Abstract
Objective:
This study describes racial differences in postoperative mortality following 8 cardiovascular and cancer procedures and assesses possible explanations for these differences.
Summary Background Data:
Although racial disparities in the use of surgical procedures are well established, relationships between race and operative mortality have not been assessed systematically.
Methods:
We used national Medicare data to identify all patients undergoing one of 8 cardiovascular and cancer procedures between 1994 and 1999. We used multiple logistic regression to assess differences in operative mortality (death within 30 days or before discharge) between black patients and white patients, controlling for patient characteristics. Adding hospital indicators to these models, we then assessed the extent to which racial differences in operative mortality could be accounted for by the hospital in which patients were cared for.
Results:
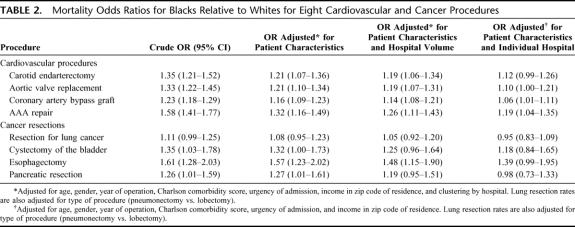
Black patients had higher crude mortality rates than white patients for 7 of the 8 operations, including coronary artery bypass, aortic valve replacement, abdominal aortic aneurysm repair, carotid endarterectomy, radical cystectomy, pancreatic resection, and esophagectomy. Among these 7 procedures, odds ratios of mortality (black versus white) ranged from 1.23 (95% confidence interval, 1.18–1.29) for CABG to 1.61 (95% confidence interval, 1.28–2.03) for esophagectomy. Adjusting for patient characteristics had modest or no effect on odds ratios of mortality by race. However, there remained few clinically or statistically significant differences in mortality by race after we accounted for hospital. Hospitals that treated a large proportion of black patients had higher mortality rates for all 8 procedures, for white as well as black patients.
Conclusions:
Black patients have higher operative mortality risks across a wide range of surgical procedures, in large part because of higher mortality rates at the hospitals they attend.
This paper describes racial differences in postoperative mortality for 8 cardiovascular and cancer procedures. For nearly all procedures, black patients had higher mortality than white patients. Racial differences in mortality were due in large part to higher mortality rates at hospitals typically attended by black patients.
Understanding and reducing racial disparities in health care have been highlighted as a national priority.1,2 In surgery, most research to date has focused on race-related differences in the utilization of procedures. For example, after adjusting for severity of disease and other factors, blacks are considerably less likely than whites to undergo coronary revascularization, knee arthroplasty, and hysterectomy for endometrial cancer.3–7 Race also seems to influence which operation is offered for a given condition.8 For example, black patients are significantly more likely than whites to receive a permanent colostomy after surgery for rectal cancer.9
In addition to differences in the use of surgery, there may also be racial disparities in surgical outcomes. For some procedures, it is well established that black patients are more likely than white patients to undergo surgery urgently or emergently,10,11 a well-known risk factor for operative mortality. In addition, it is possible that blacks receive their care in poorer quality hospitals. For example, blacks are up to twice as likely to undergo high-risk procedures in very low-volume hospitals.12,13 Several studies have suggested that black race is an independent marker for operative mortality.10,14,15 However, most studies to date have focused on individual procedures, based on data from different time periods and study populations.
To assess relationships between race and operative mortality more systematically, we used national data to examine mortality in Medicare patients undergoing one of 8 cardiovascular and cancer procedures. We were particularly interested in exploring whether higher mortality rates in blacks could be attributed more to patient-level factors (eg, higher acuity or comorbidity) or to factors associated with the hospitals where they receive their care.
METHODS
As with our previous work,12,16 we used the Medicare Provider Analysis and Review (MEDPAR) files and denominator files from the national Medicare database for the years 1994 through 1999. Because Medicare managed care patients do not appear in the MEDPAR files, such patients (6%–18%, depending on year and region) are excluded from our study, as are patients less than 65 years of age or over 99.
