Abstract
Objective:
To determine the adequacy of sentinel node (SN) concept based on micrometastasis using immunohistochemistry (IHC) and reverse transcription-polymerase chain reaction (RT-PCR) in gastric cancer.
Summary Background Data:
The SN concept has recently been introduced in gastrointestinal tract cancers. The precise detection of lymph node metastasis including micrometastasis is important for SN navigation surgery.
Methods:
Sixty-one patients with gastric cancer who were preoperatively diagnosed with T1-T2 (cT1-T2) and N0 (cN0) were enrolled. They underwent standard radical gastrectomy with lymph node dissection. One day before surgery, 4 mCi of 99mTechnetium-tin colloid was endoscopically injected into the submucosa around the tumor. During surgery, radioisotope uptake in the lymph node was measured using Navigator GPS. All dissected lymph nodes were examined by RT-PCR in addition to hematoxylin and eosin staining and IHC.
Results:
Sentinel nodes were identified in all patients (100%). The incidences of metastasis determined by hematoxylin and eosin and IHC were 8.2% (5 of 61) and 13.1% (8 of 61), respectively. Micrometastases undetectable by IHC were identified in 14 patients (23.0%) by RT-PCR. Only 1 patient had micrometastasis detectable by RT-PCR in lymph nodes other than SN, but this patient had a cT2 tumor. In patients with cT1 and cN0 tumors, the false negative and accuracy rates were 0% and 100%, respectively.
Conclusions:
Although the incidence of micrometastasis detected by RT-PCR was quite high, SN navigation identified such metastasis in all patients except one. Thus, the SN concept was applicable to patients with cT1 and cN0 gastric cancer, even when micrometastasis was detectable by RT-PCR.
Sentinel node concept was applicable to patients with cT1 and cN0 gastric cancer, even in the presence of micrometastasis determined by immunohistochemistry and reverse transcription-polymerase chain reaction.
Lymph node metastasis is one of the most important prognostic factors in patients with gastric cancer.1–5 Therefore, D2 lymph node dissection for gastric cancer has become a standard procedure that has increased the long-term survival of patients with lymph node metastasis.6 The 5-year survival rates of patients with mucosal and submucosal gastric cancer are 95% to 100% and 85% to 95%, respectively.7–9 However, the incidence of lymph node metastasis determined by histology in mucosal and submucosal gastric cancer is 2% to 4% and 13% to 20%, respectively.8–12 Thus, standard lymph node dissection may be unnecessary for patients without lymph node metastasis. However, it is difficult to precisely diagnose lymph node metastasis, especially micrometastasis, using preoperative examinations such as endoscopic ultrasonography (EUS) and computed tomography (CT).
The sentinel node (SN) concept was first advocated by Morton et al13 in patients with melanoma. Sentinel node navigation surgery (SNNS) for breast cancer and malignant melanoma can accurately asses lymph node dissection areas.14,15 The SN concept has recently been applied to cancers of the gastrointestinal tract.16–20 If SNNS could be applied to such patients, then minimally invasive surgery with personalized lymphadenectomy might be possible. At present, lymph node metastasis, including micrometastasis, must be identified when performing SNNS.21 Some authors have reported the clinical significance of lymph node micrometastasis detected by immunohistochemistry (IHC).21–23 Others have found that real-time reverse transcription-polymerase chain reaction (RT-PCR) can detect lymph node micrometastasis more sensitively than IHC.24,25 A few reports have examined micrometastasis of SN using RT-PCR in gastric cancer. Thus, whether the SN concept is applicable in the presence of lymph node micrometastasis should be investigated. The present study determines the applicability of the SN concept to gastric cancer based on lymph node micrometastasis determined by IHC and RT-PCR.
