Abstract
Objective:
To review the literature with regard to the incidence and prognostic significance of peritoneal seeding during surgery for primary colorectal cancer (CRC), the incidence of intraperitoneal recurrence of CRC, and the current treatment strategies of established PC of colorectal origin, with special focus on cytoreductive surgery and intraperitoneal chemotherapy (IPEC).
Summary Background Data:
Although hematogenous dissemination forms the greatest threat to patients with CRC, peritoneal carcinomatosis (PC), presumably arising from intraperitoneal seeding of cancer cells, is a relatively frequent event in patients with recurrent CRC.
Methods:
The PubMed and Medline literature databases were searched for pertinent publications regarding the incidence and prognostic significance of exfoliated tumor cells in the peritoneal cavity during curative surgery for primary CRC, the incidence of intraperitoneal recurrence of CRC, and the therapeutic results of systemic chemotherapy or cytoreductive surgery followed by IPEC.
Results:
The incidence of peritoneal seeding during potentially curative surgery for primary CRC, as reported in 12 patient series, varied widely, from 3% to 28%, which may be explained by differences in methods to detect tumor cells. PC is encountered in approximately 7% of patients at primary surgery, in approximately 4% to 19% of patients during follow-up after curative surgery, in up to 44% of patients with recurrent CRC who require relaparotomy, and in 40% to 80% of patients who succumb to CRC. The reported median survival after systemic 5-fluorouracil-based chemotherapy for PC varies from 5.2 to 12.6 months. Median survival after aggressive cytoreductive surgery followed by (hyperthermic) IPEC in selected patients, as reported in 16 patient series, tends to be better and varies from 12 to 32 months at the cost of morbidity and mortality rates of 14% to 55% and 0% to 19%, respectively. One randomized controlled trial has been published confirming the superiority of aggressive surgical cytoreduction and intraperitoneal chemotherapy over strictly palliative treatment.
Conclusions:
Peritoneal seeding of cancer cells possibly leading to PC is a rather common phenomenon in patients with CRC. Cytoreductive surgery and adjuvant (hyperthermic) IPEC have been shown to be efficacious in selected patients and should therefore be considered in patients with resectable PC of colorectal origin.
This paper reviews the incidence of intraperitoneal tumor cell seeding during curative surgery of colorectal cancer (CRC), the incidence of intraperitoneal recurrence, and current treatment strategies for established peritoneal carcinomatosis of colorectal origin. Intraperitoneal seeding and peritoneal carcinomatosis are relatively frequent phenomena in patients with CRC. There is a trend toward more aggressive treatment, consisting of surgical cytoreduction and intraperitoneal chemotherapy, which has been shown to be beneficial in selected patients.
Besides the lymphatic and hematogenous routes of dissemination, colorectal cancer (CRC) frequently gives rise to transcoelomic spread in the peritoneal cavity, which, ultimately, may cause peritoneal carcinomatosis (PC). During the course of disease of a patient with a gastrointestinal cancer, it is thought that there are 2 time points at which intraperitoneal spread or seeding of cancerous tumor emboli may occur.1 First, intraperitoneal spread may occur preoperatively as a result of full-thickness invasion of the bowel wall by an invasive cancer. Preoperative seeding may also occur as a result of the rupture of a structure by a noninvasive tumor, such as the mucus-producing cystadenocarcinoma of the appendix. This leads to the clinical syndrome of pseudomyxoma peritonei, characterized by massive intraperitoneal accumulation of mucus in the absence of organ metastases. This rare clinical entity has been reviewed elsewhere and is beyond the scope of this paper.2,3 Second, intraperitoneal spread may be induced iatrogenically during surgery, when in-transit tumor cells or emboli escape from dissected lymph vessels, the bowel lumen or reach the peritoneal cavity through blood spill from the surgical field.
Until recently, most oncologists considered PC a terminal condition, only to be palliated with systemic chemotherapy. However, it has been estimated that, in approximately 25% of patients with recurrent CRC, the peritoneal cavity seems to be the only site of metastatic disease, even after a detailed diagnostic workup of the liver and lungs.4,5 Because of this observation, some surgical oncologists hypothesized that, similar to liver metastases, PC may be a first step of dissemination and should therefore not necessarily be interpreted as generalized disease.6,7 In recent years, a new therapeutic approach has been investigated in a dozen medical centers worldwide, aimed at locoregional control and long-term survival.8 This approach is based on aggressive cytoreductive surgery after which the peritoneal cavity is perfused with chemotherapeutics, sometimes under hyperthermic conditions, to sterilize (minimal) residual disease.
Here, the available literature on the incidence and prognostic significance of microscopic peritoneal dissemination of tumor cells as well as on the incidence of true intraperitoneal recurrence in CRC is reviewed. Finally, the results of clinical trials investigating the feasibility and efficacy of cytoreductive surgery followed by intraperitoneal chemotherapy (IPEC) for the treatment of PC of colorectal origin are discussed.
Incidence and Prognostic Significance of Intraperitoneally Seeded Tumor Cells in CRC
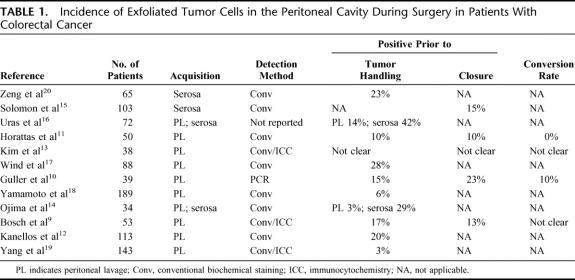
Between 1980 and 2004, 12 papers have been published reporting the results of clinical studies on the incidence and/or prognostic relevance of microscopic peritoneal dissemination of tumor cells in patients undergoing curative surgery for primary CRC.9–20 These trials focused on patients with primary CRC who underwent surgery with curative intent and excluded patients with evidence of hematogenous metastases, PC, or ascites or patients who had undergone emergency surgery for obstructive of perforated cancers. The relevant trial characteristics are summarized in Table 1.
