Abstract
Objective:
For patients with laparoscopic stage M1 gastric adenocarcinoma, no resection of the primary tumor, and systemic chemotherapy, this study investigated the incidence of subsequent palliative intervention and survival.
Summary Background Data:
Laparoscopy was performed for patients with computed tomography scan stage M0 disease and no significant obstruction or bleeding.
Methods:
A prospectively maintained database for 1993 to 2002 was used to identify 165 patients (median age, 63 years) with laparoscopic M1 disease in the peritoneum (P1, adjacent to stomach, 9%; P2, few distant sites, 35%; or P3, disseminated, 30%) or liver (10%) or both (16%). Functional performance status (FPS, Eastern Cooperative Oncology Group) was 0 to 1 (84%) or 2 (16%).
Results:
Subsequent intervention was performed on 50% of patients, at median interval of 4 months (range, 1–35 months) after laparoscopy. Intervention was performed on the stomach for obstruction (33%), bleeding (8%), or perforation (1%) or on a distant site for a metastasis-related complication (20%). More than one intervention (maximum, 4) was performed in 21%. Laparotomy was necessary in 12%; the remainder had endoscopic or radiologic procedures or radiation therapy only. There was one intervention-related death. Median survival was 10 months, with 1-year survival of 39%. On multivariate analysis, better FPS (0–1; odds ratio, 4; P = 0.001) and limited peritoneal metastasis (P1 or P2; 2; P = 0.01) were independently associated with improved survival.
Conclusions:
The incidence of subsequent intervention was 50%, but few patients had laparotomy. Intervention-related mortality was minimal. The burden of metastatic disease and functional performance status were important prognostic factors.
Of 165 patients with M1 disease and no gastrectomy, 50% subsequently required a palliative intervention. The majority of intervention was endoscopic or radiologic, and only 12% had laparotomy. Median survival was 10 months. Metastatic burden and functional performance status were important prognostic factors
An average of 25% of patients with newly diagnosed gastric adenocarcinoma have subradiologic, intra-abdominal M1 disease (metastasis to peritoneum, liver or nonregional lymph nodes) that is detected at surgical staging by laparoscopy or laparotomy.1–7 The majority is incurable and is treated with palliative strategies. Limited prior data indicate that laparotomy is rarely necessary for symptom palliation, subsequent to laparoscopic detection of M1 disease.1,2 Entire clinical outcomes for patients with laparoscopic M1 disease have not been systematically examined. For effective and appropriate palliative planning, a wide range of clinical situations, multidisciplinary treatment options, and technical factors need to be carefully considered.8 The present study aimed to examine the outcomes associated with a treatment strategy of systemic chemotherapy without gastric resection, for patients with laparoscopic stage M1 disease. The value of this approach was determined through exploration of the need for subsequent palliative intervention and its associated morbidity and mortality. Survival and prognostic characteristics were analyzed to provide information on the durability of this treatment paradigm.
PATIENTS AND METHODS
The study population was identified from a prospectively maintained database of patients with gastric adenocarcinoma admitted to Memorial Sloan-Kettering Cancer Center (MSKCC), from April 1993 to May 2002. During this period, clinical staging routinely included computerized tomography (CT) scanning of the abdomen and pelvis and plain chest x-ray. Chest CT scan, magnetic resonance imaging of the liver, or endoscopic ultrasound was used in selected cases. Laparoscopic staging was conducted if 1) the patient was M0 at clinical and radiologic staging; 2) there were no symptoms that required immediate surgical treatment; and 3) the patient was medically fit for gastric resection. Patients who had laparoscopic stage M1 disease form the present study population. Patients were specifically excluded if: 1) they did not undergo gastrectomy because there was a loco-regionally advanced primary tumor, but no M1 disease; and 2) highly selected patients with limited M1 disease who underwent gastrectomy with potentially curative intent on investigational protocols.
