Abstract
Objective:
To prospectively evaluate the oncologic and functional outcomes of laparoscopic total mesorectal excision (TME) with colonic J-pouch reconstruction.
Background:
TME is considered the established gold standard in rectal cancer surgery. However, data on laparoscopic sphincter-preserving TME are limited.
Methods:
Patients with mid or low rectal cancer underwent laparoscopic TME with colonic J-pouch reconstruction by a single surgical team. Clinical and oncologic data were prospectively recorded and analyzed.
Results:
From March 1999 to September 2004, 105 patients underwent laparoscopic TME with colonic J-pouch reconstruction. The mean operating time was 170.4 minutes and mean blood loss was 91.5 mL. The mean anastomotic distance from the anal verge was 3.9 cm. Conversion was required in 2 cases. The mean circumferential and distal margins were 17.1 mm and 3.4 cm, respectively. There was 1 case of microscopic circumferential margin involvement and 1 case of microscopic distal margin involvement. There was no 30-day mortality, and 6 patients underwent reoperation for major complications. There was no port-site metastasis. The mean follow-up time was 26.9 months (range, 1.3–65.6 months). The actuarial 5-year cancer-specific survival and local recurrence rates were 81.3% and 8.9%, respectively. Erectile dysfunction occurred in 13.6% of males, while 2 patients developed incomplete bladder denervation. Bowel function after ileostomy closure was satisfactory, with an average bowel motion of less than 3 times per day at 2 years after ileostomy closure.
Conclusions:
Laparoscopic TME with colonic J-pouch reconstruction is a safe procedure with reasonable operating time and does not appear to pose any threat to the oncologic and functional outcomes.
Laparoscopic TME with colonic J-pouch reconstruction is a safe procedure with reasonable operating time and favorable oncologic and functional outcomes.
Thirteen years after the first report of laparoscopic colorectal surgery,1 the spotlight has drifted from technical feasibility to oncologic adequacy in cancer. While recently published randomized trials lend support to laparoscopic resection of colon cancer,2–4 skepticism prevails in laparoscopic resection of rectal cancer, in particular sphincter-preserving resection for mid and low rectal cancer.5 Laparoscopic resection of mid and low rectal cancer must conform to the current standard in rectal cancer surgery that has resulted in the lowest reported local recurrence rate,6,7 ie, total mesorectal excision (TME). Procedural complexity has limited the widespread penetration of laparoscopic sphincter-preserving TME. While there are accumulating reports that confirms technical feasibility of the procedure,8–14 its oncologic safety remains controversial.
The present study reports the outcomes in 105 cases of laparoscopic TME with colonic J-pouch anal anastomosis in a 5-year period starting in 1999.
PATIENTS AND METHODS
Patient Selection and Preoperative Preparation
Since March 1999, patients with histologically proven adenocarcinoma of the mid and lower rectum, defined preoperatively as lower tumor margin within 11 cm from the anal verge as measured by rigid sigmoidoscopy, were recruited. Preoperative colonoscopy was performed in all patients, to obtain tissue for histology and to exclude synchronous tumors. Incomplete colonoscopy was supplemented with barium enema examination. Chest x-ray, abdominal ultrasonography, and endorectal ultrasonography (ERUS) were routinely performed. Patients with stenotic tumors that precluded ERUS were evaluated with computed tomography (CT). Since January 2002, patients with radiologic T3, T4, or node positive diseases were given neoadjuvant chemoradiation (50.4 Gy in 28 fractions together with systemic 5-fluorouracil and leucovorin) with a reassessment CT at 4 weeks after completion of radiation. Operation was scheduled 6 weeks after completion of radiotherapy.
Informed consent was obtained from each patient. Exclusion criteria included the presence of distant metastasis, locally advanced disease with invasion into adjacent pelvic organs, the sacrum or the pelvic sidewall (ie, T4 tumor) on postneoadjuvant imaging, or when the patient was considered unfit for major operation. In the case of tumors less than 3 cm from anal verge, abdominoperineal resection was performed, except for small, well-differentiated, radiologic T1 tumors, which were excised transanally. For tumors at 3 cm from anal verge or above, sphincter-preserving TME was routinely attempted unless there was clinical or radiologic involvement of the external anal sphincter.