TABLE 1. Incidence of Exfoliated Tumor Cells in the Peritoneal Cavity During Surgery in Patients With Colorectal Cancer
Although the inclusion and exclusion criteria were similar, the studies differed with respect to design, methods of collecting the specimens, and pathologic staining techniques. Four trials explicitly mentioned the inclusion of consecutive patients.12,15,17,19 In 8 trials, material for cytologic analysis was harvested by pouring normal saline onto the serosal surface of the tumor bed immediately after entering the abdominal cavity and, in some studies, again after the resection prior to closure of the abdomen.9–13,17–19 In 2 trials, material was obtained by gently touching, scraping, or brushing the serosa overlying the tumor mass using a slide glass or brush,15,20 whereas in 2 trials both aforementioned techniques were used.14,16 In all but one trial, an attempt was made to identify cancer cells using conventional cytology and/or immunocytochemistry. One trial used the sensitive polymerase chain reaction (PCR) technology to detect the presence of free cancer cells in the peritoneal cavity.10
In 3 trials, an attempt was made to determine the sensitivity and/or specificity of the used method by searching for tumor cells in peritoneal lavage fluid obtained from patients with well-known peritoneal metastases and/or benign diseases undergoing surgery.9,10,17 In 2 of the 3 trials, specificity was 100%,10,17 whereas in the third trial nonspecific immunocytochemical staining in one of 6 control patients with diverticular disease resulted in a specificity of 83%.9 One trial included a well-defined positive control group of 22 patients with well-established PC, 19 of whom had tumor-positive cytology, corresponding to a “sensitivity” of 86%.17
Incidence of Peritoneal Seeding
Peritoneal lavage fluid obtained prior to resection proved tumor positive in 3% to 28%,9–14,16–20 whereas scraping the serosa overlying the primary tumor site or pressing slide glasses on the serosa overlying the tumor generally resulted in somewhat higher incidence rates of 15% to 42%.14,16,20 Interestingly, Uras et al16 and Ojima et al14 tested the accuracy of serosal stamp cytology by including serosal stamps of bowel surfaces far away from the primary tumor. These control stamps were tumor positive in 3%.
In 4 trials, peritoneal lavage was performed prior to as well as after resection of the tumor. Where reported, the conversion rate from tumor-negative preresection to tumor-positive postresection lavage fluid, apparently as a result of the surgery, varied considerably from 0% to 10%.10,11
Prognostic Significance of Peritoneal Seeding
Several authors have made an attempt to (univariately) correlate the finding of tumor-positive cytology with various clinicopathologic parameters, including pathologic TNM stage, malignancy grade, locoregional recurrence rate, and/or survival. Although in 10 trials the incidence of tumor-positive cytology correlated significantly with more advanced stages of disease, especially T-stage,11,12,16,17,19,20 Solomon et al15 could not confirm this correlation in a large prospective series of 105 patients with CRC. Instead, Solomon et al found tumor cells on the serosal surface of resection specimens more frequently after resections of distal tumors, which required anterior or abdominoperineal resections.15
Interestingly, Yamamoto et al18 and Kanellos et al12 found tumor-positive cytology to be significantly correlated with the risk of intraperitoneal recurrence. Both groups used similar techniques of instilling normal saline in the peritoneal cavity immediately after entering the abdomen in 113 and 189 patients undergoing curative resection for CRC, respectively. Conventional Papanicolaou and Giemsa staining of the peritoneal lavage revealed tumor cells in 5.8% and 20% of patients, respectively. The relative risk of intraperitoneal recurrence in those patients with tumor-positive cytology as opposed to patients with tumor-negative cytology was 16.5 (95% confidence interval [CI], 4.8–57.5, P = 0.0004) and 2.9 (95% CI, 1.0–8.2, P = 0.047), respectively.
Two trials, finally, reported that tumor-positive cytology correlated with impaired overall or disease-free survival.10,17 However, in none of the studies were multivariate analyses carried out to determine whether tumor-positive cytology has an independent prognostic impact or is merely a confounding prognostic indicator.
Incidence of Intraperitoneal Recurrence in CRC
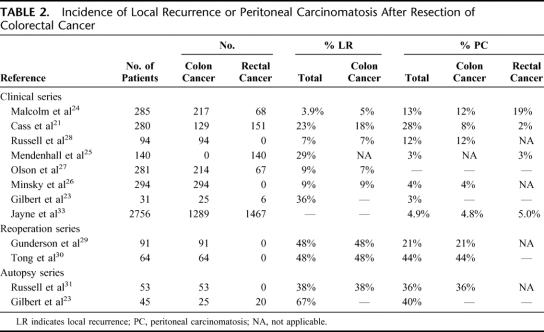
Since hematogenous metastases have always been the main cause of disease-related death in CRC, the intraperitoneal route of dissemination has long been regarded as less important. In the 1970s and 1980s, however, several authors published retrospective studies concerning the patterns of failure in patients with CRC, recognizing the peritoneal cavity as a common site of recurrence after potentially curative surgery. These patient series were either retrospective clinical follow-up studies,21–28 reoperation series,29,30 or autopsy studies of patients who had died of CRC.23,31 Table 2 summarizes the incidence rates of intraperitoneal failure reported in these studies. It should be noted that since the late 1980s the surgical technique of resection in rectal cancer has changed toward total mesorectal excision and preoperative radiotherapy, the local recurrence rates after surgery for rectal cancer have dramatically decreased to less than 3%.32 Whether or not the introduction of the total mesorectal excision technique has affected the incidence of PC is unknown.
TABLE 2. Incidence of Local Recurrence or Peritoneal Carcinomatosis After Resection of Colorectal Cancer
Clinically evident locoregional failure, in most studies defined as recurrence in the bowel anastomosis or in the resection bed, was reported in 5% to 18% after curative resection of colon cancer. PC was reported in 4% to 12% after curative resection of colon cancer and in 2% to 19% after curative resection of rectal cancer. Recently, Jayne et al33 retrospectively analyzed a large series of 3019 patients with CRC: 214 patients (7%) had synchronous PC at the time of resection of the primary tumor, whereas another 135 patients (4.5%) developed metachronous carcinomatosis. Of the patients with synchronous PC, 58% did not seem to have systemic metastatic disease.
Gunderson et al29 studied the areas of failure in 91 patients with extrapelvic Dukes B or C colon cancer who underwent a planned second-look laparotomy 6 to 12 months after a potentially curative resection. Locoregional recurrence either alone or as a component of failure was confirmed in 48% of patients. PC as the sole pattern of recurrence or as a component of failure was found in 4% and 21%, respectively. Tong et al30 mapped the sites of failure in patients who required relaparotomy for suspected recurrent proximal colon cancer and found local recurrence in 47% and diffuse PC in 44% of patients.
Gilbert et al23 showed that in a autopsy series of 45 patients who had died of CRC, 18 patients had PC. In a similar autopsy series of 53 patients who died of colon cancer, Russell et al31 reported a local recurrence rate of 38%, whereas 36% of patients had PC.
These data indicate that, in patients with CRC, intraperitoneal recurrence is a rather common phenomenon with important clinical consequences for both medical and surgical oncologists.