MATERIALS AND METHODS
Patients
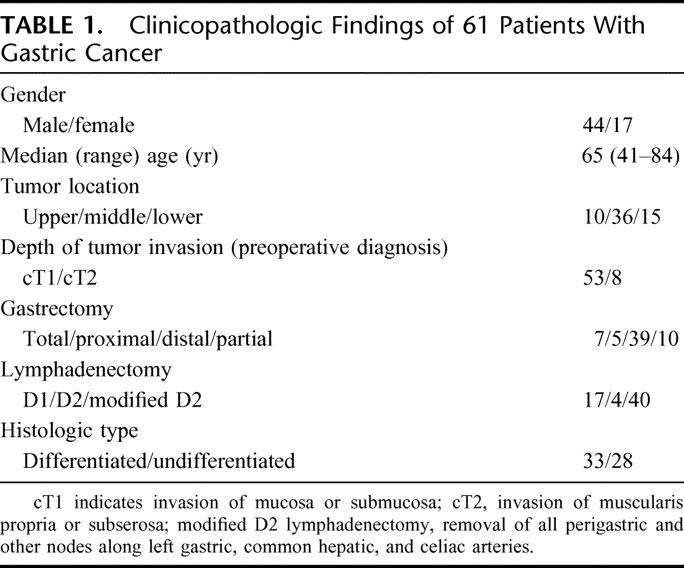
Sixty-one patients with gastric cancer, who were preoperatively diagnosed with cT1-T2 (T1, tumor invasion of the mucosa or the submucosa; T2, muscularis propria or subserosa) and cN0 (N0, free of lymph node metastasis), provided written, informed consent to participate in all procedures associated with this study. The patients were clinically diagnosed before surgery based on gastrointestinal fiberscopy, double contrast gastrography, EUS, and CT. All underwent curative gastrectomy with lymphadenectomy at the Department of Surgical Oncology and Digestive Surgery, Kagoshima University Hospital, between February 2003 and August 2004. None of the patients had received preoperative radiation therapy or chemotherapy. Table 1 shows the clinicopathologic data of the patients assessed according to the Japanese classification of gastric cancer.26 Histologically, 57 and 4 patients had T1 (33 mucosal and 24 submucosal) and T2 tumors, respectively.
TABLE 1. Clinicopathologic Findings of 61 Patients With Gastric Cancer
Detection of Sentinel Nodes
One day before surgery, 4 mCi (2 mL) of 99mTechnetium (99mTc)-tin colloid was endoscopically injected into the submucosa of the gastric wall at 4 sites (0.5 mL each) around the tumor using a disposable 23-gauge needle (MAJ-75, Olympus) and lymphoscintigraphy was performed 2 hours later. During surgery, the uptake of the radioisotope (RI) in each lymph node was counted using Navigator GPS (TYCO HEALTHCARE, Ltd., Tokyo, Japan) (window setting above 95 keV, measurement period, 1 second). After surgery, all dissected lymph nodes were mapped and RI uptake was measured again. Lymph nodes that absorbed 10 times more RI than background levels or more than 10% of the value of the node with the highest RI uptake in each patient were classified as hot (sentinel) nodes.
Lymph Nodes
We examined 1410 lymph nodes from 61 patients. The mean number of dissected lymph nodes was 23 (range, 2–59). Positive controls were lymph nodes from 10 gastric cancer patients with histologically evident metastasis. Negative controls were lymph nodes from15 patients without cancer (gall bladder stone, n = 6; gastric adenoma, n = 4; gastric ulcer, n = 4 and Crohn's disease, n = 1). The lymph nodes were separated into 2 blocks at the plane of the largest dimension. One half of each lymph node was mixed in 1 mL of Isogen (Nippon Gene, Toyama, Japan) and immediately stored at −80°C until used for RT-PCR. The other half was fixed in 10% formaldehyde, embedded in paraffin, cut into 3-μm sections, and stained with hematoxylin and eosin. Other sections were stained for IHC.
Immunohistochemistry
All lymph nodes were stained for IHC using a monoclonal anticytokeratin (CK) antibody cocktail (AE1/AE3, 20:1 mixture of AE1 to AE3; Boehringer Mannheim) that reacts with a broad spectrum of human CKs. The sections were incubated at 60°C overnight, deparaffinized in xylene, and rehydrated with a graded series of ethanol. After heating in citrate buffer solution (pH 6.0) for 6 minutes in a pressure cooker, the sections were incubated with CK monoclonal antibody diluted 1:100. Reactions for CK were visualized using alkaline phosphatase-antialkaline phosphatase.
Cell Lines
MKN-45, an adenocarcinoma cell line that produces carcinoembryonic antigen (CEA) derived from a gastric cancer and the MCF-7 breast cancer cell line were cultured in RPMI 1640 (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 10% fetal calf serum (Mitsubishi Kasei, Tokyo, Japan), 100 units/mL penicillin, and 100 units/mL streptomycin.