Laparoscopic staging was conducted in a standard manner, as described previously.2 The location and extent of peritoneal metastasis were prospectively recorded according to the classification of the Japanese Research Society for Gastric Cancer.9 Peritoneal disease was categorized as: P1, metastasis limited to the peritoneum of the lesser sac and the lesser or greater omenta; P2, a few metastasis to the distant peritoneum; P3, numerous metastasis to the distant peritoneum. Hepatic metastasis were recorded separately. Para-aortic lymph nodes were not routinely biopsied but samples were obtained from enlarged, nonregional lymph nodes when clinically indicated. The diagnosis of M1 disease was histopathologically confirmed in all cases. Laparoscopy findings for 65 consecutive, recent patients who had high-quality, spiral CT scans performed at MSKCC and reported by an attending radiologist with expertise in gastrointestinal malignancy are presented separately, to evaluate the role of laparoscopy in the current era of radiologic staging.
Patient records were reviewed and subsequent invasive procedures, including radiation therapy, that were performed with explicit palliative intent were identified using previously described criteria.10 Intervention was recorded in 2 categories: 1) procedures on the gastroesophageal junction (GEJ) or stomach, and 2) procedures on distant sites for metastasis-related complications. Feeding jejunostomy for supplemental nutrition was classified as a procedure on the stomach. Procedures were further subcategorized according to the indication for intervention.
Review and reporting of these data were approved by the Institutional Review Board of MSKCC.
Statistical Analysis
Data were analyzed using SPSS version 11 statistical software, and summary statistics were expressed as percentage or median (range). The survival time was measured from staging laparoscopy to death or date of last follow-up for patients who were alive at the time of analysis. Alive patients were treated as censored observations for survival analyses. The survival rate at 1 year or 2 years was computed by the method of Kaplan-Meier, and survival characteristics were compared using the log-rank test. A forward and backward Cox proportional hazards regression model was used to identify factors that were independently predictive of survival. P values less than 0.05 were considered significant.
RESULTS
Patient and Disease Characteristics
During the study period, 1748 patients with gastric adenocarcinoma were admitted to MSKCC and staging laparoscopy was performed on 718 (41%) patients. The following were excluded from this study: 1) patients (n = 7) who did not undergo gastrectomy because of unacceptably high risk of multiorgan resection for a T4 primary tumor but had laparoscopic M0 disease; and 2) patients (n = 20) with limited, peritoneum-only disease (P1, n = 9; P2, n = 11) at laparoscopy, who were treated with intent to prolong survival with combinations of systemic chemotherapy, laparoscopic restaging, radical gastrectomy, and intraperitoneal chemotherapy.
There were 165 patients who were eligible for inclusion in the present study, 100 men and 65 women, with a median age of 63 years (range, 27–88 years) The primary tumor was located at the GEJ (44, 27%), proximal stomach (26, 16%), gastric body (38, 23%), gastric antrum (27, 16%), or diffusely involved the whole stomach (30, 18%). The histologic type was undifferentiated (including poorly differentiated or signet-ring cell carcinoma) in 129 (78%) cases or well-differentiated (including papillary or tubular adenocarcinoma) in the remaining 36 cases. Presenting symptoms included weight loss (88 patients, 53%), abdominal pain (53, 32%), anemia (27, 16%), dysphagia (34, 21%), or vomiting (3, 2%). The Eastern Cooperative Oncology Group (ECOG)11 performance status at diagnosis was available for 101 patients, and it was 0 (fully active, 31%), 1 (restricted in strenuous activity, 53%), or 2 (unable to carry out any work activities, 16%).
Laparoscopic staging was false-negative, with detection of peritoneal disease at laparotomy, in 18 cases. No patient had any intra-abdominal procedure or laparotomy-related complication. All 18 patients have been included in the present study on an “intention to treat” basis. Six patients had placement of a feeding jejunostomy tube with laparoscopic assistance, subsequent to detection of M1 disease. These patients have been retained in the study, but the jejunostomy procedure was not counted as an intervention for symptom-palliation. There was no laparoscopy-related mortality, and a single patient sustained a major complication (pneumothorax).
Metastatic disease was limited to the peritoneum in 121 (73%) patients and was subcategorized as P1 (13), P2 (58), and P3 (50). In 16 (10%) patients, metastasis were limited to the liver, and in the remaining 28 (17%) patients there was a combination of peritoneum, liver, and nonregional lymph node metastasis (Fig. 1). Sixteen patients had T4 primary tumors at laparoscopy, in addition to M1 disease, and have been retained in this study.
