Abstract
Objective:
The aim of the study was to assess the clinical value of contrast-enhanced intraoperative ultrasound (CE-IOUS) as a novel tool in the hepatic staging of patients undergoing liver resection.
Methods:
Sixty patients scheduled to undergo liver resection for metastatic disease were studied. Preoperative staging with contrast-enhanced CT and/or MR scans was performed within 2 to 6 weeks of operation. Following exploration, intraoperative ultrasound (IOUS) was performed using an HDI-5000 scanner (Philips) and a finger-probe with pulse inversion harmonic (PIH) capability. CE-IOUS in the PIH mode was performed in a standardized protocol (low MI: 0.02–0.04) after intravenous injection of 3–4 mL of SonoVue (Bracco spa, Milan); all detected lesions on precontrast and postcontrast scans were counted and mapped. Any alteration in surgical management was documented following CE-IOUS compared with IOUS.
Results:
Three patients were excluded due to disseminated disease on exploration. CE-IOUS was significantly more sensitive than CT/MR and IOUS in detecting liver metastases (96.1% versus 76.7% and 81.5%, respectively) (P < 0.05); it altered surgical management in 29.8% (17 of 57) of cases, due to 1) additional metastases in 19.3% (11 of 57), 2) less metastases in 3.5% (2 of 57), 3) benign lesions wrongly diagnosed as metastasis on IOUS/CT in 5.3% (3 of 57), and 4) vascular proximity in 1.8% (1 of 57). Management was unchanged in 70.2% (40 of 57) despite additional lesions detected in 3.5% (2 of 57) and benign lesion wrongly diagnosed on IOUS and CT as metastasis in 1.8% (1 of 57). CE-IOUS altered combined IOUS/CT/MR staging in 35.1%.
Conclusion:
These preliminary results suggest CE-IOUS is an essential tool prior to liver resection for metastases.
Preliminary results suggest contrast-enhanced intraoperative ultrasound should be the gold standard staging tool prior to surgical resection of hepatic metastases with significant implications on surgical management at time of operation. Future long-term outcome studies will determine its true value in clinical practice.
Colorectal cancer is the second commonest cause of death from cancer in the Western World, accounting for 14% and 16% of cancer deaths in men and women, respectively; approximately 25% of patients have liver involvement at the time of initial presentation and up to 50% will develop hepatic metastases during the course of their disease.1,2 Surgical resection is the treatment of choice in patients with hepatic metastases, and it is sometimes combined with other physical ablative techniques; following liver resection, the 5-year survival rates vary from 25% to 40%.3,4 A total of 75% of those who undergo liver resection will develop recurrence and, of these, the liver is involved in 50%; 65% to 85% of all recurrences appear within the first 2 years.4 Results of growth rate studies of hepatic metastases would support the hypothesis that these metastases had been present at the time of liver resection but remained “occult” ie, undetected by intraoperative ultrasound (IOUS), CT, and/or MRI scans.5
Traditionally, contrast enhanced (CE) CT and MRI have been used to stage metastatic hepatic colorectal disease. IOUS has been shown to yield significant new information, not identified on preoperative imaging, which determines resectability or changes the operative plan in up to 50% of patients; it is considered the gold standard, thereby achieving universal usage.6–9 However, of those who develop recurrence following apparently curative liver resection, the 50% hepatic recurrence rate underlines the limitation of IOUS itself; clearly, small lesions would easily be missed if they had acoustic characteristics similar to those of the adjacent hepatic parenchyma. It is well recognized that patient outcome is highly dependent upon the ability to define the true extent of the metastatic disease; therefore, the need for a more accurate imaging technique cannot be overemphasized, as it would enable more precise as well as aggressive treatment of the liver metastases by adjunctive therapies.
More recently, there has been increasing interest in the use of contrast agents during the extracorporeal sonography of the liver to improve the detection of liver metastases. Ultrasound contrast agents consist of microbubbles of air or gases of low solubility, stabilized by a lipid, surfactant, or polymer shell. Analogous to CT or MR, it is the relative distribution of the contrast agents between normal tissue and the lesion, which makes the lesion more visible and easier to characterize.10,11 Recent advances in harmonic imaging, combined with the development of contrast agents with liver specificity, has markedly improved the sensitivity of extracorporeal sonography in the detection of small metastases, which may be equal to or even superior to that of CT or MR in some cases.12 We hypothesize that the extension of these new technologies to IOUS may improve the detection of “occult” liver metastases.
