bypass in 155 patients.5 Our study demonstrated that patients who underwent laparoscopic gastric bypass experienced less postoperative pain, decreased impairment of postoperative pulmonary function, shorter length of hospital stay, more rapid return to work and activities of daily living, and a decreased rate and severity of wound infections.5,6 In another randomized trial of laparoscopic versus open gastric bypass, Lugan et al7 similarly found that patients who underwent laparoscopic gastric bypass had a shorter length of hospital stay and lower incidence of incisional hernia. Early weight loss was similar between the 2 groups of patients.5,7
Despite the favorable results from these randomized trials and other cohort studies, third-party payers have been reluctant to provide coverage for laparoscopic gastric bypass and some insurance payers have considered the laparoscopic approach to be experimental.5,7,8 In September 2003, the Blue Cross and Blue Shield Association's Technology Evaluation Center reported that there is insufficient evidence to form conclusions about the relative efficacy and morbidity of laparoscopic gastric bypass.9 One of the reasons for their assessment was the paucity of high-quality clinical trials comparing long-term outcomes of laparoscopic versus open gastric bypass. Since physiologic principles of open Roux-en-Y gastric bypass procedure are similar to the laparoscopic procedure, we hypothesized that long-term weight loss, resolution of comorbidities, and improvement in quality of life should be equivalent between the 2 groups. This article reports the 3-year results of our randomized trial comparing laparoscopic versus open gastric bypass with emphasis on weight loss, changes in comorbidities and quality of life, and late complications.
METHODS
We conducted a prospective randomized trial comparing the outcomes of laparoscopic versus open gastric bypass for the treatment of morbid obesity in 155 patients from May 1999 to March 2001.5 Eligibility for inclusion in this study included patients with a body mass index (BMI) of 40 to 60 kg/m2 undergoing evaluation for bariatric surgery, who were 21 to 60 years of age, and had failed at all previous medical interventions for weight loss. Exclusion criteria included patients who had previous bariatric surgery, previous gastric surgery, a large abdominal ventral hernia, a history of deep venous thrombosis or pulmonary embolism, and severe cardiovascular, respiratory, hepatic, or renal disease. Randomization was performed by the use of “sealed envelope” technique stratified by BMI (<50 versus ≥50) and in blocks of 6 patients. Two patients initially allocated to open gastric bypass were excluded from the study after randomization: 1 patient withdrew consent and underwent laparoscopic gastric bypass and the other patient had intraoperative splenic injury requiring iatrogenic splenectomy and did not complete the gastric bypass procedure. The decision to perform a cholecystectomy concomitantly with the gastric bypass procedure was based on the presence of gallstones on preoperative ultrasound examination and at the discretion of the surgeon if the patient was randomized to the laparoscopic approach. Patients with gallstones undergoing laparoscopic gastric bypass could also have the cholecystectomy as a staged procedure several months after the gastric bypass procedure. Prophylactic ursodeoxycholic acid (Ursodiol) was given postoperatively as a preventive measure against gallstone formation. Before entering into the trial, written informed consent was obtained from all patients. This study was approved by the Institutional Review Board of the University of California, Davis Medical Center.
Surgical Technique
The anesthetic methods and techniques were similar between the 2 groups. All patients were given a single dose of preoperative and postoperative antibiotics. Sequential pneumatic compression devices were placed on both lower extremities for deep venous thrombosis prophylaxis. A 15- to 20-mL transected gastric pouch was created in both groups; a 75-cm Roux limb was constructed for patients with a BMI of 40 to 49 kg/m2 and a 150-cm Roux limb was used for patients with a BMI ≥ 50 kg/m2. The gastrojejunostomy anastomosis was performed with a circular stapler.
Laparoscopic gastric bypass was performed through 5 abdominal trocars. Our technique of laparoscopic gastric bypass has been described previously.5 Briefly, intra-abdominal pressure was maintained at 15 mm Hg. The dissection began directly on the lesser curve of the stomach to gain entrance into the lesser sac. Through an anterior gastrotomy, the anvil of the circular stapler was inserted into the stomach and positioned through the anterior gastric wall approximately 1 cm below the gastroesophageal junction. The linear staplers were applied around the anvil to create a 15- to 30-mL gastric pouch. The jejunum was divided 30-cm distal to the ligament of Treitz. The Roux limb length was measured and the jejunojejunostomy anastomosis constructed with a 60-mm linear stapler. The small bowel mesenteric defect was closed with sutures. The Roux limb was tunneled along a retrocolic, retrogastric path and positioned near the transected gastric pouch. The transverse colon mesenteric defect and Petersen defect were closed with interrupted sutures. The circular stapler was inserted transabdominally and positioned into the end of the jejunal Roux limb to create an end-to-side gastrojejunostomy anastomosis. The jejunal opening was closed with a linear stapler. The anastomosis was oversewn with interrupted sutures and tested endoscopically for air leaks. The 12-mm trocar sites were closed with interrupted sutures.
