Abstract
Objective:
To analyze the penetrance and clinical course of isolated nonfunctioning tumors of the pancreas (NFTP) in MEN 1 patients, and to propose a strategy for managing them.
Summary Background Data:
Pancreaticoduodenal tumors develop in a majority of MEN 1 patients and are a major cause of death. The natural history of NFTP is poorly defined, and no clear-cut guidelines have been widely accepted regarding treatment.
Methods:
Data on 108 patients with isolated NFTP among 579 MEN 1 patients from the French Endocrine Tumor Study Group (GTE) were analyzed. Survival rates were calculated using the Kaplan-Meier method.
Results:
The penetrance of NFTP was 34% at age 50, making it the most frequent pancreaticoduodenal tumor in MEN 1 patients. Forty-three patients (40%) underwent surgery, 32 of them curatively. No patient died because of surgery. Average life expectancy for patients with NFTP was shorter than that for MEN 1 patients who did not have pancreaticoduodenal tumors. Thirteen patients died during follow-up, 10 due to NFTP. Tumor size was correlated with the risks of metastasis and death. These risks were low for patients with tumors ≤20 mm.
Conclusions:
NFTP are currently the most common tumors of the pancreaticoduodenal region in patients with MEN 1. Prevention of tumor spread by surgery should be balanced with potential operative mortality and morbidity. We do not recommend routine surgery for NFTP ≤20 mm.
Nonfunctioning tumors of the pancreas (NFTP) are currently the most frequent pancreaticoduodenal tumors in MEN 1 patients. Among the 108 patients with NFTP in this study, 10 patients died from NFTP. A significant correlation between tumor size, metastasis, and reduced survival was demonstrated.
Multiple endocrine neoplasia type 1 (MEN 1) is a rare autosomal dominant condition characterized by the development of endocrine parathyroid, pancreaticoduodenal, and pituitary tumors. In addition, MEN 1 patients are also prone to developing adrenal tumors, neuroendocrine tumors (in particular of the thymus or bronchus), dermal lesions, thyroid disease, and meningeal tumors.1–7
Pancreaticoduodenal tumors are often multiple, have been shown at autopsy to have developed in up to 80% of patients with MEN 1, and are a major cause of premature death in these patients.8–12 Most pancreaticoduodenal tumors are functioning tumors, the most frequent of which are gastrinomas and insulinomas, followed by the rare glucagonomas, VIPomas, GRFomas, and somatostatinomas. Surgical resection is the treatment of choice for functioning tumors of the pancreas, although controversy exists regarding the timing of surgery for gastrinomas.13–18 In the absence of hormonal symptoms, nonfunctioning tumors of the pancreas (NFTP) have been recognized as a separate entity whose penetrance in the MEN 1 population is not well known.19,20 In addition, because large surgical series of patients presenting with isolated NFTP are lacking and few clinical series have followed patients with NFTP, no clear-cut treatment guidelines have been widely accepted. Some authors have recommended a conservative approach for asymptomatic NFTP less than 1, 2, or 3 cm.21–24 Others have recommended early surgical excision of all tumors as soon as they are found on imaging studies, or even earlier, when they are biochemically proven by an increase in human pancreatic polypeptide (hPP).11,12,25–27
To determine the penetrance and clinical course of isolated NFTP in MEN 1 patients and to propose a strategy for managing these tumors, we analyzed data from a cohort of 579 MEN 1 patients, 108 of whom had isolated NFTP.
PATIENTS AND METHODS
MEN 1 Patients
Among 579 patients with MEN 1 included in the registry of the French Endocrine Tumor Study Group [Groupe des Tumeurs Endocrines, (GTE)] who were diagnosed from June 1956 to April 2003, 108 patients with isolated NFTP were identified. MEN 1 was diagnosed in patients presenting with at least 2 of the 3 major MEN 1 specific disorders (primary hyperparathyroidism, endocrine pancreaticoduodenal tumor, or pituitary tumor), and in patients presenting with one clinical disorder and positive familial history or a germline mutation on the menin locus, according to international guidelines.13 Genetic analysis was performed as previously described.28 Because of the close collaboration between the registry and the 2 French reference genetic laboratories, patient inclusion in the registry has been prospective since 1997 for all patients tested in France. After initial MEN 1 diagnosis, patient follow-up was conducted according to previously published protocols.24
NFTP were diagnosed when one or more pancreatic solid nodules were evidenced by any imaging studies and after excluding gastrinomas (hypergastrinemia confirmed by increased gastric acid output measurements under basal conditions and increasing under stimulation with secretin),29,30 insulinomas (elevated insulin/glucose ratio, confirmed by a fasting test),30 glucagonomas (glucagon level >2 times normal values), VIPomas (VIP level >2 times normal values), or somatostatinomas (somatostatin level >2 times normal values).
Data for the 108 NFTP patients were retrieved from the registry and additional data were requested from physicians and surgeons in charge of the patients, if necessary. Patients who underwent surgery were separated in 2 groups whether the surgery was curative or palliative (incomplete tumor excision or presence of unresectable metastasis).
Statistical Analysis
Current penetrance of NFTP in the MEN 1 population was estimated using the data on patients diagnosed with MEN 1 since 1997.
Results are presented as mean values ±SD with range indicated in parentheses unless otherwise stated. Comparisons between groups were made using the χ2 test, Fisher exact test, or Student t test. Correlations were calculated using Spearman rank correlation. Penetrance of NFTP in the MEN 1 patients was evaluated using the Kaplan-Meier method. For survival analysis, data are expressed as the time from NFTP diagnosis to end of follow-up (or death) unless otherwise stated. Survival data were analyzed using the Kaplan-Meier method and groups were compared using the Log-Rank test. Patients with more than one tumor of the pancreas, including gastrinoma, were integrated in the gastrinoma group for survival analysis. P values <0.05 were considered statistically significant. Statistical analyses were performed with SPSS software (SPSS Inc., Chicago, IL).
