Abstract
Objective:
To evaluate the predictive factors, the therapy, and the prognosis of intrahepatic recurrence (IR) after surgery for hepatocellular carcinoma (HCC).
Summary Background Data:
The predictive factors of IR are debated. To class the recurrence according to the modality of presentation may help to find a correlation and to select the right therapy for the recurrence.
Methods:
A total of 213 patients were evaluated. Risk factors for recurrence were related to time (<2 years and >2 years) and type of presentation (marginal, nodular, and diffuse). Prognosis and therapy for the recurrence were studied in each group of patients.
Results:
IR was observed in 143 patients; 109 were early (group 1) and 34 late recurrences (group 2). Cirrhosis, chronic active hepatitis (CAH) and HCV positivity were independently related to the risk of recurrence with a cumulative effect (92.5% of recurrences in patients with 3 prognostic factors). For group 1, the neoplastic vascular infiltration together with cirrhosis, HCV positivity, CAH, and transaminases were significant; all the 11 patients with 5 negative prognostic factors showed an early recurrence. On the contrary, only cirrhosis was related to a late recurrence. Survival rate was significantly better in late than in early recurrence (61.9%, 27.1% and 25.7%, 4.5% at 3–5 years); a curative procedure was performed in 67.6% in group 1 and 29.3% in group 2. After a radical treatment of IR, the survival was comparable with the group of patients without recurrence.
Conclusions:
Early and late recurrences are linked to different predictive factors. The modality of presentation of the recurrence together with the feasibility of a radical treatment are the best determinants for the prognosis.
Predictive factors for hepatic recurrence after surgery for hepatocellular carcinoma are not primary related to the aggressiveness of cancer. Time and the type of presentation of the recurrence are the best determinants of the prognosis. When treated with a curative procedure, the survival of the patients who recurred is quite satisfying.
Hepatic resection is a well-accepted therapy for hepatocellular carcinoma (HCC), but many patients develop a cancer recurrence, which is the main cause of death in long-term evaluations.1–3 Prevention and therapy for recurrence could further improve the data of survival and support the value of surgery when compared to non surgical procedures such as percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA), or to liver transplantation (OLT).
The identification of the predictive factors of recurrence is the first step of this process. Different from other common gastrointestinal tumors, the pathologic features related to cancer presentation are not always related to the final results, expressed as overall survival (OS) and disease-free survival (DFS).4–7 This is not surprising; the recurrence of HCC includes some entities that are different for pathogenesis and clinical value, such as intrahepatic metastases of primary HCC or metachronous primary lesions.
Another factor to be considered is the time of presentation of recurrence; generally, shorter is the free interval time, poorer is the prognosis, but a clear line between early and late recurrences cannot be driven “a priori.”7
The aim of this study is: 1) to identify the factors influencing the risk and the type of recurrence and to verify if the type of presentation of the recurrence, including the morphology and the free interval time can identify groups of patients with a homogeneous behavior; and 2) to foresee the results that can be expected from an aggressive radical treatment of the different type of recurrence.
MATERIALS AND METHODS
Patients, Surgery, and Follow-up
Between June 1986 and December 2003, 264 patients underwent liver resection for HCC at the Surgical Clinic of the University of Brescia, Italy. Sixteen cirrhotic patients died after surgery (in-hospital mortality, 6%). Seven patients who underwent a noncurative resection and 28 patients with less than 1 year follow-up were excluded.
The remaining 213 patients are the object of this study; 179 patients were males and 34 females; the median age was 64.6 years (range, 34–83 years). Liver cirrhosis was present in 132 cases; a chronic hepatitis, classified as chronic aggressive hepatitis (CAH) or chronic persistent hepatitis (CPH),8 was documented respectively in 38 and 6 patients, respectively, this last group being considered together with 37 patients who presented an undamaged liver.
We performed 28 major resections (≥3 segments according to Couinaud classification)9 and 185 minor resections, including 82 limited resections (<1 segment) in cirrhotic patients.
After surgery, 8 patients underwent adjuvant chemotherapy. No patients were lost during follow-up (median, 39.5 months; range, 12–175 months).