Using appropriate ICD-9 codes, we identified all Medicare patients undergoing one of 8 cardiovascular and cancer procedures during the study period (carotid endarterectomy, aortic valve replacement, coronary artery bypass graft, elective abdominal aortic aneurysm repair, resection for lung cancer, cystectomy of the bladder, esophagectomy, and pancreatic resection). Procedures were selected based on previous work showing a relatively strong association between volume and mortality and to ensure representation of different subspecialties. To ensure relatively homogeneous cohorts of patients for outcomes analysis, we required an appropriate cancer diagnosis for patients in cancer resection cohorts. Abdominal aortic aneurysm patients were excluded if diagnosis or procedure codes suggested aneurysm rupture or suprarenal aneurysms. Patients undergoing concurrent valve procedures were excluded from the CABG cohort.
We determined patient race from the denominator file. Because of small numbers of patients of other races, we limited our analysis to patients identified as black or white. Hospitals were identified using their Medicare institution codes. To calculate hospital volumes for each procedure, we first assessed the total number of Medicare procedures for each hospital over the study period. All procedures performed in Medicare beneficiaries during the study period, not just those performed for our more carefully selected analytic cohorts, were included in the volume calculation. We then estimated the total annual volume for each hospital using an inflation factor estimated from the Nationwide Inpatient Sample, all payor database. For each hospital, we also calculated the proportion of patients undergoing each procedure who were black.
Statistical Analysis
We used multiple logistic regression to assess relationships between race and operative mortality. Operative mortality was defined as death within 30 days of the operation or prior to hospital discharge. (Because a large proportion of operative deaths occur after protracted hospital stays, 30-day mortality alone would not adequately reflect operative mortality.) Separate regression models were derived for each procedure, controlling for age, gender, year of procedure, urgency of admission, and mean Social Security income in the patient's zip code. We used the Charlson comorbidity score17 to adjust for comorbidities, identified from the surgical admission as well as from admissions during the previous 6 months. To explore the role of hospital-level characteristics, we then added hospital procedure volume to the models. We adjusted for the effect of clustering of mortality within hospitals by using overdispersed binary logistic models, clustering by hospital.18
Finally, we assessed “within hospital” race effects by including hospital indicator variables in the models. These fixed-effects models control for any hospital-level factor (observed or unobserved) that affects the risk of mortality of all patients, so that any remaining racial disparity reflects within-hospital differences in the risk of mortality of blacks compared with whites. If the race effect is no longer significant after including hospital indicator variables, blacks and whites from the same hospital have similar outcomes, so that any