Abstract
Background:
The management of acute pancreatitis (AP) is still based on speculative and unproven paradigms in many centers. Therefore, we performed an evidence-based analysis to assess the best available treatment.
Methods:
A comprehensive Medline and Cochrane Library search was performed evaluating the indication and timing of interventional and surgical approaches, and the value of aprotinin, lexipafant, gabexate mesylate, and octreotide treatment. Each study was ranked according to the evidence-based methodology of Sackett; whenever feasible, we performed new meta-analyses using the random-effects model. Recommendations were based on the available level of evidence (A = large randomized; B = small randomized; C = prospective trial).
Results:
None of the evaluated medical treatments is recommended (level A). Patients with AP should receive early enteral nutrition (level B). While mild biliary AP is best treated by primary cholecystectomy (level B), patients with severe biliary AP require emergency endoscopic papillotomy followed by interval cholecystectomy (level A). Patients with necrotizing AP should receive imipenem or meropenem prophylaxis to decrease the risk of infected necrosis and mortality (level A). Sterile necrosis per se is not an indication for surgery (level C), and not all patients with infected necrosis require immediate surgery (level B). In general, early necrosectomy should be avoided (level B), and single necrosectomy with postoperative lavage should be preferred over “open-packing” because of fewer complications with comparable mortality rates (level C).
Conclusions:
While providing new insights into key aspects of AP management, this evidence-based analysis highlights the need for further clinical trials, particularly regarding the indications for antibiotic prophylaxis and surgery.
This evidence-based analysis provides the currently best available treatment of patients with acute pancreatitis regarding nutrition, antibiotic prophylaxis, and medical treatments, as well as the indications for endoscopic interventions in biliary pancreatitis and surgery for necrotizing pancreatitis.
Acute pancreatitis (AP) is predominantly caused by symptomatic gallstone disease and excessive alcohol intake.1,2 Because of improvements in the management including better diagnostics and treatment modalities, disease-related mortality has declined during the past 2 decades despite an increase in the overall incidence of AP in many countries.3–5 Most AP episodes do not require a particular intervention, since they are mild and self-limiting. In contrast, about one fifth of patients develop a severe form of AP, which is still associated with a mortality rate exceeding 30%.1,6,7 This type of AP is usually accompanied by necrosis of the pancreas and the surrounding tissue (necrotizing AP). Such necrosis formation is best assessed by contrast-enhanced computed tomography (ceCT),8,9 and the Balthazar score is most commonly used to define the extent of necrosis.7,10,11 Alternatively, magnetic resonance imaging (MRI) can be used, eg, in case of contraindications for intravenous CT contrast.12 According to the Atlanta classification, AP is predicted severe if it is accompanied by single or multiorgan failure (MOF), local complications, 3 or more Ranson criteria,13 or an APACHE II score of ≥8 points.14
Over decades, the management of AP has been biased by unproven paradigms, which were generated by theories on the pathophysiology of AP. These paradigms have been increasingly questioned over the past 2 decades, resulting in treatment changes that were again based on personal experience and opinions of experts rather than convincing scientific evaluations. As a result, the management of AP still differs from center to center, and many physicians declare their management the standard of care.
The aim of this study was to assess the clinical value of different newer treatment modalities by reviewing the current literature on the treatment of AP. To secure the highest level of objectivity, we used the evidence-based approach of Sackett to analyze the literature of the last decade.15
METHODS
Study Design
Since the treatment of AP involves many different procedures, important clinical questions were defined in a roundtable discussion. As a result, we focused on the values of antibiotic prophylaxis, various medical treatments, enteral nutrition (EN), and endoscopic and surgical interventions. We decided to exclude review articles, retrospective analyses as well as studies that were only reported as abstracts. Only articles published in the English language between January 1990 and October 2004 were included.
Literature Research
An electronic search of the Medline database was performed using different key words that covered selected topics of AP. The search terms were identified in the title, abstract, or medical subject heading. Key words other than acute pancreatitis are listed in each section. In addition, we searched the Cochrane Library for publications on these topics. Summaries and abstracts of each identified publication were screened for exclusion criteria. Only publications that fulfilled the inclusion criteria and addressed the clinical questions of this analysis were further assessed. Each of these publications was independently and thoroughly reviewed by 2 of the authors (S.H., M.S.). Relevant data, including authors, title, study design, methodology, main results, and conclusions, were extracted and documented on a separate data sheet for each publication.
Literature Classification
The level of evidence of each publication was ranked in accordance to a modified Sackett's classification (Table 1). 15 According to this classification, meta-analyses were accepted and classified as level I. Randomized trials that did not provide or fulfill clear study endpoints and sample size calculations were ranked as level II. As a general rule, only studies of the 2 highest available levels of evidence were used for the final data analysis. The grade of recommendation based on the available literature for each clinical question was also determined as proposed by Sackett (Table 1).15
TABLE 1. Modified Classification of the Level of Evidence According to Sackett11
Statistical Analysis
If several level I and II trials were available for a specific topic, we performed own meta-analyses. This was done if identified trials were not included in previous meta-analyses or if preexisting meta-analyses reported controversial results.
All meta-analyses are performed on studies, which compare 2 groups with respect to a dichotomous endpoint (like mortality or the risk for sepsis). Thus, each study provides estimates of 2 proportions, one in each group. The goal was to obtain global estimates of these proportions and to test whether they significantly differ. Whereas a global estimate of a proportion can be obtained by simply pooling together the data of each study, a test for significance cannot be applied to such pooled data because the studies are usually heterogeneous with respect to study population and treatment protocols. Heterogeneity between studies is evaluated using the χ2-based Q statistic proposed by Cochrane.16 In addition, we use the random-effects model to take into account the between-studies variability. Thus, we consider the true treatment effect to differ from study to study, and we test for significance of the average treatment effect. The treatment effects are characterized by the logarithm of the odds ratio such that values smaller than zero indicate a positive treatment effect. To test whether an odds ratio is significantly different from zero, we use the standard methodology described, eg, in Whitehead and Whitehead.17 P values smaller than 0.05 are considered statistically significant. In addition, we provide the number “k” of studies included in the meta-analysis.
RESULTS
Does Medical Treatment Influence the Course of Established AP?
Uncontrolled activation of pancreatic proteases and platelet activating factor, a potent phospholipid mediator, are considered key features of pancreatic necrosis development.18 To find a causative treatment of AP, several drugs have been tested in clinical trials, which interfere with these putative mechanisms. In this section, we focus on the most frequently evaluated medical treatments of AP since 1990.
Does Gabexate Mesilate Decrease Morbidity or Mortality of Patients With Severe AP?
One level I trial,19 2 meta-analyses (level I),20,21 and one level II trial22 were eligible for this analysis (Table 2). The Valderrama et al23 and one level III trial24 were excluded since data for patients with severe AP were not separately reported23 or did not meet the inclusion criteria24 of this analysis. The meta-analysis by Andriulli et al includes 8 studies on gabexate, but only 5 of these trials were randomized, one compared gabexate with aprotinin, and one study was only reported as abstract.20 The meta-analysis by Messori et al included the study of Valderrama et al and one trial comparing gabexate mesilate and aprotinin.21 Both meta-analyses were excluded because of these methodologic limitations.
TABLE 2. Randomized Trials on Medical Treatment for Severe AP
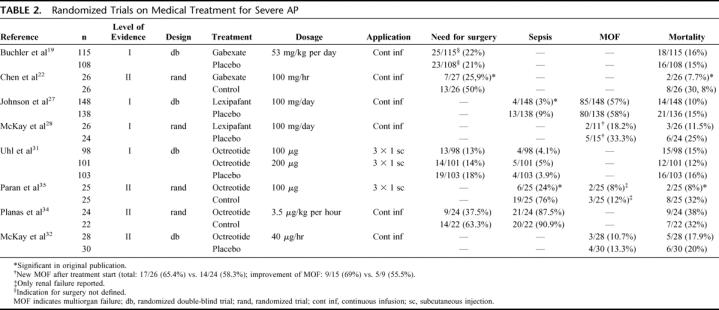
We performed a meta-analysis on the trials of Buchler et al19 and Chen et al.22 Neither the need for surgery (26.9% versus 22.7%, P = 0.46, k = 2) nor mortality rates (17.9% versus 14.2%, P = 0.46, k = 2) were significantly reduced by gabexate treatment.
From this analysis, we conclude that gabexate mesilate does not improve the outcome of patients with severe AP, and its routine use in patients with severe AP is not recommended (level A).
Does Aprotinin Decrease Morbidity or Mortality of Patients With Severe AP?
One double-blind randomized trial compared the intraperitoneal aprotinin versus saline application (level I),25 and one randomized study compared intravenous aprotinin versus gabexate mesilate (level II).26
No difference was detected between intraperitoneal aprotinin and the control group except for the need for surgery, which was defined as symptomatic necrosis and persisting organ failure.25 In addition, intravenous aprotinin was significantly less effective than gabexate mesilate regarding the systemic complication rate and need for surgery.26
We conclude that neither intraperitoneal nor intravenous aprotinin improve the outcome of patients with severe AP; therefore, its routine use in patients with severe AP is not recommended (level A).
Does Lexipafant Decrease Morbidity or Mortality of Patients With Severe AP?
