Abstract
Introduction:
Laparoscopic esophagomyotomy is the preferred approach to patients with achalasia of the esophagus, However, there are very few long-term follow-up studies (>10 years) in these patients.
Objective:
To perform a very late subjective and objective follow-up in a group of 67 patients submitted to esophagomyotomy plus a partial antireflux surgery (Dor's technique).
Material and Methods:
In a prospective study that lasted 30 years, 67 patients submitted to surgery were divided into 3 groups: group I followed for 80 to 119 months (15 patients); group II, with follow-up of 120 to 239 months (35 patients); and group III, with follow-up more than 240 months (17 patients). They were submitted to clinical questionnaire, endoscopic evaluation, histologic analysis, radiologic studies, manometric determinations, and 24-hour pH studies late after surgery.
Results:
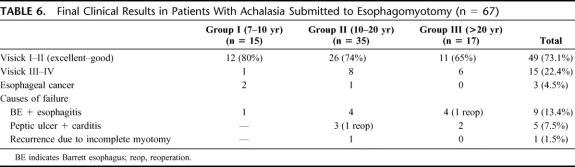
Three patients developed a squamous cell esophageal carcinoma 5, 7, and 15 years after surgery. At the late follow-up, Visick III and IV were seen in 7%, 23%, and 35%, according to the length of follow-up of each group. Endoscopic examination revealed a progressive nonsignificant deterioration of esophageal mucosa, histologic analysis distal to squamous-columnar junction showed a significant decrease of fundic mucosa in patients of group III, with increase of intestinal metaplasia, although not significant time. Lower esophageal sphincter showed a significant decrease of resting pressure 1 year after surgery, which remained similar at the late control. There was no return to peristaltic activity. Acid reflux measured by 24-hour pH studies revealed a progressive increase, and the follow-up was longer. Nine patients developed Barrett esophagus: 6 of them a short-segment and 3 a long-segment Barrett esophagus. Final clinical results in all 67 patients demonstrated excellent or good results in 73% of the cases, development of epidermoid carcinoma in 4.5%, and failures in 22.4% of the patients, mainly due to reflux esophagitis. Incomplete myotomy was seen in only 1 case.
Conclusion:
In patients with achalasia submitted to esophagomyotomy and Dor's antireflux procedure, there is a progressive clinical deterioration of initially good results if a very long follow-up is performed (23 years after surgery), mainly due to an increase in pathologic acid reflux disease and the development of short- or long-segment Barrett esophagus.
A total of 67 patients with achalasia submitted to esophagomyotomy and Dor's technique were followed for 190 months. Clinical results were excellent or good in 73%, epidermoid carcinoma developed in 4.5% and failures were present in 22.4%, due to progressive appearance of reflux esophagitis and Barrett esophagus.
Achalasia is a primary motor disorder characterized by a hypertensive lower esophageal sphincter, which fails to relax completely after swallowing and by aperistalsis of the thoracic esophagus,1–4 due to a loss of Auerbach's plexuses.3,4 As there is no medical or surgical cure for this disease, all treatments are directed to obtain a good esophageal emptying into the stomach by disrupting this hypertensive lower esophageal sphincter. Surgical approach seems to have much better long-term results than pneumatic dilatation5–9 and is the preferred treatment at this moment, due to the fact that it can be performed by laparoscopic instead of laparotomic approach.10–14 There are many reports concerning results of open15–20 or laparoscopic10 esophagomyotomy (up to 5 years), with near 90% of satisfactory results. However, only very few papers have evaluated by a long-term follow-up (over 10 years) the final results, concerning to the presence of gastroesophageal reflux and the appearance of esophageal carcinoma.
The purpose of the present prospective study was to perform a very late subjective and objective evaluation of a group of 67 patients submitted to esophagomyotomy plus a partial antireflux procedure.
MATERIALS AND METHODS
Patients Studied
The present prospective study started on March 1972, when a prospective randomized study was designed to compare surgery versus dilatation.5,6 The end of the follow-up was December 2002 and therefore is a 30-year prospective study. A total of 106 patients were included during this period of time and were operated on by 2 of the authors (A.C., I.B.). During the follow-up, 25 (23.4%) patients died due to nonrelated diseases with a mean age of 72 years at the moment of their death (range, 30–97 years). Fourteen patients (19.0%) were lost from the late control, which means that a total of 67 patients were included in this follow-up (81% of follow-up). They were divided into 3 groups, according to the length of follow-up: group I, with 80 to 119 months of follow-up after surgery (15 patients); group II, with 120 to 239 months follow-up (35 patients); and group III, with more than 240 months follow-up (17 patients).
In the same period of time, 5 patients were submitted to esophagectomy due to sigmoid-like achalasia but were not included in the present investigation.
Clinical Questionnaire
A careful clinical assessment was performed by questionnaire in each patient before and late after surgery, asking about the presence of:
Heartburn, graded as absent, occasional (less a week), and frequent (more than once a week).
Dysphagia, graded as absent, occasional (once a week or less), and frequent (more than once a week).