RESULTS
Penetrance of NFTP in Patients With MEN 1
Among the 579 patients diagnosed with MEN 1 since 1956, 108 (18.7%) were diagnosed with NFTP. In the 134 patients diagnosed with MEN 1 since 1997 (when patient inclusion in the GTE registry became prospective), 68 patients (51%) had no pancreatic tumor, 33 (25%) had NFTP, 18 (13%) had gastrinomas, 11 (8%) had insulinomas, 2 (2%) had gastrinomas and insulinomas, and 1 (1%) had VIPoma. The penetrance of pancreaticoduodenal tumors in MEN 1 patients at age 20, 50, and 80 years was 9%, 53%, and 84%, respectively. NFTP were the most frequent type of pancreaticoduodenal tumors; occurring in 3%, 34%, and 53% of patients at age 20, 50, and 80 years (Fig. 1).
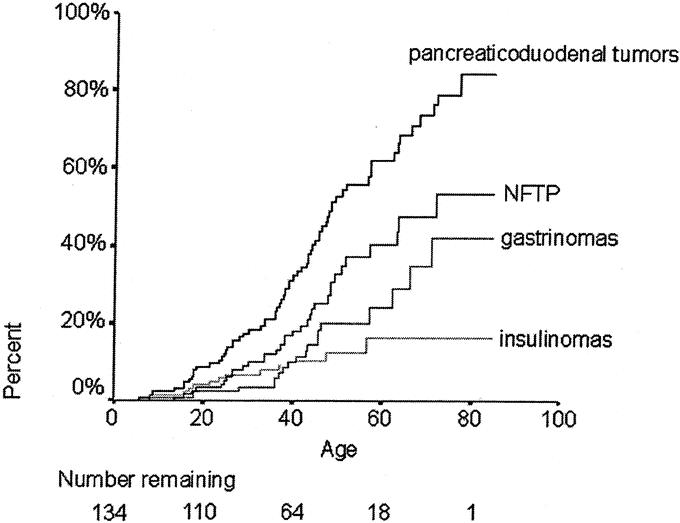
FIGURE 1. Penetrance of pancreaticoduodenal tumors by age in MEN 1 patients. The number of patients at risk at each time point is shown below the graph. Pancreaticoduodenal tumors: any kind of pancreaticoduodenal tumor.
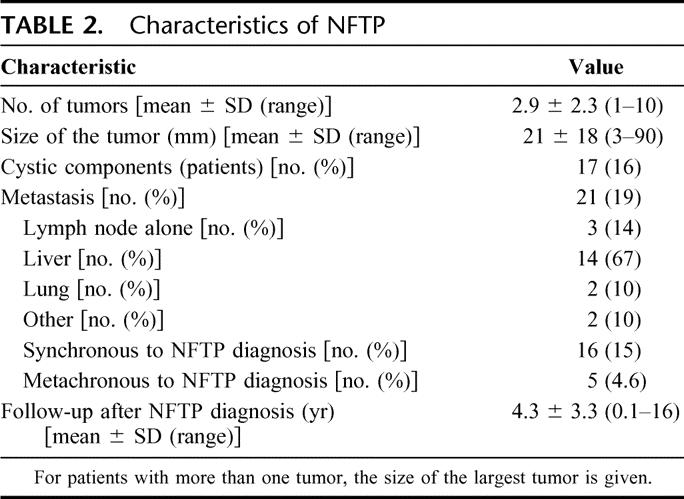
Patient and Tumor Characteristics
Characteristics of patients with NFTP are summarized in Table 1. NFTP characteristics are presented in Table 2. NFTP was diagnosed in 65% of patients by imaging studies, of whom 69% had CT scan, 42% had endoscopic ultrasonography (EUS), 33% had abdominal ultrasonography, 26% had Octreoscan, and 5% had MRI.
TABLE 1. Characteristics of the 108 Patients With NFTP
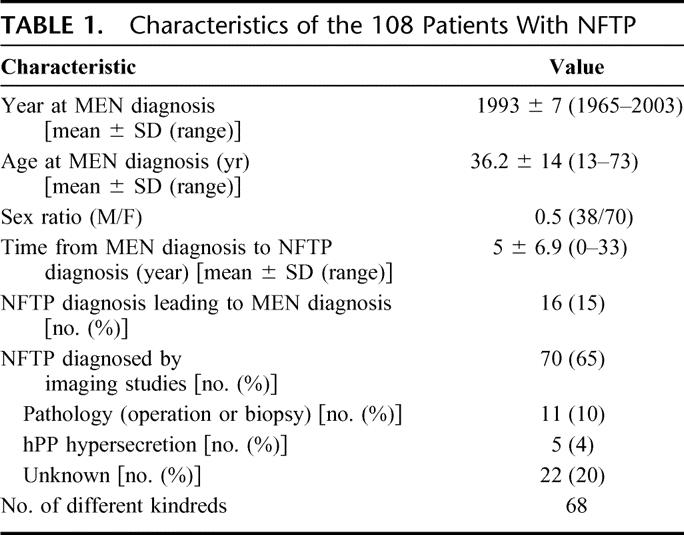
TABLE 2. Characteristics of NFTP
NFTP size was significantly correlated with metastasis (Spearman correlation coefficient 0.33, P < 0.01). One (4%) of 25 patients with tumors ≤1 cm had synchronous metastasis, as did 4 (10%) of 40 patients with tumors 11 to 20 mm, 2 (18%) of 11 patients with tumors 21 to 30 mm, and 9 (43%) of 21 patients with tumors >30 mm (Fig. 2).