Two randomized double-blind, placebo-controlled studies qualified for this analysis (level I).27,28 The study of Kingsnorth et al was excluded because results for severe AP (<50%) were not reported separately.29
Johnson et al found a significantly lower incidence of sepsis, but MOF and local complications remained unaffected27 (Table 2). McKay et al demonstrated a significantly higher reduction in the MOF score during lexipafant treatment. However, the incidence of MOF, length of hospital stay, and mortality rates were not improved by lexipafant treatment.28
The trials by Johnson et al27 and McKay et al28 were meta-analyzed. Incidence of MOF and mortality rates could be extracted from both trials. Our meta-analysis failed to detect a significant difference in MOF (27.7% versus 21.7%, P = 0.37, k = 2) or mortality (17.3% versus 10.3%, P = 0.07, k = 2).
Current literature does not provide enough evidence to recommend lexipafant for routine use in patients with severe AP (level A).
Does Octreotide Decrease Morbidity and Mortality of Patients With Severe AP?
One meta-analysis,20 3 placebo-controlled randomized studies,30–32 3 randomized open-labeled studies,33–35 one prospective matched,36 and 3 prospective nonrandomized trials were identified.37–39 In addition, 2 randomized trials compared different dosages of octreotide with an untreated control group.40,41 The trial of Uhl et al was graded as level I.31 Because the studies of McKay et al,32 Planas et al,34 and Paran et al35 did not provide a sample size calculation, they were graded as level II. Seven trials were excluded since the outcome was not provided separately for severe AP,30,33,38–40 due to methodologic limitations,41 and because trials of 2 higher levels of evidence were available.37
In contrast to Uhl et al,31 Paran et al35 found significantly lower incidences of sepsis and acute respiratory distress syndrome, as well as a shorter hospital stay and decreased mortality.31,35 The studies by Planas et al35 and McKay et al32 did not reveal any effect on morbidity or mortality (Table 2).
Fiedler et al treated 39 patients with postoperative pulmonary failure following surgery for necrotizing AP with intravenous octreotide (3 × 100 μg/day) in a prospective study.36 By matching these patients to a historical control group of 54 patients without octreotide treatment (level II), they showed a significantly lower mortality rate and incidences of acute respiratory distress syndrome and septic shock in the treatment group. Since this trial was not randomized, it was excluded from our meta-analysis.
Our meta-analysis of 4 eligible trials31,32,34,35 reveals that octreotide does not reduce surgical interventions (23.3% versus 16.3%, P = 0.09, k = 3), sepsis (28.7% versus 21.1%, P = 0.25, k = 3), mortality (20.6% versus 17.7%, P = 0.34, k = 4), or overall complication rates (70.6% versus 63.2%, P= 0.2, k = 2). Furthermore, we did not detect any significant difference for either application (s.c. versus i.v.) regarding these parameters (data not shown).
From this analysis, we conclude that the routine use of octreotide is not recommended for patients with severe AP (level A). Although one level II trial shows that a subgroup of patients might benefit from intravenous octreotide, this treatment is not recommended outside of clinical trials.
Does Early Nasojejunal Nutrition Influence Morbidity or Mortality of Patients With AP?
Suppression of pancreatic exocrine secretion by bowel rest used to be an important strategy to stabilize AP, and total parenteral nutrition (TPN) was therefore advocated.1,42 After postoperative EN was shown to be safe and to decrease infectious complications,43 EN was also introduced in the management of AP. As shown in animal experiments as well as in human studies, the intestinal mucosa atrophies during fasting periods while it is preserved by EN.44,45 Since infection of pancreatic necrosis is thought to derive from the gastrointestinal tract, EN might thereby decrease the incidence of this severe complication. In contrast to initial concerns, EN does not stimulate the exocrine function of the pancreas, if the feeding tube is positioned in the jejunum.46 As newer data contravene historic concerns against EN, there is still an ongoing debate about the indication of EN in AP.
Two level I,47,48 6 level II,49–54 and 2 level III trials,50,55 including patients with AP, were identified. All studies used nasojejunal feeding within 48 hours of admission except Eatock et al, who used nasogastric feeding.55
One meta-analysis on EN versus TPN on the trials of Kalfarentzos et al and McClave et al found a reduced relative mortality risk in patients receiving EN, but this difference did not reach statistical significance.47 McClave et al included 30 patients with mild AP but terminated the trial before the required number of patients was recruited, and no significant differences in morbidity or mortality rates were found.49 Olah et al randomized 89 patients with mild to severe AP to EN or TPN in the first phase of the trial.50 Thereafter, 14 patients received EN in a prospective study. Abou-Assi et al included 53 patients with mild to severe AP and found significantly less metabolic complications (eg, hyperglycemia) and line infections as well as lower hospital cost in patients under EN.54 Mortality rates for patients with severe AP (n = 26) were not provided in the original publication, but these data were available after personal communication with the authors (23.1% versus 23.1%).54 Windsor et al included patients with mild (n = 21) to severe disease (n = 13), but did not provide results for severe disease separately, and the observed complication rates were not significantly different.52 Kalfarentzos et al performed the only randomized trial, for which only patients with severe AP (n = 38) were eligible. They applied equal amounts of calories (24.1 kcal/kg versus 24.5 kcal/kg) and proteins (1.43 versus 1.45 g/kg) by EN and TPN and found significantly lower overall and septic complication rates for EN.53 In the prospective trial of Eatock et al,55 23% (6 of 26) of the patients required surgery for infected necrosis and 15.4% (4 of 26) died. This study was not included into our analysis because it was not randomized.
Three trials provided cost for EN and TPN, and all demonstrated significantly lower cost for EN versus TPN.49,53,54
Olah et al performed a randomized double-blind trial on patients with mild and severe AP, in which patients received either active or inactivated lactobacillus plantarum in addition to fiber containing EN (109/day) (level I).48 The addition of active lactobacillus significantly reduced the infection rate and indication for surgery.
We included data from 6 level II trials in our own meta-analysis.49,50,52–54 MOF (11.5% versus 19.8%, P = 0.3, k = 3) and mortality (10.3% versus 11.6%, P = 0.38, k = 5) were not significantly different, but central line infections (3.5% versus 26.1%, P = 0.01, k = 2) and sepsis (12.9% versus 27.9%, P = 0.02, k = 4) were significantly lower in the EN group. In addition, we separately evaluated results for mild and severe AP. Mortality rates were the only uniform parameter and did not differ significantly between EN and TPN, neither for mild (8.9% versus 5.4%, P = 0.38, k = 3) nor for severe AP (15.8% versus 20.9%, P = 0.6, k = 3).
Since 3 trials have independently shown lower cost and our meta-analysis has shown equal mortality rates but less (infectious) complications for EN, we conclude that patients with AP should preferably receive EN (level A). Also, a nasogastric application for EN appears feasible (level C) but requires further investigation. Supplementation of EN with probiotics (such as lactobacillus plantarum) may further decrease septic complications (level A), although this finding was documented in only one study.48
Larger trials are necessary to confirm these results and to define the optimal content of nutrients (amount of calories and protein, immuno-nutrition, etc.).
Antibiotic Prophylaxis in Necrotizing AP
Infection of pancreatic necrosis with consecutive sepsis belongs to the most serious complications of severe AP with a high mortality rate.7 Although the prevention of this complication by antibiotic prophylaxis is appealing to decrease mortality, the actual benefit of antibiotic prophylaxis is controversial.56 Since necrosis formation is best assessed by ceCT or MRI,8–10,12,57 only series of necrotizing AP proven by ceCT findings were included in this analysis.
Does Antibiotic Prophylaxis Reduce Morbidity and Mortality of Necrotizing AP?
Two meta-analyses (level I)58,59 and 7 level II trials60–66 compared prophylactic antibiotic treatment with an untreated control group in patients with necrotizing AP. Golub et al included all studies on antibiotic prophylaxis published from 1966 to 1997 into a meta-analysis.59 Early studies using penicillin were separately evaluated and did not show any beneficial effect in this meta-analysis. The analysis of the remaining studies60,62–65 revealed a significant reduction in mortality rates, while septic complications remained unchanged. Even after exclusion of the Luiten et al trial62 (see below), antibiotic prophylaxis significantly decreased mortality rates. Sharma et al meta-analyzed 3 trials63–65 and found significantly reduced risks for sepsis and mortality.58
The Delcenserie et al trial was excluded from our analysis since pancreatic necrosis was not convincingly demonstrated.60 Luiten et al applied oral and rectal antibiotics to achieve intestinal decontamination, as most pancreatic infections are caused by gram-negative bacteria of the intestinal flora. They found less infected pancreatic necrosis without a difference in mortality62 (Table 3). Since no study endpoints were defined, this trial is level II.