Gain or loss of weight, in kilograms, after surgery.
-
They were graded on a scale of 1 to 3 according to the criteria described by DeMeester's group.21 For the late clinical evaluation, a modified Visick gradation was used with the following criteria:
Visick I = asymptomatic.
Visick II = presence of mild or episodic symptoms; easily controlled by medical treatment or diet adjustment with no need of permanent medication.
Visick III = presence of frequent, daily symptoms; easily controlled by medical treatment or diet adjustment with no need of permanent medication.
Visick IV = failure of the operation with severe symptoms, which requires reoperation or produces a serious metabolic disturbance.
Endoscopic Evaluation
All endoscopic procedures were performed by 2 of us (A.C. and I.B.) using an Olympus GIF XQ-20 endoscope. Special care was taken to measure the exact location of the squamocolumnar junction at the beginning and at the end of the procedure, avoiding the “push” and “pull” effects of the endoscope. It was done in all patients before and late after surgery, at the time of the last control. In the final procedure, 2 biopsy samples were taken from the antrum; and, in all patients as well as in patients with suspected short-segment Barrett esophagus (length <29 mm), 4-quadrant biopsy specimens were taken 5 mm distal to the squamocolumnar junction (juxtacardial biopsies). In patients with suspected long-segment Barrett esophagus (length >30 mm), 4-quadrant biopsy specimens were taken every 2 cm from the squamocolumnar junction until the lower esophageal sphincter. The presence of a peptic ulcer was carefully determined, as well as the presence of proximal erosions.
Radiologic Evaluation
A complete upper radiologic study of the GI tract was performed in all patients before and 1 month after surgery. No long-term radiographic evaluation was routinely performed. This examination was performed after an overnight fast, using a low-density barium sulfate suspension (45% weight in volume). Patients were instructed to drink the amount of barium that they could tolerate without regurgitation or aspiration (usually between 150 and 200 mL). With the patient in upright position, 4 radiographs (35 × 35 cm) were taken between 1 and 4 minutes after the last swallow of barium. Then the patient was placed in a supine position and the other 4 films were taken. Two parameters were evaluated and measured by an independent radiologist who did not know the precedence and the name of the patient: 1) internal diameter of the esophagogastric junction (identified by the bird's break appearance of the esophagogastric junction) measured in millimeters; and 2) internal diameter of the middle third of the esophagus, approximately at the level of the bifurcation of the trachea in millimeters.
Histologic Evaluation
All biopsy samples were immediately submerged in a 10% formalin solution and sent for histologic examination. They were stained with hematoxylin and eosin and Alcian blue stain at pH 2.5. The type of epithelium lining the distal esophagus was carefully determined, including the presence of intestinal metaplasia. Fundic mucosa was identified by the presence of parietal and chief cells at the deep glandular layer. Cardiac mucosa was identified by the presence of mucus-secreting columnar cells. Specialized intestinal metaplasia was defined by the presence of well-defined goblet cells, confirmed by positive staining with Alcian blue at pH 2.5.
The presence of Helicobacter pylori was investigated at the juxtacardial biopsies and at the antrum.
Manometric Studies
Manometric testing was carried out after a 12-hour fast with the patient in the supine position. The complete details of this procedure have been fully explained in previous reports.22–24 The resting pressure of the lower esophageal sphincter was measured. The location of the distal and proximal ends of the lower esophageal sphincter was also measured in centimeters from the incisors to measure the total length of the sphincter. The percentage of relaxation after swallowing was also carefully evaluated. The amplitude of the distal esophageal contractile waves was also determined in mm Hg. This test was performed in all patients 3 times: before surgery, 1 year after surgery, and at the late control, when the last evaluation was performed.
24-Hour Intraesophageal pH Studies
This test was performed after a 12-hour fast,25,26 introducing the catheter through the nose until the stomach was reached (Digitrapper; Synectics, Sweden). The catheter was then placed 5 cm above the manometric upper border of the lower esophageal sphincter (manometry is always done before this procedure). Among the 6 different parameters that can be evaluated, the most useful and practical is the total percentage of time during which the intraesophageal pH remains below 4, being our normal value less than 4% of the time during a 24-hour period (55 minutes). This test was carried out in all patients included in the present investigation at the late control, when the last evaluation was performed.
Statistical Evaluation
For the calculation of statistical significances, the χ2 test, the Fisher exact test, and the Mann-Whitney U test were used, taking a P < 0.05 as significant. All statistical calculations refer to comparison between the groups or within the groups, according to the specific data.
Surgical Procedure
All operations were performed by the authors at the Department of Surgery, University Hospital. After laparotomy and careful abdominal exploration, the abdominal esophagus and esophagogastric junction was mobilized.5–8 The myotomy was 5 to 6 cm long at the esophagus and was extended 1 cm onto the gastric wall. Short gastric vessels were divided to avoid tension on the anterior fundoplication. An anterior 180° Dor fundoplication was used in all patients.5–8,15 None of these patients was operated on by the laparoscopic approach.