Open gastric bypass was performed through an upper midline incision. A Thompson abdominal wall retractor (Thompson Surgical Instruments, Inc., Traverse City, MI) was used to provide exposure of the operative field. An anterior gastrotomy was created, and the anvil of the circular stapler was inserted into the stomach and positioned through the anterior gastric wall. A 15- to 30-mL gastric pouch was created with the linear staplers. The jejunum was divided 30 cm distal to the ligament of Treitz. The Roux limb length was measured as previously stated. The jejunojejunostomy anastomosis was constructed with a 60-mm linear stapler. The small bowel mesenteric defect was closed. The Roux limb was placed in the retrocolic and retrogastric position. The transverse mesocolon defect and Petersen defect were closed with interrupted sutures. The circular stapler was positioned into the end of the jejunal Roux limb to create an end-to-side gastrojejunostomy anastomosis. The jejunal opening was closed with a linear stapler. The anastomosis was oversewn with interrupted sutures and tested endoscopically for air leaks. The midline fascia layer was closed with continuous running nonabsorbable sutures. The skin was approximated with staples.
Follow-up
Patients were seen in the University of California, Davis Medical Center multidisciplinary bariatric clinic for follow-up at 1, 3, 6, 9, and 12 months, then yearly thereafter. Follow-up data were collected by interview of the patient at the bariatric clinic visit, chart review, and phone interview with the primary care physician and the patient. All telephone and mail surveys included inquiries regarding changes in comorbidities and quality of life, weight loss, and the presence of late complications. Multiple attempts were made to contact patients with lost to follow-up, including phone call at home and work, e-mail, and by routine mail.
Weight Loss
Weight loss was expressed as the percentage of excess body weight loss. The ideal body weight was derived from the “Metropolitan Life” tables of height and weight using the middle weight of a medium-framed person.
Changes in Comorbidities
Changes in comorbidities were analyzed according to the following scale: a designation of worse was assigned if the patient had worsening of symptoms such as requiring higher doses of medication or the need for new medication. A designation of unchanged was assigned if the patient had no change in symptoms or medication use. A designation of improved was assigned if the patient experienced decreased severity or frequency of symptoms or decreased dosage or number of medications. A designation of resolved was assigned if the patient had resolution of symptoms and did not require medication for treatment. In the case of sleep apnea, the use of continuous positive airway pressure mask was considered treatment in lieu of medication.
Quality of Life Assessment
All patients completed the Moorehead-Ardelt Quality of Life Questionnaire administered at 1, 2, and 3 years after surgery. The Moorehead-Ardelt Questionnaire assesses 5 categories, including self-esteem, physical activity, social life, work conditions, and sexual interest/activity. Points are added for positive changes and subtracted for negative changes. The Moorehead-Ardelt Quality of Life Questionnaire is also a part of the Bariatric Analysis and Reporting Outcome System (BAROS).
The BAROS takes into account 3 main outcome categories: percent of excess body weight loss, changes in comorbidities, and the Moorehead-Ardelt Quality of Life Questionnaire. A maximum of 3 points is given for each category. In the BAROS, points are deducted for complications and reoperations from the subtotal scores of the 3 categories. The BAROS scores are classified as excellent (>7–9 points), very good (>5–7 points), good (>3–5 points), fair (>1–3 points), and failure (1 point or less).
Late Complications
Late complications that occurred more than 3 months after laparoscopic and open gastric bypass were recorded. All patients were examined at follow-up for the presence of an incisional hernia, which was defined as the presence of fascial defect(s) at the incision site resulting in herniation of abdominal contents. Other late complications that were recorded included the development of gallstones, marginal ulceration, chronic nausea or vomiting, chronic abdominal pain, anemia, and metabolic deficiencies.