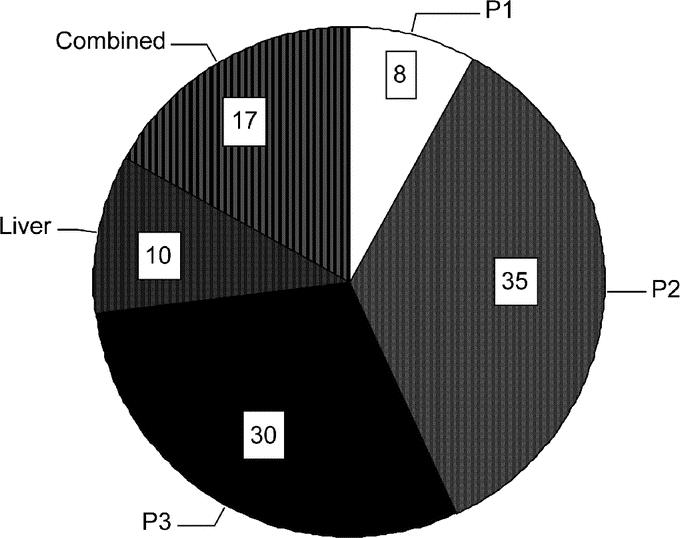
FIGURE 1. Percentage distribution of M1 disease at staging laparoscopy: P1, metastasis limited to peritoneum adjacent to the stomach; P2, few metastasis to the distant peritoneum; P3, numerous metastasis to the distant peritoneum; Liver, metastasis limited to the liver; Combined, metastasis to any combination of peritoneum, liver, or nonregional lymph nodes.
Of the 65 recent patients with spiral CT scans, M1 disease was laparoscopically detected in 21 (32%) patients and was limited to the peritoneum in 95% (n = 20) or involved both liver and peritoneum in 5% (n = 1).
Outcomes
Palliative Intervention
Following diagnosis of M1 gastric cancer, 97 (59%) patients continued their entire treatment at MSKCC. Continued treatment at MSKCC or elsewhere was not related to performance status (ECOG score 0–1, 84% versus 81%, respectively; P = 0.7) or extent of disease (P1/P2, 41% versus 49%; P = 0.4). To ensure data accuracy, the analysis of need for subsequent intervention was limited to patients who continued treatment at MSKCC. All 97 patients received systemic chemotherapy, which was commenced at median interval of 14 days (range, 1–69 days) after laparoscopy. At the last follow-up (median, 8 months), 8 patients were alive and the remaining 89 patients had died of progressive systemic disease. Combination chemotherapy was administered to 76 patients and comprised cisplatin combined with 5-fluorouracil (5-FU) or irinotecan (CPT-11) or paclitaxel for 64 patients; 5-FU, adriamycin, and methotrexate (FAMTX) for 8 patients; 5-FU and mitomycin for 2 patients, and other combinations for 2 patients. The remaining 21 patients received single agent therapy with 5-FU (11), paclitaxel (8), or cisplatin. (2) Forty-seven patients received second-line therapy because of disease progression or unacceptable toxicity, and 17 patients received third-line chemotherapy.
A palliative intervention was performed on 48 (50%) of the patients (Fig. 2). Twenty-nine (30%) patients had a procedure performed on only the GEJ or stomach. Seven patients (8%) underwent a procedure on only a distant site to manage symptoms due to metastatic disease. Twelve patients (12%) had an intervention on the stomach and also on a distant site. In total, 41 (42%) patients had intervention on the GEJ or stomach and 19 (20%) patients had intervention on distant sites. These patients were admitted to hospital on a median of 3 occasions (range, 1–15 occasions) for a median total duration of 19 days (range, 2–64 days). The median interval between staging laparoscopy and the first palliative intervention was 4 months (range, 1–35 months). More than one intervention (maximum, 4) was performed in 21% of cases. The median survival between the first intervention and death was 3 months (range, <1–28 months). The median survival between performance of a percutaneous endoscopic gastrostomy (PEG) and death was 5 months (range, <1–7 months). The need for intervention was not altered by primary tumor location, distribution of metastatic disease, age, performance status, or histology.