Follow-up
All patients were carefully followed by the main authors 1 month, 6 months, 1 year, and then each 5 years after surgery by clinical questionnaire. Manometric studies were performed in each patient 3 times after surgery, while endoscopy was done in all at the end of the study and occasionally before; 24-hour pH studies were done only at the end of the study.
RESULTS
Clinical Outcome
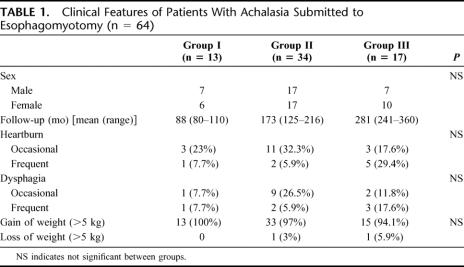
There was no operative mortality. Two patients (1.9%) had postoperative fistula, one in June 1973 and other case operated on October 1983. Both were reoperated and had an uneventful recovery. There were no other postoperative complications or splenectomy in any of the patients. The usual hospital stay for 105 uncomplicated patients was 5.4 days (range, 4–7 days). Three patients (4.5%) developed an esophageal carcinoma 5, 7, and 15 years after surgery, which gives a rate of 1 case per 120 patient/year. These patients were excluded from the following tables, leaving only 64 patients for the long-term follow-up. The main clinical features are shown in Table 1. The gender distribution was similar in all groups, as well as the presence of heartburn. Dysphagia increased late after surgery, but without statistical significance between the groups. More than 94% of all patients gained weight after surgery.
TABLE 1. Clinical Features of Patients With Achalasia Submitted to Esophagomyotomy (n = 64)
Anatomic Studies
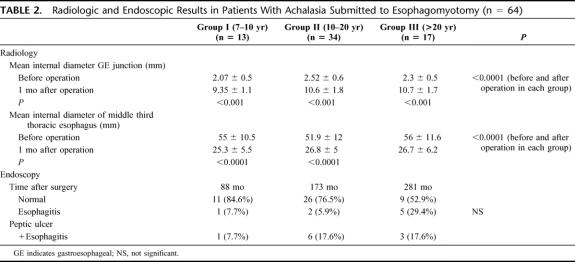
Table 2 demonstrates radiologic and endoscopic results in these patients. Radiologic examinations showed a significant increase of the internal diameter of the GE junction and a significant decrease of the internal diameter of the thoracic esophagus 1 month after surgery (P < 0.001). Endoscopic examination before surgery showed normal distal esophagus in all. Late after operation, there was a progressive deterioration of esophageal mucosa with time but without statistical significance between the groups.
TABLE 2. Radiologic and Endoscopic Results in Patients With Achalasia Submitted to Esophagomyotomy (n = 64)
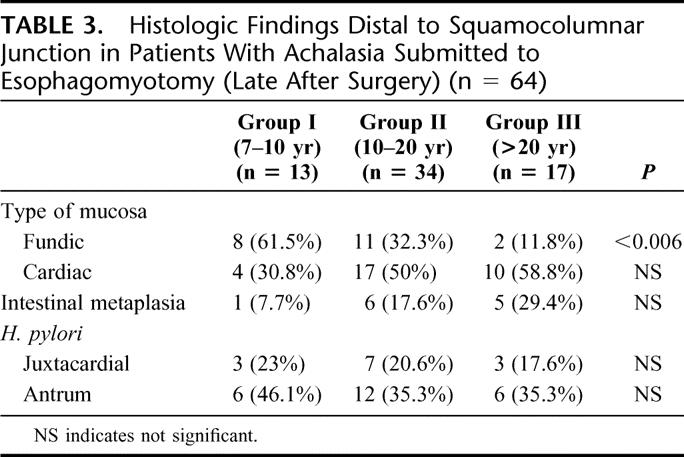
The main histologic findings at the GE junction and the antrum are shown in Table 3. There was a significant decrease in the presence of fundic mucosa in patients of group III compared with group I. Although an increase was demonstrated in the presence of intestinal metaplasia at the GE junction and distal esophagus in patients group III compared with group I, this value did not reach statistical significance. The presence of Helicobacter pylori was similar in all 3 groups of patients, as well as its presence in the antrum. Among the 13 patients who had H. pylori at the GE junction, 9 (69.2%) was located in fundic mucosa and 4 (30.8%) in cardial mucosa. No H. pylori was present in the presence of intestinal metaplasia.