TABLE 3. Randomized Trials (level II) on Antibiotic Prophylaxis for Patients With ceCT Proven Necrosis
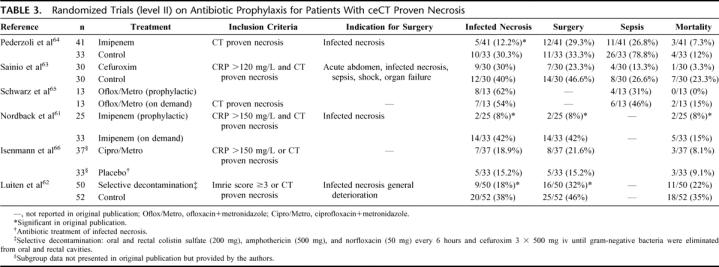
Two open labeled studies (level II) randomly compared antibiotic prophylaxis with control treatment and defined infected necrosis as indication for surgery.63,64 In addition, one level II trial compared antibiotic prophylaxis with antibiotic treatment65 in patients with proven necrosis (Table 3). Patients received either ciprofloxacin + metronidazole or no treatment, and rates of infected necrosis were equal.65 However, 5 fine needle aspirations were performed in all patients within the first 10 days after study inclusion.
The study endpoint in the Nordback et al trial was the indication for surgery as defined by infected necrosis.61 Patients were randomized to prophylactic imipenem or observation. Infected necrosis was the indication for surgery in the imipenem group, while patients in the observation group first received imipenem (treatment) after developing infection of necrosis, and surgery was only performed if antibiotic treatment failed (Table 3). The mortality rate was significantly higher in the observation group than in patients receiving prophylactic imipenem (P = 0.04). However, 64% of the patients with infected necrosis did not require surgery in the observation group due to antibiotic treatment with imipenem.
The only double-blind randomized trial was recently reported by Isenmann et al on 114 patients with predicted severe AP.66 The sample size calculation was based on the incidence of infected pancreatic necrosis, but only 67% of the patients eventually had necrosis (level II). The outcome of patients who entered this study with documented necrosis was not analyzed in the publication but provided by the authors upon personal communication (Isenmann, R). Only these data were included in our analysis (Table 3). The incidence of infected necrosis was not significantly different between both groups (13.5% versus 9.1%, P = 0.67). Since the treatment of infected necrosis was not uniform and included a variety of antibiotic treatments with or without surgery depending on the centers’ preference (Isenmann R, personal communication), we excluded mortality data from our analysis.
Because of inconclusive results and low power of the available studies, we performed a new meta-analysis on 5 trials.61,63–66 Overall, antibiotic prophylaxis significantly reduced sepsis and mortality but did not prevent infection of necrosis. However, a subgroup analysis demonstrates a significant reduction in infected necrosis for patients receiving prophylactic imipenem (36.4% versus 10.6%, P = 0.002) in contrast with those under chinolones + metronidazole (Fig. 1).
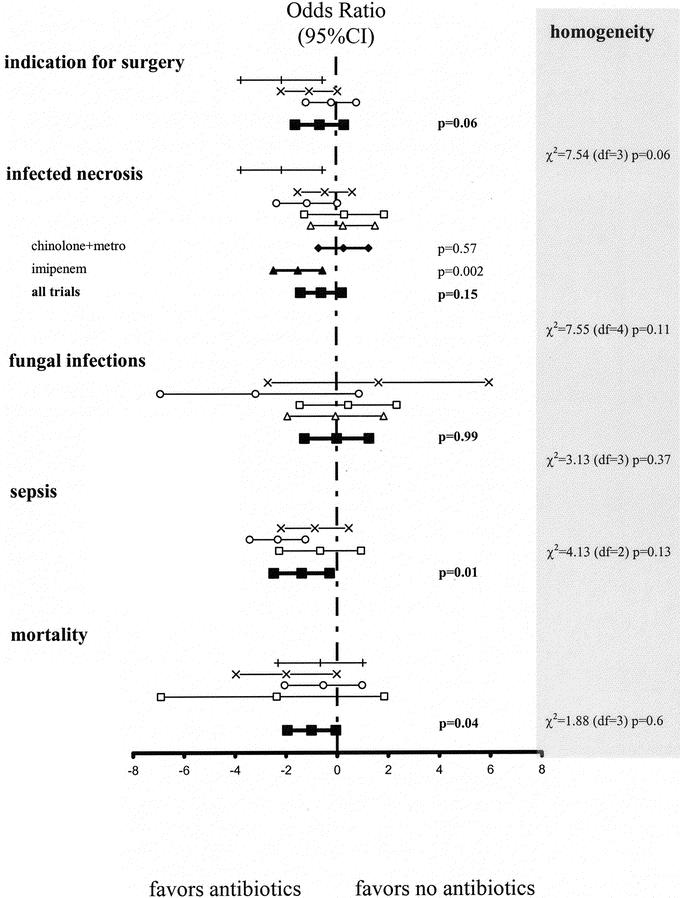
FIGURE 1. Antibiotic prophylaxis for necrotizing pancreatitis. The 95% confidence intervals (95% CI) for the logarithm of the odd ratios for indication for surgery, infected necrosis, sepsis, fungal infections, and mortality are plotted (P values presented on the right side). Heterogeneity between studies was evaluated using the χ2 based Q statistic, and the results are provided in the right column of this figure: □, Schwarz et al;65 ○, Pederzoli et al;64 +, Nordback et al;61 x, Sainio et al;63 ▵, Isenmann et al;66 ▪, meta-analysis [all]; •, meta-analysis [chinolone + metronidazole]; ▴, meta-analysis [imipenem].
From this analysis, we conclude that antibiotic prophylaxis is superior to antibiotic treatment in necrotizing AP (level B). Patients with proven pancreatic necrosis should receive antibiotic prophylaxis using imipenem or meropenem (see below) (level A).
Which Is the Best Regimen for Antibiotic Prophylaxis?
One level I67 and 2 level II trials68,69 compared different antibiotic regimen in patients with necrotizing AP.
Bassi et al randomized 60 patients with necrotizing AP to perfloxacin (2 × 0.4 g) or imipenem (3 × 0.5 g) over 14 days and found less infected necrosis for imipenem (34% versus 10%, P = 0.03), but the difference in mortality was not significant (24% versus 10%, P = 0.18).69 Eleven and 4 resistant bacteria were isolated from 10 and 3 patients in the perfloxacin and imipenem groups, respectively.70 Also, 21% of bacteria isolated from patients in the placebo group of the Isenmann et al trial were resistant to ciprofloxacin + metronidazole questioning the efficiacy of this regimen.66
Manes et al randomized 176 patients to meropenem (3 × 0.5g) or imipenem (4 × 0.5g) for at least 14 days and did not find any significant difference regarding septic complications, indication for surgery or mortality (13.6% versus 11.4%).67 Also no difference was found in the Maravi-Poma & al. study regarding morbidity and mortality rates. Patients (n = 101) were randomized to imipenem (4 × 0.5g) for either 14 days or until recovery of any major systemic complication.68 However, neither the number of patients requiring prolonged antibiotic prophylaxis nor the period until occurrence of infection were provided.
From this analysis, we conclude that imipenem is superior to perfloxacin (level B) and is equally effective to meropenem (level A). The combination of chinolones and metronidazole is not an effective antibiotic prophylaxis (level A). Fourteen days of intravenous antibiotic prophylaxis appear efficient (level B).
Does Antibiotic Prophylaxis Promote Fungal Infections?
Antibiotic prophylaxis has been claimed to promote fungal infection.71,72 However, up to 25% of patients with necrotizing AP who do not receive antibiotics also develop fungal infection with a mortality rate of up to 84%.73–75 The incidence of fungal infection correlates with the extent of necrosis as well as the disease severity on admission in these patients.75
Four of the randomized trials on intravenous antibiotic prophylaxis provided the incidence of fungal superinfection.63–65,72 They were all classified as level II, since fungal infection was not their primary endpoint. The incidence of fungal infections was below 7% in 3 trials,63,64,72 while it exceeded 20% in one small study.65 Patients in the control group of the Luiten et al trial (see above) had a higher rate of fungal infections than those receiving prophylactic antibiotics (19.2% versus 4%).62
These trials were meta-analyzed63–65,72 (Fig. 1): the fungal infection rate was not different between patients receiving antibiotics 4.9% and those in the control group 6.7% (P = 0.99, k = 4).
From this analysis, we conclude that antibiotic prophylaxis does not result in an increased incidence of fungal infections (level B).
It should be emphasized that none of the available trials on antibiotic prophylaxis was sufficiently powered to detect significant differences in mortality. In addition, different antibiotic regimens were used in the past. It appears of utmost importance to adjust the antibiotic regimen to the center's resistance spectrum to achieve a sufficient antibiotic prophylaxis. Because of their broad spectrum, imipenem and meropenem appear particularly attractive. However, a randomized trial should be performed on EN to versus prophylactic antibiotics, since both reduce septic complications, and EN is not associated with the potential risks of antibiotic prophylaxis such as resistance of bacteria.
Should Emergency Erc and Sphincterotomy Be Performed for Biliary AP?
Gallstones passing the papilla of Vater represent the initial step for biliary AP.76 Endoscopic interventions have widely replaced open surgical bile duct exploration during recent years. But endoscopic sphincterotomy (ES) and injection of contrast medium into the pancreatic duct inherit the risk to worsen AP by additional complications. Therefore, we evaluated the indication of ERC in biliary AP.
