Abstract
Objective:
To determine if routine follow-up by magnetic resonance imaging (MRI) improves the detection of resectable local recurrences from colorectal cancer.
Summary Background Data:
Surgical treatment offers the best prospect of survival for patients with recurrent colorectal cancer. Unfortunately, most cases are often diagnosed at an unresectable stage when traditional follow-up methods are used. The impact of MRI surveillance on the early diagnosis of local recurrences has yet to be ascertained.
Methods:
Patients who underwent curative surgery for rectal and left-sided colon tumors were included in a program of pelvic surveillance by routine MRI, in addition to the standard follow-up protocol. Cases were then analyzed for mode of diagnosis, resectability, and overall survival.
Results:
Pelvic recurrence was found in 30 (13%) of the 226 patients studied. MRI detected 26 of 30 (87%) and missed 4 of 30 (13%) cases with local recurrence. Of the latter, 3 were anastomotic recurrences. In 28 (14%) patients, local recurrence was suspected by an initial MR scan but cleared by subsequent MRI or CT-guided biopsy. Recurrent pelvic cancer was diagnosed by MRI with 87% sensitivity and 86% specificity. In 19 (63%) cases, CEA was abnormally elevated, and 9 patients (30%) were symptomatic. Surgical resection was possible in only 6 patients (20%). There was no difference between MRI and conventional follow-up tests in their ability to detect cases suitable for surgery.
Conclusions:
Pelvic surveillance by MRI is not justified as part of the routine follow-up after a curative resection for colorectal cancer and should be reserved for selectively imaging patients with clinical, colonoscopic, and/or biochemical suspicion of recurrent disease.
The optimal composition of intensive follow-up programs after curative surgery for colorectal cancer still needs defining. MRI surveillance has little impact on the detection of local recurrence amenable to further surgery when compared with more traditional follow-up policies and should not be used routinely.
Despite the widespread use of total mesorectal excision and perioperative radiotherapy, between 2.6% and 32% of patients with rectal cancer with will go on to develop a pelvic recurrence after initial curative surgery.1 Additionally, local recurrence complicates the postoperative history of up to 20% of colonic cancers.2 The development of local recurrence after surgery for colorectal cancer is responsible not only for significant mortality and morbidity,3 but also for impaired quality of life.4
Surgical resection alone or, more often, combined with radiation therapy and chemotherapy has been advocated as offering survival5–7 and cost8 advantages, as well as a better quality of life6,8 for patients with locally recurrent colorectal cancer. Using an aggressive therapeutic approach, overall 5-year survival rates of 20% to 30% can be obtained in highly selected patients.9–11 However, only 20% to 30% of patients with local recurrences detected by traditional follow-up strategies12 have tumors that are deemed resectable at the time of diagnosis.11,13 Considering that surgical treatment has significantly more chances of success in asymptomatic cases with limited disease,9,14 the early detection of local recurrence could increase the proportion of resectable cases and confer survival advantages. Because the majority of local recurrences have their origin in the tumor bed,15 incipient recurrent disease could easily be missed if only endoscopy is used at follow-up. This underlines the importance of being able to visualize the perirectal tissues as part of the postoperative surveillance strategy.
Magnetic resonance imaging (MRI) is one of the most accurate methods of diagnosing pelvic recurrences from colorectal tumors,16,17 although it cannot always reliably differentiate between scar tissue and recurrent disease;18,19 hence, repeat scans and/or computed tomography (CT)-guided biopsies may be necessary. Recent studies have shown that MRI may be superseded in the future by the use of 18-fluoro-2-deoxyglucose-positron emission tomography, which has the advantage of overall better specificity, sensitivity, and the ability to detect synchronous distant metastases with great accuracy.20,21 Nevertheless, MRI remains one of the best imaging tests for diagnosing colorectal recurrence in the pelvis.22
The aim of this study was to determine if a policy of pelvic surveillance by routine MRI after curative surgery for colorectal tumors has any measurable impact on the detection of resectable local recurrent disease and overall patient survival.
PATIENTS AND METHODS
Patients
A cohort of 226 patients (Table 1) who underwent curative surgery for colorectal cancer between 1995 and 1999 were included in a program of intensive follow-up using routine MR scanning at 3- to 6-month intervals, in addition to standard colonoscopic, clinical, and blood tests. All specimens had clear resection margins on histopathologic examination. Only patients with rectal tumors or left-sided colon cancers in whom an anastomosis within the pelvis was fashioned were considered for this study.