Statistics
Continuous data are expressed as mean ± SD. Analyses of differences between groups for demographics and comorbidities were performed using 2-sample t tests and Fisher exact test tests for categorical data. Mann-Whitney tests were performed for nonparametric data. The data in this trial were analyzed on an intention-to-treat basis so that results of patients randomized to laparoscopic gastric bypass who required conversion to laparotomy were included in the laparoscopic group. Differences in improvement of comorbidities and complications between groups were analyzed by Mantel-Haenszel χ2 tests. Repeated measures of analysis of variance were used to analyze the changes in the percent of excess body weight loss at follow-up. The Moorehead-Ardelt Quality of Life scores were compared between groups using unpaired t tests. Statistical evaluations were performed using the SPSS statistical software, version 12.0 (SPSS Inc., Chicago, IL) by a senior statistician in the Department of Statistics at the University of California, Davis (Mitchell Watnik, PhD). A P value of less than 0.05 was considered significant.
RESULTS
Patient Characteristics
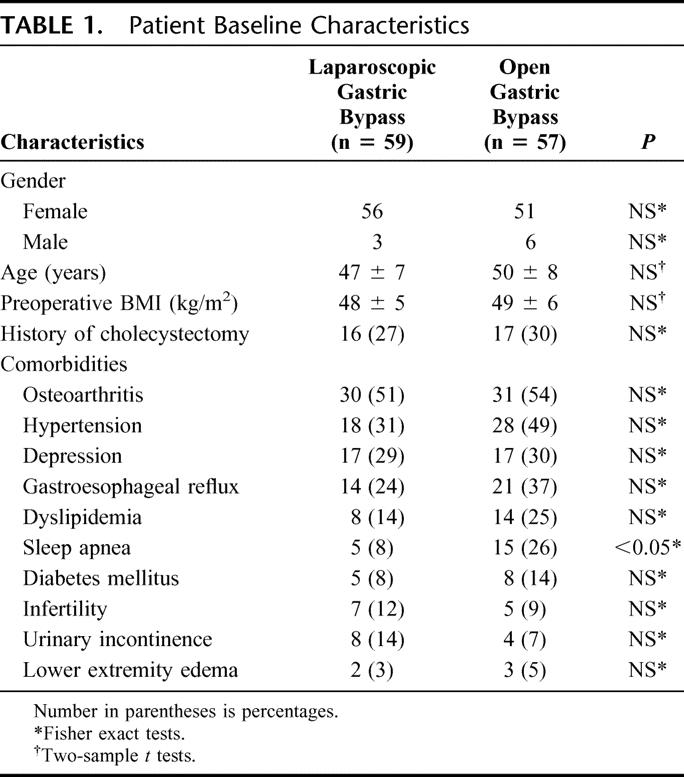
The short-term results and cost analysis of 155 patients randomized to laparoscopic or open gastric bypass have been previously published.5 A total of 116 (75%) of the original 155 patients were available for follow-up: 59 of 79 patients who underwent laparoscopic gastric bypass and 57 of 76 patients who underwent open gastric bypass. The mean follow-up was 39 ± 8 months (range, 24–58 months). Two patients in the laparoscopic group required conversion to open surgery. The groups were well matched for age, BMI, and gender. Baseline comorbidities did not differ significantly between groups except for sleep apnea, which was more prevalent in the open group (Table 1). A cholecystectomy had been done previously in 27% of laparoscopic gastric bypass patients and 30% of open gastric bypass patients. Concomitant cholecystectomy for gallstones was performed in 3 of 59 patients in the laparoscopic group and 14 of 57 patients in the open group.
TABLE 1. Patient Baseline Characteristics
Weight Loss
The percent of excess body weight loss at 3-years was 77% ± 22% for laparoscopic gastric bypass and 67% ± 21% for open gastric bypass (Fig. 1). There were no significant differences in percent of excess body weight loss after laparoscopic and open gastric bypass at 4-year follow-up (76% ± 19% versus 71% ± 25%, respectively).
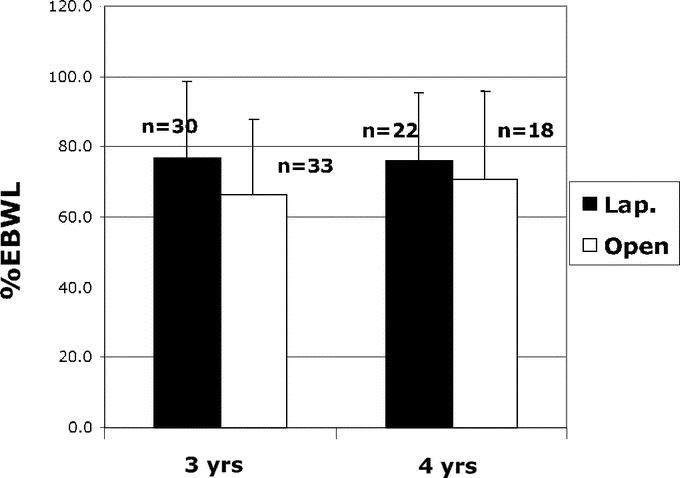
FIGURE 1. Percentage of excess body weight loss (EBWL) at 3-year and 4-year follow-up after laparoscopic and open gastric bypass.