One meta-analysis77 and 4 randomized trials78–81 were identified, of which only one fulfilled the estimated sample size calculation (level I).80 The trial by Neoptolemos et al was included, although it was published before 1990.80 Although patient inclusion was not restricted to biliary AP, the study by Fan et al was included in our analysis because the outcome of these patients was reported separately.78 One study was excluded because it was only published as abstract,81 and one because patients did not have biliary AP.78
The eligible studies compared emergency ERC + ES (within 24–72 hours) with conservative treatment79 or planned interval ERC78,80 in patients with biliary AP. In these 3 trials, ES and stone extraction were only performed if common bile duct stones were found during ERC.
Neoptolemos et al demonstrated significantly lower morbidity rates following emergency ERC.80 Eleven patients (9%) with cholangitis were equally distributed to both treatment groups, and the complication rate was significantly lower after ERC (15% versus 60%, P = 0.003) even after exclusion of these patients.80 Patients with biliary obstruction were excluded in the Fölsch et al trial,79 and median bilirubin levels were equal in both groups in the Fan et al78 trial (2.2 mg/dL). These 2 trials failed to demonstrate significant effects on morbidity and mortality rates.78,79 Of note, the Fölsch et al trial was the only multicenter trial.79
Only Neoptolemos et al80 and Fan et al78 evaluated the outcome for severe disease separately. Neither found a significant difference in complication and mortality rates in patients with mild biliary AP.78 In contrast, both trials detected a significantly lower complication rate in patients with severe AP, but differences in mortality rates did not reach statistical significance. Furthermore, Fan et al found a reduction in biliary sepsis in patients with severe biliary AP.78
The meta-analysis by Sharma and Howden77 included 4 randomized trials78–81 and demonstrated significantly lower morbidity (38.5% versus 25%; P < 0.001) and mortality (9.1% versus 5.2%; P < 0.05) rates following early ERC compared with interval ERC. Patients with severe AP were not evaluated separately in this meta-analysis.77
By meta-analyzing the trials of Fan et al,78 Neoptolemos et al,80 and Fölsch et al,79 we found that emergency ERC + ES significantly reduced the overall complication rate (41.8% versus 31.3%, P = 0.03, k = 3) without a significant effect on the mortality rate (7.2% versus 6.4%, P = 0.46, k = 3) (Fig. 2). Subgroup analyses revealed no differences in overall complications (14.5% versus 14.7%, P = 0.97, k = 2) or mortality (0.7% versus 0.7%, P = 0.99, k = 2) in patients with mild biliary AP. In contrast, ERC significantly reduced both the overall complication (57.1% versus 18.2%, P = 0.0001, k = 2) and mortality (17.9% versus 3.6%, P = 0.03, k = 2) rates in patients with severe biliary AP (Fig. 2).
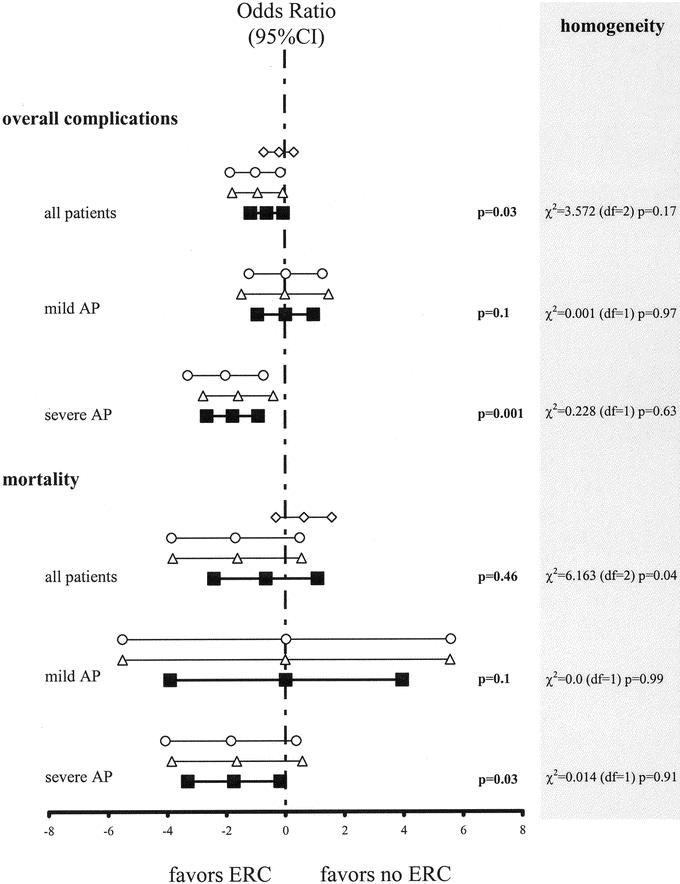
FIGURE 2. Emergency ERC for acute AP. The 95% confidence intervals (95% CI) for the logarithm of the odd ratios for mortality and local complications of emergency ERC in patients with acute AP. Heterogeneity between studies was evaluated using the χ2 based Q statistic, and the results are provided in the right column of this figure: ⋄, Fan et al;78 ○, Fölsch et al;79 ▵, Neoptolemos et al;80 ▪, meta-analysis.
We conclude that emergency ERC does not influence on the course of mild biliary AP (level A). Since 2 randomized trials demonstrated less morbidity and our meta-analysis showed lower mortality rates, we conclude that emergency ERC + ES should be strongly considered in patients with severe biliary AP (level A) as well as in patients with standard indications for ERC + ES such as cholangitis.
Role of Surgery for AP
The role of surgery in AP includes prevention of recurrence (cholecystectomy) and treatment of complications (necrosectomy) of AP. The management of biliary AP has changed since the successful advent of ERCP and laparoscopic cholecystectomy (LC).82 As shown above, patients with severe biliary AP should be treated with emergency ERC. However, there are some ongoing controversies about whether cholecystectomy is mandatory following ERC + ES to prevent recurrent episodes of AP and other biliary complications, and if so, when it should be performed.83,84
What Is the Best Treatment in Mild AP: Primary Cholecystectomy Or ERC + ES?
One level I,85 but no level II or III trials have compared ERC + ES with primary cholecystectomy in patients with mild biliary AP. Chang et al randomized patients to either ERC + ES followed by LC or to LC followed by ERC + ES.85 If LC was performed first, ERC was only performed when common bile duct (CBD) stones were detected intraoperatively. Study endpoint was hospital cost. Hospital stay and overall cost were significantly lower if LC was performed first.85 But cost for anesthesia were not included in this analysis, and laparoscopic bile duct clearance was never attempted, so that the need for postoperative ERC could probably be further reduced.
Another 3 level I86–88 and one level II89 studies compared ERC + ES with cholecystectomy in patients with symptomatic CBD stones (not exclusively biliary AP). In 3 trials,86,87,89 patients with symptomatic CBD stones were randomized to open cholecystectomy or ERC + ES. Two of these trials demonstrated significantly less recurrent biliary symptoms in the cholecystectomy group;86,87 the late mortality was increased in the ERC group in one trial89 and equal in 2 trials.86,87
Cuschieri et al compared LC + CBD clearance with ERC + ES followed by LC during the same hospitalization88 but do not provide long-term results. However, ductal stone clearance, morbidity, and mortality were not significantly different between the 2 groups.88
We conclude from this analysis that patients with mild biliary AP are best treated by primary LC with intraoperative cholangiography. ERC should be performed postoperatively if intraoperative cholangiography reveals CBD stones and laparoscopic bile duct clearance has failed (level B).
Is Cholecystectomy Indicated After Successful ERC + ES?
No level I or II, but 3 level III90–92 trials have assessed the indication for cholecystectomy after ES for biliary AP. Therefore, the current literature does not support a well-based statement. For this reason, we also analyzed studies evaluating the indication of cholecystectomy after ES for CBD stones. One level I trial compared ERC + ES versus ERC + ES followed by LC in patients with ASA scores I to III.93 If LC was performed within 6 weeks after ES, recurrent biliary symptoms occurred less often within 2 years (47% versus 2%, P < 0.0001).93 These results are supported by 2 prospective nonrandomized trials in patients with biliary AP, in which recurrent biliary symptoms occurred in 15% to 52%, if cholecystectomy was omitted.91,92 Similarly, recurrent biliary symptoms occurred in 16% of patients who did not undergo LC compared with 7.6% of patients who underwent LC after ERC + ES in a prospective cohort study (level III).
We conclude that cholecystectomy is indicated after ES for symptomatic CBD stones or biliary AP in patients with ASA scores I to III, since biliary AP represents one major complication of CBD stones (level A). The current literature does not support a well-based statement on LC in patients with ASA scores IV and V, but a “wait-and-see” policy after ERC + ES appears to be reasonable in these patients deemed too sick for surgery (level C).
What Is the Optimal Timing for Cholecystectomy After ERC + ES: Early or Late?
ERC + ES should be performed in patients with severe AP, cholangitis, and persistent cholestasis (see above), and might be performed in selected cases with mild AP. If ES has been performed, LC should be performed within 6 weeks (see above).93 However, the optimal timing for cholecystectomy is still under debate.
No randomized trial, but 4 prospective trials (level III)94–97 evaluated the optimal timing for cholecystectomy after biliary AP. Late cholecystectomy (8-12 weeks) was performed in one trial,95 and early LC in 3 trials after ERC for mild AP.94,96,97 In addition, one randomized trial compared ERC + ES + LC versus LC,88 and one randomized trial compared ERC + ES versus ERC + ES + LC93 in patients with CBD stones. Since only one arm of these trials was evaluable for this analysis (ERC + ES + LC), both trials were classified as prospective trials (level III).