All patients received mechanical bowel preparation (polyethylene glycol) the night before surgery and prophylactic antibiotics (cefuroxime 1.5 g and metronidazole 500 mg) on induction of anesthesia.
Operative Technique
A single surgical team consisting of 2 surgeons (M.K.W.L. and C.C.C.) and one camera assistant performed all operations with a standardized technique; details of the technique were reported previously.13 As a routine, colonic J-pouch reconstruction was performed after tumor exclusion and distal cytocidal washout, followed by defunctioning loop ileostomy. A 5-cm pouch length was chosen to minimize evacuation difficulties while maintaining the advantages of the pouch.15 Conversion was defined as when any part of the procedure other than the delivery of specimen and extracorporeal construction of the J-pouch had to be completed with an open technique. Patients were discharged once they were ambulatory, capable of independent stoma care, and had no major complications.
Histopathology
Surgical specimens, fixed in 10% formalin, were processed according to a standard protocol.16 Circumferential, distal, and proximal margins were marked with India ink. Particular emphasis was put on the integrity of the mesorectum and circumferential margin involvement, by means of macroscopic and microscopic examination of representative tissue blocks. Tissue blocks from the proximal margin, distal margin, nontumorous colon, and the tumor were examined. If the tumor lied within 15 mm of the distal resection margin, a longitudinal block was taken to examine the relationship of the tumor to the marked resection margin. Circumferential margins were assessed by microscopic examination of representative blocks from the areas of deepest tumor penetration. Tissue blocks were processed by auto-processor and embedded in paraffin wax. Four-micrometer sections were stained with hematoxylin and eosin for light microscopy. Presence of microscopic disease within 1 mm of any resection margin was taken as an involved margin.
Follow-up
Closure of the ileostomy was performed 2 to 3 months after resection, after radiologic verification of the integrity of the pouch-anal anastomosis with a water-soluble contrast (urograffin) enema.
Patients with T3, T4, and/or node-positive disease received postoperative adjuvant chemotherapy.
Two authors (W.W.C.T. and S.Y.K.) followed up all patients for the first time at 6 weeks after surgery to assess stoma care and to arrange ileostomy closure, then every 3 months after ileostomy closure for 1 year and every 6 months thereafter. Carcinoembryonic antigen was checked at each visit. Colonoscopy was performed at 1 year after surgery, and thereafter every 3 years. Chest x-ray and abdominal ultrasonography were performed annually.
Measured Outcomes
The primary outcome was patient survival, with tumor recurrence as the secondary outcome. Other endpoints included operating time, blood loss, conversion rate, intraoperative complications, distance of the anastomosis from anal verge (measured by rigid rectoscope at the first outpatient visit), wound length, time to return of bowel function (defined as time to functioning of the ileostomy), time to resumption of oral diet, time to ambulation, length of postoperative hospital stay, early (within 30 days) and late (after 30 days) procedure-related complications, distal and circumferential margin, and bowel function after ileostomy closure.
Postoperative bowel function was recorded with a standard proforma. Erectile function was assessed with a visual analogue scale before and at 6 months after surgery. Erectile dysfunction was defined as a 50% or more decrease in the score.
Statistical Analysis
All data were collected prospectively. Results were expressed as means. Kaplan-Meier survival curves with 95% confidence interval were used to calculate the survival and recurrence rates.
RESULTS
From March 1999 to September 2004, 135 patients presented with nonmetastatic adenocarcinoma of the mid and low rectum. Thirty (22%) patients were excluded: 2 patients with locally advanced (T4) tumors unresponsive to neoadjuvant therapy, 15 patients undergoing abdominoperineal resection, 9 patients who did not consent to laparoscopic surgery, 2 patients refusing any treatment, and 2 patients unfit for major surgery. A total of 105 patients underwent laparoscopic TME with colonic J-pouch reconstruction.