Changes in Comorbidities
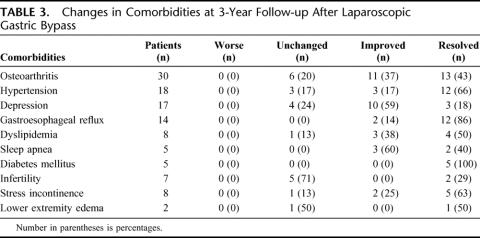
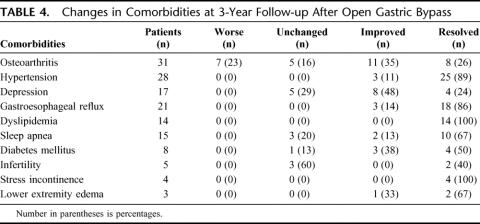
Differences in resolution or improvement of obesity-related comorbid conditions between laparoscopic and open gastric bypass are reported in Table 2. There was no significant difference in the percent of patients with improvement or resolution of comorbidities between the 2 groups except for osteoarthritis and dyslipidemia. Laparoscopic gastric bypass patients had a significantly greater improvement of arthritic symptoms, whereas open gastric bypass patients had a significantly greater improvement of dyslipidemia. Details of the number of patients affected by the various comorbidities and the percentage of patients with resolution of symptoms, improvement of symptoms, no change in symptoms, or worsening of symptoms after laparoscopic and open gastric bypass are reported in Tables 3 and 4, respectively.
TABLE 2. Improvement or Resolution of Comorbidities at 3-Year Follow-up After Laparoscopic and Open Gastric Bypass
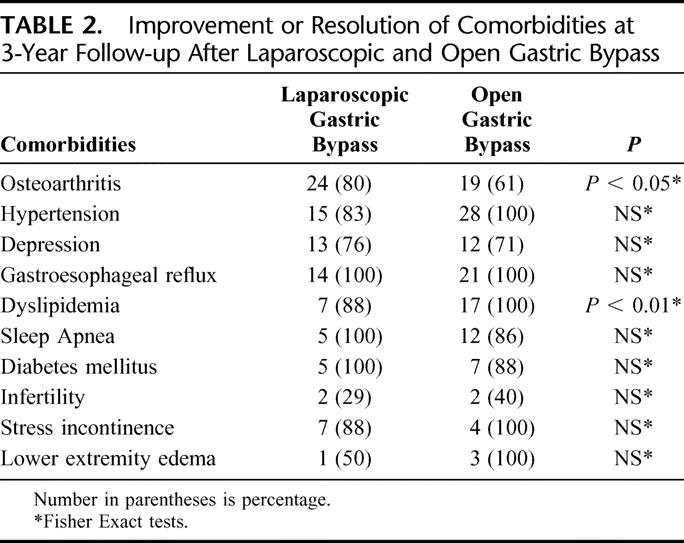
TABLE 3. Changes in Comorbidities at 3-Year Follow-up After Laparoscopic Gastric Bypass
TABLE 4. Changes in Comorbidities at 3-Year Follow-up After Open Gastric Bypass
Quality of Life Assessment
Changes in Moorehead-Ardelt Quality of Life scores after laparoscopic (n = 22) and open (n = 22) gastric bypass are depicted in Figure 2. Positive changes were observed in all 5 categories of the Moorehead-Ardelt Quality of Life Questionnaire and there were no significant differences in the scores between groups. The Moorehead-Ardelt Quality of Life scores are as follows: self-esteem (0.89 for laparoscopic versus 0.88 for open), physical activity (0.40 for laparoscopic versus 0.36 for open), social life (0.34 for laparoscopic versus 0.33 for open), labor or work conditions (0.33 for laparoscopic versus 0.25 for open), and sexual interest/activity (0.20 for laparoscopic versus 0.24 for open).