Liver recurrence was classed as early (group 1) and late (group 2) using 24 months as cutoff.
With regard to the type of recurrence, we considered as marginal the recurrence located near the resection margin (type 1), as nodular the single or double lesion located more than 3 cm from the surgical scar (type 2), and as multinodular or diffuse the recurrence consisting of many nodules scattered throughout the remaining liver or in an infiltrative lesion (type 3).
Risk Factors for Outcome
Prognostic factors for OS and DFS were evaluated among those related to the host, tumor, and treatment. With regard to the host, we considered sex, age (under or over 65 years), liver state (cirrhosis, CAH and “normal” liver), positivity for HCV and HBV, liver function (Child A and B), and transaminases value, using twice the normal value as cutoff.
Of the tumor-related factors, we studied the number of nodules (1 or more), the diameter (more or less than 5 cm), the histologic differentiation (well, moderately, and poorly differentiated), and the presence or absence of capsule, satellitosis, and microscopic and macroscopic vascular infiltration. In relation to the treatment, we considered the extent (major and minor) and the type of hepatic resection (anatomic and nonanatomic), the width of the surgical free margin (more or less than 10 mm), and the preoperative transarterial chemotherapeutic embolization (TACE) (yes and no).
The whole group of recurrent patients and then the subgroups of early and late recurrences were compared with the nonrecurrent patients, taking these 20 parameters into account. As regards the type of recurrence, the same prognostic factors were considered to compare marginal, nodular, and multinodular recurrences.
Therapy Analysis and Prognosis of Hepatic Recurrence
When liver recurrence was diagnosed, the therapeutic strategy was evaluated regardless of the time and type of recurrence, according to the general rules for treatment of HCC. Briefly, any lesion was considered for surgery if a low-risk curative resection was possible in a Child A patient. Of these situations, PEI and RFA were both considered as curative therapies; TACE was considered as a palliative procedure. Global survival and DFS were evaluated, starting from either the time of primary surgery or the diagnosis of recurrence and referring to the time and type of recurrence.
Statistical Analysis
All the data were recorded on a computed database. Statistically significant differences were analyzed by the χ2 test for categoric variables and the Student t test for continuous variables. All the significant predictors of recurrence in the univariate analysis were analyzed in a logistic regression model to show an independent value at the multivariate analysis. The Cox model with the determination of the hazard ratio was applied to evaluate the risk connected with the prognostic variables, assessed as single and in association; a 95% confidence interval was adopted. The prognostic factors were studied using a Kaplan-Meier method with a log-rank test to detect the statistical differences in the computed survival and disease-free rates. To identify the cutoff between early and late recurrence, we first evaluated the distribution in time of recurrence and then divided the 2 periods according to the variation of the slope of the curves identified with 2 linear regression lines; the function of the 2 lines, which represents the occurrence of recurrence in the first and the subsequent period of follow-up, were, respectively, y = 105.48 − 2.75x and y = 49.08 − 0.33x. The cutoff to separate early and late recurrence was stated at the intercept value of the 2 curves (Fig. 1).

FIGURE 1. Hepatic resection for HCC. Distribution in time of intrahepatic recurrences (disease free survival) and classification of early and late recurrences.
The statistical analysis was made using SPSS 9 Windows (SPSS Inc., Chicago, IL). A value of P < 0.05 was considered as significant.
RESULTS
Incidence and Characteristics of Cancer Recurrence
At the time of the final evaluation, December 2003, 120 patients were died. The 1-, 3-, and 5-year cumulative global survival was 87.5%, 61.1%, and 41.9%, respectively. In 99 patients (82.5%), the cause of death was a neoplastic recurrence; 11 patients died of liver failure or complications of portal hypertension with (2 patients) or without (9 patients) recurrence while 10 died for non-liver-related causes. Fifty-four patients are living with cancer recurrence; so, as a whole, 155 patients suffered of cancer relapse with a global recurrence rate of 72.7% and a DFS rate of 68.0%, 33.6%, and 26.3%, respectively at 1, 3, and 5 years.