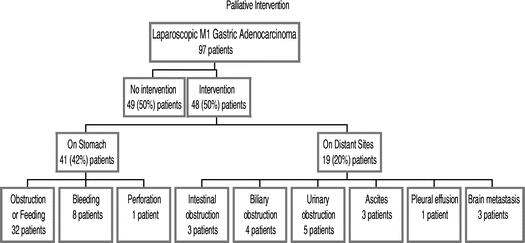
FIGURE 2. Incidence of palliative intervention subsequent to laparoscopic detection of M1 gastric adenocarcinoma. Of the 48 patients who had intervention, 12 patients had procedures on both the stomach and on a distant site.
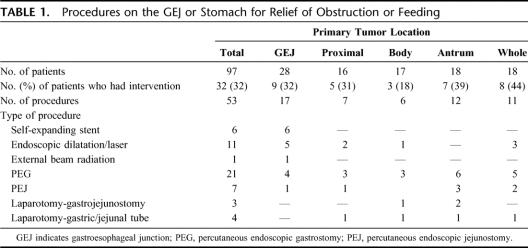
In 32 (33%) cases, procedures were performed for relief of GEJ or gastric obstruction, gastric decompression, or provision of supplemental nutrition (Table 1). These 32 patients underwent a total of 53 invasive procedures. Tumors in the gastric body appeared least likely to require intervention. The majority of patients underwent only endoscopic procedures. Seven patients underwent laparotomy for gastrostomy or jejunostomy, following technical failure at endoscopy, or gastrojejunostomy. No patient had a gastric resection for relief of obstruction. Eight (8%) patients developed an intervention-related complication, which required prolonged intravenous therapy (2) or endoscopic reintervention (6). There was one intervention-related death, due to peritonitis following PEG.
TABLE 1. Procedures on the GEJ or Stomach for Relief of Obstruction or Feeding
Thirty-two (33%) patients received blood transfusion of median of 3 units (range, 1–17 units). Eight (8%) patients had a single intervention each for bleeding from the primary tumor. Of these, 3 patients received external beam radiation therapy and 5 patients had endoscopic Nd-YAG laser therapy. There was no difference in blood transfusion according to primary tumor location.
One (1%) patient had intervention for perforation of the primary tumor during chemotherapy. The perforated tumor was initially suture-repaired at another hospital. This patient subsequently underwent 2 further laparotomies, including gastrectomy and colectomy, and ultimately survived this event.
Nineteen patients had intervention on a distant site for complications related to metastatic disease (Fig. 2). Four patients required management of biliary obstruction due to malignant lymphadenopathy and were treated with endoscopic stenting (3) or percutaneous drainage (1). Five patients required ureteral stenting for unilateral (4) or bilateral (1) ureteral obstruction due to retroperitoneal lymphadenopathy. Three patients had intervention for intestinal obstruction due to peritoneal disease. One patient underwent laparotomy and enteroenterostomy. Another patient had an endoluminal stent for left colonic obstruction and subsequently required a colostomy. A third patient had a sigmoid colostomy for obstruction of the rectum and subsequently had a second laparotomy for small bowel obstruction. Three patients had abdominal paracentesis for tense ascites. One patient had a tube thoracostomy and chemical pleurodesis. Three patients had external beam radiation therapy for brain metastasis. There was no treatment-related mortality.
Survival
Of 165 patients, 9 were entirely lost to follow-up. Of the remaining 156 patients, 138 patients were dead and 18 were alive at the time of this report. The median survival was 10 months (range, 1–39 months) with actuarial survival of 39% at 1 year and 4% at 2 years (Fig. 3). For the 18 patients who were alive at last follow-up, the median follow-up duration was 6 months (range, 3–17 months) and 3 patients were alive for more than 12 months. Of the 138 patients who had died, 32% had survived at least 1 year and 4% had survived at least 2 years, with median survival of 8 months and maximum survival of 37 months.
