Abstract
Objective:
To compare the results of percutaneous local ablative therapy (PLAT) with surgical resection in the treatment of solitary and small hepatocellular carcinoma (HCC).
Summary Background Data:
PLAT is effective in small HCC. Whether it is as effective as surgical resection in the long-term survivals remains unknown.
Methods:
We conducted a prospective randomized trial on 180 patients with a solitary HCC ≦5 cm to receive either PLAT or surgical resection. The patients were regularly followed up after treatment with physical examination, blood, and radiologic tests.
Results:
Of the 90 patients who were randomized to PLAT, only 71 received PLAT because 19 withdrew their consent. Of the 90 patients who were randomized to surgical resection, a single Couinaud liver segment resection was carried out in 69 patients, 2 segments in 16 patients, and 3 or more segments in 3 patients. Ethanol injection was given during open surgery in 2 patients. Only 1 patient died after surgical resection within the same hospital admission. Posttreatment complications were more often and severe after surgery than PLAT. The 1-, 2-, 3-, and 4-year overall survival rates after PLAT and surgery were 95.8%, 82.1%, 71.4%, 67.9% and 93.3%, 82.3%, 73.4%, 64.0%, respectively. The corresponding disease-free survival rates were 85.9%, 69.3%, 64.1%, 46.4% and 86.6%, 76.8%, 69%, 51.6%, respectively. Statistically, there was no difference between these 2 treatments.
Conclusion:
PLAT was as effective as surgical resection in the treatment of solitary and small HCC. PLAT had the advantage over surgical resection in being less invasive.
A prospective randomized trial on 180 patients with a solitary hepatocellular carcinoma ≦ 5 cm showed there was no significant difference in the long-term overall and disease-free survivals between treatment with percutaneous local ablative therapy or partial hepatectomy. Percutaneous local ablative therapy has the advantage over surgery in being less invasive.
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world.1 Although the majority of cases are still found in Asia and Africa, recent evidence has shown that the incidence and mortality rate of HCC are rising in North America and Europe.2,3 The prognosis of HCC is generally poor. Partial hepatectomy remains the best hope for a cure but is suitable for only 9% to 27% of patients.4,5 The presence of significant background cirrhosis often precludes liver resection in patients with HCC. Recurrence of tumor within the liver remnant is also common in patients who have undergone “curative” liver resection.6
In the past 2 decades, percutaneous local ablative therapy (PLAT) has emerged to be a safe and effective treatment of small HCC.6 Unfortunately, the use of percutaneous ethanol injection (PEI) was shown to be associated with a high post-treatment recurrence within the liver, exceeding 50% in 2 years.7,8 Recently, various thermal ablative therapies have been developed, of which percutaneous radiofrequency ablation (RFA) has attracted the greatest interest and popularity because of its efficacy and safety.6 Cohort studies have shown RFA to give encouraging results in terms of tumor control, with complete tumor ablation rates of 90% to 95%, and low local recurrence rate of 5% to 10%.9–13 The treatment has also been shown to be safe, with a 3-year survival rate of 62% to 68%.10,13 Prospective randomized studies have shown RFA to be better than PEI in producing a higher rate of complete tumor necrosis with fewer number of treatment sessions.14,15 The local recurrence rate was lower14 and the survival better.16 In a recent review article on PLAT, a plea was made to conduct a prospective randomized study to compare RFA with surgical resection for small HCC.6
PATIENTS AND METHDOS
Between November 1999 and June 2004, 180 patients with solitary HCC ≦5 cm in diameter were randomized to receive either PLAT or surgical resection. During this period, there were 3775 patients with HCC hospitalized in our hospital.
The diagnosis of HCC was based on the diagnostic criteria for HCC used by the European Association for the Study of the Liver.17 The diagnosis of HCC was made when 2 radiologic imaging techniques showed typical features of HCC in a patient with an elevated alpha fetoprotein level >400 ng/mL, or in the absence of the criteria cytologic/histologic diagnosis of HCC.
Inclusion criteria for this study were as follows:
age 18 to 75 years
a solitary HCC smaller than 5 cm in diameter
no extrahepatic metastasis
no radiologic evidence of invasion into the major portal/hepatic vein branches
good liver function with Pugh-Child Class A, with no history of encephalopathy, ascites refractory to diuretics, or variceal bleeding
indocyanine green retention at 15 minutes (ICG-R15) <30%
a platelet count of >40,000/mm3
no previous treatment of HCC
patient should be suitable to be treated by either surgical resection or PLAT
The decision whether to include the patient into this study was made by a multidisciplinary team of doctors coming from different departments. For patients who met the inclusive criteria, informed consent was obtained from the patient before randomization. This study was approved by the Ethical Committee of Cancer Center, Sun Yat-Sen University.