Table 1 shows the patient demographic data and tumor characteristics. Thirty patients received neoadjuvant treatment and 61 patients had adjuvant chemotherapy. Postoperative chemo-radiation was given in 3 patients who did not receive neoadjuvant therapy: microscopic involvement of the distal margin in one, microscopic involvement of the circumferential margin in another and a metastatic nodule in the perirectal fat of the distal doughnut despite a 2-cm tumor-free distal mesorectal margin in the third patient. The mean follow-up time was 26.9 months (range, 1.3–65.6 months).
TABLE 1
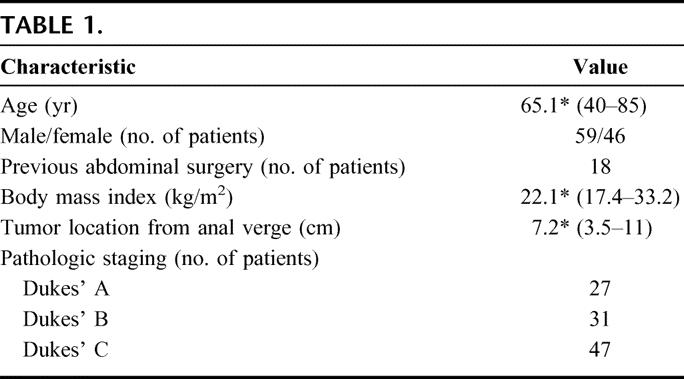
Primary and Secondary Outcomes
Five (4.8%) patients developed local recurrence, 4 of whom also presented later with distant dissemination. Fifteen other patients developed distant metastases. There was no port-site metastasis. There were 11 cancer-related deaths and 4 unrelated deaths during the study period. The actuarial 5-year overall survival and disease-free rates were 76.9% (Fig. 1a) and 64.4% (Fig. 1b) respectively. The actuarial 5-year cancer-specific survival rate was 81.3% for all stages (Fig. 2a); 100% for Dukes’ A, 94.1% for Dukes’ B, and 65.2% for Dukes’ C disease (Fig. 2b). The actuarial overall local recurrence rate at 5 years was 8.9% (Fig. 3a), while those for Dukes’ A, B, and C disease were 0%, 5.3%, and 16.1%, respectively (Fig. 3b).
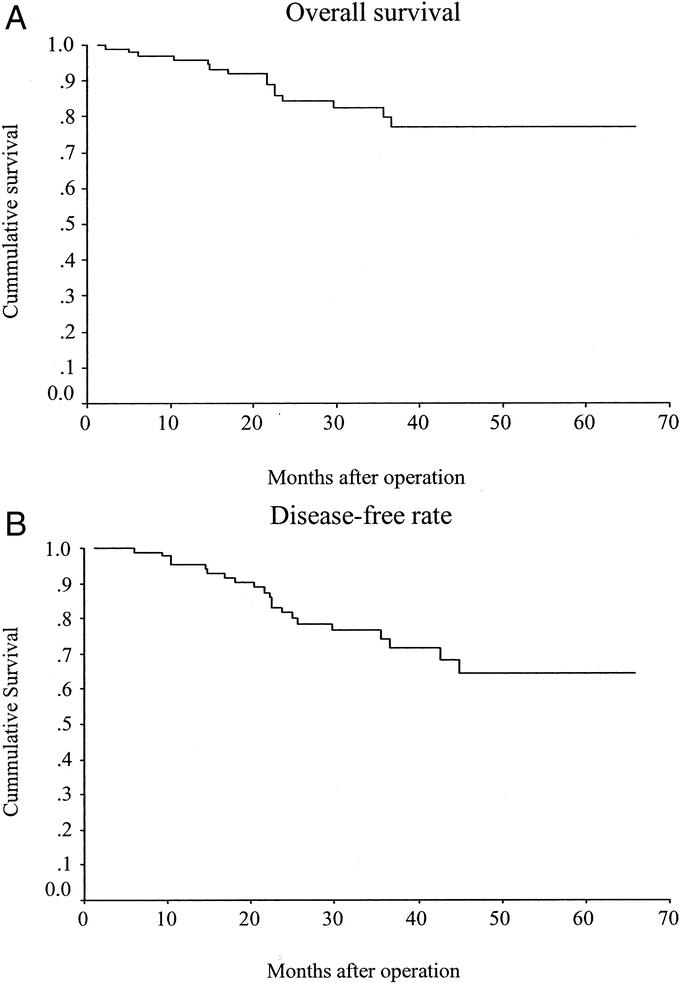
FIGURE 1. A, Overall survival. B, Disease-free rate.