In 143 of 155 cases (92.2%), the recurrence was in the remnant liver, 8 patients presented both a hepatic and extrahepatic recurrence, and 4 just an extrahepatic recurrence; these last 12 patients were excluded from any further analysis of survival.
Among the 143 patients, 109 (76.2%) recurred in the first 24-month period (group 1: early recurrence) and 34 (23.8%) after 24 months (group 2: late recurrence); group 3 comprises the 58 patients without recurrence.
Regarding the type, a marginal recurrence (type 1) was observed in 12 cases (8.4%), a nodular one (type 2) in 52 (36.3%) and a multinodular-diffuse (type 3) in 79 patients (55.3%). The distribution in time of the different lesions shows a significant correlation, types 1 and 3 being diagnosed mainly as early recurrence (respectively, in 83.3% and 88.8%), whereas type 2 recurrences were scattered during the follow-up (55.7% early and 44.3% late).
Predictive Factors of Cancer Survival and Recurrence
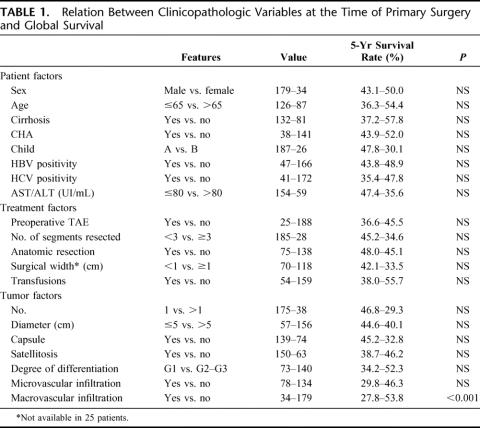
At the univariate analysis, the only factor related to a poor overall survival was the macroscopic vascular infiltration, present in 16% of cases. When 5-year recurrence rate was considered, the cirrhosis, the CAH and the HCV positivity showed a prognostic value at the univariate and multivariate analysis (Tables 1, 2). When none of these 3 factors was present, the rate of recurrence was 21.6%; and when liver resection was performed in a HCV-positive cirrhotic patient with CAH, the incidence of recurrence was 92.5% (Table 3).
TABLE 1. Relation Between Clinicopathologic Variables at the Time of Primary Surgery and Global Survival
TABLE 2. Relation Between Clinicopathologic Variables at the Time of Primary Surgery and 5 Years

TABLE 3. Cumulative Effect of Prognostic Factors on the Relative Risk of Recurrence

When groups 1 and 2 were compared separately to the patients without recurrence, only the presence of cirrhosis was significantly related both to early and late recurrence at the same time. For group 1, the following resulted significant at the univariate (Table 4) and multivariate analysis: macroscopic and microscopic vascular infiltration, transaminases values, HCV positivity, cirrhosis, and CAH. The incidence of early recurrence was very low (4.2%) when none of these prognostic factors was present (1 recurrence in 24 patients) and increased to 22.4% when a single factor was present (11 recurrences in 49 patients); this rate reached 48.9%, 66.0%, and 87.9% when, respectively, 2, 3, and 4 factors were present at the same time. Any difference between 0 factor and the other risk groups is significant (P < 0.001). All the 14 patients presenting 5 or 6 negative prognostic factors had an early recurrence.