Aim
The purpose of this study was to assess the clinical impact of CE-IOUS in patients undergoing liver resection compared with CT/MR/IOUS combined findings.
MATERIALS AND METHODS
This was a 2-center prospective study of 60 consecutive patients (mean age, 66.65 years; SD, 9.74 years; range, 40–82 years; 31 females and 29 males) undergoing liver resection for metastatic disease on the basis of preoperative CE 2-phase CT and/or MRI scans. Fifty-one patients have had previous resection of the primary colorectal cancer, whereas the remaining 9 patients had synchronous primary bowel cancer and apparently resectable liver metastasis.
CT and MR Imaging
In all patients, CT and/or MR staging was performed in a standardized manner. Two-phase CT examination was performed using 2 multidetector CT scanners (Siemens, Sensation 4 and Sensation 16, Erlangen, Germany) with 2- to 4-mm slice thickness/16 × 1.5 mm collimation and enhanced with 150 mL of Omniscan 300 (Amersham, UK), injected at 3 to 5 mL/s. Scanning was performed at 25 to 30 seconds for the arterial phase and 55 to 60 seconds for the portal venous phase following the start of the bolus contrast injection in a peripheral vein.
MR imaging was performed using 2 1.5T MRI scanners (Philips, Gyroscan, Eindoven, Netherlands, Siemens Vision, Erlangen, Germany) with body and phase array coils. Two different liver-specific MR contrast agents, MultiHance (Bracco spa, Milan, Italy; dose 1 mL/kg BW) and Resovist (Schering AG, Berlin, Germany; 8 μmol Fe/kg BW) were used. Precontrast axial T2- and T1-weighted 2-dimensional scans with and without fat saturation were first performed. MultiHance enhanced axial 3-dimensional scans were carried out at 17 seconds, 45 seconds, 120 seconds, and 60 minutes following a bolus peripheral venous injection (at 2 mL/s followed by 20 mL flush at 2 mL/s), while Resovist-enhanced axial T2 weighted scan was performed 10 minutes after a bolus injection.
Preoperative CT scans alone, combined CT and MRI scans, and MRI scans alone were performed in 40, 4, and 16 patients, respectively.
IOUS and CE-IOUS Examination
Surgery was performed within 2 to 6 weeks (mean, 3.5 weeks) after the CT/MR imaging. At operation, all patients underwent thorough abdominal and pelvic exploration for extrahepatic disease, and the liver was subsequently mobilized off the diaphragm for improved sonographic visualization of the liver. Bimanual palpation of the liver was then carried out followed by hepatic sonography using an HDI 5000 scanner and a high-frequency finger probe CT8-4 with pulse inversion harmonic (PIH) imaging capability (Philips Bothell, Washington, USA). The IOUS and CE-IOUS scans were performed by an experienced surgeon and radiologist together. IOUS was performed in a systematic fashion, inspecting the liver parenchyma for previously diagnosed as well as additional lesions and for any major vascular or biliary involvement.
Following the baseline fundamental (no Doppler mode) and PIH mode scans, a bolus intravenous injection of 3 to 4 mL of contrast agent, SonoVue (Sulfur hexafluoride gas stabilized by phospholipid shell) (Bracco spa) was administered via the central venous line, followed by 10 mL of normal saline flush and repeat ultrasound scanning performed in the PIH mode. SonoVue (Bracco spa) is a new second-generation microbubble contrast agent stabilized by phospholipid shell containing sulfur hexafluoride gas (mean size, 2.5 μm; 90% measuring less than 8 μm in diameter). The ultrasound gain, focal zone, and output power (Mechanical index, 0.02–0.04) settings were standardized. Scanning of both lobes of the liver before and after contrast administration was performed in a standardized fashion systematically in axial, sagittal, and oblique sweeps to ascertain complete liver coverage. Following contrast administration, the normal liver enhances uniformly, and the hepatic metastases were easily identified appearing as a dark contrast free, filling defect during the portal venous and delayed sinusoidal phases as illustrated in Figure 1. The persistent enhancement of the normal liver parenchyma in the late sinusoidal phase lasted up to 3 minutes.