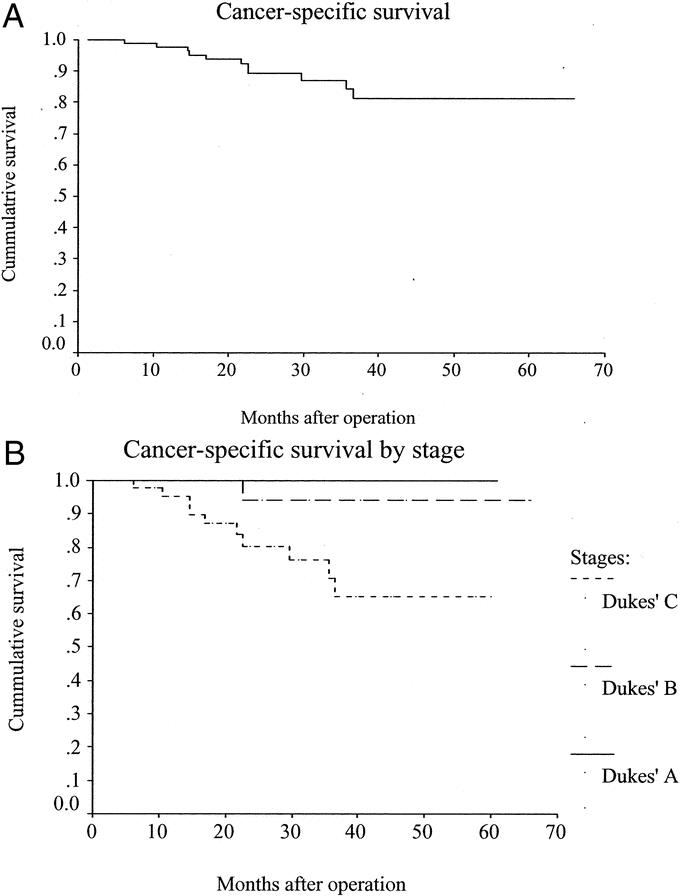
FIGURE 2. A, Cancer-specific survival. B, Cancer-specific survival by stage.
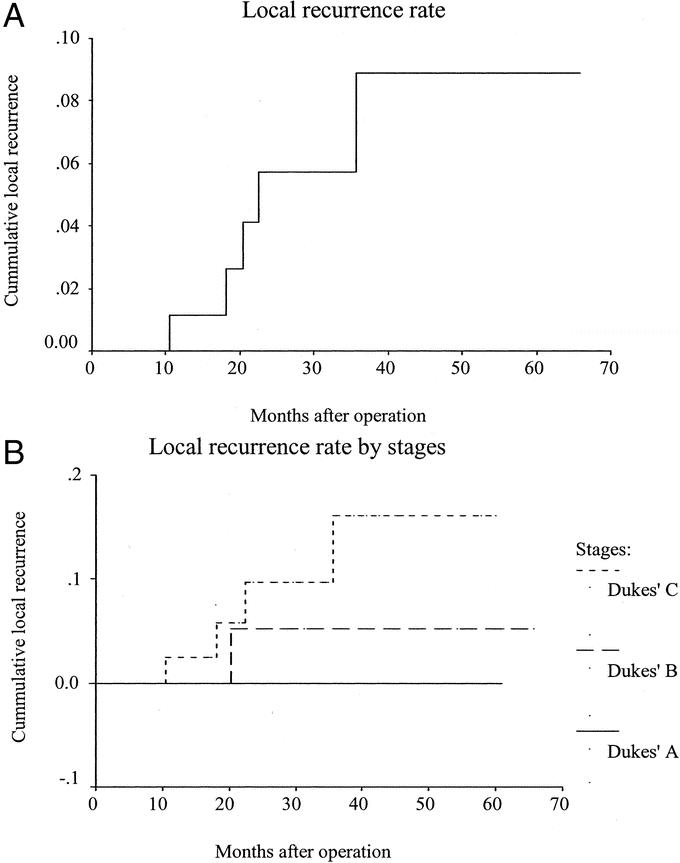
FIGURE 3. A, Local recurrence rate. B, Local recurrence rate by stages.