TABLE 4. Relation Between Clinicopathologic Variables and Recurrence Rate According to Time (< or >2 yr) of Recurrence Presentation
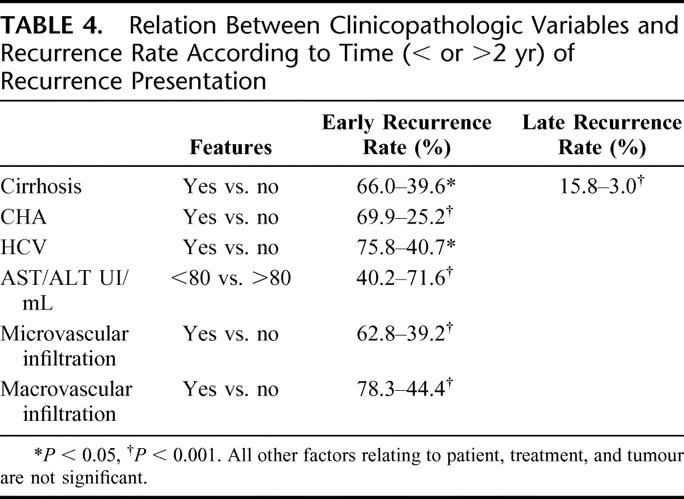
On the contrary, cirrhosis was the only risk factor for late recurrence. Regarding the type of recurrence, no factor was linked to a marginal recurrence while the multinodular primary cancer was statistically related to a nodular recurrence. Macroscopic and microscopic vascular infiltration and multinodular primary cancer were related to a multinodular recurrence at the univariate analysis; at the multivariate evaluation, only macroscopic (P < 0.05) and microscopic vascular infiltration (P < 0.001) maintained a statistic value.
Therapy and Prognosis After Recurrence
As stated above, cancer recurrence was the main reason of death after surgery. In the group of patients who did not show recurrence, global survival at 1, 3, and 5 years was 89%, 75.3%, and 72.9%, respectively, significantly better than the corresponding recurrence group (87.2.0%, 53.8%, and 30.9%) (P < 0.001). Considering survival from time when recurrence was ascertained, these last percentages were, respectively, 67.9%, 36.1%, and 12.8%. A significant gain was observed in patients with late (81.5%, 61.9%, and 27.1% at 1, 3, and 5 years) when compared with the early recurrences (63.7%, 25.7%, and 4.5%) (P < 0.0001) (Fig. 2).
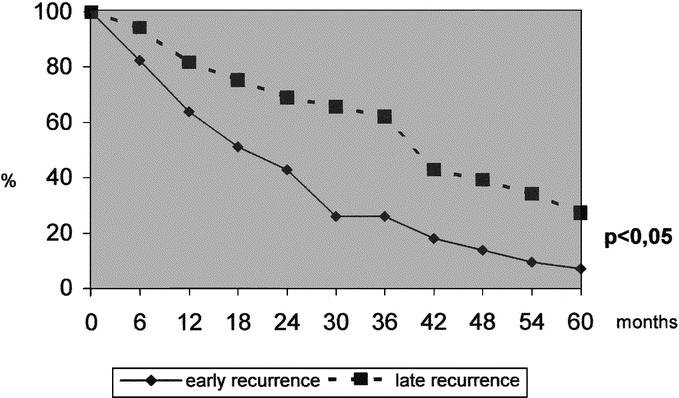
FIGURE 2. Long-term survival from the diagnosis of intrahepatic recurrence according to time of presentation (earlier or later than 24 months).
In the presence of an early recurrence, a curative treatment was feasible in 32 cases (29.3%), including iterative surgery (15 cases), PEI (13 cases), and RFA (4 patients); when facing a late recurrence, it was possible in 23 cases (67.6%, P < 0.05), including surgery (6 cases), PEI (15 cases), and RFA (2 cases).
After curative treatment of the recurrence, the global survival was 94.1%, 65.3%, and 40.4% at 1, 3, and 5 years, respectively, without any difference among the different therapeutic procedures; when accomplished from the time of primary resection, the data of survival (98.1%, 81.9%, 64.3%) were completely comparable to the 58 patients who never recurred (Fig. 3). A trend toward a better survival was noted in late recurrences (95.2%, 75.1%, and 43.8% at 1, 3, and 5 years) compared with the early recurrences (93.3%, 61.3%, and 23.3%) (not significant); DFS at 1 year was significantly higher in the late recurrences (94.4% and 59.3% versus 75.5% and 30%) (P < 0.05).