prior racial effect is explained by the hospital where blacks were treated rather than differences in risk. To further clarify the role of hospital-level factors, we stratified hospitals based on the proportion of patients undergoing each procedure who were black (0%–9% versus 10% or more) and included “% blacks in hospital” in the models as a hospital-level factor. These models served to indicate whether “% blacks in hospital” served as a marker of high mortality rate hospitals, after controlling for hospital volume.19
RESULTS
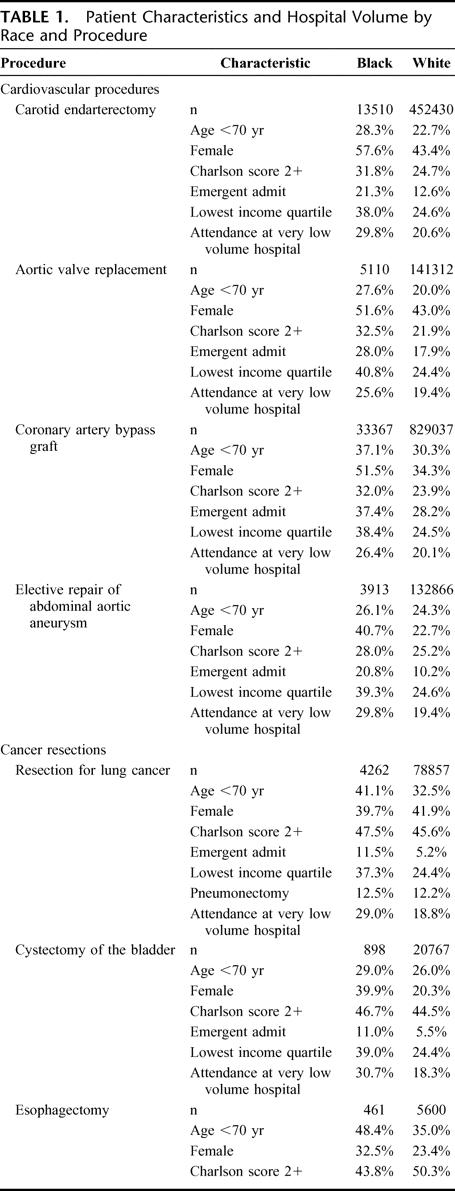
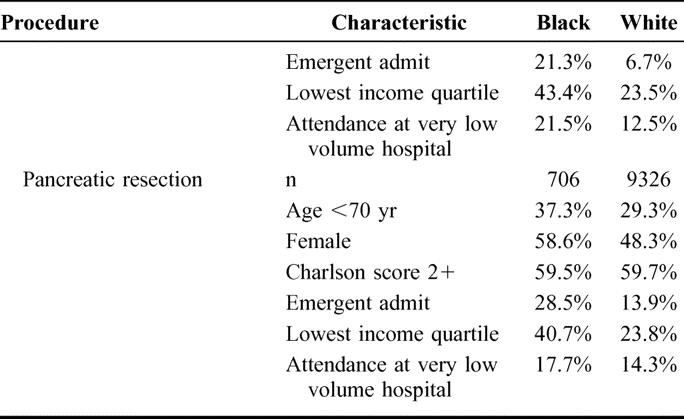
For all procedures, black patients were younger, more likely to be admitted emergently, and more likely to live in a low income zip code than white patients (Table 1). For most procedures, a higher proportion of black patients than white patients were female. For cardiovascular but not cancer procedures, black patients had higher comorbidity scores than white patients. For most cancer procedures, comorbidity scores were similar in black patients and white patients, but white esophagectomy patients had higher comorbidity scores than black patients. Compared with white patients, black patients were much more likely to undergo surgery at very low-volume hospitals for all 8 procedures.
TABLE 1. Patient Characteristics and Hospital Volume by Race and Procedure
Table
Black patients had higher observed mortality rates than white patients for all 8 procedures (Fig. 1). After controlling for patient characteristics, black patients had significantly higher mortality than white patients for 7 of the 8 procedures (Table 2), with odds ratios of mortality from 1.08 (95% confidence interval, 0.95–1.23) for lung resection to 1.57 (95% confidence interval, 1.23–2.02) for esophagectomy. After accounting for hospital volume, significant mortality differences by race remained for all cardiovascular procedures as well as for esophagectomy. However, after the final adjustment for individual hospital, odds ratios of mortality by race were further attenuated. Racial differences in operative mortality remained statistically significant for only CABG and AAA repair.
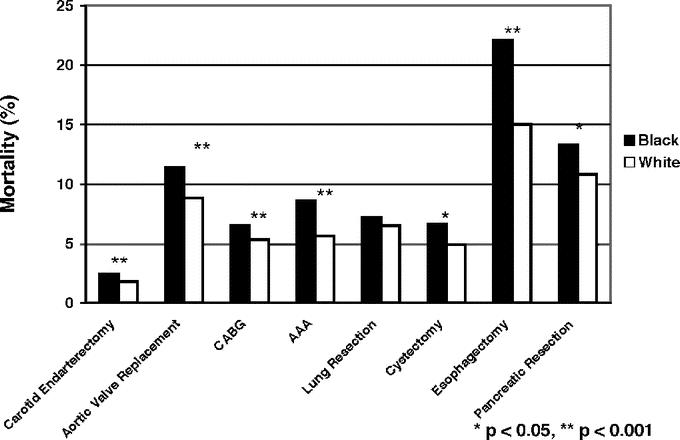
FIGURE 1. Crude operative mortality rates by race for 8 cardiovascular and cancer procedures.
TABLE 2. Mortality Odds Ratios for Blacks Relative to Whites for Eight Cardiovascular and Cancer Procedures
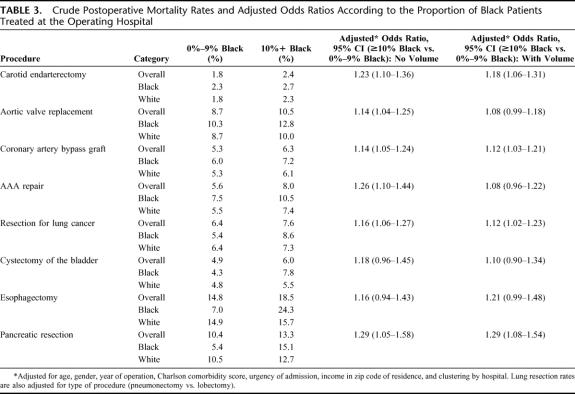
Hospitals treating a higher proportion of black patients (10% or more) had higher mortality rates for each procedure (Table 3). These hospitals had higher mortality rates independent of race: both black patients and white patients had higher mortality in hospitals disproportionately treating black patients than their racial counterparts in other centers. After adjusting for differences in patient factors in hospitals treating a large proportion of black patients and those treating only a few, the proportion of black patients at the hospital was a significant predictor of increased mortality for all cardiovascular procedures as well as for resection for lung cancer and pancreatic resection. Accounting for differences in hospital volume as well as patient characteristics attenuated the odds ratios for some, but not all, procedures.