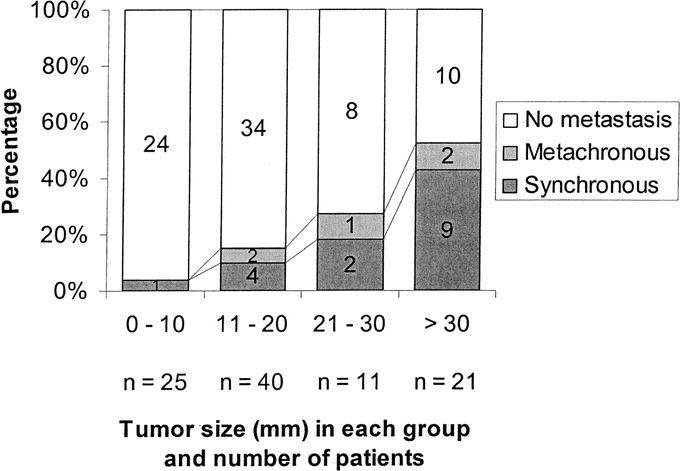
FIGURE 2. Proportions of patients without metastasis, with synchronous metastasis, or with metachronous metastasis according to tumor size. The number of patients in each group is given in each box.
Surgical and Long-term Outcomes
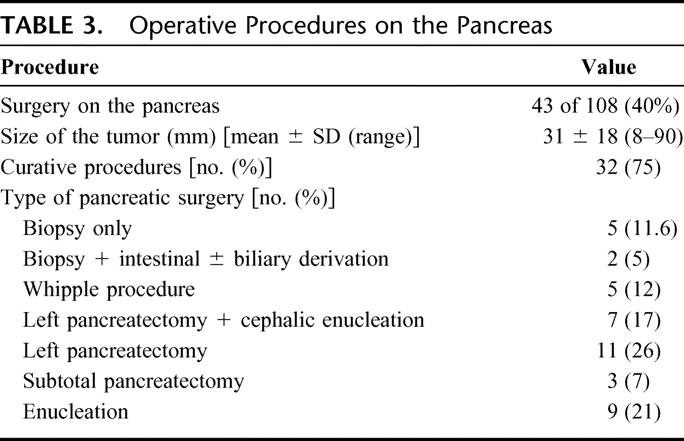
Forty-three patients with NFTP had surgery (40%). The surgical procedures performed are detailed in Table 3. No patient died following surgery.
TABLE 3. Operative Procedures on the Pancreas
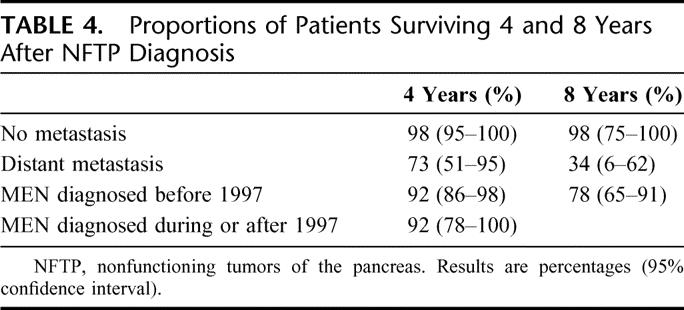
Thirteen patients died during follow-up: 10 (9%) due to NFTP and 3 (3%) to other causes. The mean age at death was significantly lower in MEN 1 patients with NFTP than in those without pancreatic tumors (43 ± 13 versus 61 ± 14 years, P < 0.01). Patients with NFTP had an average life expectancy similar to that of patients with gastrinomas (68.5 years; 95% confidence interval, 64–72.9 years versus 71.4 years; 95% confidence interval, 68–74.8 years, P = 0.99) and significantly shorter than that of MEN 1 patients without pancreatic tumors (77.1 years; 95% confidence interval, 74.4–79.9 years, P < 0.01) (Fig. 3). Survival time was significantly shorter for patients with bigger tumors (Fig. 4), for patients who underwent noncurative surgery (Fig. 5), and for patients with distant metastasis (Table 4). Survival time did not differ significantly for patients diagnosed with MEN 1 before 1997 and those diagnosed after 1997 (Table 4) or for patients who underwent curative surgery and those who did not undergo surgery (Fig. 5).
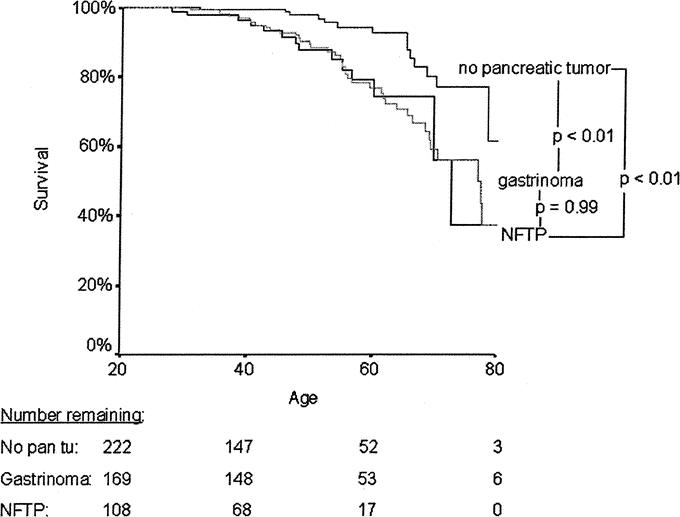
FIGURE 3. Kaplan-Meier representation of life expectancy according to the type of pancreaticoduodenal tumor. Data are expressed as age at the end of follow-up. The number of patients at risk at each time point is shown below the graph.