In general, LC after AP is reported to be feasible but is more difficult and has an increased conversion rate to open surgery in all trials.94–96 The conversion rates to open surgery are equal or even slightly lower for early LC, and morbidity of early LC after mild AP is reported to be low (Table 4). Similarly, conversion rates to open surgery were lower after early LC in patients with symptomatic CBD stones (not exclusively biliary AP).88,93
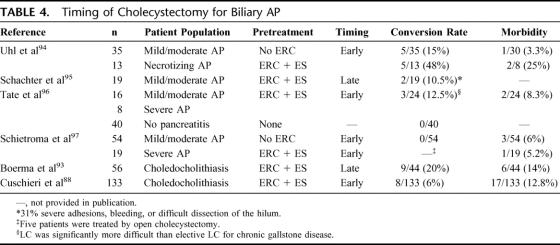
TABLE 4. Timing of Cholecystectomy for Biliary AP
Of note, open surgery for necrotizing AP is necessary in up to 20% of patients with AP dependent on the proportion of patients with severe AP,94,97 and cholecystectomy is routinely performed by most surgeons during this intervention without additional morbidity.
In conclusion, early LC after ES should be preferred in patients with mild to moderate AP (level C). Since mild AP can continuously aggravate over time, LC with bile duct exploration on admission should be evaluated as a treatment option for patients with biliary AP. In patients with severe biliary AP who did not require surgery for necrotizing AP, cholecystectomy appears to be favorable after full recovery from AP (level C).
The role of surgery for necrotizing AP has changed from extensive pancreatic resections to a more conservative treatment aiming at preservation of the gland. However, the optimal timing and type of surgery for AP are unknown. Some authors prefer reexplorations in 2-day intervals (“open packing”), whereas others perform a single necrosectomy followed by continuous postoperative lavage of the lesser sac. Following this trend to less invasiveness, the feasibility of retroperitoneal necrosectomy as well as laparoscopic98 and endoscopic99 interventions has been demonstrated, but not prospectively evaluated yet.
In the past, the main indications for surgery were pancreatic necrosis and deterioration of the patients general status. With the development of the concept of sterile and infected pancreatic necrosis, evidence arose that patients with sterile necrosis might recover without surgical intervention.6
Should All Patients With Necrotizing AP Be Operated?
So far, no level I or II trial has evaluated the benefit of surgery for sterile or infected necrosis. One prospective (level III)100 and one randomized trial (level II)61 assessed the indication of antibiotic prophylaxis and surgery for infected necrosis, and one level II trial compared early versus late surgery for severe AP.101 In addition, 6 prospective trials (level III) evaluated surgery for necrotizing AP.6,100,102–105 We decided to include both publications of Beger et al since the publication in 1991 provided information about the surgical complications of the original publication in 1988.102,106
In 2 trials, patients with necrotizing AP underwent necrosectomy after failure of conservative treatment independent of infection of these necrosis (see below), and the outcome was separately analyzed for patients with sterile and infected necrosis.102,104,106 (Table 5). In the remaining 4 studies, surgery was only performed for proven infection of necrosis, and outcome of patients with sterile and infected necrosis was again separately analyzed.6,100,103,105
TABLE 5. Results From Surgery for Necrotizing Pancreatitis
To evaluate whether all patients with necrotizing AP (sterile and infected necrosis) require surgery, we compared outcome data of patients with sterile necrosis who were operated102,104,106 with those who were not operated.6,100,103,105 A meta-analysis to show statistical significance is not possible since these trials were not randomized. The surgical treatment of sterile necrosis appears to have a higher mortality rates (11.9%; 95% confidence interval, 5.3–22.2) than the conservative treatment (2.3%; 95% confidence interval, 0.3–8.2) in patients with sterile necrosis (Table 5).
Therefore, the detection of necrosis itself is not an indication for surgery unlike proposed in the 1980s and early 1990s1,42 (level C). However, some patients will require surgery for reasons secondary to necrosis formation (eg, compartment syndrome or failure of conservative treatment), although infection has not been proven.
Do Patients With Infected Necrosis Require Immediate Surgery?
Mier et al randomized patients with an indication for surgery to either early (within 48–72 hours, n = 25) or late necrosectomy (more than 12 days, n = 15).101 The indication for surgery was defined as MOF with clinical deterioration despite maximal intensive care. All patients received antibiotic prophylaxis, but infection of necrosis was never proven prior to surgery. Of the 15 patients in the group of late necrosectomy, 3 improved during a 12-day period of conservative treatment and did not require surgery. Unfortunately, these patients were excluded from the final analysis. The remaining 12 patients were operated. Although the difference in mortality between early (56%) and late (27%) surgery was not statistically significant, the authors terminated this study based on an odds ratio of 3.4 (95% confidence interval, 0.74–15.9).
Infection of necrosis was an absolute indication for surgery in all studies on surgery for necrotizing AP with mortality rates ranging from 14% to 26%. In contrast, infection of necrosis was not considered a strict indication for surgery in only 2 studies.61,100 In the first Nordback et al trial,100 antibiotic treatment was started, when surgery was indicated (proven infection of necrosis, MOF, or recurrent inflammatory variables). Three of 25 patients (12%), who initially fulfilled criteria for surgery, recovered without surgery, and 5 patients (23%) died despite surgical treatment. In the follow-up trial, patients with sterile necrosis were randomized to the observation group or to receive prophylactic imipenem (see antibiotics).61 Prophylactic imipenem resulted in a significantly lower need for surgery (infected necrosis), but mortality rates were comparable (8% versus 15%). Furthermore, 64% of patients with infected necrosis in the control group did not require surgery because of imipenem treatment. Since follow-up data are not provided, it remains unclear whether these patients required surgery for infected necrosis at a later stage.
Based on these results, surgery should preferentially not be performed in the early phase of AP (level B), and most patients with infected necrosis require surgery. However, in case of suspected or proven infection of necrosis, adjusted antibiotic treatment could be primarily applied, if compatible with the general status of the patient (level B). This algorithm might save some patients from unnecessary surgery and postpones surgery in those patients who will require definite surgical treatment.
In most studies published during the past decade, indication for surgery was defined by necrosis formation on ceCT and positive fine-needle aspiration irrespective of secondary signs of infection on ceCT.6,103 In general, an intra-abdominal abcesses is a generally accepted indication for surgery, endoscopic, or percutaneous drainage. Since the results of the Nordback et al trial suggest that not all patients with suspected infection of necrosis require surgery if treated with adequate antibiotics, the question arises whether the definition of infected necrosis should be adjusted.
Which Surgical Technique Should Be Used?
Only one randomized trial has compared pancreatic resection versus continuous peritoneal lavage on 11 versus 10 patients.107 Pancreas resection was associated with increased perioperative morbidity, and normal pancreatic parenchyma was unnecessarily removed. Since long-term outcome of patients is closely related to the amount of preserved pancreatic tissue, treatment policy has widely changed to limited necrosectomy.108 Mainly 2 techniques aiming at maximal tissue preservation are currently used. First, the “open packing” technique, in which repeated necrosectomies are performed in 48-hour intervals until all necrosis has resolved and granulation tissue has developed. Thereafter, continuous lavage is often performed.6 Second, a single necrosectomy with continuous postoperative lavage (8–10 L/day) through surgically placed drainages has been proposed by Beger.106 As outlined above, less invasive procedures have been tested but not prospectively evaluated yet. Since sterile necrosis per se does not appear to be an indication for surgery (see above), we focus on patients with infected necrosis in this analysis.
Five prospective trials (level III) used “open packing,”6,100,101,104,105 whereas 2 studies investigated on the technique described by Beger et al (level III).102,106 Complication rates after surgical treatment were high in all trials, and in absence of randomized trials, a meta-analysis of the 2 techniques is impossible (Table 5). Of note, 25% of patients treated by the procedure reported by Beger et al required one or more reoperations during the course of their disease for fistulae, intra-abdominal abscesses, or bleeding.
“Open packing” is accompanied by a higher morbidity rate mainly due to higher incidences of fistulae, bleeding, and incisional hernias. In addition, mortality rates were slightly higher in the reports on “open packing” (Table 5).
We conclude from these low-ranked studies that careful single necrosectomy and postoperative lavage without planned relaparotomies are less harmful and should be preferred for surgical treatment of necrotizing AP, when applicable (level C).
Only a few prospective trials on the surgical treatment of AP have been published, and none of them was randomized. Therefore, the level of evidence is generally very low regarding recommendations on the surgical treatment. Further studies are mandatory to define the optimal indications, procedures, and timing for surgery. Newer approaches such as laparoscopic, endoscopic, or retroperitoneal procedures might decrease morbidity and mortality in these patients.