TABLE 3. Histologic Findings Distal to Squamocolumnar Junction in Patients With Achalasia Submitted to Esophagomyotomy (Late After Surgery) (n = 64)
Physiologic Studies
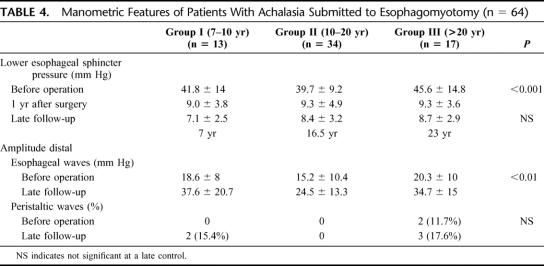
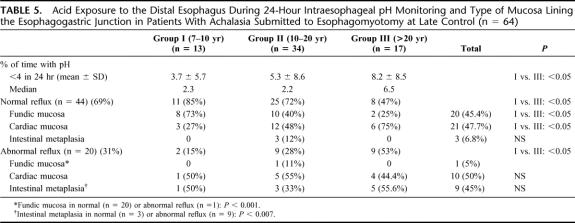
The manometric features of the lower esophageal sphincter and of the distal esophageal waves are shown in Table 4. Before surgery, the resting lower esophageal sphincter pressure was hypertensive in all groups. One year after surgery, there was a significant decrease in resting pressure (P < 0.0001). At the late follow-up, sphincter pressure remained in similar low values, as is seen in Figure 1. The percentage of relaxation before surgery ranged between 20% to 70%, with a mean of 40%. Late after surgery, sphincter relaxation varied between 90% and 100% after swallowing. The amplitude of the distal esophageal waves increased significantly late after surgery in all patients, but aperistalsis remained almost similar. The results of 24-hour pH monitoring at the distal esophagus are shown in Table 5. There was an increase in the prevalence of acid reflux as the follow-up of patients increases, which was significant (P < 0.05) comparing patients of group I with group III. A normal acid reflux test was observed in 69% of all patients. However, this test was normal in 85% of patients in group I and only in 47% of patients in group III, as can be seen in Figure 2 (P < 0.05). This table also shows the correlation between normal or abnormal acid reflux values and the type of mucosa lining the GE junction and distal esophagus. Among patients with normal acid reflux, there was a significant decrease of fundic mucosa and a significant increase in cardiac mucosa as the follow-up is longer. Three patients of group II values showed the presence of intestinal metaplasia at the GE junction. Among patients with abnormal acid reflux values, only cardiac mucosa or intestinal metaplasia were present at the GE junction, with only 1 case with fundic mucosa. Fundic mucosa was significantly lower in patients with abnormal reflux (n = 1) compared with those with normal acid reflux (n = 20). Intestinal metaplasia was present significantly higher (4.5%) in patients with abnormal reflux (n = 9) compared with patients with normal reflux (6.8%) (n = 3). The correlation with the presence of heartburn and a normal or abnormal acid reflux test was also evaluated. There were 9 patients who developed a short-segment Barrett esophagus, having only 6 of them a pathologic acid reflux with a mean value of 17.3%. The 3 patients who developed a long-segment Barrett esophagus, all had pathologic reflux with a mean value of 24.7%.
TABLE 4. Manometric Features of Patients With Achalasia Submitted to Esophagomyotomy (n = 64)
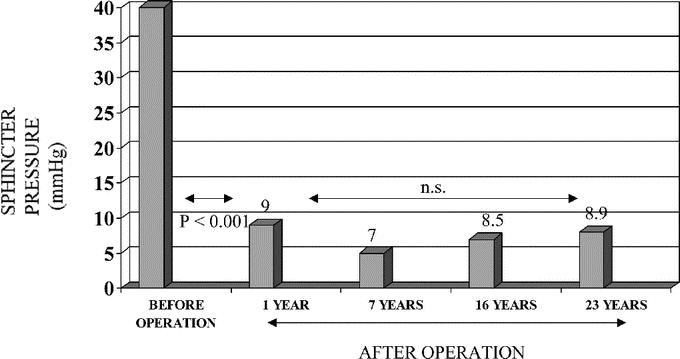
FIGURE 1. Lower esophageal sphincter pressure before operation and 1 year and at late control after surgery.
TABLE 5. Acid Exposure to the Distal Esophagus During 24-Hour Intraesophageal pH Monitoring and Type of Mucosa Lining the Esophagogastric Junction in Patients With Achalasia Submitted to Esophagomyotomy at Late Control (n = 64)
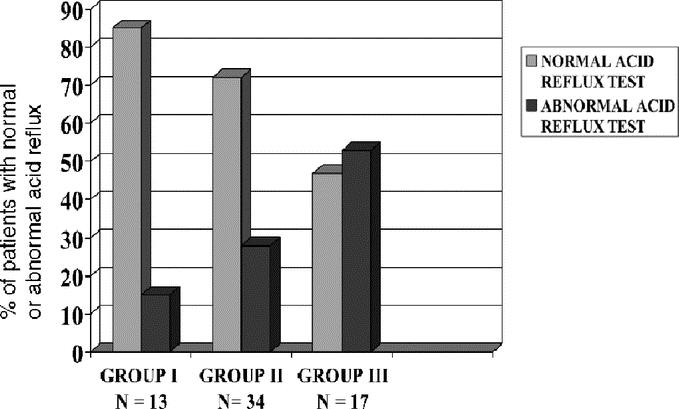
FIGURE 2. Percent of patients with normal or abnormal acid reflux late after esophagomyotomy for achalasia.