TABLE 1. Characteristics of Patients Included in the MRI Surveillance Program
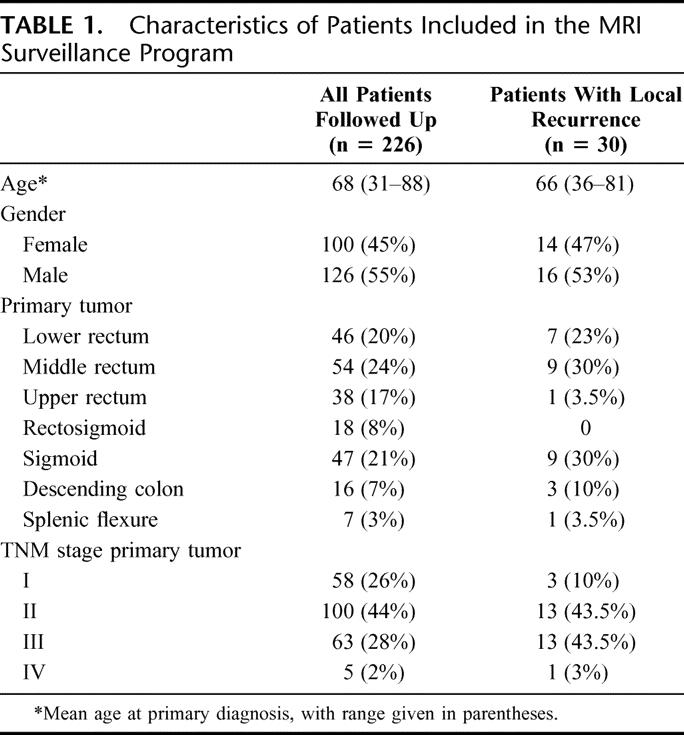
The median clinical follow-up was 42 months (interquartile range, 28–53 months), with a median MRI surveillance period of 21 (interquartile range, 12–27 months). The median number of MR scans performed per patient was 3 (interquartile range, 2–4).
Methods
Hospital records were analyzed retrospectively to identify patients who developed local recurrences during the follow-up period. The separate contribution of MRI, colonoscopy, CEA monitoring, and clinical examination to the final diagnosis was assessed for all cases with disease recurrence in the pelvis, as well as for the subgroup that were considered suitable for surgical resection. Relevant outcome data were also recorded.
Results are reported as confirmed metastases either with histologic diagnosis from CT-guided or open excision biopsy. Progression of disease both on clinical and biochemical follow-up, as well as on serial MRI (at lest 2 further scans 3 months apart) was also accepted as confirmatory of malignancy. Diagnosis as benign was based on biopsy or at least 1 year's clinical follow-up with no symptoms of recurrence and normal laboratory results, and no progression of the suspicious mass on subsequent scans.
Statistical Analysis
Results are expressed as mean (standard deviation) for normal, and median (interquartile range) for skew distribution. Comparison of proportions was made using the 2-sided Liddell's test for matched pairs. Statistical analysis was performed using the SPSS package version 9.0 (SPSS Inc., Chicago, IL). P values <0.05 were considered significant.
MR Scanning Technique
Scans were undertaken using a 1.5-T MR scanner (Signa Advantage, IGE Medical Systems, Coolidge House, Slough, UK) with a pelvic phased-array coil (IGE Medical Systems, Coolidge House) for image acquisition. Standard scan sequences were used, namely, T1-weighted axial spin echo (TR 600, TE 11, slice thickness 8 mm, gap 2 mm, NEX 3, matrix 256 × 224), T2-weighted axial fast spin echo (TR 3600–4000, TE 102, slice thickness 8 mm, gap 2 mm, NEX 3, matrix 256 × 224) and fat-suppressed T2-sagittal (TR 3000, TE 85, slice thickness 5 mm, gap 2 mm, NEX 3, matrix 256 × 224).
Any suspicious case underwent a repeat dynamic contrast-enhanced MRI within 1 to 3 months of the initial examination. A bolus intravenous injection of gadolinium-DTPA (Magnevist, Schering Health Care Ltd, Burgess Hill, UK) at a dose of 0.2 mmol/kg was administered via an antecubital vein immediately following acquisition of the first image in each slice.