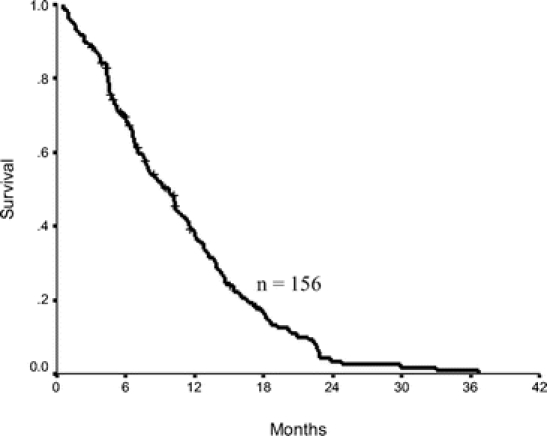
FIGURE 3. Kaplan-Meier survival plot for patients with laparoscopic stage M1 gastric adenocarcinoma (1-year survival, 39%; median survival, 10 months).
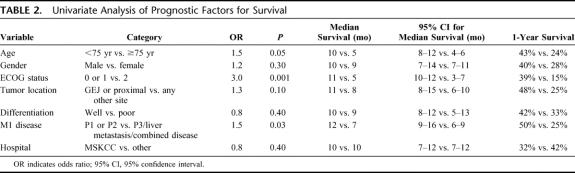
On univariate analysis, age <75 years, good performance status (ECOG performance status 0 or 1), and limited peritoneum-only metastasis (P1 or P2) were associated with a significantly longer survival (Table 2). There was no significant difference in prognosis according to gender, histologic type, tumor location or follow-up after laparoscopy at MSKCC or elsewhere (Table 2). On multivariate analysis, ECOG performance status 0 or 1 and P1 or P2 retained independently significant prognostic value (Table 3).
TABLE 2. Univariate Analysis of Prognostic Factors for Survival
TABLE 3. Multivariate Analysis of Prognostic Factors for Survival
DISCUSSION
This study deals with the natural history of progression of gastric adenocarcinoma in patients who received systemic chemotherapy but did not undergo resection of the primary tumor following laparoscopic detection of M1 disease. The management strategy argues that noncurative resection is unlikely to alter disease progression, and preemptive surgical palliation is unnecessary. Nonoperative treatment of advanced gastric cancer is not a novel concept, and a similar approach has been proposed for management of selected patients with stage IV colorectal adenocarcinoma and radiology-determined unresectable liver metastasis.12 However, patients who have laparoscopic stage M1 disease and no resection represent a population that is unique to the era of minimal access surgery and has not been adequately described. Typically, this population has CT scan stage M0 disease, no significant obstruction or bleeding, and is fit for surgical resection.2 In the present study, such selection is reflected by the high proportion of patients with initial symptoms of weight loss or abdominal pain and good functional performance status. These patients are distinct from others who do not undergo resection because of clinical or radiologic M1 disease, technical reasons, or poor physiologic risk.
Satisfactory palliation and quality of life are the principal objectives in the care of patients with incurable gastric cancer. The wisdom of resecting the stomach in the face of metastatic disease would require that this strategy avoids substantial operative morbidity, mortality, and recovery while not delaying inevitable palliative interventions to the detriment of the patient. Randomized trials to address the value of this approach do not exist and will likely not occur. Thus, we must evaluate retrospective data from reports such as this to determine the value of this treatment paradigm.
Several confounding issues need to be carefully considered during interpretation of data on palliative intervention. It is difficult to retrospectively evaluate the quality of symptom assessment that preceded patient selection for laparoscopic staging. Furthermore, decision-making in palliative care can be complex, and there is a critical triangle between the patient, family member, and surgeon that clarifies and defines the goals of each patient's individual treatment. Through the dynamics of this interaction, the patient's symptoms, values, social and emotional support are considered against the medical and surgical alternatives.10 A selection bias is thus likely to be inherent at multiple stages. Retrospective study is also limited by difficulty to distinguish disease-related symptoms from chemotherapy-related toxicity and to accurately evaluate the role of supportive care or the success of intervention. To maximize accuracy in evaluation of the need for palliative intervention subsequent to laparoscopy, this study focused on surgical, endoscopic, radiologic, or radiation interventions.