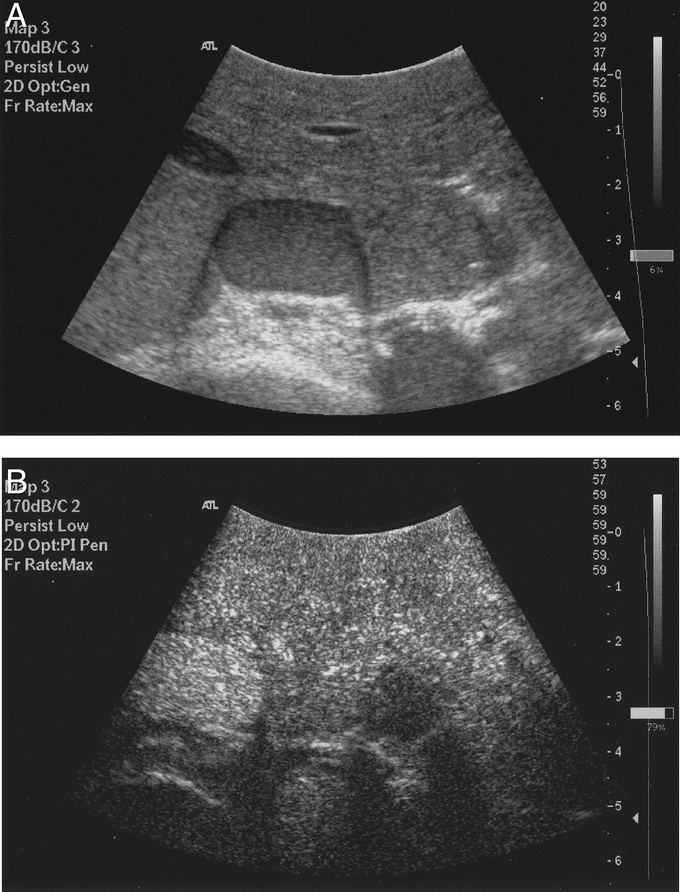
FIGURE 1. Legend to Figure 1 goes here.
The number of metastases identified on CT and/or MR, IOUS, and CE-IOUS were counted, sized, and mapped according to Couinaud classification on a liver schematic chart for each modality and in real-time for all the sonographic examinations. Benign cysts were not included in the counts. The excised liver segments or lobes were sectioned at pathology to obtain a true pathologic gold standard of the lesions. Correlation with resection/biopsy histopathology findings obtained was also carried out. Statistical analysis was performed using Mann Whitney and Wilcoxon Sign Rank tests. Changes in surgical management following CE-IOUS compared with those made after IOUS were documented (eg, abandoned resection, more extensive resection, limited resection, or combined resection with radiofrequency ablation).
RESULTS
CE-IOUS was not performed in 3 patients, and they were excluded from the analysis; 1 patient was found to have more than 10 liver metastases in a background of extensive fatty liver on IOUS and the other 2 patients had peritoneal metastases.
A total of 107 lesions were identified on histopathology findings of biopsies and resected tissues; and of these, 103 were confirmed metastases and 4 hemangiomas. The results are summarized in Table 1. The number of correctly identified metastases on CT and MRI combined, IOUS, and CE-IOUS was 79, 84, and 101, respectively. There was a statistically significant increase in the number of detected metastases on CE-IOUS compared with IOUS and also with combined CT/MRI (P = 0.029 and P = 0.047, respectively). No statistical difference was observed in the number of metastases detected between IOUS and combined CT/MRI (P = 0.53) For CT/MRI, IOUS, and CE-IOUS, the sensitivity was 76.7%, 81.5%, and 96.3%, respectively; accuracy was 73.8%, 78.5%, and 96.3% respectively; the positive predictive value was 95.2%, 95.5%, and 98.0% respectively.
TABLE 1. Summary of Results
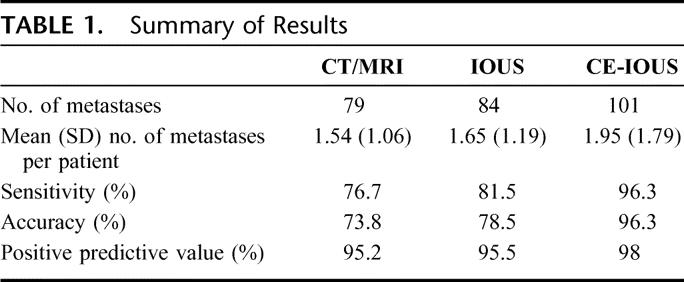
The demographics of those patients with additional lesions on CE-IOUS are summarized in Table 2.