RESULTS
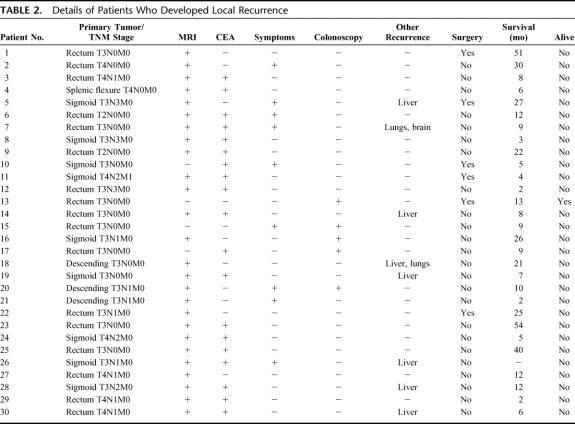
Local recurrence was observed in 30 of 226 (13%) patients who underwent regular pelvic surveillance by MRI (Table 2). Of these, 8 (41%) also had distant metastatic disease. In 26 cases, the recurrent tumor was localized in the perirectal tissues, whereas 4 patients were found to have anastomotic recurrence. Pelvic recurrence had a significantly lower incidence in patients with UICC stage I tumors, compared with those with more advanced disease (P = 0.03, χ2 test). However, UICC stage II, III, and IV cases behaved in similar manner (P = 0.19 for stage II versus stage III, P = 1 for stage III versus stage IV and P = 1 for stage II versus stage IV). Similarly, there was no difference between the incidence of local recurrence in patients with rectal compared with those with colonic cancer (P = 0.59). The median interval between surgery for the primary tumor and diagnosis of local recurrent disease was 15 months (interquartile range, 12–26 months).
TABLE 2. Details of Patients Who Developed Local Recurrence
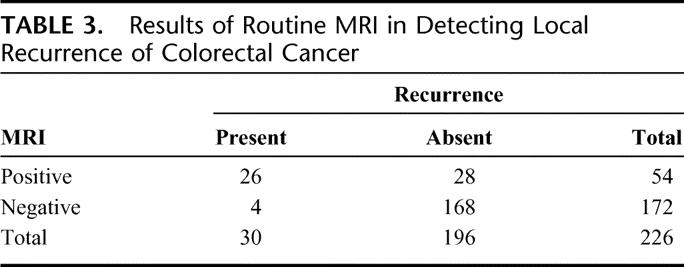
MRI diagnosed 26 (87%) and missed 4 (13%) patients with local recurrence from colorectal cancer. Interestingly, 3 of 4 (75%) anastomotic recurrences were missed by MRI, although all were diagnosed at colonoscopy. In 28 (14%) cases, an initial MRI scan suspected the presence of pelvic recurrence, but this was not confirmed by a repeat MR scan (n = 24) or CT-guided biopsy (n = 4). Overall, MRI diagnosed local recurrence with 87% sensitivity and 86% specificity, with a likelihood ratio of 6.21; the positive and negative predictive values were 48% and 98%, respectively (Table 3).
TABLE 3. Results of Routine MRI in Detecting Local Recurrence of Colorectal Cancer
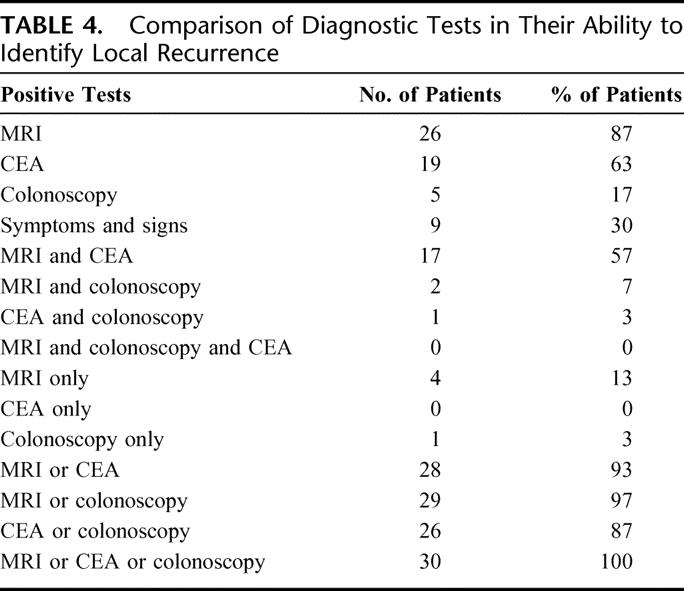
MRI was the only positive diagnostic test in 4 (13%) patients with pelvic recurrence. In all these patients, the recurrent tumor was located within the perirectal tissues. However, only 2 of these cases were deemed to have a resectable tumor. CEA was elevated in 19 (63%) patients, and 9 (30%) cases were symptomatic at the time of diagnosis (Table 4). Colonoscopy was positive in all 4 cases with anastomotic recurrence. MRI failed to confer any statistically significant advantage over the combination of colonoscopy, clinical examination, and blood tests for the diagnostic of locally recurrent colorectal tumors (P = 1, Liddell's test for matched pairs).