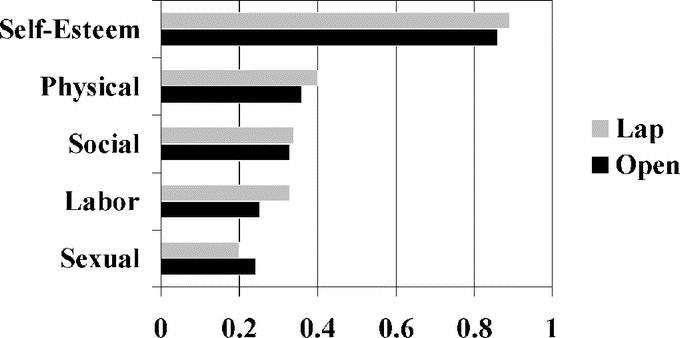
FIGURE 2. Moorehead-Ardelt Quality of Life scores at 3-year follow-up after laparoscopic and open gastric bypass. *The zero point mark represents no changes compared with baseline. Points are added for positive changes and subtracted for negative changes.
The BAROS were evaluated on 22 patients who underwent laparoscopic gastric bypass and 22 patients who underwent open gastric bypass (Fig. 3). The overall failure rate for the 44 patients who underwent laparoscopic or open gastric bypass was 2.3%. Fair results were observed in 4.5% of laparoscopic gastric bypass patients and 9.1% of open gastric bypass patients. There were no significant differences in the percentage of patients who reported “good,” “very good,” and “excellent” results after laparoscopic versus open gastric bypass (95.5% versus 86.4%, respectively).
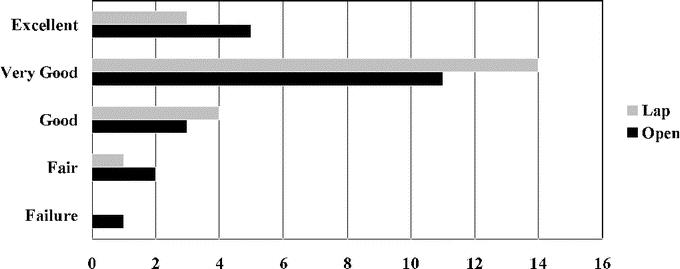
FIGURE 3. BAROS data at 3-year follow-up after laparoscopic and open gastric bypass.
Late Complications
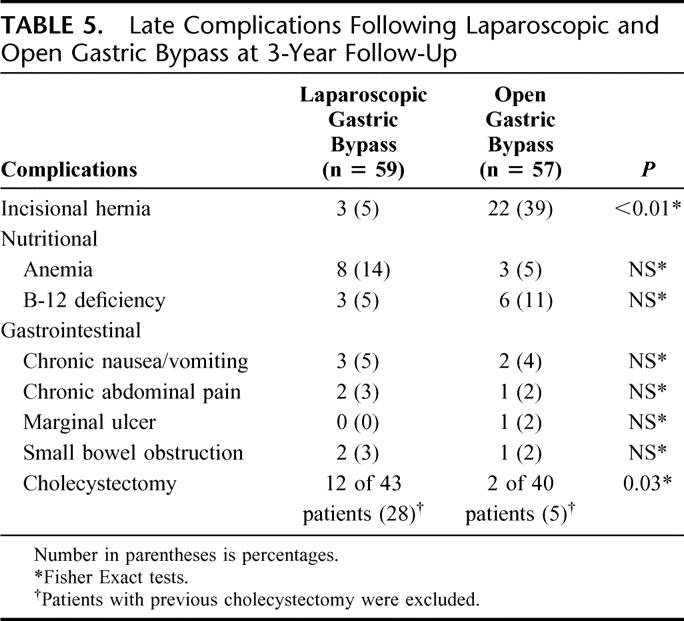
There was no statistical difference in the proportion of patients who experienced chronic nausea and vomiting, abdominal pain, marginal ulceration, anemia, and B12 deficiency between groups (Table 5). A greater proportion of patients developed incisional hernia after open gastric bypass (39% versus 5%, P < 0.01). Of the 22 patients who developed an incisional hernia after open gastric bypass, 11 patients had already undergone herniorrhaphy with 6 of these 11 patients developing a recurrence. Two of the 3 patients in the laparoscopic group who developed an incisional hernia were patients who were converted to an open procedure. Of the patients with an intact gallbladder at the time of the gastric bypass procedure, 12 (28%) of 43 patients in the laparoscopic group and 2 (5%) of 40 patients in the open group underwent postoperative cholecystectomy (P = 0.03). Reoperation for small bowel obstruction occurred in 3% of patients in the laparoscopic group and 2% of patients in the open group. There were no perioperative deaths and no late deaths.