Final Results
The final clinical results in all 67 patients submitted to esophagomyotomy are shown in Table 6. Excellent or good results were observed in 73% of all patients at a mean follow-up of 190 months (15.8 years). However, Visick gradation was lower as the follow-up was longer, but without statistical significance. Esophageal carcinoma appeared in 3 patients: 2 of them located at the proximal portion of the esophagus and 1 at the distal portion. All 3 patients had squamous cell carcinoma. This means a rate of 1 patient per 120 patient/year. All of them were operated, but died 12 to 24 months after surgery. Poor or failure results were seen in 22.4% of the patients. It is important to stress that from the 15 patients with poor results, only 1(6.7%) corresponded to a recurrence due to incomplete myotomy, who was reoperated. The other 14 patients (92.3%) had some complications related to severe reflux disease, but only 2 of them were reoperated performing a total duodenal diversion. The rest of them (12 patients) are managed with proton pump inhibitors.
TABLE 6. Final Clinical Results in Patients With Achalasia Submitted to Esophagomyotomy (n = 67)
DISCUSSION
The results of the present investigation have some unique features. They represent the longest follow-up ever reported with the largest number of patients evaluated; endoscopic examination was performed in all cases, taking several biopsies at the GE junction; manometric evaluation of the lower esophageal sphincter and thoracic esophagus was performed before operation and early and very late after surgery in the same patient, and 24-hour pH studies were also performed in all patients to objectively measure abnormal acid reflux into the esophagus.
The results of our study suggest the following: 1) there is a clinical deterioration of initially good clinical results if a very long-term follow-up is performed; 2) late failures are mainly due to an increase in severe pathologic acid reflux; 3) endoscopic esophagitis increases as well as the presence of short- or long-segment Barrett esophagus; 4) several important histologic changes occur at the mucosa just distal to squamous columnar epithelium, with decrease of the presence of fundic mucosa, increase of cardiac mucosa, and progressive appearance of intestinal metaplasia as the follow-up is longer; 5) there is no change of lower esophageal sphincter pressure after surgery, remaining in low value independent of the length of follow-up; and 6) acid reflux measured by 24-hour pH studies shows a parallel increase of abnormal reflux as follow-up is longer.
Our surgical technique has not changed over the last 3 decades, and the only difference is that we are performing this operation by laparoscopic approach since the last 6 years. However, none of the patients included in the investigation has a laparoscopic approach. The main surgical principles are:
To perform an anterior esophagomyotomy 5 to 6 cm long, which should be called properly as Zaaijer's technique27 and not a Heller's procedure,28 which truly is an anterior and posterior esophagomyotomy.
This myotomy should be prolonged at least 10 mm onto the stomach, but not more than 20 mm, because severe reflux may appear in almost all patients.29
To perform an anterior hemifundoplication or Dor's technique.30
The present study suggests that there is a progressive deterioration of initially 95% good results (at 5 years),7,15 due to late development (over 10 years) of disabling reflux disease. The review of the literature of the last over 20 years has shown us that there are only 6 studies31–36 who have reported clinical results over 10 years of follow-up. Chen et al,31 from the group of Dr. Duranceau, reported 32 patients submitted to anterior esophagomyotomy plus a partial Belsey fundoplication, followed for 7.2 years, but 16 of them were followed for 7 to 16 years. In this group, heartburn was present in 13% of patients at the 3-year follow-up and 19% at 7 to 16 years of follow-up. Malthauer et al,32 from the group of Dr. Pearson, reported a very careful clinical and endoscopic follow-up in 22 patients operated by them with a mean of 10 years of follow-up. Visick I and II (excellent and good results) were present in 95% of the patients at 1 year, 77% at 5 years, 68% at 10 years, and 67% at 20 years or more, which is very close to our 65% of results at 23 years. They performed mainly clinical evaluation, while endoscopy was performed in 13 patients. They concluded that there is a deterioration of the good results due to development of severe reflux disease, but only if patients are controlled 15 or 20 years after surgery. Mattioli et al33 compared 3 groups of patients: a) 83 were submitted to long abdominal myotomy alone, with a mean follow-up of 193 months; b) 30 patients were submitted to thoracic myotomy and followed 137 months; and c) 72 patients had a long abdominal myotomy plus a Dor fundoplication, and were followed by for 87 months. Final excellent and good results were seen in only 36% of group a and 44% of group b, while group c had 87% of good results. Probably these results mainly reflect the differences in the long-term follow-up (193 versus 87 months) more than the surgical technique itself. Liu et al34 reported on 145 patients submitted to anterior Heller (Zaaijer) myotomy plus a Belsey antireflux technique compared with myotomy alone. They concluded that early improvement is seen in 98.4%, while at the late control clinical improvement is only present in 55.6%, showing a progressive deterioration of the operated patients with time, due to incomplete myotomy, fused or healed myotomy, and gastroesophageal reflux. Ellis et al, from England,35,36 reported in 2 papers almost the same patients and results. They followed 57 patients with a long-term follow-up, including 67% of them for a mean follow-up of 13.6 years. Excellent and good results were reported on 68% of the patients, a value almost identical to that reported by Malthauer et al32 and by us in the present study. The incidence of clinical reflux reported by them was low (5%), but no endoscopic evaluation was performed. Finally, Ellis,36 from the United States, reported 67 patients followed for 10 years. Excellent and good results were seen in 63% while fair and poor results were present in 37%, similar to our values. As a conclusion of these publications, we can presume that 20 years after esophagomyotomy plus some partial antireflux surgery, two thirds to three fourths of patients may have excellent or good responses, while one third to one fourth present poor results or failure due mainly to severe reflux disease. In our study, only 1(1.5%) presented an incomplete myotomy, while the rest of patients with failure had severe reflux disease.