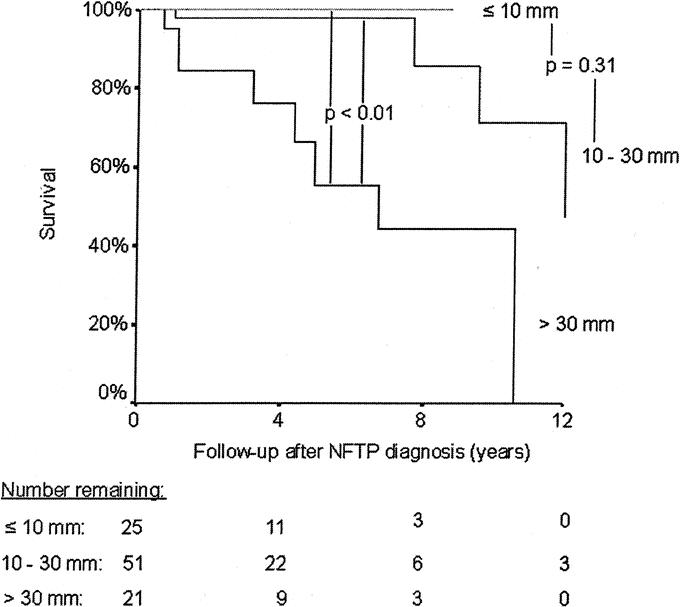
FIGURE 4. Kaplan-Meier representation of survival after NFTP diagnosis according to the size of the NFTP. The number of patients at risk at each time point is shown below the graph.
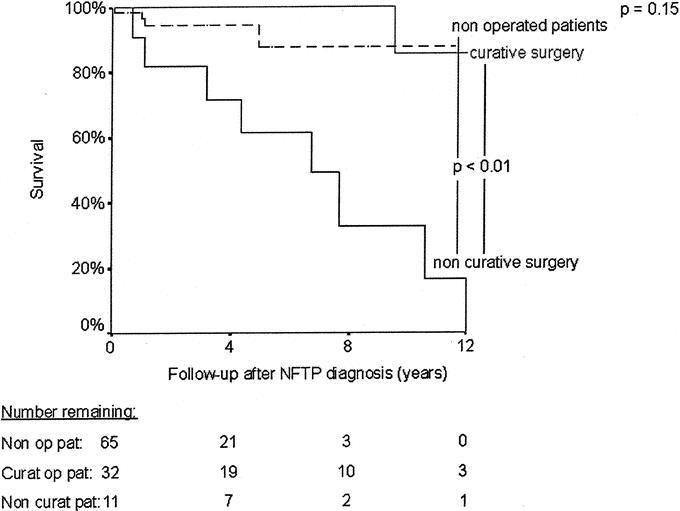
FIGURE 5. Kaplan-Meier representation of survival after NFTP diagnosis according to the type of operation. The number of patients at risk at each time point is shown below the graph.
TABLE 4. Proportions of Patients Surviving 4 and 8 Years After NFTP Diagnosis
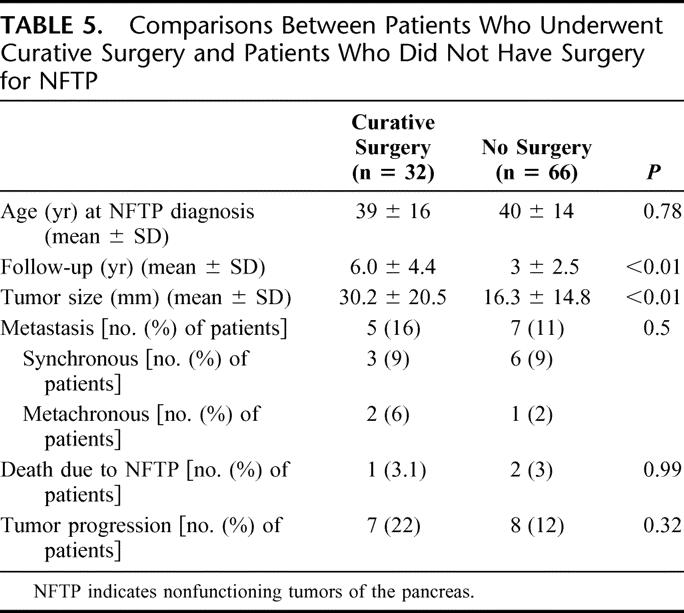
Patients who underwent potentially curative operations had significantly larger tumors and longer follow-up times than patients who did not undergo surgery, but tumor progression (22% in the patients who underwent curative resection and 12% in the patients who did not undergo surgery) and death due to NFTP (3.1% and 3%, respectively) were not significantly different between the 2 groups (Table 5). Two patients in the curative surgery group underwent reoperation for new NFTP during follow-up.
TABLE 5. Comparisons Between Patients Who Underwent Curative Surgery and Patients Who Did Not Have Surgery for NFTP
DISCUSSION
To determine the penetrance and clinical course of isolated NFTP in MEN 1 patients and to propose a strategy for managing these tumors, we analyzed data from a cohort of 579 MEN 1 patients,108 of whom had isolated NFTP. We found that NFTP are the most frequent type of pancreaticoduodenal tumor in MEN 1 patients. The penetrance of NFTP increases with age and reaches 34% by age 50 years, whereas the penetrance of any kind of pancreaticoduodenal tumors is 50% at that age. This is probably the best estimate of the actual penetrance of NFTP in MEN 1 patients because it is the only one that is based on data from a population-based registry. NFTP formerly were undetected because of the lack of symptoms and reliable biologic markers, but they are being diagnosed more frequently as a result of genetic testing and acceptance of national and international recommendations on screening programs.13,24 Previous studies have already suggested that NFTP were the most frequent type of pancreatic tumor in MEN 1 patients.12,22,25 Moreover, the numbers presented in this study only account for patients without any functioning tumor. As most, if not all, patients with pancreatic involvement have multiple tumors,31,32 the penetrance of NFTP alone or in association with functioning tumors should be considered similar to that of pancreatic involvement overall. The high penetrance of NFTP in MEN 1 patients makes it difficult for clinicians to recommend a major pancreatic resection to a high number of often young and otherwise healthy MEN 1 patients. Because the treatment approach for patients with an association of functioning and nonfunctioning tumors of the pancreas is dictated by the functioning component and because much controversy exists about how to manage isolated NFTP, only patients with isolated NFTP have been considered in this study.