TABLE 5. Late Complications Following Laparoscopic and Open Gastric Bypass at 3-Year Follow-Up
DISCUSSION
The rationale for laparoscopic gastric bypass is that the efficacy of the Roux-en-Y procedure can be obtained through small access incisions thereby reducing the extent of surgical injury to the host. We previously reported the initial outcomes of our randomized trial comparing laparoscopic versus open gastric bypass in 155 patients.5 Advantages of the laparoscopic approach include less postoperative pain, shorter hospitalization, improved pulmonary function, and an early return to normal activity.5,6 In addition, we previously demonstrated that laparoscopic gastric bypass reduces the operative trauma to the host compared with open gastric bypass.10 To date, there have been 3 published randomized trials comparing the outcomes of laparoscopic versus open gastric bypass; however, none of these studies has provided long-term follow-up.5,7,11 This study presents the 3-year results of patients who participated in our randomized trial of laparoscopic versus open gastric bypass between May 1999 and March 2001. In this study, we found that laparoscopic gastric bypass was equally effective as open gastric bypass with regard to weight loss and improvement of comorbidities and quality of life. An important advantage of laparoscopic gastric bypass at long-term follow-up was the lower rate of incisional hernia.
We found that weight loss was similar between laparoscopic and open gastric bypass patients at 3-year (77% for laparoscopic versus 67% for open) and 4-year (76% for laparoscopic versus 71% for open) follow-up. Since the anatomic and physiologic principles of the gastric bypass operation were identical between the 2 techniques, it is not surprising that long-term weight loss was similar. Lugan et al7 also reported equivalent weight loss between laparoscopic and open gastric bypass patients at a mean follow-up of 23 months. Higa et al12 reported a similar result with a 62% excess body weight loss at 3-year follow-up of a small cohort of 19 patients who underwent laparoscopic gastric bypass.
Improvement or resolution of obesity-related comorbidities after gastric bypass has been well documented and tends to be directly related to the extent of weight reduction. In a landmark paper entitled “Who would have thought of it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus,” Pories et al13 found an 83% resolution of type II diabetes at 14-year follow-up. Schauer et al14 similarly reported an 83% resolution of type II diabetes in a cohort of 191 patients who underwent laparoscopic gastric bypass. Since the weight loss between laparoscopic and open gastric bypass patients was similar in our trial, we hypothesized that improvement or resolution of comorbidities should be equivalent between the 2 groups. At the 3-year follow-up, we found that both techniques (laparoscopic and open) were equally effective in improvement of obesity-related comorbidities, including type II diabetes, sleep apnea, gastroesophageal reflux, urinary stress incontinence, lower extremity edema, and hypertension. The greater improvement of symptoms of osteoarthritis after laparoscopic gastric bypass and amelioration of dyslipidemia after open gastric bypass may be a function of the small number of patients who had these conditions.
Improvement in the impaired quality of life brought about by morbid obesity has been observed as early as 3 months following bariatric surgery. Continued weight loss results in further improvement in quality of life, often reaching that of the national “normal” population within 6 months postoperatively.5 Our current study demonstrates that the improvement in quality of life persisted at 3-year follow-up, and there was no significant difference between the 2 groups. The Moorehead-Ardelt Quality of Life Questionnaire demonstrated positive changes compared with baseline scores in all 5 categories of the questionnaire. When the 3 categories of weight loss, changes in comorbidities, and quality of life were taken into account, the BAROS demonstrated that 86% of open gastric bypass patients and 95% of laparoscopic gastric bypass patients reported “good,” “very good,” or “excellent” results at the 3-year follow-up.