Systemic Chemotherapy for PC of Colorectal Origin
To date, 4 clinical studies have been published dedicated to the efficacy of chemotherapeutic treatment of patients with PC of colorectal origin. In 1989, Chu et al34 prospectively analyzed a series of 100 patients with PC of nongynecologic cancers, among whom 45 patients with CRC, with the aim to identify prognostic factors. In those patients with CRC, of whom the majority was treated with 5-fluorouracil (5-FU) and leucovorin (LV), median survival was 6 months. Shorter disease-free interval, the presence of lung metastases, and the presence of ascites correlated significantly with decreased survival. In 2000, Sadeghi et al35 reported the results of a French prospective multicenter study of 370 patients with PC of nongynecologic malignancy. The 118 patients with PC of colorectal origin in this study had a median survival of only 5.2 months. Jayne et al33 recently published an extensive series of 3019 patients with CRC. The 392 patients who presented with PC had a median survival of 7 months. Finally, Verwaal et al36 conducted a phase III randomized controlled trial (RCT), comparing ultraradical cytoreductive surgery and adjuvant hyperthermic intraperitoneal chemotherapy and systemic 5-FU-based chemotherapy with systemic chemotherapy and palliative surgery in patients with PC of colorectal or appendiceal origin, but without evidence of extraperitoneal, metastatic disease. Overall median survival of the 50 patients who were treated with systemic chemotherapy and palliative surgery was 12.6 months, with a 2-year survival rate of approximately 18%. Median time to disease progression was 7.6 months. The better survival of the patients that received chemotherapy and conventional surgery within this RCT as compared with the above-mentioned results reported by other authors is probably due to patient selection, since patients had to be medically fit to undergo cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and were not allowed to have hematogenous metastases.
Thus, the reported median survival of patients with PC of colorectal origin, with of without hematogenous metastases, after chemotherapy-based treatment, varies between 5.2 and 12.6 months.
Historical Perspective of Cytoreductive Surgery
As mentioned above, the presence of peritoneal metastases has for long been regarded as a lethal disease, characterized by “contamination” of the entire abdomen, for which complete R0 resection was considered not feasible and, consequently, any attempt thereto futile. In the 1930s, Meigs, an American gynecologist, was the first to advocate cytoreductive surgery followed by adjuvant radiotherapy in patients with ovarian cancer, a disease with a very high propensity to disseminate to the peritoneum.37 The survival rate after cytoreductive surgery for the treatment of ovarian cancer, however, remained poor. As a result, treatment of ovarian cancer mainly depended on chemotherapy, and surgical strategies were not optimized until the late 1960s and 1970s, when Munnell and Griffiths independently demonstrated that better survival rates could be achieved by more extensive surgery with the size of residual disease being the most important prognostic factor.38–40 Thus, the concept of ultraradical cytoreduction of PC was introduced.
In view of the improved results of ultraradical cytoreductive surgery in ovarian cancer, surgical oncologists regained a renewed interest in the subgroup of patients with colorectal carcinomatosis without evidence of hematogenous metastases. In 1979, after testing the technique of hyperthermic peritoneal perfusion in 15 dogs, Spratt et al were the first to report the results of cytoreductive surgery followed by hyperthermic IPEC using thiothepa in a 35-year-old patient with pseudomyxoma peritonei.41,42 Except for minor pulmonary atelectasis with bacteremia, the patient's postoperative course was uneventful. In the early 1980s, this approach was adopted and optimized by Sugarbaker, who investigated its therapeutic efficacy in patients with peritoneal metastases of various gastrointestinal cancers.43,44 Since then, cytoreductive surgery and adjuvant (hyperthermic) IPEC have been further developed and applied by 30 medical centers worldwide in patients with various kinds of peritoneal surface malignancy, including PC, sarcomatosis, and peritoneal mesothelioma.8 Several reviews have been published describing the techniques of cytoreductive surgery,45 the rationale of hyperthermia,46 and the various methods of the intraperitoneal administration of chemotherapy.47
Cytoreductive Surgery and (Hyperthermic) IPEC in PC of Colorectal Origin
To date, 20 papers have been published reporting the toxicity, complications, and survival results of trials investigating the morbidity, mortality, and therapeutic efficacy of surgical cytoreduction followed by IPEC, either with or without hyperthermia, in patients with PC of colorectal origin.36,48–66 Relevant trial characteristics, as summarized in Table 3, differed with respect to design, patient selection, and treatment protocol. Thirteen studies were nonrandomized, single-arm, retrospective patient series, whereas 3 studies were comparative trials, 2 of which were randomized.36,50
TABLE 3. Characteristics of Trials Investigating the Efficacy of Cytoreductive Surgery and Intraperitoneal Chemotherapy in Patients With Peritoneal Carcinomatosis of Colorectal Origin
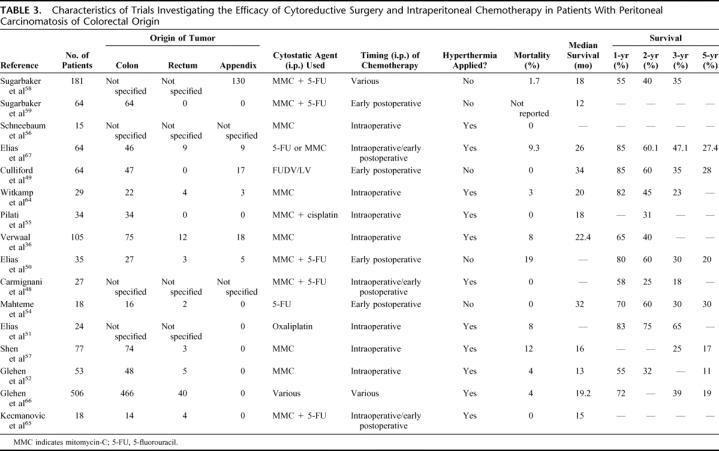
In 9 trials, only patients with PC of colorectal origin were included,48,51,52,54–57,59,65 whereas in the remaining 6 trials patients with PC of appendiceal origin were also included.49,50,58,64,67,68 Since in the latter trials, the results were analyzed for patients with colorectal carcinomatosis separately, these reports were included in this review. Furthermore, in 7 trials patients with hematogenous metastases were eligible for inclusion.48,50,51,57,58,65,66 IPEC was administered intraoperatively in 6 trials,36,51,55–57,64 early postoperatively in 4 trials,49,50,54,59 both intraoperatively and early postoperatively in 2 trials,53,65 whereas in 4 patient series various protocols of administering chemotherapy intraperitoneally were used.48,58,66,67 In 10 trials, IPEC was given under hyperthermic conditions,36,48,51,53,55–57,64–67 whereas in 5 trials it was not.49,50,54,58,59 Mitomycin-C was the most frequently used cytostatic agent, either alone,36,56,57,64 or in combination with 5-FU48,50,53,58,59,65,67 or cisplatin.55 Two trials, finally, used 5-FU alone,49,54 whereas in one trial oxaliplatin was administered.51
The clinical outcomes with respect to long-term survival varied considerably. In short, median survival varied from 12 to 32 months. One-year, 2-year, 3-year and, when reported, 5-year survival rates varied from 65% to 90%, 25% to 60%, 18% to 47%, and 17% to 30%, respectively.