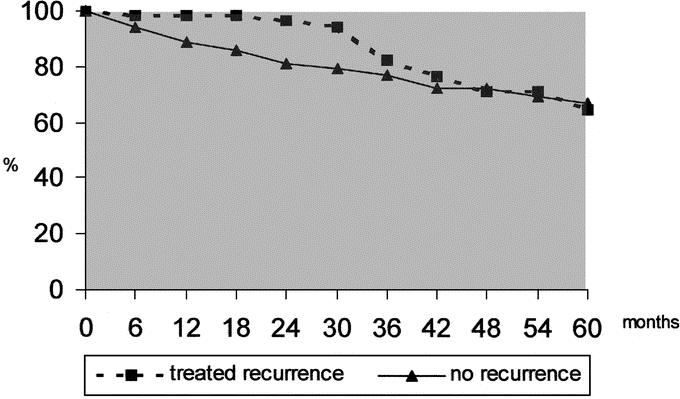
FIGURE 3. Survival from the primary resection of the patients without recurrence and of the patients with intrahepatic recurrence treated with radical purpose.
In the group of patients submitted to palliation, the survival rate was 52.2%, 17.4%, and 0%; the results were better after TACE (77.7%, 35.0%, and 0%) than after a supportive therapy (33,6%, 5%, and 0%) (P < 0.0001).
With regard to the type of recurrence, the global survival at 1, 3, and 5 years was, respectively, 91.6%, 55.0%, and 41.2% for type 1 recurrence, 91.8%, 62.5%, and 20.8% for type 2 (not significant), and 49.3%, 13.5%, and 0% for type 3 recurrence, no patient surviving more than 48 months (P < 0.0001). A curative treatment was feasible in 10 of 12 marginal recurrences (83.3%), in 43 of 52 nodular recurrences (82.7%), and only in 2 patients (2.5%) with a multinodular-diffuse recurrence.
When time and type of recurrence were considered together, the best prognosis was observed in 30 patients with a late nodular recurrence; a curative treatment was feasible in 91.3% of cases and 3-year survival was 68.6%. On the contrary, the lowest survival was in presence of an early multinodular recurrence; in these patients, the median survival was 10 months.
In the group of 21 patients submitted to 28 iterative resection (3 patients were submitted to a third, 2 to a fourth hepatic resection), a limited curative resection was performed in every case. No patient died in the postoperative period. The median survival was 40 months after iterative surgery.
DISCUSSION
Cancer recurrence, generally in the hepatic remnant, occurs in 70% to 100% of cases after resective surgery for HCC.1,2,10 In our experience, this was the cause of death in 82.5% of the patients while only 9% of deaths were due to hepatic failure. Consequently, the analysis of the factors carrying a high risk of recurrence may improve the selection of the patients for surgery, reserving the poorest for other less invasive treatments.
Factors concerning the patient, the condition of the extranodular liver parenchyma, the tumor, and the treatment of the neoplastic disease are considered in the literature.1,2,11,12 In many experiences, the parameters linked to a high risk of recurrence do not have the same significance in the prediction of a poor prognosis.3,13 In our experience, among the cancer-related factors only, the vascular involvement of a major portal branch has a negative prognostic value; this feature can be considered a limit to the indication or a relative contraindication to resective surgery. Looking for other usual factors, no single feature is clearly related to long-term survival, apart the recurrence itself. The presence of cirrhosis, a CAH, and the seropositivity for HCV are correlated with a high risk of recurrence. Every predictive factor increases the relative efficacy of the others; the rate of recurrence increases up to 92.5% when all 3 are present in the same patient.
The lack of coincidence among factors conditioning recurrence and survival corresponds to the evidence that long-term survival is possible after recurrence: only 13 of 47 5-year survivors after surgery did not experience a radical treatment of the recurrence during follow-up (data not shown). The recurrence limits the probability of survival, but a group of patients survives after recurrence; so it is important to assess, together with the factors predictive for recurrence, those factors conditioning the prognosis of the patients presenting recurrence.
The extension and the type of resection do not show any correlation with the risk of recurrence provided that surgery is radical. The same consideration arises from other surgical experiences; the low rate of marginal recurrence supports this conclusion.2,14
As in our other experiences, no single macroscopic factor related to the tumor has been clearly assessed as negative regarding the possibility of liver recurrence.2,15–18 This consideration primary reflects a bias in the assessment of the recurrences, which do not have to be considered as a single entity but have to be divided in subgroups with the same pathogenesis and biologic significance.