DISCUSSION
The treatment of AP remains challenging, and many aspects are still controversial in the literature. This systematic review provides the best evidence for actual treatment modalities and helps defining the optimal treatment strategy for patients with AP. Moreover, it reveals weaknesses in the current literature and should help designing novel trials. Our approach is substantially different from classic review articles by its meticulous methodology, since it was performed to assess the current evidence for specific clinical questions. The literature search was conducted under strictly defined terms, and all literature identified was classified according to Sackett's classification for evidence-based medicine.15 A major challenge for such analyses is the comparability of the included studies, which have been performed in different patient populations with respect to patients’ characteristics and inclusion criteria, and differences in the standard of care of the participating hospitals. Moreover, definitions of disease severity have not uniformly been used before the consensus conference of Atlanta in 1992,14 and the lack of uniform and widely accepted definitions of complications represents a common problem of medical trials.109–111 Therefore, mortality rates often represent the only objective and convincing parameter.
We addressed these methodologic limitations by applying strict inclusion criteria and by using the random-effects model in the statistical analysis, which takes into account this interstudies variability. Although significant heterogeneity was only detected once, we used the random-effects model for all meta-analyses, since the lack of significant heterogeneity is due to the small number of studies rather than homogeneous study populations. Moreover, the random-effects model is valid in homogeneous and heterogeneous populations, although a treatment effect may be slightly underestimated in homogeneous populations.
Meta-analyses increase the level of evidence when trials provide different results or when an observed difference is not significant due to small sample sizes in individual studies.15 However, they may still fail to identify significance (type II error), when the number of patients remains too small (eg, EN). For this reason, the number of patients included must be considered in a negative meta-analysis before rejecting a particular treatment. Another possible shortcoming of meta-analyses is a negative publication bias.112 This type of error typically occurs when randomized trials are not published due to insignificant results, while small studies with significant results are published. Possible methods to assess such bias are the funnel plot or the Spearman correlation between estimated effects and sample sizes. But these mathematical approaches also require the availability of a large number of studies.113 In the field of AP, it is difficult to draw conclusions from these tests as only 2 or 3 studies are available for most meta-analyses. However, publication bias appears unlikely in our current study as most published studies used for our analyses reported negative results, and publication bias would favor positive results.
In providing the highest level of evidence for each clinical question, we excluded retrospective analyses due to their methodologic shortcomings. According to the evidence-based medicine, they only provide a low level of evidence, and treatment recommendation should always be based on the highest evidence. Retrospective analyses are only valuable in the absence of higher level studies, although they may provide important data for further prospective trials. We excluded publications before 1990, since crucial treatment modalities (eg, ICU management) have markedly changed over the past 20 years. Therefore, differences in outcome among trials of a larger period might mainly be related to improved supportive treatment rather than the evaluated therapy. Abstract publications were excluded, since comparability of results cannot be ascertained without the availability of complete inclusion criteria and patients characteristics. Also, one may assume that the majority of high-quality and relevant studies will subsequently be published in full within a reasonable time period to allow a detailed review by others. Trials published in a language other than English were excluded to ascertain strict inclusion criteria for all analyses. A recent literature analysis focused on this topic and found no statistical difference between meta-analyses excluding languages other than English and those being not restrictive to languages.114 Finally, all trials were excluded from meta-analysis comparing a treatment group with a group other than untreated control or placebo, since ineffective or deleterious treatments might overestimate the other evaluated treatment.
Mainly because of differences in criteria for the literature search and study inclusion, some of our results are in contrast to those from earlier meta-analyses and structured reviews.115–117 In addition, some concluding guidelines were eventually raised from the consensus conferences rather than from evidence-based criteria in 2 of these analyses.116,117 We would caution that a comprehensive literature research with reproducible inclusion criteria and proper statistical analysis best prevents bias and provides the highest level of evidence.
Finally, we want to emphasize that the application of these evidence-based recommendations to an individual clinical case needs to be performed in a multidisciplinary manner by physicians experienced in AP. Thus, an important factor, not always apparent in evidence-based studies, is that patients with severe or complex diseases should be referred to specialized centers.
ACKNOWLEDGMENTS
The authors thank Dr. R. Isenmann for providing us with the raw data of the German Antibiotics in Severe Acute Pancreatitis (ASAP) Study Group trial (Gastroenterology. 2004;126:997–1004).
Footnotes
Reprints: Pierre-Alain Clavien, MD, PhD, FACS, FRCS, Department of Visceral and Transplantation Surgery, University Hospital of Zurich, Raemistrasse 100, 8091 Zurich, Switzerland. E-mail: clavien@chir.unizh.ch.
REFERENCES
- 1.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198–1210. [DOI] [PubMed] [Google Scholar]
- 2.Lankisch PG. Epidemiology of acute pancreatitis. In: Malfertheimer P, ed. Acute Pancreatitis: Novel Concepts in Biology and Therapy, 1st ed. Berlin: Blackwell Science, 1999:145–153. [Google Scholar]
- 3.Bank S, Singh P, Pooran N, et al. Evaluation of factors that have reduced mortality from acute pancreatitis over the past 20 years. J Clin Gastroenterol. 2002;35:50–60. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Raraty M, Finch M, et al. Acute pancreatitis: the substantial human and financial costs. Gut. 1998;42:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soran A, Chelluri L, Lee KKW, et al. Outcome and quality of life of patients with acute pancreatitis requiring intensive care. J Surg Res. 2000;91:89–94. [DOI] [PubMed] [Google Scholar]
- 6.Bradley EL 3rd, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg. 1991;161:19–24. [DOI] [PubMed] [Google Scholar]
- 7.Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412–1417. [DOI] [PubMed] [Google Scholar]
- 8.London NJ, Neoptolemos JP, Lavelle J, et al. Contrast-enhanced abdominal computed tomography scanning and prediction of severity of acute pancreatitis: a prospective study. Br J Surg. 1989;76:268–272. [DOI] [PubMed] [Google Scholar]
- 9.Clavien PA, Hauser H, Meyer P, et al. Value of contrast-enhanced computerized tomography in the early diagnosis and prognosis of acute pancreatitis: a prospective study of 202 patients. Am J Surg. 1988;155:457–466. [DOI] [PubMed] [Google Scholar]
- 10.Balthazar EJ, Ranson JH, Naidich DP, et al. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767–772. [DOI] [PubMed] [Google Scholar]
- 11.Ranson JH, Balthazar E, Caccavale R, et al. Computed tomography and the prediction of pancreatic abscess in acute pancreatitis. Ann Surg. 1985;201:656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvanitakis M, Delhaye M, De Maertelaere V, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715–723. [DOI] [PubMed] [Google Scholar]
- 13.Ranson JH, Rifkind KM, Turner JW. Prognostic signs and nonoperative peritoneal lavage in acute pancreatitis. Surg Gynecol Obstet. 1976;143:209–219. [PubMed] [Google Scholar]
- 14.Bradley EL 3rd. A clinically based classification system for acute pancreatitis: Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. [DOI] [PubMed] [Google Scholar]
- 15.Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95(suppl):2–4. [PubMed] [Google Scholar]
- 16.Cochrane WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 17.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–1677. [DOI] [PubMed] [Google Scholar]
- 18.Konturek SJ, Dembinski A, Konturek PJ, et al. Role of platelet activating factor in pathogenesis of acute pancreatitis in rats. Gut. 1992;33:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchler M, Malfertheiner P, Uhl W, et al. Gabexate mesilate in human acute pancreatitis: German Pancreatitis Study Group. Gastroenterology. 1993;104:1165–1170. [DOI] [PubMed] [Google Scholar]