TABLE 4. Comparison of Diagnostic Tests in Their Ability to Identify Local Recurrence
Surgical resection of local recurrent disease was possible in 6 (20%) cases. MRI correctly diagnosed 4 of 6 (67%) patients, the same proportion as that diagnosed by conventional follow-up tests. Two of these patients had adjuvant chemo-radiotherapy, 2 had adjuvant radiotherapy, 1 adjuvant chemotherapy, and 1 surgery alone. One patient, who was also diagnosed with liver metastases, underwent a simultaneous liver resection. The median survival time for the 6 cases treated surgically was 19 months (interquartile range, 7–27 months); only 1 of these patients was alive at the time of data collection, 13 months after the recurrence was diagnosed. All deaths in this group were due to rerecurrent tumor in the pelvis.
Of the 24 patients who had unresectable pelvic recurrences, 10 (42%) were treated with a combination of chemotherapy and radiotherapy, 8 (34%) with chemotherapy alone, 2 with radiotherapy alone (8%), and 4 (16%) refused or were unsuitable for any further treatment. For this group, the median survival time was 9 months (interquartile range, 6–16 months). None of these patients was alive at the time of data collection. The majority of deaths were the consequence of recurrent cancer, with the exception of 2 cases, who died with chest infection and stroke, respectively.
DISCUSSION
Our results show that routine pelvic MRI examination after curative surgery for colorectal tumors helped identify 4 (13%) patients with local recurrence in which this diagnosis would have been delayed or even missed if only clinical examination, endoscopy, and blood tests were performed at follow-up. However, of these 4 patients, only 2 were suitable for surgical resection and had a postoperative survival time of 25 and 51 months, respectively; unfortunately, none of them is still alive. In absolute terms, one can affirm that routine MRI pelvic surveillance offered a potential advantage in terms of management and survival to less than 1% of the patients included in this program.
Acknowledging the relatively low number of patients with pelvic recurrence and the limitations of a retrospective study, these data strongly question the use of routine pelvic imaging in the postoperative follow-up of colorectal cancer patients. If one considers that 576 MR scans have been performed in 226 patients only to detect 4 (<2%) cases with local recurrence that would potentially have been missed by other tests, this does not seem to be an efficient use of an expensive and scanty resource; moreover, only 2 of those were suitable for surgical resection.
Unfortunately, although the value of patient follow-up after curative surgery for colorectal cancers has been addressed by several studies,23–26 work done on the impact of routine imaging as part of follow-up programs is limited,27 and there are as yet no reports on the routine use of MRI for postoperative pelvic surveillance.
In an earlier study, 216 patients who underwent curative surgery for rectal cancer were entered into an intensive follow-up program, while 89 similar cases were followed in the community by their general practitioner.28 The composition of the follow-up program was similar to that used by us, with the difference that the abdomen and pelvis were routinely imaged by ultrasonography. After a median follow-up of 42 months, there was no significant difference either in the rate of curative-intent surgery or clinical outcome between the 2 groups for patients that developed pelvic recurrence. The observed local recurrence (26.8%) and reoperation (30.5%) rates, and the median survival (15 months) were similar to those found in our cohort of patients.