Toxicity and Complications of Cytoreductive Surgery and (Hyperthermic) IPEC
Cytoreductive surgery followed by (hyperthermic) IPEC carries a high morbidity and a substantial mortality rate. Postoperative grade III and IV toxicity and complications after cytoreductive surgery and adjuvant IPEC varied from 14% to 55%, whereas treatment-related mortality varied from 0% to 19%.
Morbidity of cytoreductive surgery and (hyperthermic) IPEC can be categorized as surgery- or chemotherapy-related. Five studies have specifically addressed the toxicity and complications related to cytoreductive surgery and (hyperthermic) IPEC, 2 of which in patients with colorectal or appendiceal cancer only.53,63,69–71
Esquivel et al69 reported on the complication rate associated with cytoreductive surgery and early postoperative IPEC using mitomycin-C (MMC) and 5-FU in 44 patients with PC of appendiceal, colonic, small bowel, or fallopian tube origin. Twenty-two patients had been treated with induction IPEC prior to cytoreductive surgery and postoperative IPEC. The median duration of postoperative ileus was 21 days, which was related to age and the extensiveness of the surgical cytoreduction. In 4 patients, postoperative hemorrhage required surgical reintervention, whereas 2 patients developed pneumonia and respiratory failure requiring orotracheal intubation. Enteric complications, including small bowel fistulas, anastomotic disruption, bile leak, and pancreatitis occurred in 7 patients (16%), of whom 6 had been treated with induction IPEC. The authors concluded that, since induction IPEC carries an increased risk for postoperative enteric complications, this treatment modality should only be reserved for patients with small volume disease.
Jacquet et al53 reported the morbidity and mortality rate of cytoreductive surgery and (hyperthermic) IPEC (MMC) in 60 patients with PC of colorectal or appendiceal origin. Serious complications were encountered in 35% of patients, with anastomotic leakage or bowel perforations being the most frequent and significantly correlated to the number of peritonectomy procedures and the duration of the operation. Three patients (5%) died of treatment-related causes.
Verwaal et al63 reported the toxicity of cytoreductive surgery and (hyperthermic) IPEC (MMC) in a series of 102 patients with PC of colorectal or appendiceal origin that had been treated according to the same protocol in prospective phase I, II, or III trials. Grade 3, 4, or 5 toxicity, as recorded in accordance to the Common Toxicity Criteria published by the National Cancer Institute, was observed in 65%. Surgical failures were encountered in 35 patients (35%), resulting in abdominal sepsis in 16 patients (16%). Twenty-one patients (21%) developed intra-abdominal abscesses that could be drained percutaneously. A total of 8 patients (8%) died due to treatment-related causes, 6 of whom due to abdominal sepsis. Patients with higher tumor loads, incomplete cytoreductions, blood loss exceeding 6 L, and patients with 3 or more anastomoses had an increased risk of having a complicated postoperative course.
Stephens et al71 described a series of 183 patients with PC of gastrointestinal origin who underwent 200 cytoreductive surgeries followed by (hyperthermic) IPEC (MMC). Combined grade 3 or 4 toxicity was noted in 27% of patients, peripancreatitis being the most frequent complication in 6%, followed by fistulization (4.5%), hemorrhage (4.5%), and hematologic toxicity (4%). Three patients (1.5%) died of treatment-related toxicity, 2 of whom due to severe hematologic toxicity. Treatment-related mortality was 1.5%. The duration of the surgery, the number of peritonectomy procedures, resections of anastomoses were significantly associated with the occurrence of grade 3, 4, or 5 toxicity, whereas (hyperthermic) IPEC-related variables were not.
Glehen et al70 analyzed the morbidity and mortality rates following 216 consecutive cytoreductive surgeries and (hyperthermic) IPEC in 207 patients with PC. Most patients suffered from ovarian, colorectal, or gastric cancer. Grade III/IV toxicity was encountered in 51 patients (23.6%), digestive fistulization (6.5%), and hematologic toxicity (4.5%) being the most frequent complications. Seven patients (3.4%) died of treatment-related complications.
Prognostic Indicators of Survival
Univariate and multivariate analyses of most series of patients with PC of colorectal origin revealed several clinical (preoperative), surgical (intraoperative and postoperative), and pathologic factors predictive of survival. Clinical characteristics that have been univariately correlated to an improved survival are female gender,66 younger age,66 and clinical performance status.57
The most important surgical factors that have been identified as predictors of survival are: the extent of carcinomatosis encountered at laparotomy36,48,49,51,52,58–60,65–67 and the completeness of resection.36,48,49,51,52,54,57–60,64–66 Various scoring system were used to assess the extent of carcinomatosis. Most authors used the semi-quantitative peritoneal cancer index, described by Jacquet and Sugarbaker.72 The peritoneal cancer index relies on the distribution and size of the cancer lesions throughout the abdomen. More extensive carcinomatosis or higher peritoneal cancer index was invariably associated with decreased survival. After the surgical cytoreduction, the size of residual disease was usually expressed as R1, meaning no macroscopic residual disease, R2a, meaning macroscopic residual disease less than 2.5 or 5mm, or R2b, meaning macroscopic residual disease more than 2.5 or 5 mm in diameter. When reported, median survival following complete R1 resection of all macroscopic disease varied from 17.8 months to 39.0 months,52,54,57,60,65,66 whereas the reported 5-year survival rates varied from 20% to 54%.49,52,57,66 Median survival after incomplete R2a resection resulted in median survival times of 12.5 months to 24 months,36,52,54,57,60,66 with 5-year survival rates of between 10% and 29%.52,59,66 When macroscopic disease of more than 5 mm in diameter had to be left behind, the reported median survival varied between 5 months and 12 months.36,52,57,59,60 None of these patients survived for 5 years.59,66 Finally, bowel obstruction,57 the presence of ascites,57 and the presence and resection of metastatic disease to the liver were reported to be significantly correlated with impaired survival.57,66
Several authors have made an attempt to correlate the site of the primary tumor (colon versus appendix versus rectum) with prognosis.36,48,49,58,60,66 Verwaal et al analyzed a series of 102 consecutive patients with PC of colorectal or appendiceal origin who had been treated with cytoreductive surgery followed by hyperthermic IPEC. The 5 patients with PC of rectal origin had a median survival of 16.0 months, whereas those 82 patients with PC of colonic origin had a median survival of 21.6 months (relative risk, 3.14; 95% CI, 1.11–8.91, P = 0.069). Similar results were reported by Culliford et al, although in their patient series 6 of 17 patients with appendiceal carcinomatosis had pseudomyxoma peritonei.49 Other authors, however, did not confirm worse survival of patients with PC of rectal origin as compared with patients with PC of colonic origin.36,66 Sugarbaker et al reported on the efficacy of cytoreductive surgery and IPEC in a relatively large patient series of 51 patients with colorectal carcinomatosis and 130 patients with PC of appendiceal origin. Three-year survival of the patients with PC of appendiceal origin was significantly better than that of the patients with PC of colorectal origin (73% versus 36%, P = 0.0001). Other authors, however, did not find a significant survival benefit for patients with appendiceal carcinomatosis as compared with patients with PC of colonic or rectal origin.36,60,66 Finally, other pathologic factors that have been correlated with impaired survival include poor tumor differentiation,52,55,58–60,66 signet cell histology,60 and lymph node involvement.58,66