Real-Time RT-PCR
Thawed lymph nodes were homogenized using FastPrep (Qbiogene, Inc., Carlsbad, CA) and then total RNA extracted according to the manufacturer's instructions was dissolved in 20 μL of water treated with diethylpyrocarbonate. The concentration, purity, and amount of total RNA were determined by measuring absorption at 260 and 280 nm using a GeneQuant pro UV/Vis Spectrophotometer (Amersham Pharmacia Biotech, Cambridge, England). To avoid contamination with genomic DNA, 0.5 μg of total RNA was digested with 1 unit of DNase-I (Invitrogen, Life technologies, Foster City, CA) at 37°C for 15 minutes. The DNase-I was then inactivated by heating with 1 μL of 25 mmol/L ethylenediamine tetra-acetic acid (EDTA) at 65°C for 15 minutes. Complementary DNA (cDNA) was synthesized using the Advantage RT-for PCR Kit (Clontech Laboratory. Inc., Palo Alto, CA) according to the manufacturer's protocol and then stored at −20°C. A CEA-specific oligonucleotide primer was designed based on the that described by Gerhard et al27: sense, 5′-TGTCGGCATCATGATTGG-3′; and ante-sense, 5′-GCAAATGCTTTAAGGAAGAAGC-3′. The donor and acceptor probe sequences for CEA identification were: 5′-CCTGAAATGAAGAAACTACACCAGGGC-fluoresceinand 5′-LC-Red640-GCTATATCAGAGCAACCCCAACCAGC-phosphorylation. Amplification of CEA by PCR using a quantitative fluorescence LightCycler (Roche Diagnostics, Mannheim, Germany) proceeded in a 20-μL reaction mixture containing 2 μL of LightCycler FastStart DNA Master Hybridization Probes (Roche), 3.0 mmol/L MgCl2, 0.5 μmol/L sense and antisense primers, 0.4 μmol/L fluorescent probe, 0.2 μmol/L LC-Red probe, and 5 μL of undiluted template cDNA in LightCycler capillaries (Roche). Before amplification, 0.32 μL of anti-Taq DNA polymerase antibody (Taq Start antibody, Clontech Laboratory. Inc.) was added to the reaction mixture, which was then incubated at room temperature for 5 minutes to avoid primer prolongation. The amplification profile consisted of denaturation for 1 cycle at 95°C for 10 minutes followed by 35 cycles of 95°C for 10 seconds, 60°C for 15 seconds, and 72°C for 5 seconds. Real-time PCR was monitored by measuring fluorescent signals at the end of the annealing phase for each cycle. We quantified and confirmed the integrity of the RNA by comparison with real-time RT-PCR of the amplified glyceraldehyde-3-phosphatase dehydrogenase (GAPDH) housekeeping gene. The sense and antisense primers for GAPDH were 5′-TGAACGGGAAGCTCACTGG-3′ and TCCACCACCCTGTTGCTGTA-3′. The donor and acceptor probes for GAPDH were 5′-TCAACAGCGACACCCACTCCT-3′-fluoresceinand 5′-LC-Red640-CACCTTTGACGCTGGGGCT-3′-phosphorylation. The GAPDH gene was amplified in 20 μL of the same reaction mixture as described above in a LightCycler capillary (Roche). The amplification profile consisted of denaturation for 1 cycle at 95°C for 10 minutes followed by 45 cycles of 95°C for 15 seconds, 60°C for 15 seconds, and 72°C for 12 seconds. All primers and probes were synthesized and purified by reverse-phase high-performance liquid chromatography, and the optimal reagent concentrations and PCR cycling conditions were established by the Nihon Gene Research Laboratories (Sendai, Japan). External standards for CEA and GAPDH mRNA were prepared by 5-fold serial dilutions of cDNA synthesized from MKN-45 cells. Standard curves were constructed for each assay according to the LightCycler software (Roche). Real-time RT-PCR assays were repeated in triplicate.
We tested the sensitivity of the RT-PCR assay by spiking a series of 10-fold dilutions of MCF-7 cells (106–100) into 1 × 107 peripheral blood mononuclear cells (PBMCs) from a normal healthy volunteer who did not express CEA mRNA. Total RNA extracted as described above was assayed by real-time RT-PCR. The RT-PCR product was identified by 2% agarose-gel electrophoresis in Tris-acetate EDTA buffer and visualized after staining with ethidium bromide.
Sensitivity of RT-PCR
We detected CEA mRNA at a frequency of 101 tumor cells/107 PBMCs in MCF-7 cancer cell lines (Fig. 1). Ten lymph nodes from 10 patients with gastric cancer and who had histologically evident metastasis expressed positive bands (positive control; Fig. 2). On the other hand, none of 15 lymph nodes from the 15 patients without cancer expressed CEA mRNA under the same conditions (negative control; Fig. 2).