The frequency of palliative interventions was examined in 97 patients who had their entire follow-up care in the authors’ institution. One half of the patients did not undergo any palliative intervention subsequent to laparoscopy. A total of 88% of patients did not have laparotomy and 58% had no stomach-related procedure. Of those who did have a stomach-related procedure, two thirds were managed nonoperatively, most commonly with a PEG to decompress the stomach in patients with gastric outlet obstruction, gastric stasis due to dysmotility, or intestinal obstruction due to peritoneal disease.13 Thus, some patients with intestinal obstruction are included under the category of “procedures on the gastroesophageal junction (GEJ) or stomach.” Owing to the complex nature of decision making in palliative surgery, the indications for PEG could not be precisely categorized in each case. PEG may be also used for feeding in selected cases with GEJ or proximal gastric tumors, but there is risk of laryngotracheal aspiration and direct percutaneous endoscopic jejunostomy is more effective.14 A small number of patients had preemptive placement of jejunostomy tubes at staging laparoscopy, and their impact on incidence of subsequent intervention is unclear. Supplemental enteral nutrition often becomes necessary because of a combination of gastric luminal obstruction by the primary tumor as well as debilitating systemic effects of disseminated disease and patients may require such interventions during the course of their lifetime.
The role of self-expanding endoluminal stents for obstructing GEJ tumors is well established.15 Endoscopic dilatation of obstructing tumors is associated with risk of perforation but can provide successful short-term relief in well-selected patients.15 Gastrojejunostomy was performed in only a minority of patients. Recently developed self-expanding gastroduodenal stents were not used in the authors’ institution during this study period. Such stents are reported to satisfactorily relieve malignant gastric outlet obstruction in a large proportion of cases16 and may avoid the need for gastrojejunostomy in some cases.
One third of the patients received blood transfusion. In addition to tumor bleeding, anemia may have been caused by chemotherapy-related toxicity, nutritional deficiencies, hemolysis, or suppressed hematopoiesis.17 Radiation therapy or endoscopic laser therapy was used to control tumor bleeding in 8% of cases. A short course of high-dose per fraction radiation therapy can satisfactorily control bleeding in most cases, although it may not be effective if a large vessel has been eroded.18 Variable results have been previously reported with endoscopic argon plasma coagulation or Nd:YAG laser for bleeding gastric tumors.15 In this series, no patient required gastric resection for bleeding.
Only 1 patient had perforation of the gastric tumor during chemotherapy. It is rare for gastric adenocarcinoma to perforate, and less than 2% of cases present with this complication.19 Thus, it does not appear justifiable to perform a noncurative gastric resection because of concern about tumor perforation during currently used chemotherapy.
Nineteen (20%) patients had intervention for symptoms due to metastatic disease. Of these, laparotomy was performed in 3 patients for intestinal obstruction due to peritoneal disease. The decision to operate for intestinal obstruction requires careful consideration. Up to 50% of patients with obstruction due to peritoneal metastasis from gastric adenocarcinoma are not improved by operation, and 40% of these patients suffer recurrent obstruction.20 Nonoperative measures, including decompression by PEG, can relieve symptoms in many circumstances.21
In the present study, the median survival was 10 months; and the estimated survival at 1 year was 39%. Palliative intervention-related mortality was 1%. A contemporaneous series of patients that was not selected for laparoscopy and underwent noncurative gastrectomy at MSKCC has been previously reported.22 These patients had a median survival of 10.6 months. The perioperative mean hospital stay was 18 days, a complication was identified in 54% of patients, and the mortality rate was 6%. Furthermore, after the initial operation, a subsequent gastric-related procedure was required in 9% of patients.22 Other series of patients, who had M1 disease detected at laparotomy and proceeded to gastrectomy, report median survival of 8 to 12 months, 1-year survival of 28% to 50%, and perioperative mortality of 2% to 19%.23–27