The results of multivariate analyses on the above-mentioned clinicopathologic factors were reported in 5 publications.49,52,57,60,66 In 4 of these, the extent of disease and/or the completeness of resection were the factors most prominently related to treatment success and survival.49,52,60,66 Shen et al found the presence of ascites or bowel obstruction to have an even greater impact on survival.57 In the large multi-institutional patient series of 506 patients with PC of colorectal origin, Glehen et al furthermore identified treatment by a second procedure, age less than 65 years, and use of adjuvant chemotherapy as positive independent prognostic indicators, whereas the use of neoadjuvant chemotherapy, lymph node involvement, presence of liver metastasis, and poor histologic differentiation were negative independent prognostic indicators.66
Quality of Life After Cytoreductive Surgery and (Hyperthermic) IPEC
In recent years, there is an increased interest in the impact of a disease as well as its treatment on the quality of life in patients with cancer.73 In ovarian cancer, cytoreductive surgery and adjuvant (intravenous) chemotherapy have clearly been shown to result in better survival and improved quality of life.74,75 Only 2 interrelated studies have been published focusing on quality of life after cytoreductive surgery followed by (hyperthermic) IPEC for the treatment of PC in nongynecologic malignancy. McQuellon et al76 investigated the quality of life of 64 patients with various peritoneal surface malignancies, 16 of whom of colonic origin, in the first year after cytoreductive surgery and (hyperthermic) IPEC. Quality of life was assessed by means of the Functional Assessment of Cancer Therapy-Colon scale, analysis of various activities of daily living, the Brief Pain Inventory, the Center for Epidemiologic Studies-Depression scale, and the Eastern Cooperative Oncology Group performance status rating scale. Before surgery, patients with ascites had a significantly lower quality of life as compared with patients without ascites. However, patients with ascites reported an improved overall quality of life immediately after surgery, whereas those patients without ascites reported a decreased quality of life during the first 3 months after the surgery. From 3 months postoperatively onwards, quality of life improved relative to baseline. At 1 year after surgery, 58% of patients reported a normal performance status rating, whereas 14% had to spend extra time in bed during the day due to disease- or treatment-related symptoms. The mean scores at activities of daily living, however, were still lower than the general population norm, even after successful treatment and symptom reduction.
In a second publication, McQuellon et al77 reported the quality of life of 17 patients who had survived more than 3 years after cytoreductive surgery and (hyperthermic) IPEC for PC. Sixteen patients reported no limitations on moderate activities, whereas 10 patients described their health as very good or excellent.
Controlled Studies
To date, 3 controlled studies have been published verifying the efficacy of cytoreductive surgery and (hyperthermic) IPEC. Of these studies, 2 were randomized.
Mahteme et al54 compared 18 patients with PC of colorectal origin, who had been treated with cytoreductive surgery and early postoperative IPEC (5-FU, cisplatinum, or irinotecan) as well as intravenous 5-FU-based chemotherapy, with 18 matched control patients with PC, who received intravenous chemotherapy only. The median survival as well as the 2-year and 5-year survival rates of the patients who were treated with cytoreductive surgery and IPEC were significantly better than those of the control group (32 months versus 14 months, and 60% versus 10% and 28% versus 5%, respectively, P = 0.01). The authors concluded that, although selection bias may have influenced the results, the results indicate that cytoreductive surgery followed by IPEC can be beneficial and result in complete remission of the disease for a prolonged period of time.
The seemingly better results of the aggressive surgical approach with respect to survival led Verwaal et al36 to conduct a RCT, investigating the efficacy of this treatment as compared with merely palliative treatment, consisting of systemic chemotherapy and surgery when indicated. A total of 105 patients with established PC of colorectal or appendiceal origin, without hematogenous metastases, were randomized to be treated with either cytoreductive surgery and hyperthermic IPEC using MMC followed by systemic 5-FU/LV based chemotherapy or systemic chemotherapy alone and palliative surgery when necessary. Despite the rather high postoperative mortality of 8%, median survival after surgical cytoreduction and (hyperthermic) IPEC was 22.3 months, which was significantly better (P = 0.032) than the median survival of 12.6 months, obtained in the control arm. Several comments on this RCT seem justified. First, in both treatment arms, patients had surgical interventions, the effect of which remains unknown. Second, patients in the standard treatment arm were treated with 5-FU-based chemotherapy. Several new cytostatic agents, such as irinotecan and oxaliplatin, have been introduced. When combined with 5-FU/LV, both irinotecan and oxaliplatin have been shown to be superior to 5-FU/LV alone in advanced CRC.78–81 Therefore, it cannot be excluded that the observed survival difference between the patients treated with cytoreductive surgery, (hyperthermic) IPEC, and systemic 5-FU/LV versus the patients treated with systemic 5-FU/LV and palliative surgery may become less pronounced, when chemotherapy is changed to a combination of 5-FU/LV and irinotecan and/or oxaliplatin.
The second RCT was conducted by Elias et al,50 who randomized patients with established PC to be treated with cytoreductive surgery with or without early postoperative IPEC. Unfortunately, due to difficulties in patient recruitment, the trial was prematurely terminated after 35 patients. Two-year survival rate after cytoreductive surgery was 60%. Early postoperative IPEC had no measurable effect on treatment outcome.
CONCLUSION
Improved insights into the mechanisms and incidence of intraperitoneal spread of CRC have contributed to a better understanding and a different perception of the pathologic basis of PC. Even though the reported incidence rates of intraperitoneally exfoliated cancer cells during resection of primary tumors varied widely, the presence of free tumor cells in the peritoneal cavity of some patients was repeatedly demonstrated in all studies. Although the presence of exfoliated tumor cells in the peritoneal cavity, similar to micrometastases in blood or bone marrow,82–84 may not be an independent prognostic factor, it seems plausible that these tumor cells may indeed contribute to intraperitoneal treatment failure. Indeed, in 2 studies, a correlation was found between the presence of free tumor cells in the peritoneal cavity and intraperitoneal tumor recurrence.12,18
Despite the favorable results of cytoreductive surgery and adjuvant (hyperthermic) IPEC in patients with PC of colorectal origin, the results should be interpreted with caution for several reasons. First, the reported trials differed significantly with regard to patient selection. In some trials, patients with hematogenous metastases were eligible for inclusion, whereas other patient series also included patients with appendiceal carcinomatosis or pseudomyxoma peritonei. The latter 2 disease entities may have a relatively favorable prognosis as compared with PC of colorectal origin. Second, the design of the combination treatment inflicted on the patients differed widely with regard to the timing of the IPEC (preoperative, intraoperative, postoperative, or combinations), the method of administration of the chemotherapy (open or closed abdomen), the cytostatic agent(s) used and whether or not chemotherapy was given under hyperthermic conditions. Third, the patient series published to date all originate from specialized tertiary referral centers.