TABLE 3. Crude Postoperative Mortality Rates and Adjusted Odds Ratios According to the Proportion of Black Patients Treated at the Operating Hospital
DISCUSSION
Black patients are consistently more likely to die after major surgery than whites. Confirming the results of earlier, smaller studies,5,7,14,15,20 we noted racial differences in crude operative mortality rates for 7 of the 8 procedures examined. For most procedures, these differences were clinically as well as statistically significant. With the exception of surgery for lung cancer, crude odds of operative mortality were more than 20% higher for black patients than for whites.
Our study suggests potential reasons for apparent racial disparities in operative mortality. First, black patients have higher baseline risks than whites. Although they were younger and tended to have similar comorbidity scores for some procedures, black patients were considerably more likely to be admitted to the hospital emergently, an important risk factor for operative mortality.21 They also tended to reside in low-income areas, another independent risk factor.4,22 For cardiovascular procedures, but not cancer procedures, adjusting for these patient characteristics attenuated the observed associations between race and crude mortality, ie, reduced odds ratios of mortality by race. Although these attenuations were relatively modest in magnitude, they may have been larger had we access to more detailed information about illness severity and acuity (from clinical data) or patient-level data on socioeconomic status.
Second, black patients were more likely to undergo surgery in hospitals with higher mortality rates, independent of race. As suggested previously,12,23,24 black patients were considerably more likely to receive their care in very low volume hospitals: more than 20% more likely for all procedures. For most of these procedures, low hospital procedure volume is a well-established risk factor for increased operative mortality.25,26 Moreover, surgical volume is not the only contributor to worse mortality rates; for some procedures, hospitals that treated a large proportion of black patients had higher mortality rates independent of their procedure volume. Therefore, relationships between race and mortality may be confounded by other factors influencing hospital mortality rates.
In most cases, adjusting for hospital attenuated the odds ratios of mortality by race more than did adjusting for patient characteristics and more than adjusting for hospital volume alone. Race remained an independent predictor of increased operative mortality for only 2 procedures after accounting for hospital. For one of these (CABG), residual differences in mortality by race were small and not clinically significant.
Although the first focusing on surgical outcomes, our study is not the first to suggest to that racial disparities in health care may be partly attributable to suboptimal systems in which black patients receive their care. Bach et al recently reported that physicians treating black patients are less likely to be board certified and have worse access to specialists and other technologic resources.27 Barnato et al have shown that racial disparities in quality of care following acute myocardial infarction are partly attributable to hospital.27a And Bradley et al have shown that racial differences in time to reperfusion following acute myocardial infarction are partly attributable to the hospitals to which minority and white patients are typically admitted.28
Our study has several important limitations. First, administrative data have obvious limitations with regards to risk adjustment.29,30 Inability to fully capture race-related differences in illness severity and acuity would bias this study toward underestimating the contribution of patient factors on increased mortality risks in black patients. However, our relatively homogeneous patient cohorts and our concentration on short-term operative mortality should mitigate problems associated with inadequate risk adjustment. In addition, we were able to account for 2 very important predictors of operative mortality, age and emergent admission. Second, we studied only Medicare patients over age 65. Although the influence of race on cancer mortality seems to be more pronounced at younger ages,31–33 there is at present no evidence that the relationship between race and short-term operative mortality are age-dependent. However, because our study was restricted to patients with insurance (ie, Medicare), its findings may underestimate racial differences in access to health care and ultimately disease severity and acuity in younger patients. Finally, our study has limitations with regard to its categorization of race. Coding of “black” versus “white” race is highly reliable in Medicare claims data, with sensitivity, specificity, and positive predictive value rates all exceeding 95%.34 However, because Medicare data do not reliably identify other racial groups34 and because the numbers of patients of other races were very small, we were unable to examine mortality rates in other important minority groups.