- 20.Andriulli A, Leandro G, Clemente R, et al. Meta-analysis of somatostatin, octreotide and gabexate mesilate in the therapy of acute pancreatitis. Aliment Pharmacol Ther. 1998;12:237–245. [DOI] [PubMed] [Google Scholar]
- 21.Messori A, Rampazzo R, Scroccaro G, et al. Effectiveness of gabexate mesilate in acute pancreatitis: a metaanalysis. Dig Dis Sci. 1995;40:734–738. [DOI] [PubMed] [Google Scholar]
- 22.Chen HM, Chen JC, Hwang TL, et al. Prospective and randomized study of gabexate mesilate for the treatment of severe acute pancreatitis with organ dysfunction. Hepatogastroenterology. 2000;47:1147–1150. [PubMed] [Google Scholar]
- 23.Valderrama R, Perez-Mateo M, Navarro S, et al. Multicenter double-blind trial of gabexate mesylate (FOY) in unselected patients with acute pancreatitis. Digestion. 1992;51:65–70. [DOI] [PubMed] [Google Scholar]
- 24.Harada H, Miyake H, Ochi K, et al. Clinical trial with a protease inhibitor gabexate mesilate in acute pancreatitis. Int J Pancreatol. 1991;9:75–79. [DOI] [PubMed] [Google Scholar]
- 25.Berling R, Genell S, Ohlsson K. High-dose intraperitoneal aprotinin treatment of acute severe pancreatitis: a double-blind randomized multi-center trial. J Gastroenterol. 1994;29:479–485. [DOI] [PubMed] [Google Scholar]
- 26.Pederzoli P, Cavallini G, Falconi M, et al. Gabexate mesilate vs aprotinin in human acute pancreatitis (GA. ME. P.A.): a prospective, randomized, double-blind multicenter study. Int J Pancreatol. 1993;14:117–124. [DOI] [PubMed] [Google Scholar]
- 27.Johnson CD, Kingsnorth AN, Imrie CW, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKay CJ, Curran F, Sharples C, et al. Prospective placebo-controlled randomized trial of lexipafant in predicted severe acute pancreatitis. Br J Surg. 1997;84:1239–1243. [PubMed] [Google Scholar]
- 29.Kingsnorth AN, Galloway SW, Formela LJ. Randomized, double-blind phase II trial of Lexipafant, a platelet- activating factor antagonist, in human acute pancreatitis. Br J Surg. 1995;82:1414–1420. [DOI] [PubMed] [Google Scholar]
- 30.Gjorup I, Roikjaer O, Andersen B, et al. A double-blinded multicenter trial of somatostatin in the treatment of acute pancreatitis. Surg Gynecol Obstet. 1992;175:397–400. [PubMed] [Google Scholar]
- 31.Uhl W, Buchler MW, Malfertheiner P, et al. A randomised, double blind, multicentre trial of octreotide in moderate to severe acute pancreatitis. Gut. 1999;45:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKay C, Baxter J, Imrie C. A randomized, controlled trial of octreotide in the management of patients with acute pancreatitis. Int J Pancreatol. 1997;21:13–19. [DOI] [PubMed] [Google Scholar]
- 33.Luengo L, Vicente V, Gris F, et al. Influence of somatostatin in the evolution of acute pancreatitis: a prospective randomized study. Int J Pancreatol. 1994;15:139–144. [DOI] [PubMed] [Google Scholar]
- 34.Planas M, Perez A, Iglesia R, et al. Severe acute pancreatitis: treatment with somatostatin. Intensive Care Med. 1998;24:37–39. [DOI] [PubMed] [Google Scholar]
- 35.Paran H, Mayo A, Paran D, et al. Octreotide treatment in patients with severe acute pancreatitis. Dig Dis Sci. 2000;45:2247–2251. [DOI] [PubMed] [Google Scholar]
- 36.Fiedler F, Jauernig G, Keim V, et al. Octreotide treatment in patients with necrotizing pancreatitis and pulmonary failure. Intensive Care Med. 1996;22:909–915. [DOI] [PubMed] [Google Scholar]
- 37.D'Amico D, Favia G, Biasiato R, et al. The use of somatostatin in acute pancreatitis: results of a multicenter trial. Hepatogastroenterology. 1990;37:92–98. [PubMed] [Google Scholar]
- 38.Karakoyunlar O, Sivrel E, Tanir N, et al. High dose octreotide in the management of acute pancreatitis. Hepatogastroenterology. 1999;46:1968–1972. [PubMed] [Google Scholar]
- 39.Beechey-Newman N. Controlled trial of high-dose octreotide in treatment of acute pancreatitis: evidence of improvement in disease severity. Dig Dis Sci. 1993;38:644–647. [DOI] [PubMed] [Google Scholar]
- 40.Binder M, Uhl W, Friess H, et al. Octreotide in the treatment of acute pancreatitis: results of a unicenter prospective trial with three different octreotide dosages. Digestion. 1994;55(suppl 1):20–23. [DOI] [PubMed] [Google Scholar]
- 41.Nikou GC, Arnaoutis TP, Giamarellos-Bourboulis EJ, et al. The significance of the dosage adjustment of octreotide in the treatment of acute pancreatitis of moderate severity. Hepatogastroenterology. 2001;48:1754–1757. [PubMed] [Google Scholar]
- 42.Ranson JH. Acute pancreatitis: pathogenesis, outcome and treatment. Clin Gastroenterol. 1984;13:843–863. [PubMed] [Google Scholar]
- 43.Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74:534–542. [DOI] [PubMed] [Google Scholar]
- 44.Alscher KT, Phang PT, McDonald TE, et al. Enteral feeding decreases gut apoptosis, permeability, and lung inflammation during murine endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2001;281:G569–G576. [DOI] [PubMed] [Google Scholar]
- 45.Hadfield RJ, Sinclair DG, Houldsworth PE, et al. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med. 1995;152:1545–1548. [DOI] [PubMed] [Google Scholar]
- 46.Vu MK, van der Veek PP, Frolich M, et al. Does jejunal feeding activate exocrine pancreatic secretion? Eur J Clin Invest. 1999;29:1053–1059. [DOI] [PubMed] [Google Scholar]
- 47.Al-Omran M, Groof A, Wilke D. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2002:CD002837. [DOI] [PubMed] [Google Scholar]
- 48.Olah A, Belagyi T, Issekutz A, et al. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103–1107. [DOI] [PubMed] [Google Scholar]
- 49.McClave SA, Greene LM, Snider HL, et al. Comparison of the safety of early enteral vs parenteral nutrition in mild acute pancreatitis. JParenter Enteral Nutr. 1997;21:14–20. [DOI] [PubMed] [Google Scholar]
- 50.Olah A, Pardavi G, Belagyi T, et al. Early nasojejunal feeding in acute pancreatitis is associated with a lower complication rate. Nutrition. 2002;18:259–262. [DOI] [PubMed] [Google Scholar]
- 51.Powell JJ, Murchison JT, Fearon KC, et al. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. Br J Surg. 2000;87:1375–1381. [DOI] [PubMed] [Google Scholar]
- 52.Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalfarentzos F, Kehagias J, Mead N, et al. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg. 1997;84:1665–1669. [PubMed] [Google Scholar]
- 54.Abou-Assi S, Craig K, O'Keefe SJ. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: results of a randomized comparative study. Am J Gastroenterol. 2002;97:2255–2262. [DOI] [PubMed] [Google Scholar]
- 55.Eatock FC, Brombacher GD, Steven A, et al. Nasogastric feeding in severe acute pancreatitis may be practical and safe. Int J Pancreatol. 2000;28:23–29. [DOI] [PubMed] [Google Scholar]
- 56.Brown A. Prophylactic antibiotic use in severe acute pancreatitis: hemlock, help or hype? Gastroenterol. 2004;126:1195–1198. [DOI] [PubMed] [Google Scholar]
- 57.Lankisch PG, Blum T, Maisonneuve P, et al. Severe acute pancreatitis: when to be concerned? Pancreatology. 2003;3:102–110. [DOI] [PubMed] [Google Scholar]
- 58.Sharma VK, Howden CW. Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta-analysis. Pancreas. 2001;22:28–31. [DOI] [PubMed] [Google Scholar]
- 59.Golub R, Siddiqi F, Pohl D. Role of antibiotics in acute pancreatitis: a meta-analysis. J Gastrointest Surg. 1998;2:496–503. [DOI] [PubMed] [Google Scholar]
- 60.Delcenserie R, Yzet T, Ducroix JP. Prophylactic antibiotics in treatment of severe acute alcoholic pancreatitis. Pancreas. 1996;13:198–201. [PubMed] [Google Scholar]
- 61.Nordback I, Sand J, Saaristo R, et al. Early treatment with antibiotics reduces the need for surgery in acute necrotizing pancreatitis: a single-center randomized study. J Gastrointest Surg. 2001;5:113–118. [DOI] [PubMed] [Google Scholar]
- 62.Luiten EJ, Hop WC, Lange JF, et al. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg. 1995;222:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sainio V, Kemppainen E, Puolakkainen P, et al. Early antibiotic treatment in acute necrotising pancreatitis. Lancet. 1995;346:663–667. [DOI] [PubMed] [Google Scholar]
- 64.Pederzoli P, Bassi C, Vesentini S, et al. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176:480–483. [PubMed] [Google Scholar]
- 65.Schwarz M, Isenmann R, Meyer H, et al. Antibiotic use in necrotizing pancreatitis: results of a controlled study. Dtsch Med Wochenschr. 1997;122:356–361. [DOI] [PubMed] [Google Scholar]
- 66.Isenmann R, Runzi M, Kron M, et al. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997–1004. [DOI] [PubMed] [Google Scholar]
- 67.Manes G, Rabitti PG, Menchise A, et al. Prophylaxis with meropenem of septic complications in acute pancreatitis: a randomized, controlled trial versus imipenem. Pancreas. 2003;27:e79–383. [DOI] [PubMed] [Google Scholar]
- 68.Maravi-Poma E, Gener J, Alvarez-Lerma F, et al. Early antibiotic treatment (prophylaxis) of septic complications in severe acute necrotizing pancreatitis: a prospective, randomized, multicenter study comparing two regimens with imipenem-cilastatin. Intensive Care Med. 2003;29:1974–1980. [DOI] [PubMed] [Google Scholar]