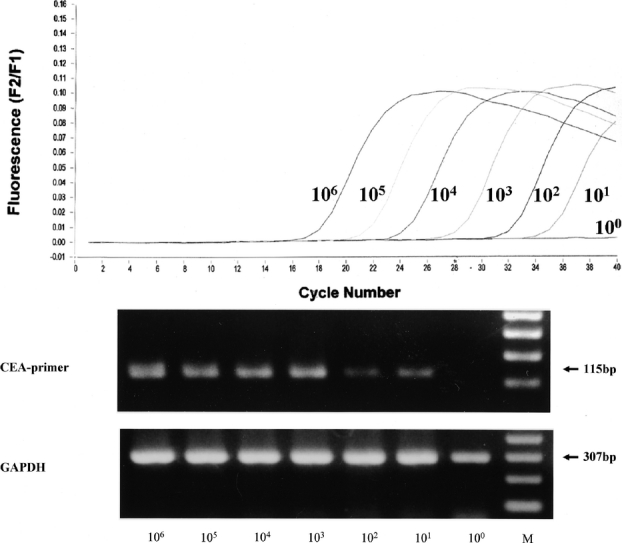
FIGURE 1. Real-time RT-PCR for CEA (carcinoembryonic antigen) mRNA. We used a LightCycler (Roche) to detect various ratios of MCF-7 cells mixed with peripheral blood mononuclear cells (PBMCs). Upper panel: fluorescence versus PCR cycles. MCF-7 cells were sequentially diluted 10-fold and mixed with PBMCs from healthy volunteers (that did not express CEA mRNA) to give 106 to 100 MCF-7 cells per 107 PBMCs. Lower panel: upper and lower portions show ethidium bromide-stained agarose gels following electrophoresis of CEA and GAPDH RT-PCR products, respectively. The RT-PCR products for CEA and GAPDH were resolved as 115 and 307 base pair fragments, respectively. bp, base pairs; M, DNA molecular weight marker.
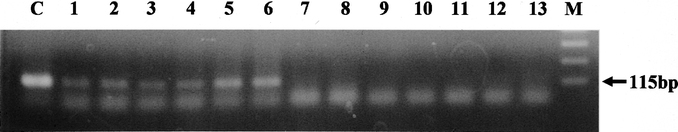
FIGURE 2. MCF-7 cell line and lymph nodes from patients with gastric cancer express CEA mRNA. Lanes 1–6, positive control lymph nodes from gastric cancer patients with histologically evident metastases, also expressed CEA mRNA (lanes 1–6). No lymph nodes from patients with benign diseases expressed CEA mRNA: lanes 7 and 8, gallbladder stone; lanes 9 and 10, gastric adenoma; lanes 11 and 12, gastric ulcer; lane 13, Crohn's disease. Lane C, MCF-7 cells; M, DNA molecular weight marker.
RESULTS
Detection of Sentinel Nodes
We identified a mean number of 4.6 (range, 1–17) SNs in all patients (100%).
Incidence of Lymph Node Micrometastasis
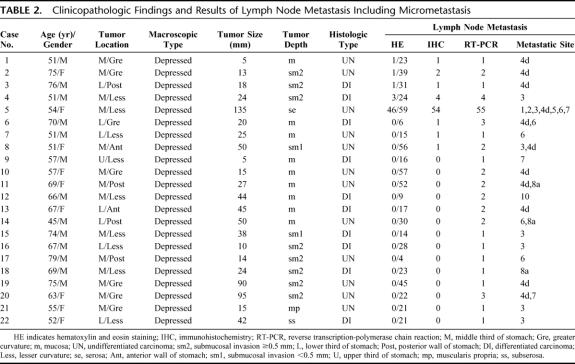
Routine histologic hematoxylin and eosin staining revealed lymph node metastasis in 5 of the 61 patients (8.2%) and in 52 of 1410 nodes (3.7%). Lymph node metastases including micrometastases were found in 8 patients (13.1%) and 65 nodes (4.6%) by IHC (Fig. 3). Ninety lymph nodes (6.4%) from 22 patients (36.1%) were positive by RT-PCR. Although 65 lymph node micrometastases detected by IHC were also positive by RT-PCR, the remaining 25 were detected only by RT-PCR (Table 2).