Endoscopic studies have been very scarcely reported by the authors who mention a late follow-up. Chen et al31 reported that erosive esophagitis was present in no patient at 1 year, while it was seen in 14% 7 to 16 years after surgery. Barrett esophagus appeared in 1 case. Malthauer et al32 performed endoscopy in 13 patients from the 22 followed at late control. In 10 symptomatic patients, 7 had ulcerative esophagitis and 3 a Barrett esophagus, while among 3 asymptomatic patients, all 3 had erosive esophagitis. Mattioli et al33 reported erosive esophagitis in 22% of patients followed 193 months and in 10% among patients followed 87 months. No mention to Barrett esophagus is done. The other authors do not mention endoscopic results. In our present investigation, we have observed that a normal upper endoscopy was present in 85% of patients followed for 10 years, a value that decreased to 76% when the follow-up was 20 years and to 53% when the follow-up was more than 20 years, with progressive appearance of erosive esophagitis and peptic ulcer of the esophagus in short or long columnar lined mucosa of the distal esophagus and Barrett esophagus. In none of the cited reports is histologic analysis of the mucosa at the GE junction and presence of Helicobacter pylori mentioned. Therefore, this is the unique report concerning this topic.
The present study could be considered as a “clinical experiment” concerning the development of histologic changes at the mucosa just distal to squamous-columnar junction due to the presence of increasing acid reflux, very similar to what the group of Dr. DeMeester has shown in patients with GERD.37 We obviously do not have any biopsy sample before operation, but it is improbable that severe histologic abnormalities could be present at that time. The beginning of progressive histologic changes can be clearly seen among patients with a short follow-up (<10 years after surgery) in whom fundic mucosa is mainly present, with less proportion of cardiac mucosa and intestinal metaplasia in only 7%. As the follow-up is longer, and parallel to the increase of reflux disease, fundic mucosa is replaced by cardial mucosa and intestinal metaplasia increases, always in cardiac mucosa. These histologic findings suggest a progressive metaplastic change from fundic mucosa to cardial mucosa and then to intestinal metaplasia and seem to confirm the hypothesis of Dr. DeMeester. The reports concerning measurements of lower esophageal sphincter pressure have shown that, at 5 to 7 years after surgery, a decreased sphincter pressure is maintained5,15,16,18 and usually is reduced to an average of 10 mm Hg.7,15 None of the authors reporting very late results after surgery has mentioned measurements of LES, except the group of Dr. Duranceau31 who performed systematic determinations of lower esophageal sphincter pressure. The mean resting lower esophageal sphincter pressure decreased from 36 mm Hg to 22 and 23 mm Hg at 7 and 16 years of follow-up, which is higher than the value that we have found in all our patients. However, measuring LES gradient pressure, they observed a decrease from 29 to 9 to 10 mm Hg. Also, they demonstrated an improvement in sphincter relaxation, exactly as our results. In our patients (Fig. 1), resting lower esophageal sphincter pressure decreases significantly 1 year after surgery, and this pressure (mean, 9 mm Hg) is maintained stable up to 23 years after surgery, suggesting at least a constant partial barrier against reflux.
Gastroesophageal reflux in the most common and important complication in patients with achalasia submitted to esophagomyotomy. Only a few papers have evaluated the presence of reflux 5 to 7 years after surgery15,16,18,38 noting 10% to 20% of acid reflux present, measured by objective evaluation. A classic review20 of the literature revealed that postoperative reflux was present in 7.4% of patients who also had an antireflux procedure compared with 13% of reflux in patients with esophagomyotomy alone. However, the methods were different, and few random patients were evaluated and in different periods of time after surgery (usually in early periods). From the authors who reported results after 10 years of follow-up,31–36 only the group of Dr. Duranceau31 has performed systematic pH evaluation. Other authors32,33 measured reflux only by symptoms or endoscopic findings, reporting “massive postoperative reflux” or “severe reflux.” On the contrary, Ellis et al35,36 reported only 5% of “reflux” based on clinical grounds. The findings reported by Chen et al31 are disturbing; they found 0% of reflux before and in several controls late after surgery, which suggests complete absence of reflux despite the decrease of sphincter pressure, appearance of erosive esophagitis in 14%, and Barrett esophagus in 1 case.