Diagnosis of NFTP
In this study, only 4% of the NFTP were biochemically diagnosed by hPP hypersecretion, whereas 65% were diagnosed by imaging studies. CT scanning was the preferred imaging study for the first detection and subsequent follow-up of NFTP in MEN 1 patients. Use of EUS, which has been shown to be more sensitive than CT for detecting small pancreatic lesions and was rapidly adopted after 1992,33–35 did not increase with time. Octreoscan, which can demonstrate biochemically proven tumors of the pancreas that are not identified by CT,36 was always performed together with another radiologic study. CT is widely available, costs less than MRI, and is less inconvenient and less invasive for patients than routine EUS. Our current recommendations for asymptomatic, biochemically negative MEN 1 patients follow the guidelines for diagnosis and therapy for MEN 1 published in 2001,13 that is, to obtain CT scans of the abdomen every 3 years. EUS should be used to detect biochemically proven lesions that are not shown on CT, for staging (locoregional extension) once a lesion is discovered by CT, and every 5 years thereafter for follow-up. Moreover, we recommend EUS immediately preoperatively because it permits better operative planification by detecting more precisely the number and extent of tumors and providing anatomic detail.37,38 Octreoscan should be performed for biochemically proven lesions that are not shown by CT, for staging (distant metastasis) once a lesion is shown on CT, and preoperatively to rule out distant metastasis. The role of MRI is not clearly defined because its superiority over EUS for locoregional extension has not been clearly demonstrated. In countries where it is widely available, MRI could replace CT with a higher sensitivity and specificity.39,40
Clinical Course of NFTP
The results of this study show that NFTP are a significant risk factor for death and that this risk is correlated with tumor size. We found that MEN 1 patients with NFTP have a significantly shorter life expectancy than MEN 1 patients without pancreatic tumors and a similar life expectancy to MEN 1 patients with gastrinoma. Moreover, when patients die of NFTP, they do so at younger age than MEN 1 patients who die of other causes. These findings confirm those from previous studies showing that islet cell tumors are a significant risk factor for death in MEN 1 patients.2,9,11 In our study, patients who died of NFTP did so because of metastatic disease, a finding that has been reported by others.12
The mean age at death for patients who died of NFTP in our study was 43 years, which is similar to the 46 years reported by Doherty et al in MEN 1 patients with pancreaticoduodenal tumors.10 We also found that tumor size was significantly correlated with the presence of metastasis and that tumor size >30 mm and the presence of metastasis were associated with a significant reduction in survival time. For patients with tumors 11 to 30 mm, the risk for death is slightly, but not significantly, higher than for patients with tumors ≤10 mm. This correlation between tumor size, distant metastasis, and reduced survival time has been the basis for the current conservative management of small (≤2 or 3 cm) gastrinomas proposed by several groups.14,16,18,41,42 However, a correlation between tumor size and metastasis was not found in another study of islet cell tumors in MEN 1 patients,43 leading the authors to recommend early surgery. Our study suggests that, similar to gastrinomas, the size of the NFTP can be used as a prognostic factor and, therefore, as a indication for delayed surgery in the case of small tumors.
Our study may underestimate the actual rate of metastasis because only 40% of the patients underwent an operation for NFTP. This means that metastases were found by imaging studies alone in 60% of the cases. CT scanning, the most frequently used imaging study, has been shown to have low sensitivity for detecting small pancreatic tumors and metastasis in MEN 1 patients.44 The metastasis rate is always higher with pathology reports than with imaging studies, as was shown in a study in which 26% of patients with radiographically demonstrated pancreatic lesions had preoperatively unidentified metastasis at operation.45 However, in our study, only one (1.5%) metachronous metastasis appeared on subsequent imaging studies in the 66 patients who did not have an operation, and only 2 (3%) of the patients who did not have an operation died of NFTP during follow-up. Both patients had synchronous distant metastasis at the time of NFTP diagnosis. If the true metastasis rate of NFTP in MEN 1 patients is much higher than the one in our study, most of the metastatic tumors have a relative indolent course. Unfortunately, because of the relative short follow-up time for NFTP patients, rate of growth for NFTP could not be predicted, and, consequently, the rate at which tumors progress from one size group to another could not be evaluated.
The rate of tumor progression found in our study (22% with a mean follow-up time of 6 years for patients who had curative surgery and 12% with a follow-up time of 3 years for those who did not) is similar to the 31% reported for pancreaticoduodenal tumors in MEN 1 patients after a median follow-up of 6.3 years by the Ann Arbor group.27 The much lower rate of reoperation in our patients (6%) than in their study (39% after a median follow-up of 9.9 years) may be due to the shorter follow-up time in our study and to the more aggressive approach used by the Ann Arbor group.
MEN 1 patients with residual pancreatic tissue are predisposed to developing new pancreaticoduodenal tumors with time. Once NFTP is diagnosed with CT scan or MRI, an EUS and an Octreoscan should be performed for staging. If these 2 examinations are negative, the patient should be monitored every year for 3 years to detect rapidly growing tumors, and then every other year with CT scan or MRI. Furthermore, they should also undergo an EUS every 5 years. The same follow-up schedule should be used for patients who have surgery for NFTP and those who do not.
Surgical Versus Nonsurgical Treatment of NFTP
With the exception of gastrinomas, the presence of a clinical syndrome caused by a functioning pancreaticoduodenal tumor is an accepted indication for surgery in MEN 1 patients. The risk of malignant spread of a tumor is also an accepted indication for surgery; however, the potential malignancy and the rate at which a tumor grows, spreads to distant sites, and finally leads to death is not clearly defined in patients with NFTP. Because of these uncertainties, the timing of interventions for NFTP in MEN 1 patients is still controversial.