Other Endpoints
The mean operating time was 170.4 minutes (range 100–305 minutes) and the mean operative blood loss was 91.5 mL (range, 10–600 mL). Conversion was necessary in 2 cases (1.9%): locally advanced disease in one and intraperitoneal fecal soiling due to instrument failure with staple line disruption in the other. The left ureter was inadvertently damaged in 1 case and was repaired laparoscopically without conversion. The mean wound length for specimen extraction was 6.4 cm (range, 4–25 cm). The mean anastomotic height was 3.9 cm (range, 2–5.5 cm). The mean distal and circumferential margins were 3.4 cm (range, 0–7 cm) and 17.1 mm (range, 1–40 mm), respectively. Microscopic margin involvement happened in 2 cases: distal margin in one (1%) and circumferential margin in another (1%). The mean time to return of bowel function was 1.8 days (range, 1–14 days), while that to resumption of oral feeding was 2.5 days (range, 2–15 days). The mean time to ambulation was 3.8 days (range, 3–17 days). The mean postoperative hospital stay was 10.1 days (range, 5–54 days).
There was no 30-day mortality. Twenty-six patients developed 28 early complications (Table 2). One patient developed anastomotic leakage on the fifth postoperative day and was managed successfully with systemic antibiotics. The routine outpatient urograffin enema revealed asymptomatic leakage in 11 patients. Twenty-two patients developed late complications. Twelve patients had anastomotic stenosis that responded well to dilatation at the time of ileostomy closure. Eight men (13.6%) had persistent erectile dysfunction at the conclusion of follow-up. Two patients developed incomplete bladder denervation as confirmed by urodynamics and were managed conservatively. One patient required pouch excision and end colostomy for pouch ischemia. Figures 4 and 5 depict the postoperative bowel function after ileostomy closure.
TABLE 2
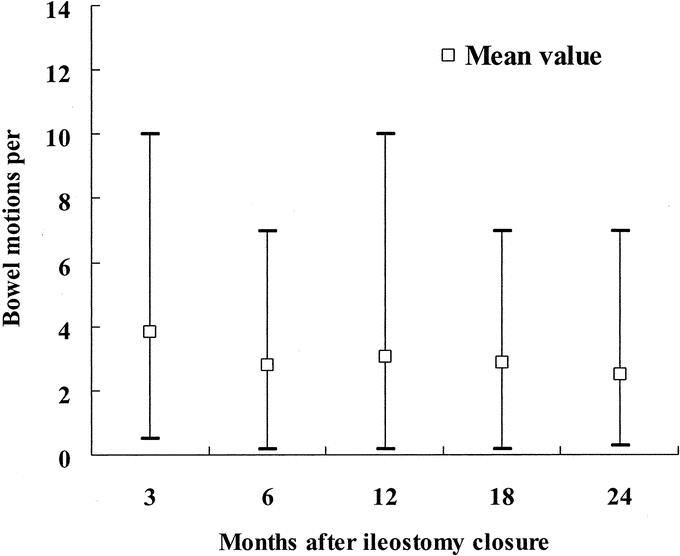
FIGURE 4. Bowel motions after ileostomy closure.