Nonetheless, the results in terms of survival after cytoreductive surgery and (hyperthermic) IPEC appear much better than those obtained in historical controls and have, indeed, been shown to be superior to conventional chemotherapy-based treatment in one RCT.36 Given the high morbidity and mortality rates of cytoreductive surgery and (hyperthermic) IPEC as well as the high failure rate of 80%, it is of the utmost importance to select only those patients for this treatment modality that could benefit the most. Univariate and multivariate analysis of several clinicopathologic parameters repeatedly showed the extent of the carcinomatosis and consequently, the extent of surgery to be the most important factors related to postoperative morbidity and mortality. In all patient series, postoperative morbidity and mortality were predominantly determined by surgery-related factors, such as the number of anastomoses, peritonectomy procedures, and the amount of blood loss. Toxicity clearly attributable to the hyperthermic IPEC was relatively rare. Furthermore, despite the differences in timing and methods of intraperitoneally administering chemotherapy, the completeness of resection has proven to be the most important prognostic factor predictive of survival in almost all patient series reported to date. Patients, in whom complete resection of all macroscopic disease was not feasible, invariably showed a dismal prognosis similar to that of historical controls.61 Interestingly, complete resection frequently resulted in survival rates comparable to those obtained after resection of liver metastases.85 Cytoreductive surgery and (hyperthermic) IPEC should therefore only be offered to patients with limited and resectable disease.
According to Sugarbaker86 as well as Verwaal,87 the time has come to accept cytoreductive surgery and (hyperthermic) IPEC as one of the standard treatments for patients with limited carcinomatosis. Sugarbaker argues that the acquisition of level I evidence by meta-analysis of data from several well-designed RCTs may not be necessary for experimental therapies to mature into standard of care. For example, while there has never been an RCT confirming the superiority of resection of liver metastases of colorectal origin over systemic chemotherapy, surgery is generally accepted as standard of care in selected patients with liver metastases of colorectal origin. Others recognize the efficacy of the cytoreductive surgery but argue that, based on only one RCT, it is too early to accept it as part of a procedure in which (hyperthermic) IPEC is applied.88 With the completeness of resection being the most important prognostic factor, it could, indeed, be questioned whether (hyperthermic) IPEC is mandatory after complete resection. For that reason, Mansfield88 recognizes the need to move forward and pleads for a new multicenter RCT, comparing cytoreductive surgery followed by (hyperthermic) IPEC and systemic chemotherapy with cytoreductive surgery and systemic chemotherapy alone, resembling the design of the RCT conducted by Elias et al.50 Indeed, such a trial is currently considered at the Netherlands Cancer Institute.
Given the small number of medical centers worldwide currently practicing cytoreductive surgery and (hyperthermic) IPEC for the treatment of PC, larger-scale application of this treatment modality may be challenging. Both the surgery and the administration of (hyperthermic) IPEC are technically demanding procedures, for which a learning curve exists.86 The further implementation of cytoreductive surgery and (hyperthermic) IPEC for the management of PC of colorectal origin should therefore be pursued with caution and should still be applied as part of controlled trials.
Footnotes
Supported in part by a grant from the Netherlands Organization for Health Research and Development (ZonMw) (Grant No. 920-03-220).
Reprints: Manuel J. Koppe, MD, Department of Surgery, Radboud University Nijmegen Medical Center, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands. E-mail: m.koppe@chir.umcn.nl.
REFERENCES
- 1.Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. 1996;82:79–100. [DOI] [PubMed] [Google Scholar]
- 2.Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin North Am. 2003;12:585–603. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee A, Parvaiz A, Cecil TD, et al. Pseudomyxoma peritonei usually originates from the appendix: a review of the evidence. Eur J Gynaecol Oncol. 2004;25:411–414. [PubMed] [Google Scholar]
- 4.Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy: a prospective study of prognostic factors. Cancer. 1989;63:364–367. [DOI] [PubMed] [Google Scholar]
- 5.Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16:83–97. [PubMed] [Google Scholar]
- 6.Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol. 1998;14:254–261. [DOI] [PubMed] [Google Scholar]
- 7.Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon's role. Langenbecks Arch Surg. 1999;384:576–587. [DOI] [PubMed] [Google Scholar]
- 8.Ceelen WP, Hesse U, De Hemptinne B, et al. Hyperthermic intraperitoneal chemoperfusion in the treatment of locally advanced intra-abdominal cancer. Br J Surg. 2000;87:1006–1015. [DOI] [PubMed] [Google Scholar]
- 9.Bosch B, Guller U, Schnider A, et al. Perioperative detection of disseminated tumour cells is an independent prognostic factor in patients with colorectal cancer. Br J Surg. 2003;90:882–888. [DOI] [PubMed] [Google Scholar]
- 10.Guller U, Zajac P, Schnider A, et al. Disseminated single tumor cells as detected by real-time quantitative polymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Ann Surg. 2002;236:768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horattas MC, Evasovich MR, Topham N. Colorectal carcinoma and the relationship of peritoneal cytology. Am J Surg. 1997;174:334–337. [DOI] [PubMed] [Google Scholar]
- 12.Kanellos I, Demetriades H, Zintzaras E, et al. Incidence and prognostic value of positive peritoneal cytology in colorectal cancer. Dis Colon Rectum. 2003;46:535–539. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Milsom JW, Gramlich TL, et al. Does laparoscopic versus conventional surgery increase exfoliated cancer cells in the peritoneal cavity during resection of colorectal cancer? Dis Colon Rectum. 1998;41:971–978. [DOI] [PubMed] [Google Scholar]
- 14.Ojima H, Sasaki S, Fujisawa T, et al. Utility of serosal stamp cytology as an indicator for high-risk peritoneal metastasis in colorectal cancer surgery. Hepatogastroenterology. 2003;50:87–90. [PubMed] [Google Scholar]