With this aim, we decided to evaluate the significance of 2 aspects easy to assess and of proven validity: the first is time of recurrence, as primarily stated by Poon et al7: the second, the site and type of recurrence (marginal, dodular, multinodular-diffuse) as proposed by Matsumata et al.19 We considered these 2 aspects separately and in association to assess the curability and the prognosis of the recurrent patients.
Time is an important prognostic factor7,18; generally, 1 year is chosen as an arbitrary time cutoff. Looking at the curve designed by the distribution in time of the events, we decided to assume 24 months as the time limit to separate the early from the late recurrences; after this time, the risk of recurrence falls and remains low for the following years. The value of this cutoff in our series of cases is confirmed by the different characteristics of the 2 groups of recurrences relating to prognostic factors, morphologic aspects, clinical course, and prognosis. The early recurrences are more often diffuse, rarely treatable, with an unsatisfactory long-term survival. Besides the predictive factors for recurrences as a whole, this type of recurrence is directly related to the neoplastic vascular infiltration, both at the macroscopic and microscopic level. When all these factors are present in association, any patient shows an early recurrence; on the contrary, the risk of recurrence is 4% and 22% when no factor or one factor is present, thus selecting a group of patients (73 of 213, 34% of our patients) with an acceptable low risk (16.4%) of an early recurrence.
Vascular infiltration is well related to the worse type of recurrence, the multinodular-diffuse, which must be considered, in our opinion, as metastases.20 This ominous type of recurrence is early in almost 90% of cases and is rarely treatable, thus being the main responsible for the low curability (29.3%) and the poor long-term survival ascribed to the early when compared with the late recurrences.
On the other side, the nodular and marginal recurrences are not clearly related to the aggressiveness of the carcinoma. These types of recurrences are suitable for a curative procedure, including surgery, PEI, and RFA, in more than 80% of cases; no difference in survival was noted relating to the different treatment employed.
The results of the radical treatment of the recurrence are quite good; if we look at the time of primary surgery, the 5-year survival rate in 55 cases of cured recurrences was 64.3%, not significantly different from 72.9% observed in the 58 patients who never recurred. A similar result was reported by only 2 published studies from eastern surgical groups, but these experiences reserved the comparison to the small group of patients submitted to an iterative resection (18 and 11 patients, respectively), thus limiting the statistic validity of the comparison with the larger group of nonrecurrent patients (38 and 139 patients).6,21
The risk deriving from this aggressive attitude in the presence of an intrahepatic recurrence, if applied in selected patients, is quite low; in our experience, including 28 resections for recurrence; the mortality was nil, as in the near totality of the series reported in the literature.15 The right selection of cases and the wide application of a limited resection as the best surgical approach are the main conditions to be respected for a good outcome.
CONCLUSION
Our study suggests that the most important prognostic factors for recurrence are not linked to the characteristics of the tumors but to the chronic damage of the liver, including the presence of cirrhosis, CAH, and infection of HCV. When none of these factors is present, the results are particularly good; postoperative mortality is nil and the risk of recurrence is about 20%. In our opinion, surgery has to be considered the best therapeutic solution in these cases. On the contrary, the contemporary presence of the risk factors increases the probability of recurrence in an exponential fashion; the recurrence is almost the rule when multiple factors are present in the same patient. Because the majority of these features are known at the time of the clinical assessment of the patient, these elements must be carefully considered in the choice among the different therapies; the indication to percutaneous ablative procedure must not be ruled out “a priori” also in a good surgical patient with multiple prognostic factors of recurrence.
Nonetheless, recurrence must not be considered a definitive failure of the therapeutic program in the patient initially addressed to resective surgery; in almost 40% of cases, recurrence may be treated with a curative intent, and a long survival may result from an aggressive attitude against the recurrent lesion, both with iterative resection and percutaneous ablation. So the relative value of every predictive factor of recurrence must not be considered for the entire group of recurrent patients, each type of recurrence being related to a different feasibility of a curative treatment. Waiting for a prospective randomized trial, the analysis of predictive factors of recurrence after surgery might help with the choice between resection and OLT in Child A patients. Our study is not able to support some other useful elements. Indeed, the ominous type of recurrence, the multinodular one, resulted at high risk after resection of tumors presenting vascular infiltration, which is unfortunately considered a contraindication for OLT.