The return or not of peristaltic activity in the thoracic esophagus has been a matter of debate. Chen et al31 reported return of peristalsis in some patients in the proximal esophagus, while the distal esophagus remained aperistaltic. Although some authors have reported return of esophageal peristalsis after surgery,39–41 especially among patients with short clinical evolution and mild esophageal dilatation, in our very late follow-up, return to partial peristaltic activity was seen in only 15% of the patients, while the great majority remained with a complete aperistalsis of the distal esophagus.
CONCLUSION
The present study is the longest follow-up ever reported in patients with achalasia of the esophagus submitted to esophagomyotomy and Dor's anterior fundoplication. Late evaluation (up to 30 years after surgery) by several objective parameters (endoscopy, histologic analysis, computerized manometry, and 24-hour pH study) has shown that 73% of the patients have excellent or good results, while 4.5% developed a squamous cell esophageal carcinoma and 21.4% had poor results or failure, mainly due to the presence of severe reflux disease, with progressive appearance of erosive esophagitis and Barrett esophagus with intestinal metaplasia.
Footnotes
Reprints: Attila Csendes, FACS, Department of Surgery, Clinical Hospital University of Chile, Santos Dumont #999, Santiago, Chile. E-mail: acsendes@machi.med.uchile.cl.
REFERENCES
- 1.Reynolds JC, Parkman HP. Achalasia. Gastroenterol Clin North Am. 1989;18:223–255. [PubMed] [Google Scholar]
- 2.Csendes A, Larraín A, Ayala M. Motility studies in 50 patients with Achalasia of the esophagus. Am J Gastroenterol. 1974;62:333–336. [PubMed] [Google Scholar]
- 3.Cohen S, Fisher R, Trich A. The site of denervation in achalasia. Gut. 1972;13:556–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csendes A, Smok G, Braghetto I, et al. Histological studies of Auerbach's plexuses of the oesophagus stomach, jejunum and color in patients with achalasia of the oesophagus: correlation with gastric acid secretion, presence of parietal cells and gastric emptying of solids. Gut. 1992;33:150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csendes A, Velasco N, Braghetto I, et al. A prospective randomized study comparing forceful dilatation and esophagomyotomy in patients with achalasia of the esophagus. Gastroenterology. 1981;80:789–795. [PubMed] [Google Scholar]
- 6.Csendes A, Braghetto I, Henríquez A, et al. Late results of a prospective randomized study comparing forceful dilatation and oesophagomyotomy in patients with achalasia. Gut. 1989;30:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csendes A. Results of surgical treatment of achalasia of the esophagus. Hepatogastroenterology. 1991;38:479–480. [PubMed] [Google Scholar]
- 8.Csendes A, Braghetto I, Burdiles P, et al. Comparison of forceful dilatation and esophagomyotomy in patients with achalasia of the esophagus. Hepatogastroenterology. 1991;38:502–505. [PubMed] [Google Scholar]
- 9.Okike N, Payne S, Neufeld DM, et al. Esophagomyotomy versus forceful dilatation for achalasia of the esophagus: results in 899 patients. Ann Thorac Surg. 1979;28:119–125. [DOI] [PubMed] [Google Scholar]
- 10.Hunter JG, Richardson WS. Surgical management of achalasia. Surg Clin North Am. 1997;75:993–1017. [DOI] [PubMed] [Google Scholar]
- 11.Yamanura MS, Gibster JC, Meyers BS, et al. Laparoscopic Heller myotomy and anterior fundoplication for achalasia results in a high degree of patients satisfaction. Arch Surg. 2000;135:902–906. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Meir A, Lubach DR, Khajanchee YS, et al. Quality of life before and after laparoscopic Heller myotomy for achalasia. Am J Surg. 2001;181:471–474. [DOI] [PubMed] [Google Scholar]
- 13.Patti MG, Pellegrini CA, Horgan S, et al. Minimally invasive surgery for achalasia: an 8 year experience with 168 patients. Ann Surg. 1999;230:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Csendes A, Burdiles P, Korn O, et al. Laparoscopic esophagomyotomy for achalasia: preliminary results in 19 patients. Rev Méd Chile. 2001;129:1142–1146. [PubMed] [Google Scholar]
- 15.Csendes A, Braghetto I, Mascaro J, et al. Late subjective and objective evaluation of the results of esophagomyotomy in 100 patients with achalasia of the esophagus. Surgery. 1988;104:469–475. [PubMed] [Google Scholar]
- 16.Bonavina L, Mosadini A, Bardini R, et al. Primary treatment of esophageal achalasia: long term results of myotomy and Dor fundoplication. Arch Surg. 1992;127:222–226. [DOI] [PubMed] [Google Scholar]
- 17.Pichiocchi A, Cardillo G, Dugo D, et al. Surgical treatment of achalasia: a retrospective comparative study. Surg Today. 1993;23:855–859. [DOI] [PubMed] [Google Scholar]