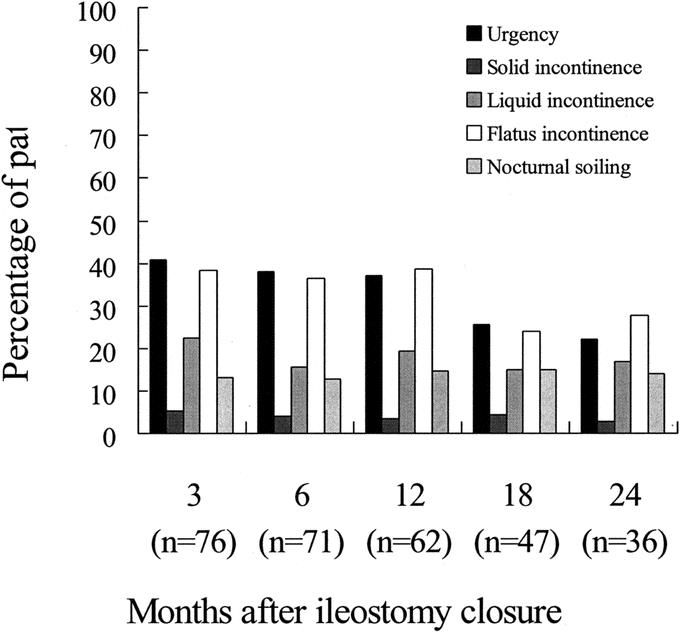
FIGURE 5. Varying levels of incontinence after ileostomy closure.
DISCUSSION
Results from recent randomized trials have provided optimism for the oncologic safety of laparoscopic resection of colon cancer.2–4 Such optimism is yet to be substantiated in laparoscopic resection for rectal cancer. Sphincter-ablating resection dominated the early literature on laparoscopic rectal cancer surgery.17–20 Technical hurdles as well as skepticism as to oncologic clearance had once confined sphincter preservation to cancers located at the rectosigmoid junction or in the upper rectum.5,21–24 However, progress in technology and skills has eventually led to the extension of minimally invasive techniques to distal rectal cancer with sphincter preservation.8–14 As a technically demanding procedure, laparoscopic sphincter-preserving TME is currently limited to a number of specialized centers.
There was no 30-day mortality and 26 (24.8%) patients experienced early complications. These results compare favorably with open TME series.6,25 In the present study, the authors practiced routine defunctioning ileostomy, and there was only 1 case of clinical anastomotic leakage. Much higher leakage rates (11%–17%) were reported in series of laparoscopic sphincter-preserving TME with a policy of selective defunctioning.8,11,12 Thus, routine defunctioning after laparoscopic TME should be recommended.26 The present study demonstrated the short-term benefits that are recognized in laparoscopic colorectal surgery in general.2–4,27–30 Moreover, this was accomplished with a reasonable operating time and a low conversion rate. Locally advanced tumor has been a common reason for conversion in the literature.8,28 All but one such tumors were excluded by preoperative imaging in the present study.
The hospital stay in the present study was comparable to other series of laparoscopic TME8–9,11 but is prolonged in comparison with other laparoscopic colorectal surgery studies.2–4 Such discrepancy may be due to difference in the magnitude of the procedures since these latter studies were concerned primarily with laparoscopic colectomy and anterior resection.2–4
Quah et al suggested that laparoscopic TME might be associated with increased male sexual dysfunction.29 In their study, however, the open technique was used to complete the distal rectal dissection during which nerve damage is most likely. Such a hybrid technique may undermine the theoretical advantage of magnified view and improved visualization of deep pelvic structures under the laparoscope.14,31 In contrast to the Quah et al study,29 the rates of erectile dysfunction and bladder denervation in the present series compare favorably to open TME series.32 The function of the “neorectum” in the present series was comparable to other series of colonic J-pouch;33,34 at 2 years after ileostomy closure, on average patients in this study had less than 3 bowel motions per day (Fig. 4), and less than 3% of patients complained of solid incontinence (Fig. 5).
There was no port-site metastasis in the present series, a finding that concurs with the recent literature.4,30,35 The mean follow-up time is too short to conclude on the long-term oncologic outcomes of laparoscopic TME. Nevertheless, the actuarial survival and local recurrence rates in the present study were comparable to other laparoscopic TME series8,11 as well as open TME series.7,36–38
With careful case selection and expertise, laparoscopic TME with colonic J-pouch reconstruction is a safe procedure within a reasonable operating time. Notwithstanding the short mean follow-up time, laparoscopic sphincter-preserving TME did not appear to confer any oncologic disadvantage. It warrants a randomized trial to clearly define the role of laparoscopic surgery in the treatment of rectal cancer.