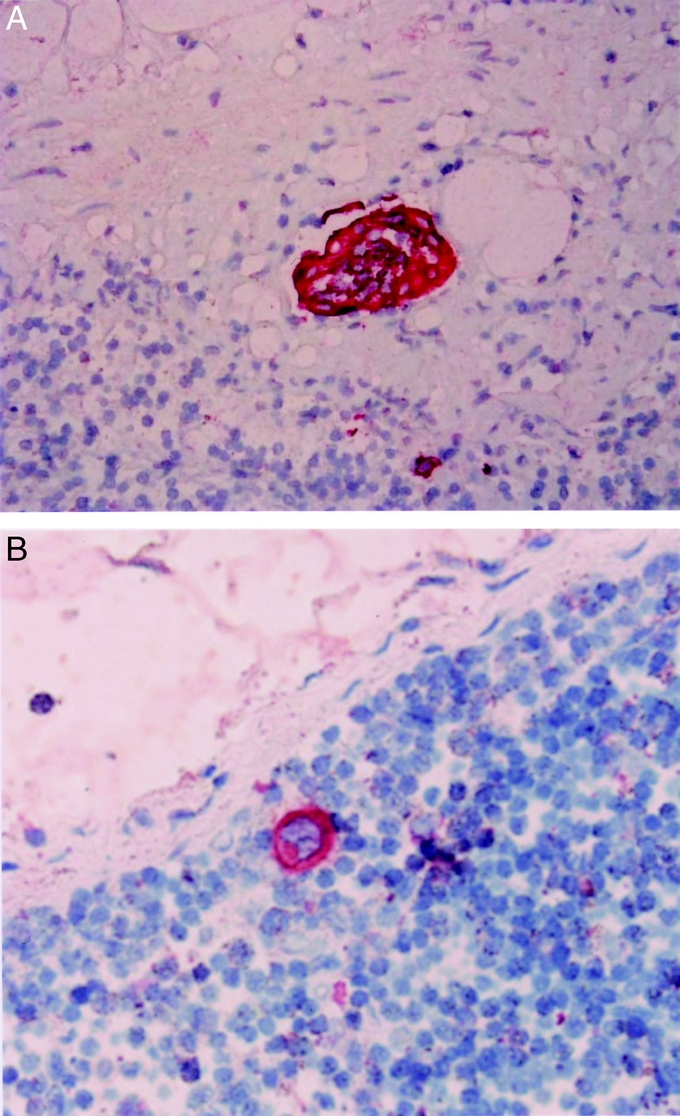
FIGURE 3. Tumor involvement in lymph nodes detected by IHC with anticytokeratin AE1/AE3. Tumor cells are evident as small clusters (A, original magnification ×∼400) and as single cells (B, original magnification ×400).
TABLE 2. Clinicopathologic Findings and Results of Lymph Node Metastasis Including Micrometastasis
Accuracy of Sentinel Node Concept Based on Findings of Lymph Node Metastasis Including Micrometastasis
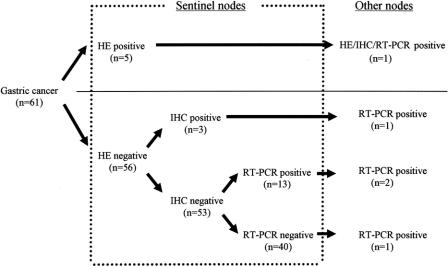
Lymph node metastasis was stained by hematoxylin and eosin in SNs from 5 patients, 1 of whom had metastasis in other nodes. Among 56 patients without metastasis in the SNs according to hematoxylin and eosin staining, 3 were IHC positive in the SN. In 1 of these patients, RT-PCR identified lymph node micrometastasis in nodes other than SNs. Thirteen patients were RT-PCR positive among 53 patients without micrometastasis in the SNs according to IHC, and 2 of these patients had micrometastasis in lymph nodes other than SNs (Fig. 4). Although RT-PCR identified micrometastasis of other nodes in 1 of the remaining 40 patients, this patient had a cT2 tumor (Fig. 4). Accordingly, the false-negative and accuracy rates were 2.5% and 98.4%, respectively.
FIGURE 4. Distribution of lymph node metastasis and micrometastasis in sentinel and other nodes.
Characteristics of Patients With Micrometastasis in the SNs
The tumors of 5 patients with lymph node metastasis detected by hematoxylin and eosin staining had penetrated beyond the submucosal layer with a single exception. Lymph node micrometastasis was found in 24.2% (8 of 33) of mucosal cancers, in 29.2% (7 of 24) of submucosal cancers, and in 50% (2 of 4) of T2 cancers. All patients had depressed type tumors. The ratios of patients with differentiated and undifferentiated tumors were 30.3% (10 of 33) and 42.9% (12 of 28), respectively, which was not significant. Although lymph node micrometastasis was limited to Compartment 1 nodes in 11 patients, the remaining 6 patients had lymph node micrometastases in Compartment 2 (Table 2).