TABLE 2. Demographics of the Patients With Additional Lesions on CE-IOUS With Fong's Clinical Risk Score at CT/MRI/IOUS
The mean (SD) size of the lesions identified on CT/MRI/IOUS combined and CE-IOUS were 2.73 (1.46) cm and 1.71 (1.57) cm, respectively.
The median size of the additional lesions identified on CE-IOUS was 0.8 cm. The smallest metastasis identified was 4 mm in diameter.
Actual Change in Surgical Management as a Result of CE-IOUS
Of the 60 patients, CE-IOUS was not performed in 3 patients; 2 patients had peritoneal metastases at exploration and 1 patient had widespread metastases in a background of fatty liver on the basis of IOUS. In 40 of the remaining 57 patients, there was no alteration in the surgical management; CE-IOUS detected no additional lesion in 37 patients; in 2 cases, there were additional lesions but they did not entail any extended resection or adjunctive surgical maneuvers; in another patient, one of the lesions was wrongly diagnosed on IOUS and CT as metastasis and was accurately identified as a benign hemangioma on CE-IOUS.
In contrast, new information identified on CE-IOUS alone altered the surgical plan in the remaining 17 of 57 patients (29.8%); additional hepatic metastases were detected in 11 cases (19.3%), which extended to a trisegmentectomy in 3 cases, additional nonsegmental wedge resection in 2 patients, and radiofrequency ablations of the additional lesions in 6 cases, as an adjunct to the planned hepatic lobectomy. Prior to radiofrequency ablation, all additional lesions were biopsied and confirmed as metastasis; all biopsies and radiofrequency ablations were performed using CE-IOUS guidance. In 2 patients (3.5%), there were fewer lesions than identified on preoperative imaging scans and could not be confidently excluded on IOUS alone, which resulted in alteration in the original surgical plan from right hepatectomy to excision of 3 segments in one and removal of segment VII/VIII plus a metastatectomy in the other. CE-IOUS also confirmed presence of an arteriovenous malformation in 1 patient (1.8%), which was not identified on IOUS and was previously diagnosed as apparent solitary metastasis on CT; CE-IOUS also accurately diagnosed a solitary benign hemangioma with characteristic peripheral nodular enhancement with progressively filling-in over the vascular and late phases in 2 patients (3.5%), which were wrongly identified as metastasis on CT and IOUS. Previously planned resections were therefore not carried out. In 1 case, the tumor margin could only be clearly visualized on CE-IOUS to be too close to the inferior vena cava for resection, and it was ablated instead. New findings on CE-IOUS alone also altered IOUS/CT/MRI hepatic staging in 35.1% (20 of 57) of patients.
DISCUSSION
CE-IOUS has not been described to date. The high-frequency intraoperative US finger-probe capable of PIH imaging to enable visualization of contrast enhancement of the hepatic microvasculature is a relatively novel tool. In PIH imaging, a sequence of 2 ultrasound pulses is transmitted instead of 1 single pulse. The first pulse is an in-phase pulse and the second is a mirror image of the first. For any linear target, the response to the second pulse is an inverted copy of the response from first pulse. These are then summated, and all linear echoes are cancelled. However, for a nonlinear target such a microbubbles, the responses to positive and negative pulse are different and therefore do not cancel each other on summation. The fundamental components of the echo are cancelled while the even harmonic components are added, resulting in twice the harmonic level of a single pulse. The result is the display of microbubbles signals and cancellation of those of liver tissue. This new technique allows the use of broader transmit and receive bandwidths with improved resolution and increased sensitivity to contrast, thereby overcoming some of the limitations of the simple harmonic modes.13
However, there are some limitations in the CE-IOUS technique. PIH capability is a prerequisite and as yet it is not widely available for the intraoperative US finger probes. Although such new technology is relatively cheap, there would be the additional cost of the contrast agent, and any upgrade from existing conventional equipment. IOUS probes with PIH capability would be a tenth of the cost of a top of the range ultrasound scanner and the cost of the US contrast agent is about half that of an MRI liver-specific contrast agent (in UK SonoVue costs £40 [$72] per vial, which is made up to 5 mL volume).