A prospective randomized study by Makela et al29 found that yearly CT scans through the site of the primary resection failed to improve the diagnosis and resection rates for locally recurrent colorectal tumors, compared with sole CEA monitoring. Similar results were reported by Ohlsson et al30 in patients followed up by pelvic CT every 3 to 6 months after an abdominopelvic resection for rectal cancer. Despite being able to identify all 4 cases with pelvic recurrence, routine CT added nothing to the diagnosis if one considers that all these patients were symptomatic when the scans were performed. Another prospective randomized study by Pietra et al31 found that yearly abdominal CT scans provided the initial diagnosis in only 1 of 26 patients with local recurrence and had no place in the routine follow-up of colorectal cancer patients. However, clinical and endoscopic examinations, and CEA monitoring every 3 months, resulted in significantly more operable cases being diagnosed and improved survival rates for patients in the intensive follow-up arm of the study.
The work published so far does not support the use of routine postoperative pelvic imaging as part of the follow-up program for colorectal cancer patients. However, the more recent studies are retrospective, and the prospective studies published so far date from an era when the treatment of pelvic recurrences was less aggressive than nowadays, providing strong support for the necessity of future prospective randomized studies on large number of patients that will address this issue in a more rigorous manner.
CONCLUSION
This study suggests that pelvic MRI has little to offer when used as a routine surveillance tool following curative surgery for colorectal tumors. The minimal benefit observed is largely superseded by cost and patient anxiety caused by the large proportion (14%) of initial false-positive examinations. In our opinion, MR examination of the pelvis should be reserved for imaging cases with suspected pelvic recurrence on the basis of clinical, endoscopic, and/or blood examinations. A final decision about the composition of an ideal follow-up program can only be reached after more prospective randomized trials are undertaken.
Footnotes
Presented as a poster at the XIXth Biennial Congress of the International Society of University Colon and Rectal Surgeons, Osaka, Japan, April 2002.
Reprints: John R. T. Monson, MD, Academic Surgical Unit, Castle Hill Hospital, Castle Road, Cottingham, East Yorkshire HU16 5JQ, UK. E-mail: J.R.Monson@hull.ac.uk.
REFERENCES
- 1.Abulafi AM, Williams NS. Local recurrence of colorectal cancer: the problem, mechanisms, management and adjuvant therapy. Br J Surg. 1994;81:7–19. [DOI] [PubMed] [Google Scholar]
- 2.Turk PS, Wanebo HJ. Results of surgical treatment of nonhepatic recurrence of colorectal carcinoma. Cancer. 1993;71:4267–4277. [DOI] [PubMed] [Google Scholar]
- 3.Miller AR. Multidisciplinary management of recurrent colorectal cancer. Surg Oncol. 1998;7:209–221. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri-Brennan J, Steele RJ. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol. 2001;27:349–353. [DOI] [PubMed] [Google Scholar]
- 5.Rodel C, Grabenbauer GG, Matzel KE, et al. Extensive surgery after high-dose preoperative chemoradiotherapy for locally advanced recurrent rectal cancer. Dis Colon Rectum. 2000;43:312–319. [DOI] [PubMed] [Google Scholar]
- 6.Sagar PM, Pemberton JH. Surgical management of locally recurrent rectal cancer. Br J Surg. 1996;83:293–304. [DOI] [PubMed] [Google Scholar]
- 7.Lowy AM, Rich TA, Skibber JM, et al. Preoperative infusional chemoradiation, selective intraoperative radiation, and resection for locally advanced pelvic recurrence of colorectal adenocarcinoma. Ann Surg. 1996;223:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AR, Cantor SB, Peoples GE, et al. Quality of life and cost effectiveness analysis of therapy for locally recurrent rectal cancer. Dis Colon Rectum. 2000;43:1695–1701. [DOI] [PubMed] [Google Scholar]
- 9.Huguier M, Houry S, Barrier A. Local recurrence of cancer of the rectum. Am J Surg. 2001;182:437–439. [DOI] [PubMed] [Google Scholar]