Development of incisional hernia is a major problem after open gastric bypass. The incidence of incisional hernia after open gastric bypass has been reported as high as 26%, and postoperative incisional hernia is a risk for incarceration or strangulation.3 Herniorrhaphy is indicated, but recurrence is common. Obesity is a well-known risk factor for development of an incisional hernia after abdominal surgery.15 The mechanism for development of incisional hernia in the morbidly obese is related to the increase intra-abdominal pressure, which is normally 2 to 3 times higher in morbidly obese individuals than nonobese subjects.16 Another risk factor for development of an incisional hernia is the presence of a wound infection at the index procedure.15 Christou et al17 reported that the rate of incisional hernia in patients who underwent gastric bypass without wound infection was 14%, whereas the rate of incisional hernia in patients with wound infection was 38%. In this trial, wound infection occurred in 1.3% after laparoscopic gastric bypass and 10.5% after open gastric bypass.5 At the 3-year follow-up, the rate of incisional hernia was significantly higher after open gastric bypass compared with laparoscopic gastric bypass (39% versus 5%, respectively). As previously stated, 2 of the 3 patients who developed an incisional hernia after laparoscopic gastric bypass were patients that had a conversion to an open procedure. The results of these 2 patients were kept within the laparoscopic group based on an intention-to-treat principle. Incisional hernia as a complication of gastric bypass increases the cost of care. The mean cost for an incisional hernia repair at our institution is $8259. In addition to the higher cost for the incisional hernia repair, there is also the risk for recurrence after the repair, which can be as high as 36%.18
Rapid weight loss after Roux-en-Y gastric bypass can lead to formation of gallstones. In a randomized trial of prophylactic ursodiol for the prevention of gallstone, Sugerman et al19 reported a 32% incidence of gallstone formation in patients receiving placebo compared with a 2% incidence of gallstone formation in patients receiving 600 mg of ursodiol. In this study, 28% of patients in the laparoscopic group required subsequent cholecystectomy for newly developed gallstone (11 patients) or had cholelithiasis preoperatively and cholecystectomy was performed as a staged procedure (1 patient) after laparoscopic gastric bypass. The higher rate of cholecystectomy performed after laparoscopic gastric bypass compared with open gastric bypass is probably related to low compliance on the use of ursodiol and the greater number of cholecystectomies performed during open gastric bypass for a diagnosis of cholelithiasis. In a randomized trial of prophylactic ursodiol or placebo in the prevention of gallstones after gastric bypass, Wudel et al20 reported that the compliance rate for the use of ursodiol was achieved in only 28% of patients. Staged cholecystectomy after laparoscopic gastric bypass appeared to be an acceptable option. In a study of concomitant gastric bypass and cholecystectomy, Hamad et al21 reported significant increases in operative time and doubling of the hospital stay in patients who underwent concomitant laparoscopic gastric bypass and cholecystectomy compared with patients who underwent laparoscopic gastric bypass alone.
The major drawback of laparoscopic gastric bypass over the open approach is the steep “learning curve.” Laparoscopic gastric bypass is currently one of the most challenging advanced laparoscopic operations. Because of its technical complexity, the learning curve for laparoscopic gastric bypass is longer than most other advanced laparoscopic operations. Many factors contribute to the extent of the learning curve for laparoscopic gastric bypass. These factors include the experience of the surgeon with other advanced laparoscopic operations, with open bariatric operations, and with laparoscopic suturing skill and intracorporeal knot tying techniques. Schauer et al22 demonstrated that operative time and technically related complications decreased with operative experience and stated that the learning curve for laparoscopic gastric bypass is 100 cases. We previously reported that the learning curve for laparoscopic gastric bypass was 75 cases.23 The surgeon's early operative experience (first 75 cases) was the major predictor of not only a longer operative time but also a higher major complication and reoperation rate, and a longer length of hospital stay.
Follow-up is imperative in any trial, particularly for a bariatric surgical trial in which long-term durability of the procedure is a key outcome; for example, Pories et al,13 in 1995, reported a 97% follow-up rate at 14 years after Roux-en-Y gastric bypass. Despite persistent efforts at contacting our patients, we were able to obtain a 75% follow-up. Our primary reason for not achieving a higher follow-up rate was the inability to contact patients who had moved out of the area and the contact information was outdated. Various methods were used to reach patients, including home and work phone, e-mail, conventional mail, and through the primary care physician. Our 75% follow-up in this trial reflects the high mobility of the California population, but we have also observed a desire on the part of successful patients to seek new job opportunities often at a new location. We suspect that only continual contact of the patients to update their contact information prior to their changes will increase the rate of follow-up. This task is difficult and remains a challenge for most bariatric practices.