Randomization was done by using random numbers generated from a computer in a central registry for this study.
PLAT
For PLAT, we first treated our patients with RFA by using a commercially available system (RF 2000; RadioTherapeutics, Mountain View, CA) and a needle electrode with a 15-gauge insulated cannula with 10 hook-shape expandable electrode tines with a diameter of 3.5 cm at expansion (LeVeen; RadioTherapeutics).
The procedure was done using local anesthetics, intravenous sedation, or lumbar epidural anesthesia if the patient preferred it. If needle biopsy needed to be done, it was performed before the RFA. RFA initiated with 10 W of power, and the power was increased 10 W per minute to 90 W. RF was applied until either there was a marked increase in impedance or 15 minutes had elapsed. If a marked increase in impedance was not achieved, a second application of RF was given. Up to 3 applications of RF were given in the treatment session. For tumors larger than 3 cm or unsatisfactory needle placement, more placement of needle was necessary. During RFA, a hyperechoic area was observed around the electrode tip on ultrasonic monitoring. The aim of the treatment was to have this hyperechoic area covering a larger area than the HCC.
Surgical Resection
Surgery was carried out under general anesthesia using a right subcostal incision with a midline extension. Intraoperative ultrasound was routinely used. We performed anatomic resection aiming at a resection margin of at least 1 cm. Pringle's maneuver was routinely used with a clamp/unclamp time of 10 minutes/5 minutes. Hemostasis on the raw liver surface was done with suturing and fibrin glue.
Follow-up
A dual-phase spiral computed tomography (CT) was done 4 weeks after treatment and thereafter every 2 monthly in the first 2 years. At each of these follow-up visits, blood tests including liver function tests and serum alpha fetoprotein were done. Chest radiography was done every 6 months. The follow-up visits were spaced out to once every 3 months after 2 years.
Residual viable tumor tissue was considered to be present on the first CT assessment at 4 weeks after treatment if enhancement areas were seen within the tumor at either the arterial or the portal venous phase. Magnetic resonance imaging was carried out if CT was uncertain about whether there was residual viable tumor tissue. Additional treatment with RFA or PEI was given. If residual viable tumor was still present after repeated treatments, transarterial chemoembolization was given.
Operative mortality was defined as death within the same hospital admission after treatment. We also compared the 30-day and 60-day mortality rates between the 2 treatment groups of patients. All adverse events after treatment were recorded. Pain was graded as absent or mild if the patient did not require any analgesics or moderate or severe if analgesics were necessary to relieve the pain.
Sample Size
We used tumor recurrence rate at 2 years after treatment to be the outcome measurement to estimate the sample size for this study. Assuming an alpha risk of 0.05, a beta risk of 0.8, post PLAT recurrence to be 30% at 2 years, and post surgical treatment to be 10%, the number of patients needed in each treatment group was estimated to be 60. Assuming a post randomization dropout of 10%, the sample size required for this study was estimated to be at least 70 patients in each study group.
Statistical Analysis
All data were prospectively entered into a computer. Comparisons between the 2 groups were done using the Student t test for continuous data and the χ2 test for categorical data. The overall and disease-free survivals were calculated using the life-table method and compared with Mantel-Cox test. The survival curves were constructed using the Kaplan-Meier method. The relative prognostic significance of the variables in predicting overall survival was assessed using multivariate Cox proportional hazards regression analysis. The statistical analyses of the data were performed using the SPSS 9.0 statistical software. Results were given as mean ± SD. All statistical tests were 2-sided, and a significant difference was considered when P < 0.05. All analyses were done on the intention-to-treat basis.
RESULTS
Patient Groups
The data on the 180 patients after randomization are given in Figure 1. Nineteen patients in the PLAT group withdrew their consent after randomization. They were all treated with surgical resection; 21 patients received additional PEI or RFA because follow-up CT showed incomplete tumor necrosis. At the end of the percutaneous treatments, 91.5% of patients showed complete necrosis on CT scan. If the initial treatment of RFA was given alone without the additional follow-up treatment with PEI or RFA, the complete necrosis on CT scan was present in only 62.0% of patients, and the subsequent intrahepatic recurrence rate would be much higher. The complete necrosis rate in tumor smaller than 3 cm was 97.3% (36 of 37). Two patients received transarterial chemoembolization to control the disease because residual tumors were found within the liver during the follow-up CT despite repeated treatments with RFA/PEI. As the tumors were increasing in size and had spread within the liver, we decided to use an alternative treatment to control it.