- 15.Solomon MJ, Egan M, Roberts RA, et al. Incidence of free colorectal cancer cells on the peritoneal surface. Dis Colon Rectum. 1997;40:1294–1298. [DOI] [PubMed] [Google Scholar]
- 16.Uras C, Altinkaya E, Yardimci H, et al. Peritoneal cytology in the determination of free tumour cells within the abdomen in colon cancer. Surg Oncol. 1996;5:259–263. [DOI] [PubMed] [Google Scholar]
- 17.Wind P, Norklinger B, Roger V, et al. Long-term prognostic value of positive peritoneal washing in colon cancer. Scand J Gastroenterol. 1999;34:606–610. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto S, Akasu T, Fujita S, et al. Long-term prognostic value of conventional peritoneal cytology after curative resection for colorectal carcinoma. Jpn J Clin Oncol. 2003;33:33–37. [DOI] [PubMed] [Google Scholar]
- 19.Yang SH, Lin JK, Lai CR, et al. Risk factors for peritoneal dissemination of colorectal cancer. J Surg Oncol. 2004;87:167–173. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Z, Cohen AM, Hajdu S, et al. Serosal cytologic study to determine free mesothelial penetration of intraperitoneal colon cancer. Cancer. 1992;70:737–740. [DOI] [PubMed] [Google Scholar]
- 21.Cass AW, Million RR, Pfaff WW. Patterns of recurrence following surgery alone for adenocarcinoma of the colon and rectum. Cancer. 1976;37:2861–2865. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg B, Decosse JJ, Harford F, et al. Carcinoma of the colon and rectum: the natural history reviewed in 1704 patients. Cancer. 1982;49:1131–1134. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert JM, Jeffrey I, Evans M, et al. Sites of recurrent tumour after ‘curative’ colorectal surgery: implications for adjuvant therapy. Br J Surg. 1984;71:203–205. [DOI] [PubMed] [Google Scholar]
- 24.Malcolm AW, Perencevich NP, Olson RM, et al. Analysis of recurrence patterns following curative resection for carcinoma of the colon and rectum. Surg Gynecol Obstet. 1981;152:131–136. [PubMed] [Google Scholar]
- 25.Mendenhall WM, Million RR, Pfaff WW. Patterns of recurrence in adenocarcinoma of the rectum and rectosigmoid treated with surgery alone: implications in treatment planning with adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:977–985. [DOI] [PubMed] [Google Scholar]
- 26.Minsky BD, Mies C, Recht A, et al. Resectable adenocarcinoma of the rectosigmoid and rectum: I. Patterns of failure and survival. Cancer. 1988;61:1408–1416. [DOI] [PubMed] [Google Scholar]
- 27.Olson RM, Perencevich NP, Malcolm AW, et al. Patterns of recurrence following curative resection of adenocarcinoma of the colon and rectum. Cancer. 1980;45:2969–2974. [DOI] [PubMed] [Google Scholar]
- 28.Russell AH, Tong D, Dawson LE, et al. Adenocarcinoma of the proximal colon: sites of initial dissemination and patterns of recurrence following surgery alone. Cancer. 1984;53:360–367. [DOI] [PubMed] [Google Scholar]
- 29.Gunderson LL, Sosin H, Levitt S. Extrapelvic colonāreas of failure in a reoperation series: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1985;11:731–741. [DOI] [PubMed] [Google Scholar]
- 30.Tong D, Russell AH, Dawson LE, et al. Second laparotomy for proximal colon cancer: sites of recurrence and implications for adjuvant therapy. Am J Surg. 1983;145:382–386. [DOI] [PubMed] [Google Scholar]
- 31.Russell AH, Pelton J, Reheis CE, et al. Adenocarcinoma of the colon: an autopsy study with implications for new therapeutic strategies. Cancer. 1985;56:1446–1451. [DOI] [PubMed] [Google Scholar]
- 32.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. [DOI] [PubMed] [Google Scholar]
- 33.Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. [DOI] [PubMed] [Google Scholar]
- 34.Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy: a prospective study of prognostic factors. Cancer. 1989;63:364–367. [DOI] [PubMed] [Google Scholar]
- 35.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. [DOI] [PubMed] [Google Scholar]
- 36.Verwaal VJ, Van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. [DOI] [PubMed] [Google Scholar]
- 37.Meigs JV. Tumors of the Female Pelvic Organs. New York: McMillan, 1934. [Google Scholar]
- 38.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–104. [PubMed] [Google Scholar]
- 39.Griffiths CT, Parker LM, Fuller AF Jr. Role of cytoreductive surgical treatment in the management of advanced ovarian cancer. Cancer Treat Rep. 1979;63:235–240. [PubMed] [Google Scholar]
- 40.Munnell EW. The changing prognosis and treatment in cancer of the ovary: a report of 235 patients with primary ovarian carcinoma 1952–1961. Am J Obstet Gynecol. 1968;100:790–805. [DOI] [PubMed] [Google Scholar]
- 41.Spratt JS, Adcock RA, Sherrill W, et al. Hyperthermic peritoneal perfusion system in canines. Cancer Res. 1980;40:253–255. [PubMed] [Google Scholar]
- 42.Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–260. [PubMed] [Google Scholar]
- 43.Sugarbaker PH, Kern K, Lack E. Malignant pseudomyxoma peritonei of colonic origin: natural history and presentation of a curative approach to treatment. Dis Colon Rectum. 1987;30:772–779. [DOI] [PubMed] [Google Scholar]
- 44.Sugarbaker PH. Surgical management of peritoneal carcinosis: diagnosis, prevention and treatment. Langenbecks Arch Chir. 1988;373:189–196. [DOI] [PubMed] [Google Scholar]
- 45.Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin North Am. 2003;12:703–727. [DOI] [PubMed] [Google Scholar]
- 46.Sticca RP, Dach BW. Rationale for hyperthermia with intraoperative intraperitoneal chemotherapy agents. Surg Oncol Clin North Am. 2003;12:689–701. [DOI] [PubMed] [Google Scholar]
- 47.Sarnaik AA, Sussman JJ, Ahmad SA, et al. Technology of intraperitoneal chemotherapy administration: a survey of techniques with a review of morbidity and mortality. Surg Oncol Clin North Am. 2003;12:849–863. [DOI] [PubMed] [Google Scholar]
- 48.Carmignani CP, Ortega-Perez G, Sugarbaker PH. The management of synchronous peritoneal carcinomatosis and hematogenous metastasis from colorectal cancer. Eur J Surg Oncol. 2004;30:391–398. [DOI] [PubMed] [Google Scholar]
- 49.Culliford AT, Brooks AD, Sharma S, et al. Surgical debulking and intraperitoneal chemotherapy for established peritoneal metastases from colon and appendix cancer. Ann Surg Oncol. 2001;8:787–795. [DOI] [PubMed] [Google Scholar]
- 50.Elias D, Delperro JR, Sideris L, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol. 2004;11:518–521. [DOI] [PubMed] [Google Scholar]