To minimize the disruption of the microbubbles to prolong enhancement and maintain uniformity, scanning is performed at a much lower ultrasound output power (mechanical index range, 0.02–0.04), which renders the ultrasound screen display dark until the arrival of contrast; this can be compensated to some extent by increasing the ultrasound gain; the spatial resolution is also lower compared with that of the fundamental mode. These require some degree of observer adaptation with an obvious learning curve, which may be up to 10 scans. Furthermore, the duration of the contrast enhancement is shorter with a mean duration of 2 to 3 minutes compared with the 4 to 5 minutes of enhancement obtained from lower frequency probe scans; this may also be related to some of the interactions with the general anesthetics as well as the positive pressure ventilation, which may disrupt the microbubbles. After the liver parenchymal enhancement has completely vanished (usually 5 minutes from the injection time), the injection of the same dose of SonoVue can be safely repeated (total safe dose per patient: 10 mL of SonoVue in 3 doses) to complete the examination, and we can anticipate future software optimization for improved sensitivity to SonoVue.
The appropriate use of contrast agents will improve the performance of any imaging modality; the value of contrast agents in CT and MR in the detection of liver metastases is predictably unquestioned in current practice, such that it would even be considered unethical if they were not applied routinely. However, the application of contrast agents to US in general is relatively new and is now gradually gaining increasing support in routine clinical practice. Its extension to IOUS is a natural pathway to improving detection and characterization. There is still some speculation as to the mechanism underlying the prolonged enhancement of the liver parenchyma in the late phase; there is some evidence that it may be due to the microbubbles being slowed or trapped within the sinusoids.14 The advantage of such an agent is that in malignant lesions, especially metastases, there is rapid wash-in and wash-out of the microbubbles through the immature microvasculature of the tumors, and they remain devoid of microbubbles in the portal and late phases. It is this differential distribution of microbubbles that leads to a marked increase in the contrast ratio between metastasis and normal liver parenchyma to improve detection of subcentimeter lesions. Indeed, in this study, the sensitivity of CE-IOUS in the detection of liver metastases is significantly higher than that of IOUS and that of CT/MR combined. CE-IOUS detected additional lesions not identified on IOUS or CT/MR in 13 patients (22.8%), and the smallest of the additional lesions measured 0.4 cm.
A key question is whether the detection of these additional lesions really matters. One might argue that small lesions measuring less than 5 mm (if left undetected) might be eradicated by adjuvant chemotherapy; however, the true value of the latter remains uncertain; as yet adjuvant chemotherapy following liver resection is not routinely administered in most centers. Surprisingly, the median size of the additional lesions identified on CE-IOUS in this study is 8 mm which is larger than the threshold size of lesions identified on conventional IOUS (5 mm); they may potentially impact on the patient eventual outcome. The median clinical risk score of those 13 patients with additional lesions was 2 before CE-IOUS and did not significantly change after CE-IOUS (median score, 2).15 One would only anticipate a change in the clinical risk score for those patients with solitary lesion at CT/MRI/IOUS; in this study, 3 of those 13 patients (23.1%) had solitary lesions before CE-IOUS. However, the numbers are far too small for any proper analysis. Clearly, follow-up studies of larger cohort of patients are needed.
There is still some debate as to the value of IOUS during hepatic resection with its impact on surgical management ranging between 7% and 44%.6–8 As previous suggested, the impact variations between studies might be explained partly by the difference in the adequacy and extent of the preoperative imaging studies; clearly, the higher the performance of CT/MR, the lower the impact of IOUS. Moreover, resectability/irresectability is no longer the sole surgical issue with the advent of extensive/adjunctive surgical/interventional maneuvers; the more aggressive the surgeon/interventional radiologist to proceed with these maneuvers, the higher the impact of IOUS. Similar considerations may be applied to the CE-IOUS impact on surgical plans; any additional information provided by CE-IOUS alone that would alter surgical management may depend on the adequacy of IOUS and also on the quality and the extent of the preoperative imaging scans. Our surgical/interventional approach might be considered as “aggressive,” and the optimum protocol for imaging had been selected using top of the range equipment. Ultimately, the true value of CE-IOUS can only be judged by the outcome of these patients.