DISCUSSION
The clinical applicability of SNNS and radio-guided SN detection to gastrointestinal tract cancer has been extensively investigated.20,28,29 However, only hematoxylin and eosin staining and IHC without RT-PCR diagnosed lymph node metastasis, including micrometastasis in these reports. Several investigators confirmed that RT-PCR can detect lymph node micrometastasis in gastrointestinal tract or breast cancers.24,25 Here, we designed a CEA primer based on that used by Gerhard et al,27 since CEA is an epithelial-specific antigen that is expressed in most cancers and in normal gastrointestinal tissue.27 Many studies have detected lymph node micrometastasis in gastrointestinal tract cancers using RT-PCR of CEA mRNA.24,25
In the injection of RI colloid into gastric wall, one important problem arose that CEA mRNA expression was related more to the injection into the tissue rather than the tumor itself. In this study, we examined all dissected lymph nodes using hematoxylin and eosin staining and IHC. However, certain epithelial cells could not be detected in multiple sections of these lymph nodes. Furthermore, in another series, we have performed SN navigation using same methods in nonepithelial tumors (2 gastrointestinal stromal tumors of the stomach, 1 esophageal malignant melanoma, and 3 rectal malignant melanomas). CEA mRNA expression was not found in these patients. Thus, we think that there are few possibilities of contamination with CEA mRNA and/or CEA expressing cells following the endoscopic injection.
Here, we examined all dissected lymph nodes by RT-PCR using CEA mRNA as the primer in addition to IHC. This strategy in turn increased the detection rates of micrometastasis by RT-PCR, IHC, and hematoxylin and eosin staining. These results were in accordance with reports proving that RT-PCR is more sensitive than IHC for detecting lymph node micrometastasis.25,30 Since the clinical significance of micrometastasis in gastric cancer remains controversial,23 the unequivocal detection of lymph node micrometastasis is essential when performing SNNS. Since none of the 61 patients enrolled in the present study died or recurred during the follow-up period within 2 years, a significant difference was not found in the survival and recurrence between the micrometastasis-positive and -negative cases. There are some possible explanations for these results: 1) the follow-up period within 2 years was short; 2) the majority of patients (93.4%) had early gastric cancer (33 mucosal and 24 submucosal tumors); and 3) standard lymphadenectomy, including these micrometastatic lymph nodes, was performed. Therefore, long follow-up in patients with micrometastasis should be necessary to clarify the clinical significance of lymph node micrometastasis.
Since the clinical significance of lymph node micrometastasis is controversial, at present, it is necessary to ascertain the presence or absence of lymph node micrometastasis during SNNS. We developed rapid IHC, which take 30 minutes to diagnose lymph node micrometastasis,31 and we have recently applied this rapid IHC to the intraoperative diagnosis of micrometastasis. An ultrarapid RT-PCR system, which can complete the detection of cancer cells within approximately 70 minutes, has been developed.32 In the near future, RT-PCR will be applied to detect micrometastasis during SNNS.
The present study investigated the adequacy of the SN concept based on lymph node micrometastasis determined by IHC and RT-PCR. In patients who were preoperatively diagnosed as having cT1-T2 tumors and cN0, the false-negative and accuracy rates were 2.5% (1/40) and 98.4% (60/61), respectively. A false-negative result was obtained from 1 of the 61 patients. The primary tumor of the one false-negative patient was located in the lesser curvature of the middle third region and 3 SNs were detected in the perigastric node near the lesser curvature, whereas one non-SN along the common hepatic artery was positive by RT-PCR. According to the anatomic classification by lymphatic drainage, this metastatic profile conformed to that of a “skip metastasis.” The reported clinical rate of skip metastasis in gastric cancer is in the range of 10%.19 However, this patient was preoperatively diagnosed as cT2 and cN0 and histologically as pT1 (sm2; submucosal invasion ≥0.5 mm) and pN0. When analyzing the data limited to the cT1 and cN0 patients, the false-negative and accuracy rates were 0% and 100%, respectively. Based on these results, we think that the SN concept is presently applicable to patients with cT1 and cN0 gastric cancer, considering lymph node micrometastasis.
We advocated that the size of the radioactively labeled colloid is an important factor in detecting SNs in early gastric cancer and that a 100-nm colloid can efficiently detect SNs.20,33 Therefore, we used 100-nm 99mTc-tin colloid in this study, and SNs were detected in all patients. Kitagawa et al28 reported that the overall accuracy rate of radio-guided SN detection in cT1-T2 gastric cancer is 98.6% (136 of 138 patients), and they reported false-negative results from 2 of 138 patients. Although radio-guidance alone can detect SNs, a combination of this procedure and dye has been recommended to decrease the false-negative findings.17
We identified lymph node micrometastasis in patients with depressed-type tumors. The presence of micrometastasis should be considered with this type of tumor. Furthermore, 6 of 17 patients had lymph node micrometastasis in Compartment 2. This result meant that such metastasis was not “skip metastasis,” which was decided artificially but natural lymphatic flows in these patients. Thus, SN detection might be useful for patients with lymph node metastasis in Compartment 2. Although tumors were classified as stage Ia (n = 53), stage Ib (n = 7), and stage IIIb (n = 1) based on the diagnosis of routine hematoxylin and eosin staining, 50 patients were diagnosed as stage Ia, 10 patients as stage Ib, and 1 patient as stage IIIb by CK staining. On the other hand, according to the RT-PCR results, the number of patients with stages Ia, Ib, II, and IIIb was 38, 14, 8, and 1, respectively. Finally, tumor upstaged in 17 patients by RT-PCR, compared with hematoxylin and eosin staining.