- 18.Parrilla P, Martinez de Haro L, Ortiz A, et al. Achalasia of the cardia: long term results of oesophagomyotomy and posterior partial fundoplication. Br J Surg. 1990;77:1371–1374. [DOI] [PubMed] [Google Scholar]
- 19.Torbey CF, Achkar E, Rice TW, et al. Long term outcome of achalasia treatment: the need for closer follow up. J Clin Gastroenterol. 1999;28:25–30. [DOI] [PubMed] [Google Scholar]
- 20.Andreollo NA, Earlaim JH. Heller's myotomy for achalasia: is an added antireflux procedure necessary? Br J Surg. 1987;74:765–769. [DOI] [PubMed] [Google Scholar]
- 21.Zaninotto G, DeMeester TR, Seenger W, et al. The lower esophageal sphincter in health and disease. Am J Surg. 1988;155:104–111. [DOI] [PubMed] [Google Scholar]
- 22.Csendes A, Braghetto I, Burdiles P, et al. A new physiological approach for the surgical treatment of patients with Barrett's esophagus. Ann Surg. 1997;226:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csendes A, Braghetto I, Maluenda F, et al. Peptic ulcer of the esophagus secondary to reflux esophagitis: clinical, radiological, endoscopic, histologic, manometric and isotopic studies in 127 patients. Gullet. 1991;1:177–189. [Google Scholar]
- 24.Csendes A, Maluenda F, Braghetto I, et al. Location of the lower esophageal sphincter and the squamous columnar mucosal junction in 109 healthy controls and 778 patients with different degrees of endoscopic esophagitis. Gut. 1993;34:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeMeester TR, Wang CI, Wernly JA. Technique indications and clinical use of 24 hour intraesophageal pH monitoring. J Thorac Cardiovasc Surg. 1980;79:656–670. [PubMed] [Google Scholar]
- 26.Csendes A, Alvarez F, Burdiles P, et al. Magnitude of gastroesophageal reflux measured by 24-hour esophageal pH monitoring according to the degree of endoscopic esophagitis. Rev Med Chile. 1994;122:59–67. [PubMed] [Google Scholar]
- 27.Zaaijer JH. Cardiospasm in the aged. Ann Surg. 1923;77:615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heller F. Extramukose kardiaplastik bein chronischen kardiospasms nut dilatation des oesophagus. Mett Grensgeb Med Chir. 1914;27:141–129. [Google Scholar]
- 29.Jara FM, Toledo-Pereyra LH, Lewis JW, et al. Long term results of esophagomyotomy for achalasia of esophagus. Arch Surg. 1979;114:935–936. [DOI] [PubMed] [Google Scholar]
- 30.Dor J, Humbert P, Paali JM, et al. Traitment du reflux par la technique dite de Heller-Nissen modifiee. Presse Med. 1967;75:256–265. [PubMed] [Google Scholar]
- 31.Chen LQ, Chugtai T, Sideris L, et al. Long term effects of myotomy and partial fundoplication for esophageal achalasia. Dis Esophagus. 2002;15:171–179. [DOI] [PubMed] [Google Scholar]
- 32.Malthauer RA, Todd TR, Miller L, et al. Long term results in surgically managed esophageal achalasia. Ann Thorac Surg. 1994;58:1343–1347. [DOI] [PubMed] [Google Scholar]
- 33.Mattioli S, Di Simone MP, Bassi F, et al. Surgery for esophageal achalasia: long term results with three different techniques. Hepatogastroenterology. 1996;43:492–500. [PubMed] [Google Scholar]
- 34.Liu HC, Huang BS, Hsu WH, et al. Surgery for achalasia: long term results in operated achalasic patients. Ann Thorac Cardiac Surg (Jpn). 1998;4:12–20. [PubMed] [Google Scholar]
- 35.Ellis FH, Watkins E Jr, Gibb S, et al. Ten to 20 year clinical results after short esophagomyotomy without an antireflux procedure (modified Heller operation) for esophageal achalasia. Eur J Cardiothorac Surg. 1992;6:86–90. [DOI] [PubMed] [Google Scholar]
- 36.Ellis FHJ. Oesophagomyotomy for achalasia: a 22 year experience. Br J Surg. 1993;80:882–885; discussion 1346–1347. [DOI] [PubMed]
- 37.Oberg S, Peters JH, DeMeester TR, et al. Inflammation and metaplasia of the cardia are manifestations of gastroesophageal reflux disease. Ann Surg. 1997;226:522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Little AG, Soviano A, Fergusson MK, et al. Surgical treatment of achalasia results with esophagomyotomy and Belsey repair. Ann Thorac Surg. 1988;45:489–494. [DOI] [PubMed] [Google Scholar]
- 39.Mellow MH. Return of esophageal peristalsis in idiopathic achalasia. Gastroenterology. 1976;70:1148–1151. [PubMed] [Google Scholar]
- 40.Ponce J, Miralles M, Ganiques V, et al. Return of esophageal peristalsis after Heller's myotomy for idiopathic achalasia. Dig Dis Sci. 1986;31:545–547. [DOI] [PubMed] [Google Scholar]
- 41.Parrilla P, Martínez de Haro F, Ortiz A, et al. Factors involved in the return of peristalsis in patients with achalasia of the cardia after Heller's myotomy. Am J Gastroenterol. 1995;90:713–717. [PubMed] [Google Scholar]