Footnotes
Reprints: Michael K.W. Li, FRCS, Department of Surgery, Pamela Youde Nethersole Eastern Hospital, 3 Lok Man Road, Chai Wan, Hong Kong. E-mail: kwokshekyuen@yahoo.com.
REFERENCES
- 1.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc. 1991;1:144–150. [PubMed] [Google Scholar]
- 2.Leung KL, Kwok SPY, Lam SCW, et al. Laparoscopic resection of rectosigmoid cancer: prospective randomized trial. Lancet. 2004;363:1187–1192. [DOI] [PubMed] [Google Scholar]
- 3.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. [DOI] [PubMed] [Google Scholar]
- 4.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. [DOI] [PubMed] [Google Scholar]
- 5.Scheidbach H, Schneider C, Baerlehner E, et al. Laparoscopic anterior resection for rectal carcinoma: results of a registry. Surg Oncol Clin North Am. 2001;10:599–609. [PubMed] [Google Scholar]
- 6.Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg. 1998;133:894–899. [DOI] [PubMed] [Google Scholar]
- 7.Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. [DOI] [PubMed] [Google Scholar]
- 8.Morino M, Parini U, Giraudo G, et al. Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg. 2003;237:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rullier E, Sa Cunha A, Couderc P, et al. Laparoscopic intersphincteric resection with coloplasty and coloanal anastomosis for mid and low rectal cancer. Br J Surg. 2003;90:445–451. [DOI] [PubMed] [Google Scholar]
- 10.Tsang WWC, Chung CC, Li MKW. Prospective evaluation of laparoscopic total mesorectal excision with colonic J-pouch reconstruction for mid and low rectal cancers. Br J Surg. 2003;90:867–871. [DOI] [PubMed] [Google Scholar]
- 11.Leroy J, Jamali F, Forbes L, et al. Laparoscopic total mesorectal excision (TME) for rectal cancer surgery: long term outcomes. Surg Endosc. 2004;18:281–289. [DOI] [PubMed] [Google Scholar]
- 12.Hartley JE, Mehigan BJ, Qureshi AE, et al. Total mesorectal excision: assessment of the laparoscopic approach. Dis Colon Rectum. 2001;44:315–321. [DOI] [PubMed] [Google Scholar]
- 13.Chung CC, Ha JPY, Tsang WWC, et al. Laparoscopic-assisted total mesorectal excision and colonic J pouch reconstruction in the treatment of rectal cancer. Surg Endosc. 2001;15:1098–1101. [DOI] [PubMed] [Google Scholar]
- 14.Weiser MR, Milsom JW. Laparoscopic total mesorectal excision with autonomic nerve preservation. Semin Surg Oncol. 2000;19:396–403. [DOI] [PubMed] [Google Scholar]
- 15.Hida J, Yasutomi M, Fujimoto K, et al. Functional outcome after low anterior resection with low anastomosis for rectal cancer using the colonic J-pouch: prospective randomized study for determination of optimum pouch size. Dis Colon Rectum. 1996;39:986–991. [DOI] [PubMed] [Google Scholar]
- 16.Compton CC. Updated protocol for the examination of specimens from patients with carcinomas of the colon and rectum, excluding carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix. Arch Pathol Lab Med. 2000;124:1016–1025. [DOI] [PubMed] [Google Scholar]
- 17.Larach SW, Salomon MC, Williamson PR, et al. Laparoscopic assisted abdominoperineal resection. Surg Laparosc Endosc. 1993;3:115–118. [PubMed] [Google Scholar]
- 18.Darzi A, Lewis C, Menzies Gow N, et al. Laparoscopic abdominoperineal excision of the rectum. Surg Endosc. 1995;9:414–417. [DOI] [PubMed] [Google Scholar]
- 19.Ramos JR, Petrosemolo RH, Valory EA, et al. Abdominoperineal resection: laparoscopic versus conventional. Surg Laparosc Endosc. 1997;7:148–152. [PubMed] [Google Scholar]
- 20.Fleshman JW, Wexner SD, Anvari M, et al. Laparoscopic vs. open abdominoperineal resection for cancer. Dis Colon Rectum. 1999;42:930–939. [DOI] [PubMed] [Google Scholar]