- 10.Wanebo HJ, Antoniuk P, Koness RJ, et al. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 1999;42:1438–1448. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg RM, Fleming TR, Tangen CM, et al. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection. Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med. 1998;129:27–35. [DOI] [PubMed] [Google Scholar]
- 12.Vernava AM 3rd, Longo WE, Virgo KS, et al. Current follow-up strategies after resection of colon cancer: results of a survey of members of the American Society of Colon and Rectal Surgeons. Dis Colon Rectum. 1994;37:573–583. [DOI] [PubMed] [Google Scholar]
- 13.Ogunbiyi OA, McKenna K, Birnbaum EH, et al. Aggressive surgical management of recurrent rectal cancer: is it worthwhile? Dis Colon Rectum. 1997;40:150–155. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Kostner F, Fazio VW, Vignali A, et al. Locally recurrent rectal cancer: predictors and success of salvage surgery. Dis Colon Rectum. 2001;44:173–178. [DOI] [PubMed] [Google Scholar]
- 15.Wiig JN, Wolff PA, Tveit KM, et al. Location of pelvic recurrence after ‘curative' low anterior resection for rectal cancer. Eur J Surg Oncol. 1999;25:590–594. [DOI] [PubMed] [Google Scholar]
- 16.Stoker J, Rociu E, Wiersma TG, et al. Imaging of anorectal disease. Br J Surg. 2000;87:10–27. [DOI] [PubMed] [Google Scholar]
- 17.Markus J, Morrissey B, deGara C, et al. MRI of recurrent rectosigmoid carcinoma. Abdom Imaging. 1997;22:338–342. [DOI] [PubMed] [Google Scholar]
- 18.Dicle O, Obuz F, Cakmakci H. Differentiation of recurrent rectal cancer and scarring with dynamic MR imaging. Br J Radiol. 1999;72:1155–1159. [DOI] [PubMed] [Google Scholar]
- 19.de Lange EE, Fechner RE, Wanebo HJ. Suspected recurrent rectosigmoid carcinoma after abdominoperineal resection: MR imaging and histopathologic findings. Radiology. 1989;170:323–328. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi O, Saito N, Koda K, et al. Clinical assessment of positron emission tomography for the diagnosis of local recurrence in colorectal cancer. Br J Surg. 1999;86:932–937. [DOI] [PubMed] [Google Scholar]
- 21.Staib L, Schirrmeister H, Reske SN, et al. Is (18)F-fluorodeoxyglucose positron emission tomography in recurrent colorectal cancer a contribution to surgical decision making? Am J Surg. 2000;180:1–5. [DOI] [PubMed] [Google Scholar]
- 22.Blomqvist L, Ohlsen H, Hindmarsh T, et al. Local recurrence of rectal cancer: MR imaging before and after oral superparamagnetic particles vs contrast-enhanced computed tomography. Eur Radiol. 2000;10:1383–1389. [DOI] [PubMed] [Google Scholar]
- 23.Howell JD, Wotherspoon H, Leen E, et al. Evaluation of a follow-up programme after curative resection for colorectal cancer. Br J Cancer. 1999;79:308–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjeldsen BJ, Kronborg O, Fenger C, et al. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84:666–669. [PubMed] [Google Scholar]
- 25.Bruinvels DJ, Stiggelbout AM, Kievit J, et al. Follow-up of patients with colorectal cancer: a meta-analysis. Ann Surg. 1994;219:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ovaska JT, Jarvinen HJ, Mecklin JP. The value of a follow-up programme after radical surgery for colorectal carcinoma. Scand J Gastroenterol. 1989;24:416–422. [DOI] [PubMed] [Google Scholar]
- 27.Declan Fleming RY. Colorectal cancer screening and follow-up. Surg Oncol. 1998;7:125–137. [DOI] [PubMed] [Google Scholar]
- 28.Secco GB, Fardelli R, Rovida S, et al. Is intensive follow-up really able to improve prognosis of patients with local recurrence after curative surgery for rectal cancer? Ann Surg Oncol. 2000;7:32–37. [DOI] [PubMed] [Google Scholar]
- 29.Makela JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer: results of a prospective randomized trial. Arch Surg. 1995;130:1062–1067. [DOI] [PubMed] [Google Scholar]
- 30.Ohlsson B, Breland U, Ekberg H, et al. Follow-up after curative surgery for colorectal carcinoma: randomized comparison with no follow-up. Dis Colon Rectum. 1995;38:619–626. [DOI] [PubMed] [Google Scholar]
- 31.Pietra N, Sarli L, Costi R, et al. Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis Colon Rectum. 1998;41:1127–1133. [DOI] [PubMed] [Google Scholar]