Not surprisingly, good functional performance status and low burden of peritoneum-only metastasis were significant indicators of longer survival. These data corroborate the prognostic importance of performance status in advanced gastric cancer.28 The prognostic value of metastatic burden has been also previously reported,29,30 but the present data emphasize the importance of systematically recording the laparoscopic category of peritoneal disease. Metastasis-related complications, such as extrahepatic biliary obstruction or ureteral obstruction, are known to be associated with a very poor outcome.31,32 Preoperative serum albumin and total body weight loss are also likely to serve as prognostic markers.8 Prognostic data may aid in the choice of palliative intervention and patient counseling. Most palliative procedures have a fixed duration of effect, and correct estimation of survival may influence choice of an appropriate procedure. Moreover, previous data imply that patients with survival times less than 3 to 6 months are substantially less likely to achieve durable palliation by surgical procedures than those with better prognosis.8,10
CONCLUSION
This study describes outcomes in patients who were selected through a management strategy that avoided noncurative gastric resection in minimally symptomatic patients with laparoscopically identified M1 disease. Although one half of the patients subsequently needed a palliative intervention during the course of a limited life span, gastrectomy-associated morbidity and mortality were avoided. The argument that preemptive palliative gastrectomy will avoid subsequent bleeding or perforation is not compelling, given the low incidence of either complication in this study. Gastric outlet obstruction is a more difficult issue and deserves careful study of health-related quality of life in parallel cohorts to further clarify the optimal use of PEG, stent, bypass, or resection. As in all circumstances in which cure is no longer possible, the obligation to care for the patient does not diminish the difficulty of the decision process. Patient-specific considerations, augmented by data from reports such as this, will allow appropriate selection of management strategies and avoid empiric or routine preemptive palliation when this is of limited value.
Footnotes
Supported by the Richard A Gelb Foundation.
Reprints: Murray F. Brennan, MD, Department of Surgery, Memorial Hospital, 1275 York Avenue, New York, NY 10021. E-mail: brennanm@mskcc.org.
REFERENCES
- 1.Lowy AM, Mansfield PF, Leach SD, et al. Laparoscopic staging for gastric cancer. Surgery. 1996;119:611–614. [DOI] [PubMed] [Google Scholar]
- 2.Burke EC, Karpeh MS, Conlon KC, et al. Laparoscopy in the management of gastric adenocarcinoma. Ann Surg. 1997;225:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith A, Finch MD, John TG, et al. Role of laparoscopic ultrasonography in the management of patients with oesophagogastric cancer. Br J Surg. 1999;86:1083–1087. [DOI] [PubMed] [Google Scholar]
- 4.Feussner H, Omote K, Fink U, et al. Pretherapeutic laparoscopic staging in advanced gastric carcinoma. Endoscopy. 1999;31:342–347. [DOI] [PubMed] [Google Scholar]
- 5.Lehnert T, Rudek B, Kienle P, et al. Impact of diagnostic laparoscopy on the management of gastric cancer: prospective study of 120 consecutive patients with primary gastric adenocarcinoma. Br J Surg. 2002;89:471–475. [DOI] [PubMed] [Google Scholar]
- 6.Asencio F, Aguilo J, Salvador JL, et al. Video-laparoscopic staging of gastric cancer: a prospective multicenter comparison with noninvasive techniques. Surg Endosc. 1997;11:1153–1158. [DOI] [PubMed] [Google Scholar]
- 7.Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. [DOI] [PubMed] [Google Scholar]