Minimally invasive surgery, including endoscopic mucosal resection34 and laparoscopy,35 has been performed in consideration of the postsurgical quality of life. From the viewpoint of detecting lymph node metastasis, minimally invasive surgery with personalized lymphadenectomy could be safely performed if SNNS is introduced for patients with early gastric cancer.
CONCLUSION
We demonstrated that RT-PCR can detect lymph node micrometastasis. However, we could evaluate that the SN concept was applicable to early gastric cancer even when IHC and RT-PCR confirmed the presence of lymph node micrometastasis. Thus, we think that SNNS is suitable for patients who are preoperatively diagnosed with cT1 and cN0 gastric cancer.
ACKNOWLEDGMENTS
The authors thank Hidehiro Nomura, MD, Hirohki Yoshii, MD, Yuzo Kawasaki, MD, Masahiro Tokushige, MD, Tamotsu Ohsako, MD, and Tsutomu Kozono, MD of Imamura Hospital, as well as Shigeho Maenohara, MD, Masahiro Hamanoue, MD, Yoshito Ogura, MD, Fumio Kijima, MD, and Akihiro Nakajo, MD, of Kagoshima Kouseiren Hospital for valuable contributions to this study.
Footnotes
Reprints: Takaaki Arigami, MD, Department of Surgical Oncology and Digestive Surgery, Field of Oncology, Course of Advanced Therapeutics, Kagoshima University Graduate School of Medical and Dental Sciences, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan. E-mail: arigami@m.kufm.kagoshima-u.ac.jp.
REFERENCES
- 1.Matsushita M, Hajiro K, Suzaki T, et al. Histopathological assessment of lymph node metastasis in patients with gastric cancer. Hepatogastroenterology. 1995;42:861–866. [PubMed] [Google Scholar]
- 2.Kwon SJ, Kim GS. Prognostic significance of lymph node metastasis in advanced carcinoma of the stomach. Br J Surg. 1996;83:1600–1603. [DOI] [PubMed] [Google Scholar]
- 3.Takagane A, Terashima M, Abe K, et al. Evaluation of the ratio of lymph node metastasis as a prognostic factor in patients with gastric cancer. Gastric Cancer. 1999;2:122–128. [DOI] [PubMed] [Google Scholar]
- 4.Kunisaki C, Shimada H, Takahashi M, et al. Prognostic factors in early gastric cancer. Hepatogastroenterology. 2001;48:294–298. [PubMed] [Google Scholar]
- 5.Nitti D, Marchet A, Olivieri M, et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077–1085. [DOI] [PubMed] [Google Scholar]
- 6.Maehara Y, Okuyama T, Moriguchi S, et al. Prophylactic lymph node dissection in patients with advanced gastric cancer promotes increased survival time. Cancer. 1992;15:392–395. [DOI] [PubMed] [Google Scholar]
- 7.Maehara Y, Orita H, Okuyama T, et al. Predictors of lymph node metastasis in early gastric cancer. Br J Surg. 1992;79:245–247. [DOI] [PubMed] [Google Scholar]
- 8.Cai J, Ikeguchi M, Maeta M, et al. Micrometastasis in lymph nodes and microinvasion of the muscularis propria in primary lesions of submucosal gastric cancer. Surgery. 2000;127:32–39. [DOI] [PubMed] [Google Scholar]
- 9.Kim DY, Joo JK, Ryu SY, et al. Factors related to lymph node metastasis and surgical strategy used to treat early gastric carcinoma. World J Gastroenterol. 2004;10:737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamao T, Shirao K, Ono H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602–606. [DOI] [PubMed] [Google Scholar]
- 11.Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery. 1999;125:148–154. [PubMed] [Google Scholar]
- 12.Folli S, Morgagni P, Roviello F, et al. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol. 2001;31:495–499. [DOI] [PubMed] [Google Scholar]
- 13.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. [DOI] [PubMed] [Google Scholar]
- 15.Edwards MJ, Martin KD, McMasters KM. Lymphatic mapping and sentinel lymph node biopsy in the staging of melanoma. Surg Oncol. 1998;7:51–57. [DOI] [PubMed] [Google Scholar]