Nevertheless, the preliminary results of this study are compelling. In this study, additional findings based on CE-IOUS alone altered the surgical plan in 29.8% of patients. Lesional characterization was also improved with CE-IOUS as a result of its ability to image contrast enhancement in real-time; it could be argued that the 4 patients with benign lesions wrongly diagnosed on CT and IOUS would have been spared of laparotomy if MRI had been performed preoperatively; on the other hand, the value of performing both MR and CT in all patients undergoing liver resection remains debatable. While the AVM lesion missed on IOUS and CT would have been diagnosed on Doppler US mode, the latter is not routinely performed. Nonetheless, even if they were to be excluded, a change in surgical management in the remaining 13 patients would still be clinically significant. Moreover, CE-IOUS alone identified additional lesions in a total of 13 patients (22.8%) and exclusively guided biopsy and radiofrequency ablation of new lesions in 10.5% of cases.
CONCLUSION
These preliminary results suggest that CE-IOUS is an essential tool prior to liver resection of metastases with implications on surgical options at time of operation. Future long-term outcome studies will determine its true value in clinical practice.
Footnotes
Reprints: Edward Leen, MD, FRCR, Department of Radiology, Queen Elizabeth Building, Alexandra Parade, Royal Infirmary, Glasgow, G31 2ER, UK. E-mail: gpda01@udcf.gla.ac.uk.
REFERENCES
- 1.O'Brien MJ. Cancer of the colon and rectum: current concepts of etiology and pathogenesis. Br J Med Sci. 1988;157:5–15. [PubMed] [Google Scholar]
- 2.McArdle CS, Hole D, Hansell D, et al. A prospective study of colorectal cancer in the West of Scotland: a ten year follow-up. Br J Surg. 1990;77:206–208. [DOI] [PubMed] [Google Scholar]
- 3.Scheele J, Strang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J. Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 4.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–46. [DOI] [PubMed] [Google Scholar]
- 5.Finlay IG, Meek D, Brunton F, et al. Growth rate of hepatic metastases in colorectal carcinoma. Br J Surg. 1988;75:641–644. [DOI] [PubMed] [Google Scholar]
- 6.Conlon R, Jacobs M, Dasgupta D, et al. The value of intraoperative ultrasound during hepatic resection compared with improved pre-operative magnetic resonance imaging. Eur J Ultrasound. 2003;16:211–216. [DOI] [PubMed] [Google Scholar]
- 7.Jarnagin WR, Bach AM, Winston CB, et al. What is the yield of intraoperative ultrasonography during partial hepatectomy for malignant disease? J Am Coll Surg. 2001;192577–582. [DOI] [PubMed]
- 8.Cervone A, Sardi A, Conaway GL. Intraoperative ultrasound is essential in the management of metastatic colorectal liver lesions. Am Surg. 2000;66:611–615. [PubMed] [Google Scholar]
- 9.Charnley RM, Morris DL, Dennison AR, et al. Detection of colorectal liver metastases using intraoperative ultrasonography. Br J Surg. 1991;78:45–48. [DOI] [PubMed] [Google Scholar]
- 10.Leen E, Horgan P. Ultrasound contrast agents for hepatic imaging with nonlinear modes. Curr Probl Diagn Radiol. 2003;32:66–87. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SR, Burns PN. Liver mass evaluation with ultrasound: the impact of microbubble contrast agents and pulse inversion imaging. Semin Liver Dis. 2001;21:147–159. [DOI] [PubMed] [Google Scholar]
- 12.Albrecht T, Blomley MJ, Burns PN, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology. 2003;227:361–370. [DOI] [PubMed] [Google Scholar]
- 13.Burns PN, Hope Simpson D, Averkiou MA. Nonlinear imaging. Ultrasound Med Biol. 2000;26(suppl 1):19–22. [DOI] [PubMed] [Google Scholar]
- 14.Kono Y, Steinbach GC, Peterson T, et al. Mechanism of parenchymal enhancement of the liver with a microbubble-based US contrast medium: an intravital microscopy study in rats. Radiology. 2002;224:253–257. [DOI] [PubMed] [Google Scholar]
- 15.Fong Y, Fortner J, Sun RL, et al. Clinical score fro predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]