Some authors recommend a conservative approach for asymptomatic NFTP less than 1, 2, or 3 cm.21–24 This approach is based on 3 principal reasons. First, even though islet cell tumors of the pancreas are a significant risk factor for death in MEN 1 patients, their relative impact in the MEN 1 population is still low. Indeed, in a summary of the 3 series documenting causes of MEN 1 mortality, only 25 deaths (15%) were due to metastatic pancreatic islet cell tumors, whereas 140 (85%) were due to other MEN 1-related or unrelated causes.9–11,46 Second, pancreatic surgery is associated with significant mortality and morbidity, as has been shown in several studies. A recent review of mortality rates for 10,530 pancreatic resections performed in the United States between 1994 and 1999 found rates ranging from 3.8% to 17.6%, according to hospital volumes of pancreatic resection.47 Doherty et al reported a 4.8% mortality rate in their series of MEN 1 patients undergoing pancreatic resection.22 Moreover, endocrine insufficiency is an important postoperative morbidity of pancreatic resection.48 This has been shown in a study of 28 healthy donors who underwent hemipancreatectomy for living donor pancreas transplantation, which found that all of them had deterioration in insulin secretion and glucose tolerance one year after hemipancreatectomy, and 7 (25%) had abnormal results on glucose-tolerance test according to the criteria of the National Diabetes Data Group.49 Endocrine insufficiency has also been reported after pancreatic resection in MEN 1 patients; 17 (81%) of the 21 patients who underwent surgery in Ann Arbor and available for follow-up were currently taking antidiabetic medication, including 10 (46%) who were taking insulin.27 Moreover, pancreatogenic diabetes resulting from pancreatic resection is particularly difficult to treat because of the associated glucagon and hPP deficiency, leading to a significant number of brain damage and deaths due to hypoglycemia.50 Pancreatic exocrine insufficiency, another possible complication of pancreatectomy, is usually well controlled with pancreatic enzyme supplementation and antiacid medication.50 The third reason for a conservative approach is that unless a total pancreatectomy is performed, the pancreatic remnant is prone to develop new tumors. The evidence for this tendency comes from surgical and autopsy series showing that preneoplastic changes are present in virtually all the MEN 1 patients and throughout the pancreas.8,32 Evidence for this tendency has also been found in the study by the Ann Arbor group of 39 MEN 1 patients with pancreaticoduodenal tumors, in which 15 (39%) required reoperation after a median follow-up time of 9.9 years, and 12 (31%) had biochemical or imaging evidence of recurrence at a median follow-up of 6.3 years.27
Other authors have recommended aggressive surgical management for NFTP as soon as they are seen on imaging studies or even before, when they are biochemically proven by an hPP increase.11,12,25,26,46 This aggressive attitude is based on the fact that up to 39% of deaths related to MEN 1 were due to metastatic pancreatic islet cell tumors,46 that the deaths due to islet cell tumor occurred at a younger age,10 and that up to 33% of MEN 1 patients with islet cell tumor of the pancreas had metastasis when primary tumor size was less than 10 mm in greatest diameter.43 Moreover, Skogseid et al reported that “prophylactic” surgery could have a beneficial effect on the survival of MEN 1 patients with NFTP.19
The type and extent of surgery for pancreaticoduodenal tumors in MEN 1 patients are also controversial.15,18,19,51 The aim of surgery is to prevent malignant spread while minimizing the mortality and morbidity associated with pancreatic resection. Enucleation reduces the long-term rate of exocrine and endocrine insufficiency but leaves a big pancreatic remnant prone to developing new tumors. Major pancreatic resection maximizes cancer prevention but increases the risk of long-term morbidity. In our study, the surgical approach was based on tumor size, number, and location, and the proportions of enucleations only versus major pancreatic resections are similar to those found in previous studies.19,22,52
In our opinion, the mortality and morbidity of pancreatic resection outweigh the low risk of metastasis and death in MEN 1 patients with NFTP <2 cm. Therefore, we recommend a conservative approach for these patients. However, patients with rapidly growing tumors or tumors ≥2 cm should be offered surgical resection. The type of surgery should be aimed at excising every tumor while preserving the spleen and as much pancreatic tissue as possible and will therefore depend on the number, size, and location (head, body or tail, distance to the pancreatic duct and vessels) of the tumors.
Study Limitations
The controversy about the timing and extent of surgery for MEN 1 patients with NFTP is reflected in our study by the wide overlap in tumor size between patients who had curative surgery and those who did not. The most extreme example was that a patient with an 8-mm tumor underwent pancreatic resection, whereas a patient with a 30-mm tumor and no sign of distant metastasis did not. Because of the difference in tumor size and the length of follow-up for patients who underwent curative surgery and those who did not, the 2 groups are probably not comparable. Moreover, patients were not randomly assigned to a conservative or aggressive treatment; the interpretation of surgery as curative or noncurative was done retrospectively, based on intraoperative findings and not on an intent-to-treat basis. This is another limitation to this study that probably overestimates the length of survival in patients who had curative surgery. Nonetheless, because it is based on population-based registry data and because it is the largest reported series of NFTP in MEN 1 patients, we think our study offers important insights regarding the current clinical management of MEN 1 patients with NFTP.
CONCLUSION
This study shows that NFTP are the most frequent type of pancreaticoduodenal tumor in MEN 1 patients. Moreover, NFTP are a significant risk factor for death, and this risk correlates with the size of the tumor. We do not routinely recommend surgery for NFTP <20 mm 1) because the risk of metastasis and death is low, 2) because doing so would result in many patients undergoing a major surgical procedure to prevent only a few deaths, and 3) because surgery is not really prophylactic for malignant spread unless a total pancreatectomy is to be performed.
ACKNOWLEDGMENTS
The authors thank Pamela Derish for her very helpful editorial comments on this manuscript as well as Drs. Abs, Altman, Baudin, Beckers, Bercovici, Berger, Borson-Chazot, Buscail, Caron, Chabre, Chabrier, Chanson, Chapuis, Chigot, Comas, Conte-Devolx, Corcuff, Corone, Cubertafond, Decoulx, Delemer, Delpech, De Micco, Denizot, Dewailly, Duron, Emperauger, Emile, Emy, Estour, Flament, Giraud, Guliana, Hamon, Hubens, Henry, Jaeck, Jais, Jaquet, Knebelmann, Kraimps, Kuhn, Lalau, Le Bodic, Lecomte-Houcke, Le Neel, Leprat, Lespinasse, Luton, Malthiery, Mantion, Meurisse, Mignon, Mitry, Modigliani, Olivier, Parneix, Partensky, Pascal, Peix, Penformis, Porchet, Pugeat, Riou, Rodier, Roger, Rohmer, Rougier, Ruszniewski, Saint-André, Samama, Sarfati, Schillo, Schlumberger, Scoazec, Tabarin, Thieblot, Thivollet, Tognarelli, Tourniaire, Trouillas, Verger, Vidal-Chazot, Visset, Warner, Waterlot, Wemeau and Wion-Barbot for their efforts in data collection and analysis; the French Endocrine Tumor Study Group exists because of their dedicated efforts.