- 16.Saha S, Wiese D, Badin J, et al. Technical details of sentinel lymph node mapping in colorectal cancer and its impact on staging. Ann Surg Oncol. 2000;7:120–124. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa Y, Kitajima M. Gastrointestinal cancer and sentinel node navigation surgery. J Surg Oncol. 2002;79:188–193. [DOI] [PubMed] [Google Scholar]
- 18.Bilchik AJ, Saha S, Tsioulias GJ, et al. Aberrant drainage and missed micrometastases: the value of lymphatic mapping and focused analysis of sentinel lymph nodes in gastrointestinal neoplasms. Ann Surg Oncol. 2001;8:82–85. [PubMed] [Google Scholar]
- 19.Aikou T, Higashi H, Natsugoe S, et al. Can sentinel node navigation surgery reduce the extent of lymph node dissection in gastric cancer? Ann Surg Oncol. 2001;8:90–93. [PubMed] [Google Scholar]
- 20.Uenosono Y, Natsugoe S, Higashi H, et al. Evaluation of colloid size for sentinel nodes detection using radioisotope in early gastric cancer. Cancer Lett. 2003;200:19–24. [DOI] [PubMed] [Google Scholar]
- 21.Siewert JR, Kestlmeier R, Busch R, et al. Benefits of D2 lymph node dissection for patients with gastric cancer and pN0 and pN1 lymph node metastases. Br J Surg. 1996;83:1144–1147. [DOI] [PubMed] [Google Scholar]
- 22.Maehara Y, Oshiro T, Endo K, et al. Clinical significance of occult micrometastasis lymph nodes from patients with early gastric cancer who died of recurrence. Surgery. 1996;119:397–402. [DOI] [PubMed] [Google Scholar]
- 23.Ishida K, Katsuyama T, Sugiyama A, et al. Immunohistochemical evaluation of lymph node micrometastases from gastric carcinomas. Cancer. 1997;79:1069–1076. [DOI] [PubMed] [Google Scholar]
- 24.Mori M, Mimori K, Inoue H, et al. Detection of cancer micrometastases in lymph nodes by reverse transcriptase-polymerase chain reaction. Cancer Res. 1995;55:3417–3420. [PubMed] [Google Scholar]
- 25.Raj GV, Moreno JG, Gomella LG. Utilization of polymerase chain reaction technology in the detection of solid tumors. Cancer. 1998;82:1419–1442. [DOI] [PubMed] [Google Scholar]
- 26.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 2nd English Edition. Gastric Cancer. 1998;1:10–24. [DOI] [PubMed] [Google Scholar]
- 27.Gerhard M, Juhl H, Kalthoff H, et al. Specific detection of carcinoembryonic antigen-expressing tumor cells in bone marrow aspirates by polymerase chain reaction. J Clin Oncol. 1994;12:725–729. [DOI] [PubMed] [Google Scholar]
- 28.Kitagawa Y, Fujii H, Mukai M, et al. Radio-guided sentinel node detection for gastric cancer. Br J Surg. 2002;89:604–608. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi H, Ochiai T, Mori M, et al. Sentinel lymph node mapping for gastric cancer using a dual procedure with dye- and gamma probe-guided techniques. J Am Coll Surg. 2003;196:68–74. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto M, Natsugoe S, Ishigami S, et al. Lymph node micrometastasis and lymphatic mapping determined by reverse transcriptase-polymerase chain reaction in pN0 gastric carcinoma. Surgery. 2002;131:630–635. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto M, Natsugoe S, Ishigami S, et al. Rapid immunohistochemical detection of lymph node micrometastasis during operation for upper gastrointestinal carcinoma. Br J Surg. 2003;90:563–566. [DOI] [PubMed] [Google Scholar]
- 32.Marutsuka T, Shimada S, Shiomori K, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003;9:678–685. [PubMed] [Google Scholar]
- 33.Higashi H, Natsugoe S, Uenosono Y, et al. Particle size of tin and phytate colloid in sentinel node identification. J Surg Res. 2004;121:1–4. [DOI] [PubMed] [Google Scholar]
- 34.Takekoshi T, Baba Y, Ota H, et al. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352–358. [DOI] [PubMed] [Google Scholar]
- 35.Ohgami M, Otani Y, Kumai K, et al. Curative laparoscopic surgery for early gastric cancer: five years experience. World J Surg. 1999;23:187–192. [DOI] [PubMed] [Google Scholar]