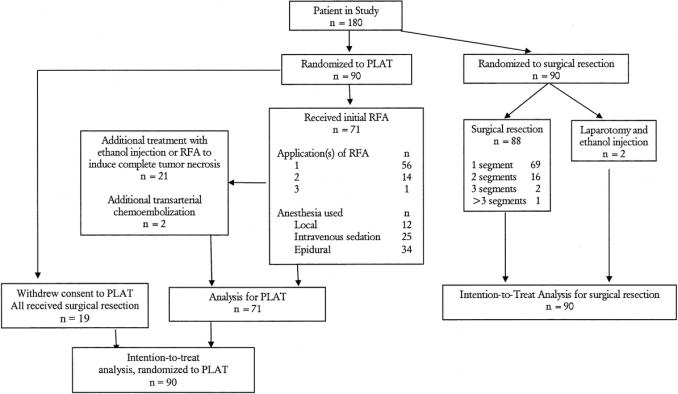
FIGURE 1. Patient numbers for the intention-to-treat analysis.
For the 90 patients who were randomized to surgical resection, 2 were found to have disseminated tumor in the liver. Both were treated with ethanol injection. Histopathology in 1 patient showed a focal nodular hyperplasia instead of HCC. The pathologic staging of the resected specimens in the remaining 87 patients correlated well with the preoperative CT staging of the disease; 81 (90%) of these patients had coexisting liver cirrhosis.
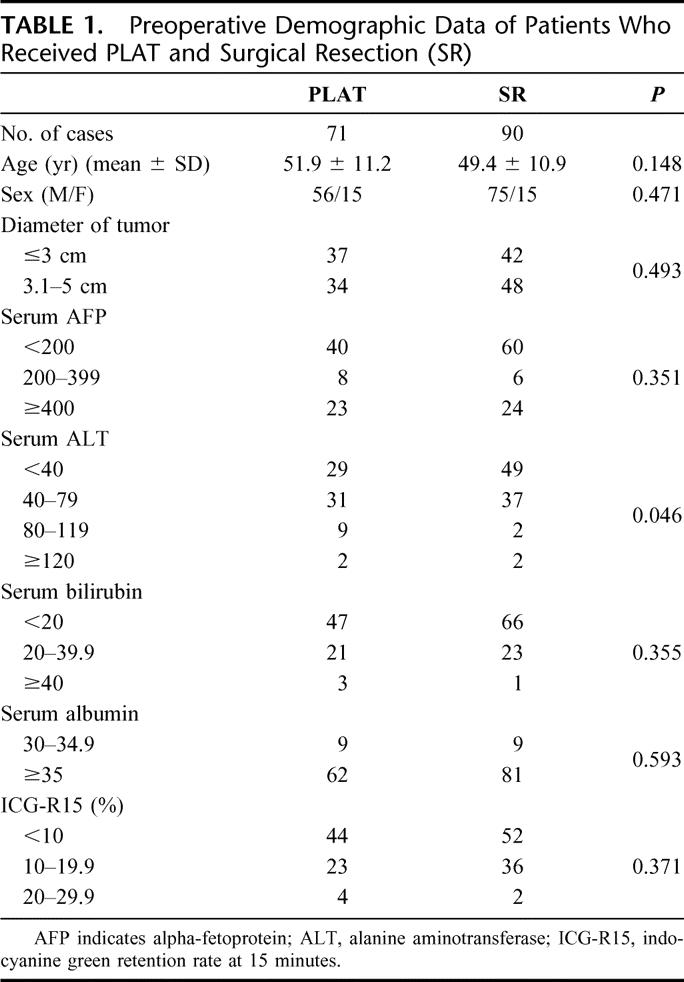
The preoperative demographic data of these 2 groups of patients are shown in Table 1. There is no significant difference between these 2 groups of patients in the factors being analyzed and listed in the table except there were significantly more patients with a raise ALT in the PLAT group. The mean ± SD for the follow-up periods for the PLAT and the surgical resection groups were 27.9 ± 10.6 months and 29.2 ± 11.9 months, respectively.
TABLE 1. Preoperative Demographic Data of Patients Who Received PLAT and Surgical Resection (SR)
Survival
We analyzed the survival data of the 2 groups of patients under 2 headings:
Intention-to-Treat Analyses
For the PLAT group, 71 patients who received PLAT were analyzed together with the 19 patients who withdrew their consent after randomization and who received surgical resection. This group of patient was compared with the 90 patients who were randomized to receive surgical resection.