Our findings, along with those of previous studies,10,35 reinforce the need to focus on why black patients are more likely to present urgently for surgical intervention. Racial differences in patient preferences about surgery may be partly responsible. For example, previous studies of patients with colorectal and prostate cancer have indicated that black patients are more likely to delay or refuse surgical care until disease has become relatively acute or advanced.36,37 However, black patients may also be getting different advice from their physicians than white patients.35 Several studies have suggested that providers are less likely to recommend or facilitate surgical care for black patients than for whites with comparable disease.38,39 In settings where earlier intervention clearly improves outcomes, efforts aimed at changing both patient and provider behavior should be sought and encouraged.
However, our study suggests that racial disparities may be as much about the system in which black patients get their care as about patient- or physician-level factors. Selective referral, moving high risk surgical patients at hospitals with high mortality rates to other centers with better outcomes, is one obvious but likely impractical approach to addressing potential problems at hospitals that disproportionately treat black patients. Large numbers of patients would be involved and their access to low mortality rate centers (even if they could be readily identified) is uncertain. Moreover, removing surgical caseloads from hospitals that disproportionately treat black patients might worsen care for other patients by further eroding resources at those centers.
For these reasons, it would make more sense to focus on quality improvement at poor performing hospitals. Achieving this goal would require a better understanding of deficits in the number of surgical subspecialists or other resources potentially underlying higher surgical mortality rates at such institutions. Quality improvement also implies that hospitals should implement programs for monitoring their processes of care and outcomes in a systematic manner (eg, with the National Surgical Quality Improvement Program). Finding adequate resources to ensure high quality surgical care may be challenging for hospitals already facing declining reimbursements and constrained budgets. To the extent that reducing racial disparities in health care has been identified as a top priority by the federal government, providing hospitals with the resources necessary to improve care for minority populations would most likely fall to public sector payers, particularly the Center for Medicare and Medicaid Services. The Center for Medicare and Medicaid Services could provide reimbursement premiums to urban hospitals and surgeons treating large medically underserved populations, much as it has recently increased reimbursements to rural healthcare providers to maintain access. Improving surgical care at hospitals that disproportionately serve minority populations would not be easy or inexpensive. However, it may be a more attractive alternative than shutting down surgical programs or, worse, accepting the high mortality rates of the status quo.
Footnotes
Supported in part by Grant No. R01 HS10141-02S1 from the Agency for Healthcare Research and Quality and Grant No. AG 0189783 from the National Institute of Aging.
Reprints: F. L. Lucas, PhD, Center for Outcomes Research and Evaluation, Maine Medical Center, 22 Bramhall Street, Portland, ME 04101. E-mail: lucasl@mmc.org.
REFERENCES
- 1.Agency for Healthcare Research and Quality. National Healthcare Disparities Report. Rockville, MD: Agency for Healthcare Research and Quality, 2003. [Google Scholar]
- 2.Smedley B, Stith A, Nelson A. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press, 2003. [PubMed] [Google Scholar]
- 3.Ford ES, Cooper RS. Racial/ethnic differences in health care utilization of cardiovascular procedures: a review of the evidence. Health Serv Res. 1995;30:237–252. [PMC free article] [PubMed] [Google Scholar]
- 4.Gornick ME, Eggers PW, Reilly TW, et al. Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med. 1996;335:791–799. [DOI] [PubMed] [Google Scholar]
- 5.Peterson ED, Shaw LK, DeLong ER, et al. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med. 1997;336:480–486. [DOI] [PubMed] [Google Scholar]
- 6.Skinner J, Weinstein JN, Sporer SM, et al. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349:1350–1359. [DOI] [PubMed] [Google Scholar]
- 7.Randall TC, Armstrong K. Differences in treatment and outcome between African-American and white women with endometrial cancer. J Clin Oncol. 2003;21:4200–4206. [DOI] [PubMed] [Google Scholar]