- 21.Leung KL, Kwok SPY, Lau WY, et al. Laparoscopic-assisted resection of rectosigmoid carcinoma: immediate and medium-term results. Arch Surg. 1997;132:761–764. [DOI] [PubMed] [Google Scholar]
- 22.Goh YC, Eu KW, Seow-Choen F. Early postoperative results of a prospective series of laparoscopic vs. open anterior resections for rectosigmoid cancers. Dis Colon Rectum. 1997;40:776–780. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes M, Rudd M, Nathanson L, et al. Laparoscopic anterior resection: a consecutive series of 84 patients. Surg Laparosc Endosc. 1996;6:213–217. [PubMed] [Google Scholar]
- 24.Schwandner O, Schiedeck TH, Killaitis C, et al. A case-control-study comparing laparoscopic versus open surgery for rectosigmoidal and rectal cancer. Int J Colorectal Dis. 1999;14:158–163. [DOI] [PubMed] [Google Scholar]
- 25.Carlsen E, Schlichting E, Guldvog I, et al. Effect of the introduction of total mesorectal excision for the treatment of rectal cancer. Br J Surg. 1998;85:526–529. [DOI] [PubMed] [Google Scholar]
- 26.Karanjia ND, Corder AP, Holdsworth PJ, et al. Risk of peritonitis and fatal septicaemia and the need to defunction the low anastomosis. Br J Surg. 1991;78:196–198. [DOI] [PubMed] [Google Scholar]
- 27.Chapman AE, Levitt MD, Hewett P, et al. Laparoscopic-assisted resection of colorectal malignancies: a systematic review. Ann Surg. 2001;234:590–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandya S, Murray JJ, Coller JA, et al. Laparoscopic colectomy: indications for conversion to laparotomy. Arch Surg. 1999;134:471–475. [DOI] [PubMed] [Google Scholar]
- 29.Quah HM, Jayne DG, Eu KW, et al. Bladder and sexual dysfunction following laparoscopically assisted and conventional open mesorectal resection for cancer. Br J Surg. 2002;89:1551–1556. [DOI] [PubMed] [Google Scholar]
- 30.Chung CC, Tsang WWC, Kwok SY, et al. Laparoscopy and its current role in the management of colorectal disease. Colorectal Dis. 2003;5:528–543. [DOI] [PubMed] [Google Scholar]
- 31.Rubino F, Leroy J, Marescaux J. Bladder and sexual dysfunction following laparoscopically assisted and conventional open mesorectal resection for cancer. Br J Surg. 2003;90:486. [DOI] [PubMed] [Google Scholar]
- 32.Nesbakken A, Nygaard K, Bull-Njaa T, et al. Bladder and sexual dysfunction after mesorectal excision for rectal cancer. Br J Surg. 2000;87:206–210. [DOI] [PubMed] [Google Scholar]
- 33.Hallbook O, Pahlman L, Krog M, et al. Randomized comparison of straight and colonic J pouch anastomosis after low anterior resection. Ann Surg. 1996;224:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazorthes F, Chiotasso P, Gamagami RA, et al. Late clinical outcome in a randomized prospective comparison of colonic J pouch and straight coloanal anastomosis. Br J Surg. 1997;84:1449–1451. [PubMed] [Google Scholar]
- 35.Zmora O, Weiss EG. Trocar site recurrence in laparoscopic surgery for colorectal cancer: myth or real concern? Surg Oncol Clin North Am. 2001;10:625–638. [PubMed] [Google Scholar]
- 36.Aitken RJ. Mesorectal excision for rectal cancer. Br J Surg. 1996;83:214–216. [DOI] [PubMed] [Google Scholar]
- 37.Arbman G, Nilsson E, Hallbook O, et al. Local recurrence following total mesorectal excision for rectal cancer. Br J Surg. 1996;83:375–379. [DOI] [PubMed] [Google Scholar]
- 38.Hainsworth PJ, Egan MJ, Cunliffe WJ. Evaluation of a policy of total mesorectal excision for rectal and rectosigmoid cancers. Br J Surg. 1997;84:652–656. [PubMed] [Google Scholar]