CONCLUSION
Before accepting the laparoscopic approach as the standard technique for morbidly obese patients undergoing gastric bypass, 2 main questions need to be addressed: 1) are the short-term outcomes more favorable than the open approach and 2) what are the long-term differences between laparoscopic and open gastric bypass? Our previous study demonstrated the short-term benefits of laparoscopic gastric bypass, including lower analgesic use, shorter length of hospitalization, improved pulmonary function, and faster recovery compared with open gastric bypass. Our current study with a 3-year follow-up demonstrated that both techniques were equally effective with regard to weight loss and improvement of comorbidities and quality of life. A major advantage of laparoscopic gastric bypass at long-term follow-up is the reduction in the rate of incisional hernia. The perioperative and long-term results from this randomized trial of laparoscopic versus open gastric bypass support our recommendation that the laparoscopic approach should be the standard technique for patients undergoing Roux-en-Y gastric bypass for the treatment of morbid obesity.
Footnotes
Presented at the Annual Meeting of the American Society for Bariatric Surgery on June 15, 2004 in San Diego, CA.
Reprints: Ninh T. Nguyen, MD, Department of Surgery, 101 The City Drive, Bldg. 55, Rm 106, Orange, CA 92868. E-mail: ninhn@uci.edu.
REFERENCES
- 1.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.See C, Carter PL, Elliott D, et al. An institutional experience with laparoscopic gastric bypass complications seen in the first year compared with open gastric bypass complications during the same period. Am J Surg. 2002;183:533–538. [DOI] [PubMed] [Google Scholar]
- 3.Sugerman HJ, Sugerman EL, Wolfe L, et al. Risks and benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4:353–357. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen NT, Lee SL, Goldman C, et al. Comparison of pulmonary function and postoperative pain after laparoscopic versus open gastric bypass: a randomized trial. J Am Coll Surg. 2001;192:469–476. [DOI] [PubMed] [Google Scholar]
- 7.Lugan JA, Frutos D, Hernandez Q, et al. Laparoscopic versus open gastric bypass in the treatment of morbid obesity: a randomized prospective study. Ann Surg. 2004;239:433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newer techniques in bariatric surgery for morbid obesity: Blue Cross and Blue Shield Association's Technology Evaluation Center. www.bluecares.com Assessment Program, 2003:18. [PubMed]
- 10.Nguyen NT, Goldman CD, Ho HS, et al. Systemic stress response after laparoscopic and open gastric bypass. J Am Coll Surg. 2002;194:557–567. [DOI] [PubMed] [Google Scholar]
- 11.Westling A, Gustavsson S. Laparoscopic vs open Roux-en-Y gastric bypass: a prospective, randomized trial. Obes Surg. 2001;11:284–292. [DOI] [PubMed] [Google Scholar]
- 12.Higa KD, Ho T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: technique and 3-year follow-up. J Laparoendosc Adv Surg Tech. 2001;11:377–382. [DOI] [PubMed] [Google Scholar]
- 13.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugerman HJ, Kellum JM, Reines D, et al. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg. 1996;171:80–84. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen NT, Lee SL, Anderson JT, et al. Evaluation of intraabdominal pressure after open and laparoscopic gastric bypass. Obes Surg. 2001;11:40–45. [DOI] [PubMed] [Google Scholar]
- 17.Christou NV, Jarand J, Sylvestre JL, et al. Analysis of the incidence and risk factors for wound infections in open bariatric surgery. Obes Surg. 2004;14:16–22. [DOI] [PubMed] [Google Scholar]
- 18.Hesselink VJ, Luijendijk RW, de Wilt JHW, et al. An evaluation of risk factors in incisional hernia recurrence. Surg Gynecol Obstet. 1993;176:228–234. [PubMed] [Google Scholar]
- 19.Sugerman HJ, Brewer WH, Shiffman ML, et al. A multicenter, placebo-controlled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg. 1995;169:91–96. [DOI] [PubMed] [Google Scholar]
- 20.Wudel LJ Jr, Wright JK, Debelak JP, et al. Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: results of a randomized controlled pilot study. J Surg Res. 2002;102:50–56. [DOI] [PubMed] [Google Scholar]
- 21.Hamad GG, Ikramuddin S, Gourash WF, et al. Elective cholecystectomy during laparoscopic Roux-en-Y gastric bypass: is it worth the wait? Obes Surg. 2003;13:76–81. [DOI] [PubMed] [Google Scholar]
- 22.Schauer PR, Ikramuddin S, Hamad G, et al. The learning curve for laparoscopic Roux-en-Y gastric bypass in 100 cases. Surg Endosc. 2003;17:212–215. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen NT, Rivers R, Wolfe BM. Factors associated with operative outcomes in laparoscopic gastric bypass. J Am Coll Surg. 2003;197:548–557. [DOI] [PubMed] [Google Scholar]