Footnotes
Supported in part by a grant from the University Hospital of Geneva, Switzerland (to F.T.).
Reprints: Frederic Triponez, MD, Thoracic and Endocrine Surgery, University Hospital of Geneva, 1211 Geneva 14, Switzerland. E-mail: frederic.triponez@hcuge.ch.
REFERENCES
- 1.Wermer P. Genetic aspects of adenomatosis of endocrine glands. Am J Med. 1954;16:363–371. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd JJ. The natural history of multiple endocrine neoplasia type 1. Highly uncommon or highly unrecognized? Arch Surg. 1991;126:935–952. [DOI] [PubMed] [Google Scholar]
- 3.Skogseid B, Eriksson B, Lundqvist G, et al. Multiple endocrine neoplasia type 1: a 10-year prospective screening study in four kindreds. J Clin Endocrinol Metab. 1991;73:281–287. [DOI] [PubMed] [Google Scholar]
- 4.Larsson C, Skogseid B, Oberg K, et al. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332:85–87. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. [DOI] [PubMed] [Google Scholar]
- 6.Lin FC, Lin CM, Hsieh CC, et al. Atypical thymic carcinoid and malignant somatostatinoma in type I multiple endocrine neoplasia syndrome: case report. Am J Clin Oncol. 2003;26:270–272. [DOI] [PubMed] [Google Scholar]
- 7.Levy-Bohbot N, Merle C, Goudet P, et al. Prevalence, characteristics and prognosis of MEN 1-associated glucagonomas, VIPomas, and somatostatinomas: study from the GTE (Groupe des Tumeurs Endocrines) registry. Gastroenterol Clin Biol. 2004;28:1075–1081. [DOI] [PubMed] [Google Scholar]
- 8.Majewski JT, Wilson SD. The MEN-I syndrome: an all or none phenomenon? Surgery. 1979;86:475–484. [PubMed] [Google Scholar]
- 9.Wilkinson S, Teh BT, Davey KR, et al. Cause of death in multiple endocrine neoplasia type 1. Arch Surg. 1993;128:683–690. [DOI] [PubMed] [Google Scholar]
- 10.Doherty GM, Olson JA, Frisella MM, et al. Lethality of multiple endocrine neoplasia type I. World J Surg. 1998;22:581–586. [DOI] [PubMed] [Google Scholar]
- 11.Dean PG, van Heerden JA, Farley DR, et al. Are patients with multiple endocrine neoplasia type I prone to premature death? World J Surg. 2000;24:1437–1441. [DOI] [PubMed] [Google Scholar]
- 12.Doherty GM, Thompson NW. Multiple endocrine neoplasia type 1: duodenopancreatic tumours. J Intern Med. 2003;253:590–598. [DOI] [PubMed] [Google Scholar]
- 13.Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. [DOI] [PubMed] [Google Scholar]
- 14.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. [DOI] [PubMed] [Google Scholar]
- 15.Thompson NW. Current concepts in the surgical management of multiple endocrine neoplasia type 1 pancreatic-duodenal disease: results in the treatment of 40 patients with Zollinger-Ellison syndrome, hypoglycaemia or both. J Intern Med. 1998;243:495–500. [DOI] [PubMed] [Google Scholar]
- 16.Cadiot G, Vuagnat A, Doukhan I, et al. Prognostic factors in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Groupe d'Etude des Neoplasies Endocriniennes Multiples (GENEM and groupe de Recherche et d'Etude du Syndrome de Zollinger-Ellison (GRESZE). Gastroenterology. 1999;116:286–293. [DOI] [PubMed] [Google Scholar]
- 17.Gibril F, Schumann M, Pace A, et al. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83:43–83. [DOI] [PubMed] [Google Scholar]
- 18.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240:757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skogseid B, Oberg K, Eriksson B, et al. Surgery for asymptomatic pancreatic lesion in multiple endocrine neoplasia type I. World J Surg. 1996;20:872–876. [DOI] [PubMed] [Google Scholar]
- 20.Skogseid B, Rastad J, Akerstrom G. Pancreatic endocrine tumors in multiple endocrine neoplasia type 1. In: Doherty GM, Skogseid B, eds. Surgical Endocrinology. Philadelphia: Lippincott Williams & Wilkins, 2001:511–524. [Google Scholar]
- 21.Mutch MG, Frisella MM, DeBenedetti MK, et al. Pancreatic polypeptide is a useful plasma marker for radiographically evident pancreatic islet cell tumors in patients with multiple endocrine neoplasia type 1. Surgery. 1997;122:1012–1019. [DOI] [PubMed] [Google Scholar]
- 22.Lairmore TC, Chen VY, DeBenedetti MK, et al. Duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg. 2000;231:909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goudet P, Calender A, Cougard P, et al. Multiple endocrine neoplasia type I or Werner syndrome: what is important to know about surgery of a rare disease. Ann Chir. 2002;127:591–599. [DOI] [PubMed] [Google Scholar]
- 24.GTE. Recommendation booklet on MEN 1. 2004. Available at: http://www.sf-endocrino.net/sfe/index.php?pageID=50c46b0ac0cee215b20b5627b461b2c3. Accessed February 09, 2005.