- 8.McCahill LE, Smith DD, Borneman T, et al. A prospective evaluation of palliative outcomes for surgery of advanced malignancies. Ann Surg Oncol. 2003;10:654–663. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 2nd English ed. Gastric Cancer. Tokyo: Japanese Gastric Cancer Association, 1998;1:10–24. [DOI] [PubMed] [Google Scholar]
- 10.Miner TJ, Jaques DP, Shriver CD. A prospective evaluation of patients undergoing surgery for the palliation of an advanced malignancy. Ann Surg Oncol. 2002;9:696–703. [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 12.Sarela AI, Guthrie JA, Seymour MT, et al. Non-operative management of the primary tumour in patients with incurable stage IV colorectal cancer. Br J Surg. 2001;88:1352–1356. [DOI] [PubMed] [Google Scholar]
- 13.Herman LL, Hoskins WJ, Shike M. Percutaneous endoscopic gastrostomy for decompression of the stomach and small bowel. Gastrointest Endosc. 1992;38:314–318. [DOI] [PubMed] [Google Scholar]
- 14.Shike M, Latkany L, Gerdes H, et al. Direct percutaneous endoscopic jejunostomies for enteral feeding. Gastrointest Endosc. 1996;44:536–540. [DOI] [PubMed] [Google Scholar]
- 15.Nash CL, Gerdes H. Methods of palliation of esophageal and gastric cancer. Surg Oncol Clin North Am. 2002;11:459–483. [DOI] [PubMed] [Google Scholar]
- 16.Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002;97:72–78. [DOI] [PubMed] [Google Scholar]
- 17.Glimelius B, Linne T, Hoffman K, et al. Epoetin beta in the treatment of anemia in patients with advanced gastrointestinal cancer. J Clin Oncol. 1998;16:434–440. [DOI] [PubMed] [Google Scholar]
- 18.Ferris FD, Bezjak A, Rosenthal SG. The palliative uses of radiation therapy in surgical oncology patients. Surg Oncol Clin North Am. 2001;10:185–201. [PubMed] [Google Scholar]
- 19.Lehnert T, Buhl K, Dueck M, et al. Two-stage radical gastrectomy for perforated gastric cancer. Eur J Surg Oncol. 2000;26:780–784. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull AD, Guerra J, Starnes HF. Results of surgery for obstructing carcinomatosis of gastrointestinal, pancreatic, or biliary origin. J Clin Oncol. 1989;7:381–386. [DOI] [PubMed] [Google Scholar]
- 21.Hardy JR. Medical management of bowel obstruction. Br J Surg. 2000;87:1281–1283. [DOI] [PubMed] [Google Scholar]
- 22.Miner TJ, Jaques DP, Karpeh MS, et al. Defining palliative surgery in patients receiving noncurative resections for gastric cancer. J Am Coll Surg. 2004;198:1013–1021. [DOI] [PubMed] [Google Scholar]
- 23.Boddie AW Jr, McMurtrey MJ, Giacco GG, et al. Palliative total gastrectomy and esophagogastrectomy: a reevaluation. Cancer. 1983;51:1195–1200. [DOI] [PubMed] [Google Scholar]
- 24.Bozzetti F, Bonfanti G, Audisio RA, et al. Prognosis of patients after palliative surgical procedures for carcinoma of the stomach. Surg Gynecol Obstet. 1987;164:151–154. [PubMed] [Google Scholar]
- 25.Hanazaki K, Sodeyama H, Mochizuki Y, et al. Palliative gastrectomy for advanced gastric cancer. Hepatogastroenterology. 2001;48:285–289. [PubMed] [Google Scholar]
- 26.Kikuchi S, Arai Y, Morise M, et al. Gastric cancer with metastasis to the distant peritoneum: a 20-year surgical experience. Hepatogastroenterology. 1998;45:1183–1188. [PubMed] [Google Scholar]
- 27.Hartgrink HH, Putter H, Kranenbarg EK, et al. Value of palliative resection in gastric cancer. Br J Surg. 2002;89:1438–1443. [DOI] [PubMed] [Google Scholar]
- 28.Jeung HC, Rha SY, Jang WI, et al. Treatment of advanced gastric cancer by palliative gastrectomy, cytoreductive therapy and postoperative intraperitoneal chemotherapy. Br J Surg. 2002;89:460–466. [DOI] [PubMed] [Google Scholar]
- 29.Maehara Y, Moriguchi S, Kakeji Y, et al. Pertinent risk factors and gastric carcinoma with synchronous peritoneal dissemination or liver metastasis. Surgery. 1991;110:820–823. [PubMed] [Google Scholar]
- 30.Bonenkamp JJ, Sasako M, Hermans J, et al. Tumor load and surgical palliation in gastric cancer. Hepatogastroenterology. 2001;48:1219–1221. [PubMed] [Google Scholar]
- 31.Donat SM, Russo P. Ureteral decompression in advanced nonurologic malignancies. Ann Surg Oncol. 1996;3:393–399. [DOI] [PubMed] [Google Scholar]
- 32.Chu KM, Law S, Branicki FJ, et al. Extrahepatic biliary obstruction by metastatic gastric carcinoma. J Clin Gastroenterol. 1998;27:63–66. [DOI] [PubMed] [Google Scholar]