- 8.Baxter NN, Virnig BA, Durham SB, et al. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. [DOI] [PubMed] [Google Scholar]
- 9.Morris AM, Billingsley KG, Baxter NN, et al. Racial disparities in rectal cancer treatment: a population-based analysis. Arch Surg. 2004;139:151–155; discussion 156. [DOI] [PubMed]
- 10.Ball JK, Elixhauser A. Treatment differences between blacks and whites with colorectal cancer. Med Care. 1996;34:970–984. [DOI] [PubMed] [Google Scholar]
- 11.Oddone EZ, Horner RD, Sloane R, et al. Race, presenting signs and symptoms, use of carotid artery imaging, and appropriateness of carotid endarterectomy. Stroke. 1999;30:1350–1356. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 13.Dardik A, Bowman HM, Gordon TA, et al. Impact of race on the outcome of carotid endarterectomy: a population-based analysis of 9,842 recent elective procedures. Ann Surg. 2000;232:704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bridges CR, Edwards FH, Peterson ED, et al. The effect of race on coronary bypass operative mortality. J Am Coll Cardiol. 2000;36:1870–1876. [DOI] [PubMed] [Google Scholar]
- 15.Godley PA, Schenck AP, Amamoo MA, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst. 2003;95:1702–1710. [DOI] [PubMed] [Google Scholar]
- 16.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 18.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 19.Berlin JA, Kimmel SE, Ten Have TR, et al. An empirical comparison of several clustered data approaches under confounding due to cluster effects in the analysis of complications of coronary angioplasty. Biometrics. 1999;55:470–476. [DOI] [PubMed] [Google Scholar]
- 20.Cooper GS, Yuan Z, Landefeld CS, et al. Surgery for colorectal cancer: race-related differences in rates and survival among Medicare beneficiaries. Am J Public Health. 1996;86:582–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–360. [DOI] [PubMed] [Google Scholar]
- 22.Polednak AP. Segregation, discrimination and mortality in U.S. blacks. Ethn Dis. 1996;6:99–108. [PubMed] [Google Scholar]
- 23.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725; discussion 726. [DOI] [PubMed]
- 24.Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. [DOI] [PubMed] [Google Scholar]
- 25.Dudley RA, Johansen KL, Brand R, et al. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. JAMA. 2000;283:1159–1166. [DOI] [PubMed] [Google Scholar]
- 26.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–520. [DOI] [PubMed] [Google Scholar]
- 27.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–584. [DOI] [PubMed] [Google Scholar]
- 27a.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Med Care. 2005;43:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley EH, Herrin J, Wang Y, et al. Racial and ethnic differences in time to acute reperfusion therapy for patients hospitalized with myocardial infarction. JAMA. 2004;292:1563–1572. [DOI] [PubMed] [Google Scholar]
- 29.Bach PB, Guadagnoli E, Schrag D, et al. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40:19–25. [DOI] [PubMed] [Google Scholar]
- 30.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40:26–35. [DOI] [PubMed] [Google Scholar]
- 31.Powell IJ, Banerjee M, Bianco FJ, et al. The effect of race/ethnicity on prostate cancer treatment outcome is conditional: a review of Wayne State University data. J Urol. 2004;171:1508–1512. [DOI] [PubMed] [Google Scholar]
- 32.Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91:178–188. [DOI] [PubMed] [Google Scholar]
- 33.Newman LA, Bunner S, Carolin K, et al. Ethnicity related differences in the survival of young breast carcinoma patients. Cancer. 2002;95:21–27. [DOI] [PubMed] [Google Scholar]
- 34.Arday SL, Arday DR, Monroe S, et al. HCFA's racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 35.Bach PB, Schrag D, Brawley OW, et al. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. [DOI] [PubMed] [Google Scholar]
- 36.Demissie K, Oluwole OO, Balasubramanian BA, et al. Racial differences in the treatment of colorectal cancer: a comparison of surgical and radiation therapy between Whites and Blacks. Ann Epidemiol. 2004;14:215–221. [DOI] [PubMed] [Google Scholar]
- 37.Shavers VL, Brown ML, Potosky AL, et al. Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. J Gen Intern Med. 2004;19:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians' recommendations for cardiac catheterization. N Engl J Med. 1999;340:618–626. [DOI] [PubMed] [Google Scholar]
- 39.Ayanian JZ, Cleary PD, Weissman JS, et al. The effect of patients' preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341:1661–1669. [DOI] [PubMed] [Google Scholar]