- 25.Akerstrom G, Hessman O, Skogseid B. Timing and extent of surgery in symptomatic and asymptomatic neuroendocrine tumors of the pancreas in MEN 1. Langenbecks Arch Surg. 2002;386:558–569. [DOI] [PubMed] [Google Scholar]
- 26.Dralle H, Krohn SL, Karges W, et al. Surgery of resectable nonfunctioning neuroendocrine pancreatic tumors. World J Surg. 2004;28:1248–1260. [DOI] [PubMed] [Google Scholar]
- 27.Hausman MS Jr, Thompson NW, Gauger PG, et al. The surgical management of MEN-1 pancreatoduodenal neuroendocrine disease. Surgery. 2004;136:1205–1211. [DOI] [PubMed] [Google Scholar]
- 28.Giraud S, Zhang CX, Serova-Sinilnikova O, et al. Germ-line mutation analysis in patients with multiple endocrine neoplasia type 1 and related disorders. Am J Hum Genet. 1998;63:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goudet P, Peschaud F, Mignon M, et al. Gastrinomas in multiple endocrine neoplasia type-1: a 127-case cohort study from the endocrine tumor group (GTE). Ann Chir. 2004;129:149–155. [DOI] [PubMed] [Google Scholar]
- 30.Wiedenmann B, Jensen RT, Mignon M, et al. Preoperative diagnosis and surgical management of neuroendocrine gastroenteropancreatic tumors: general recommendations by a consensus workshop. World J Surg. 1998;22:309–318. [DOI] [PubMed] [Google Scholar]
- 31.Le Bodic MF, Heymann MF, Lecomte M, et al. Immunohistochemical study of 100 pancreatic tumors in 28 patients with multiple endocrine neoplasia, type I. Am J Surg Pathol. 1996;20:1378–1384. [DOI] [PubMed] [Google Scholar]
- 32.Thompson NW, Lloyd RV, Nishiyama RH, et al. MEN I pancreas: a histological and immunohistochemical study. World J Surg. 1984;8:561–574. [DOI] [PubMed] [Google Scholar]
- 33.Rosch T, Lightdale CJ, Botet JF, et al. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992;326:1721–1726. [DOI] [PubMed] [Google Scholar]
- 34.Gauger PG, Scheiman JM, Wamsteker EJ, et al. Role of endoscopic ultrasonography in screening and treatment of pancreatic endocrine tumours in asymptomatic patients with multiple endocrine neoplasia type 1. Br J Surg. 2003;90:748–754. [DOI] [PubMed] [Google Scholar]
- 35.Cadiot G, Lebtahi R, Sarda L, et al. Preoperative detection of duodenal gastrinomas and peripancreatic lymph nodes by somatostatin receptor scintigraphy: Groupe D'etude Du Syndrome De Zollinger-Ellison. Gastroenterology. 1996;111:845–854. [DOI] [PubMed] [Google Scholar]
- 36.Yim JH, Siegel BA, DeBenedetti MK, et al. Prospective study of the utility of somatostatin-receptor scintigraphy in the evaluation of patients with multiple endocrine neoplasia type 1. Surgery. 1998;124:1037–1042. [DOI] [PubMed] [Google Scholar]
- 37.Anderson MA, Carpenter S, Thompson NW, et al. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271–2277. [DOI] [PubMed] [Google Scholar]
- 38.Proye C, Malvaux P, Pattou F, et al. Noninvasive imaging of insulinomas and gastrinomas with endoscopic ultrasonography and somatostatin receptor scintigraphy. Surgery. 1998;124:1134–1143. [DOI] [PubMed] [Google Scholar]
- 39.Van Nieuwenhove Y, Vandaele S, Op de Beeck B, et al. Neuroendocrine tumors of the pancreas. Surg Endosc. 2003;17:1658–1662. [DOI] [PubMed] [Google Scholar]
- 40.Owen NJ, Sohaib SA, Peppercorn PD, et al. MRI of pancreatic neuroendocrine tumours. Br J Radiol. 2001;74:968–973. [DOI] [PubMed] [Google Scholar]
- 41.Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–1649. [DOI] [PubMed] [Google Scholar]
- 42.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477–488. [DOI] [PubMed] [Google Scholar]
- 43.Lowney JK, Frisella MM, Lairmore TC, et al. Pancreatic islet cell tumor metastasis in multiple endocrine neoplasia type 1: correlation with primary tumor size. Surgery. 1998;124:1043–1048. [DOI] [PubMed] [Google Scholar]
- 44.Langer P, Kann PH, Fendrich V, et al. Prospective evaluation of imaging procedures for the detection of pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. World J Surg. 2004;28:1317–1322. [DOI] [PubMed] [Google Scholar]
- 45.Skogseid B, Oberg K, Akerstrom G, et al. Limited tumor involvement found at multiple endocrine neoplasia type I pancreatic exploration: can it be predicted by preoperative tumor localization? World J Surg. 1998;22:673–677. [DOI] [PubMed] [Google Scholar]
- 46.Doherty GM. Multiple endocrine neoplasia type 1: duodenopancreatic tumors. Surg Oncol. 2003;12:135–143. [DOI] [PubMed] [Google Scholar]
- 47.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 48.Slezak LA, Andersen DK. Pancreatic resection: effects on glucose metabolism. World J Surg. 2001;25:452–460. [DOI] [PubMed] [Google Scholar]
- 49.Kendall DM, Sutherland DE, Najarian JS, et al. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. N Engl J Med. 1990;322:898–903. [DOI] [PubMed] [Google Scholar]
- 50.Kahl S, Malfertheiner P. Exocrine and endocrine pancreatic insufficiency after pancreatic surgery. Best Pract Res Clin Gastroenterol. 2004;18:947–955. [DOI] [PubMed] [Google Scholar]
- 51.Norton JA, Alexander HR, Fraker DL, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001;234:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartsch DK, Langer P, Wild A, et al. Pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1: surgery or surveillance? Surgery. 2000;128:958–966. [DOI] [PubMed] [Google Scholar]