- 69.Bassi C, Falconi M, Talamini G, et al. Controlled clinical trial of pefloxacin versus imipenem in severe acute pancreatitis. Gastroenterology. 1998;115:1513–1517. [DOI] [PubMed] [Google Scholar]
- 70.Bassi C, Larvin M, Villatoro E. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2003:CD002941. [DOI] [PubMed] [Google Scholar]
- 71.Gloor B, Muller CA, Worni M, et al. Pancreatic infection in severe pancreatitis: the role of fungus and multiresistant organisms. Arch Surg. 2001;136:592–596. [DOI] [PubMed] [Google Scholar]
- 72.Isenmann R, Schwarz M, Rau B, et al. Characteristics of infection with Candida species in patients with necrotizing pancreatitis. World J Surg. 2002;26:372–376. [DOI] [PubMed] [Google Scholar]
- 73.De Waele JJ, Vogelaers D, Blot S, et al. Fungal infections in patients with severe acute pancreatitis and the use of prophylactic therapy. Clin Infect Dis. 2003;37:208–213. [DOI] [PubMed] [Google Scholar]
- 74.Hoerauf A, Hammer S, Muller-Myhsok B, et al. Intra-abdominal Candida infection during acute necrotizing pancreatitis has a high prevalence and is associated with increased mortality. Crit Care Med. 1998;26:2010–2015. [DOI] [PubMed] [Google Scholar]
- 75.Gotzinger P, Wamser P, Barlan M, et al. Candida infection of local necrosis in severe acute pancreatitis is associated with increased mortality. Shock. 2000;14:320–323; discussion 323–324. [DOI] [PubMed]
- 76.Forsmark CE. The clinical problem of biliary acute necrotizing pancreatitis: epidemiology, pathophysiology, and diagnosis of biliary necrotizing pancreatitis. J Gastrointest Surg. 2001;5:235–239. [DOI] [PubMed] [Google Scholar]
- 77.Sharma VK, Howden CW. Metaanalysis of randomized controlled trials of endoscopic retrograde cholangiography and endoscopic sphincterotomy for the treatment of acute biliary pancreatitis. Am J Gastroenterol. 1999;94:3211–3214. [DOI] [PubMed] [Google Scholar]
- 78.Fan ST, Lai EC, Mok FP, et al. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328:228–232. [DOI] [PubMed] [Google Scholar]
- 79.Fölsch UR, Nitsche R, Ludtke R, et al. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis: the German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1997;336:237–242. [DOI] [PubMed] [Google Scholar]
- 80.Neoptolemos JP, Carr-Locke DL, London NJ, et al. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979–983. [DOI] [PubMed] [Google Scholar]
- 81.Nowak A, Nowakowska-Dulawa E, Marek T. Final results of the prospective, randomized controlled study on endoscopic sphincterotomy versus conventionell management in acute biliary pancreatitis. Gastroenterology. 1995;108:A380. [Google Scholar]
- 82.Kelly TR. Gallstone pancreatitis: the timing of surgery. Surgery. 1980;88:345–350. [PubMed] [Google Scholar]
- 83.Ando T, Tsuyuguchi T, Okugawa T, et al. Risk factors for recurrent bile duct stones after endoscopic papillotomy. Gut. 2003;52:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schreurs WH, Vles WJ, Stuifbergen WH, et al. Endoscopic management of common bile duct stones leaving the gallbladder in situ: a cohort study with long-term follow-up. Dig Surg. 2004;21:60–65. [DOI] [PubMed] [Google Scholar]
- 85.Chang L, Lo S, Stabile BE, et al. Preoperative versus postoperative endoscopic retrograde cholangiopancreatography in mild to moderate gallstone pancreatitis: a prospective randomized trial. Ann Surg. 2000;231:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suc B, Escat J, Cherqui D, et al. Surgery vs endoscopy as primary treatment in symptomatic patients with suspected common bile duct stones: a multicenter randomized trial. French Associations for Surgical Research. Arch Surg. 1998;133:702–708. [DOI] [PubMed] [Google Scholar]
- 87.Targarona EM, Ayuso RM, Bordas JM, et al. Randomised trial of endoscopic sphincterotomy with gallbladder left in situ versus open surgery for common bileduct calculi in high-risk patients. Lancet. 1996;347:926–929. [DOI] [PubMed] [Google Scholar]
- 88.Cuschieri A, Lezoche E, Morino M, et al. E.A.E.S. multicenter prospective randomized trial comparing two-stage vs single-stage management of patients with gallstone disease and ductal calculi. Surg Endosc. 1999;13:952–957. [DOI] [PubMed] [Google Scholar]
- 89.Hammarstrom LE, Holmin T, Stridbeck H, et al. Long-term follow-up of a prospective randomized study of endoscopic versus surgical treatment of bile duct calculi in patients with gallbladder in situ. Br J Surg. 1995;82:1516–1521. [DOI] [PubMed] [Google Scholar]
- 90.Siegel JH VA, Cohen SA, Kasmin FE. Endoscopic sphincterotomy for biliary pancreatitis: an alternative to cholecystectomy in high-risk patients. Gastrointest Endosc. 1994;40:573–574. [DOI] [PubMed] [Google Scholar]
- 91.Kaw MA-AY, Kaw P. Management of gallstone pancreatitis: cholecystectomy or ERCP and endoscopic sphincterotomy. Gastrointest Endosc. 2002;56:61–65. [DOI] [PubMed] [Google Scholar]
- 92.Gislason HVM, Horn A, Hoem D, et al. Endoscopic sphincterotomy in acute gallstone pancreatitis: a prospective study of the late outcome. Eur J Surg. 2001;176:204–208. [DOI] [PubMed] [Google Scholar]
- 93.Boerma D, Rauws EA, Keulemans YC, et al. Wait-and-see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile-duct stones: a randomised trial. Lancet. 2002;360:761–765. [DOI] [PubMed] [Google Scholar]
- 94.Uhl W, Muller CA, Krahenbuhl L, et al. Acute gallstone pancreatitis: timing of laparoscopic cholecystectomy in mild and severe disease. Surg Endosc. 1999;13:1070–1076. [DOI] [PubMed] [Google Scholar]
- 95.Schachter P, Peleg T, Cohen O. Interval laparoscopic cholecystectomy in the management of acute biliary pancreatitis. Hepatobiliary Pancreat Surg. 2000;11:319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tate JJ, Lau WY, Li AK. Laparoscopic cholecystectomy for biliary pancreatitis. Br J Surg. 1994;81:720–722. [DOI] [PubMed] [Google Scholar]
- 97.Schietroma M, Carlei F, Lezoche E, et al. Acute biliary pancreatitis: staging and management. Hepatogastroenterology. 2001;48:988–993. [PubMed] [Google Scholar]
- 98.Zhu JF, Fan XH, Zhang XH. Laparoscopic treatment of severe acute pancreatitis. Surg Endosc. 2001;15:146–148. [DOI] [PubMed] [Google Scholar]
- 99.Baron TH, Thaggard WG, Morgan DE, et al. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–764. [DOI] [PubMed] [Google Scholar]
- 100.Nordback I, Paajanen H, Sand J. Prospective evaluation of a treatment protocol in patients with severe acute necrotising pancreatitis. Eur J Surg. 1997;163:357–364. [PubMed] [Google Scholar]
- 101.Mier J, Leon EL, Castillo A, et al. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173:71–75. [DOI] [PubMed] [Google Scholar]
- 102.Beger HG, Buchler M, Bittner R, et al. Necrosectomy and postoperative local lavage in necrotizing pancreatitis. Br J Surg. 1988;75:207–712. [DOI] [PubMed] [Google Scholar]
- 103.Buchler MW, Gloor B, Muller CA, et al. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsiotos GG, Luque-de Leon E, Soreide JA, et al. Management of necrotizing pancreatitis by repeated operative necrosectomy using a zipper technique. Am J Surg. 1998;175:91–98. [DOI] [PubMed] [Google Scholar]
- 105.Kalfarentzos FE, Kehagias J, Kakkos SK, et al. Treatment of patients with severe acute necrotizing pancreatitis based on prospective evaluation. Hepatogastroenterology. 1999;46:3249–3256. [PubMed] [Google Scholar]
- 106.Beger HG. Operative management of necrotizing pancreatitis: necrosectomy and continuous closed postoperative lavage of the lesser sac. Hepatogastroenterology. 1991;38:129–133. [PubMed] [Google Scholar]
- 107.Schroder T, Sainio V, Kivisaari L, et al. Pancreatic resection versus peritoneal lavage in acute necrotizing pancreatitis: a prospective randomized trial. Ann Surg. 1991;214:663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsiotos GG, Luque-de Leon E, Sarr MG. Long-term outcome of necrotizing pancreatitis treated by necrosectomy. Br J Surg. 1998;85:1650–1653. [DOI] [PubMed] [Google Scholar]
- 109.Clavien PA, Sanabria JR, Mentha G, et al. Recent results of elective open cholecystectomy in a North American and a European center: comparison of complications and risk factors. Ann Surg. 1992;216:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–526. [PubMed] [Google Scholar]
- 111.Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Terrin N, Schmid CH, Lau J, et al. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22:2113–2126. [DOI] [PubMed] [Google Scholar]
- 114.Moher D, Pham B, Klassen TP, et al. What contributions do languages other than English make on the results of meta-analyses? J Clin Epidemiol. 2000;53:964–972. [DOI] [PubMed] [Google Scholar]
- 115.Dervenis C, Johnson CD, Bassi C, et al. Diagnosis, objective assessment of severity, and management of acute pancreatitis: Santorini consensus conference. Int J Pancreatol. 1999;25:195–210. [DOI] [PubMed] [Google Scholar]
- 116.Uhl W, Warshaw A, Imrie C, et al. IAP Guidelines for the Surgical Management of Acute Pancreatitis. Pancreatology. 2002;2:565–573. [DOI] [PubMed] [Google Scholar]
- 117.Wyncoll DL. The management of severe acute necrotising pancreatitis: an evidence-based review of the literature. Intensive Care Med. 1999;25:146–156. [DOI] [PubMed] [Google Scholar]