- 51.Elias D, Pocard M, Sideris L, et al. Preliminary results of intraperitoneal chemohyperthermia with oxaliplatin in peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:455–456. [DOI] [PubMed] [Google Scholar]
- 52.Glehen O, Cotte E, Schreiber V, et al. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:747–754. [DOI] [PubMed] [Google Scholar]
- 53.Jacquet P, Stephens AD, Averbach AM, et al. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer. 1996;77:2622–2629. [DOI] [PubMed] [Google Scholar]
- 54.Mahteme H, Hansson J, Berglund A, et al. Improved survival in patients with peritoneal metastases from colorectal cancer: a preliminary study. Br J Cancer. 2004;90:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pilati P, Mocellin S, Rossi CR, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10:508–513. [DOI] [PubMed] [Google Scholar]
- 56.Schneebaum S, Arnold MW, Staubus A, et al. Intraperitoneal hyperthermic perfusion with mitomycin C for colorectal cancer with peritoneal metastases. Ann Surg Oncol. 1996;3:44–50. [DOI] [PubMed] [Google Scholar]
- 57.Shen P, Hawksworth J, Lovato J, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11:178–186. [DOI] [PubMed] [Google Scholar]
- 58.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugarbaker PH, Schellinx ME, Chang D, et al. Peritoneal carcinomatosis from adenocarcinoma of the colon. World J Surg. 1996;20:585–591. [DOI] [PubMed] [Google Scholar]
- 60.Verwaal VJ, van Tinteren H, Van Ruth S, et al. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2004;91:739–746. [DOI] [PubMed] [Google Scholar]
- 61.Verwaal VJ, Boot H, Aleman BM, et al. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: location, treatment, and outcome. Ann Surg Oncol. 2004;11:375–379. [DOI] [PubMed] [Google Scholar]
- 62.Verwaal VJ, Zoetmulder FA. Follow-up of patients treated by cytoreduction and chemotherapy for peritoneal carcinomatosis of colorectal origin. Eur J Surg Oncol. 2004;30:280–285. [DOI] [PubMed] [Google Scholar]
- 63.Verwaal VJ, van Tinteren H, Ruth SV, et al. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol. 2004;85:61–67. [DOI] [PubMed] [Google Scholar]
- 64.Witkamp AJ, de Bree E, Kaag MM, Boot H, et al. Extensive cytoreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer. 2001;37:979–984. [DOI] [PubMed] [Google Scholar]
- 65.Kecmanovic DM, Pavlov MJ, Ceranic MS, et al. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31:147–152. [DOI] [PubMed] [Google Scholar]
- 66.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. [DOI] [PubMed] [Google Scholar]
- 67.Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–76. [DOI] [PubMed] [Google Scholar]
- 68.Verwaal VJ, Van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. [DOI] [PubMed] [Google Scholar]
- 69.Esquivel J, Vidal-Jove J, Steves MA, et al. Morbidity and mortality of cytoreductive surgery and intraperitoneal chemotherapy. Surgery. 1993;113:631–636. [PubMed] [Google Scholar]
- 70.Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863–869. [DOI] [PubMed] [Google Scholar]
- 71.Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790–796. [DOI] [PubMed] [Google Scholar]
- 72.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with carcinomatosis. In: Sugarbaker PH, ed. Peritoneal Carcinomatosis: Principles of Management. Boston: Kluwer Academic, 1996:359–374. [DOI] [PubMed] [Google Scholar]
- 73.Langenhoff BS, Krabbe PF, Wobbes T, et al. Quality of life as an outcome measure in surgical oncology. Br J Surg. 2001;88:643–652. [DOI] [PubMed] [Google Scholar]
- 74.Eisenkop SM, Friedman RL, Wang HJ. Secondary cytoreductive surgery for recurrent ovarian cancer: a prospective study. Cancer. 1995;76:1606–1614. [DOI] [PubMed] [Google Scholar]
- 75.Le T, Leis A, Pahwa P, et al. Quality-of-life issues in patients with ovarian cancer and their caregivers: a review. Obstet Gynecol Surv. 2003;58:749–758. [DOI] [PubMed] [Google Scholar]
- 76.McQuellon RP, Loggie BW, Fleming RA, et al. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27:65–73. [DOI] [PubMed] [Google Scholar]
- 77.McQuellon RP, Loggie BW, Lehman AB, et al. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2003;10:155–162. [DOI] [PubMed] [Google Scholar]
- 78.Chau I, Cunningham D. Adjuvant therapy in colon cancer: current status and future directions. Cancer Treat Rev. 2002;28:223–236. [DOI] [PubMed] [Google Scholar]
- 79.Comella P, Casaretti R, Crucitta E, et al. Oxaliplatin plus raltitrexed and leucovorin-modulated 5-fluorouracil i.v. bolus: a salvage regimen for colorectal cancer patients. Br J Cancer. 2002;86:1871–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Comella P, Crucitta E, De Vita F, et al. Addition of either irinotecan or methotrexate to bolus 5-fluorouracil and high-dose folinic acid every 2 weeks in advanced colorectal carcinoma: a randomised study by the Southern Italy Cooperative Oncology Group. Ann Oncol. 2002;13:866–873. [DOI] [PubMed] [Google Scholar]
- 81.Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059–2069. [DOI] [PubMed] [Google Scholar]
- 82.Vlems FA, Ruers TJ, Punt CJ, et al. Relevance of disseminated tumour cells in blood and bone marrow of patients with solid epithelial tumours in perspective. Eur J Surg Oncol. 2003;29:289–302. [DOI] [PubMed] [Google Scholar]
- 83.Vlems FA, Wobbes T, Punt CJ, et al. Detection and clinical relevance of tumor cells in blood and bone marrow of patients with colorectal cancer. Anticancer Res. 2003;23:523–530. [PubMed] [Google Scholar]
- 84.Vlems FA, Diepstra JH, Punt CJ, et al. Detection of disseminated tumour cells in blood and bone marrow samples of patients undergoing hepatic resection for metastasis of colorectal cancer. Br J Surg. 2003;90:989–995. [DOI] [PubMed] [Google Scholar]
- 85.Gertsch P. A historical perspective on colorectal liver metastases and peritoneal carcinomatosis: similar results, different treatments. Surg Oncol Clin North Am. 2003;12:531–541. [DOI] [PubMed] [Google Scholar]
- 86.Sugarbaker PH. Clinical research to standard of care: when does the transition occur? Ann Surg Oncol. 2003;10:825–826. [DOI] [PubMed] [Google Scholar]
- 87.Verwaal VJ. Cytoreduction and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis of colorectal origin [Thesis/Dissertation]. Amsterdam, University of Amsterdam, 2004. [Google Scholar]
- 88.Mansfield PF. Management of peritoneal carcinomatosis: is an answer at hand? Ann Surg Oncol. 2003;10:827–828. [DOI] [PubMed] [Google Scholar]