Time and type of presentation are 2 interdependent prognostic factors in the recurrent patient; together with the possibility of a radical treatment, these factors are the main determinant of the prognosis.
Regardless the timing of appearance, the marginal and nodular recurrences must be considered for a radical treatment, which gives back to the patient the same life expectancy of the patients who never recurred.
Footnotes
Reprints: Stefano Maria Giulini, MD, III Chirurgia, Spedali Civili di Brescia, Piazzale Spedali Civili n° 1, 25123 Brescia, Italy. E-mail: giulini@master.cci.unibs.it.
REFERENCES
- 1.Lise M, Bacchetti S, Da Pian P, et al. Prognostic factors affecting long term outcome after resection for hepatocellular carcinoma. Cancer. 1998;82:1028–1036. [DOI] [PubMed] [Google Scholar]
- 2.Chen MF, Hwang TL, Jeng LB, et al. Postoperative recurrence of hepatocellular carcinoma: two hundred five consecutive patients who underwent hepatic resection in 15 years. Arch Surg. 1994;129:738–742. [DOI] [PubMed] [Google Scholar]
- 3.Poon RTP, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;3:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee WC, Jeng CB, Chen MF. Estimation of prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2002;89:311–316. [DOI] [PubMed] [Google Scholar]
- 5.Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon RTP, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;2:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon RTP, Fan ST, Oi-Lin Ng I, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection for hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 8.De Groote J, Desmet VJ, Gerigk P, et al. A classification of chronic hepatitis. Lancet. 1968;1:626–628. [DOI] [PubMed] [Google Scholar]
- 9.Couinaud C. Le foie: Etudes anatomiques et chirurgicales. Paris: Masson, 1957. [Google Scholar]
- 10.Belghiti J, Panis Y, Farges O, et al. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko S, Nakajima Y, Kaneiro H, et al. Significant influence of accompanying chronic hepatitis status on recurrence of hepatocellular carcinoma after hepatectomy: result of multivariate analysis. Ann Surg. 1996;224:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh CN, Lee WC, Chen MF, et al. Predictors of long-term disease free survival after resection of hepatocellular carcinoma: two decades of experience at Chang Gung Memorial Hospital. Ann Surg Oncol. 2003;10:916–921. [DOI] [PubMed] [Google Scholar]
- 13.Nagasue N, Ono T, Yamanoi A, et al. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg. 2001;88:515–522. [DOI] [PubMed] [Google Scholar]
- 14.Shirabe K, Kanematsu T, Matsumata T, et al. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991;14:802–805. [DOI] [PubMed] [Google Scholar]
- 15.Poon RTP, Fan ST, Wong J, et al. Risk factors, prevention and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itamoto T, Nakahara H, Tashiro H, et al. Indications of partial hepatectomy for transplantable hepatocellular carcinoma with compensated cirrhosis. Am J Surg. 2005;189:167–172. [DOI] [PubMed] [Google Scholar]
- 17.Poon RTP, Fan ST, Oi-Lin Ng I, et al. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimada M, Takenaka K, Gion T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology. 1996;111:720–726. [DOI] [PubMed] [Google Scholar]
- 19.Matsumata T, Kanematsu T, Takenaka T, et al. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology. 1989;9:457–460. [DOI] [PubMed] [Google Scholar]
- 20.Sonoyama T, Ochai T, Hironaka T, et al. Predictors of postoperative diffuse intrahepatic recurrence of hepatocellular carcinoma. Hepatogastroenterology. 2003;50:1078–1084. [PubMed] [Google Scholar]
- 21.Suenaga M, Sugiura H, Kokuba Y, et al. Repeated hepatic resection for recurrent hepatocellular carcinoma in eighteen cases. Surgery. 1994;115:452–457. [PubMed] [Google Scholar]