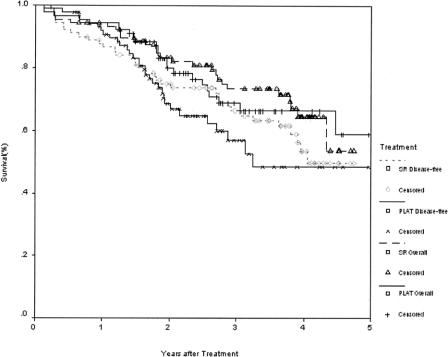
The 1-, 2-, 3-, and 4-year overall survival rates for the PLAT group and the surgical resection group were 94.4%, 79.8%, 68.6%, 65.9% and 93.3%, 82.3%, 73.4%, 64.0%, respectively. The corresponding disease-free survival rates for the 2 groups were 90.8%, 68.6%, 59.8%, 48.2% and 86.6%, 76.8%, 69.0%, 51.6%, respectively. There were no significant difference between the 2 groups in the overall survival and disease-free survival rates (Fig. 2). Also, there was no significant difference in the overall and disease-free survival rates between the 2 groups by analyzing tumors smaller than 3 cm, and between 3.1 and 5 cm, respectively (Fig. 3).
FIGURE 2. Overall and disease-free survivals for patients randomized to percutaneous local ablative therapy (PLAT, n = 90, including 19 patients who withdrew their consent after randomization and received surgical resection) or surgical resection (SR).
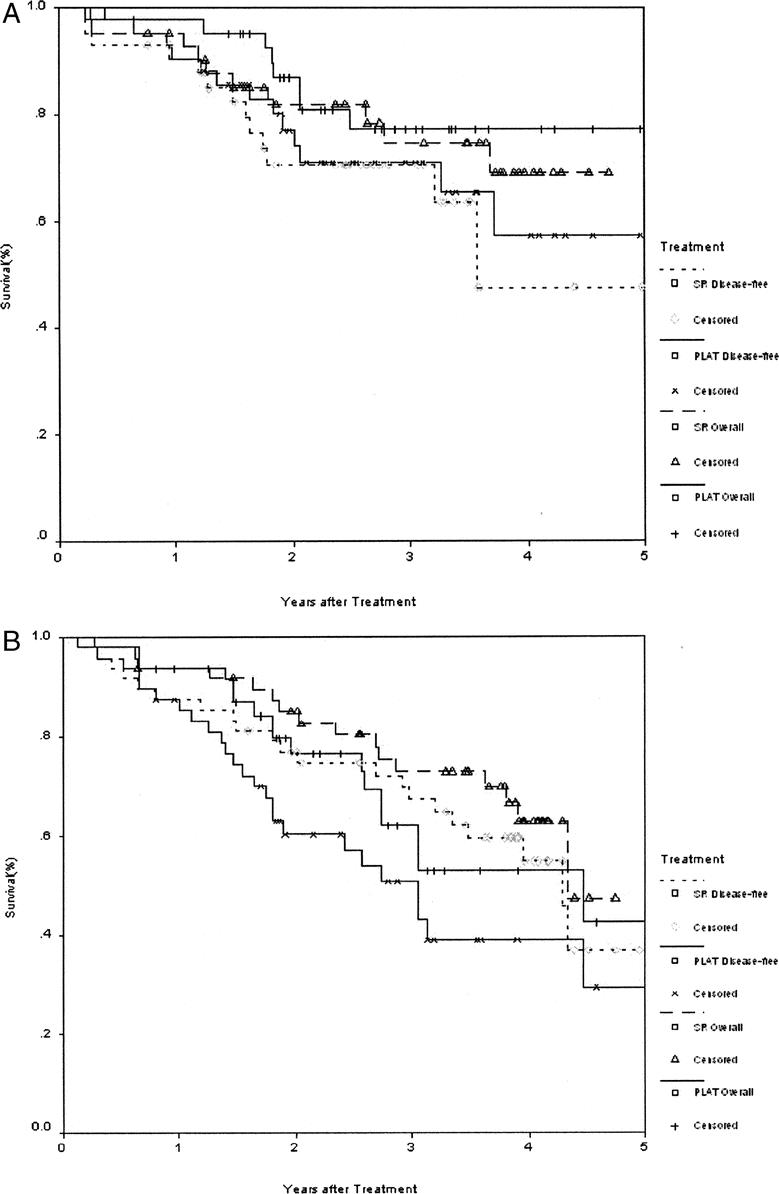
FIGURE 3. Overall and disease-free survivals for patients randomized to percutaneous local ablative therapy (PLAT, n = 90, including 19 patients who withdrew their consent after randomization and received surgical resection) or surgical resection (SR) for tumors less than 3 cm (A), and between 3.1 cm and 5 cm (B).
Analysis After Post-Randomization Exclusion
For the PLAT group, 19 patients withdrew their consent after randomization. The remaining 71 patients were analyzed with the 90 patients who were randomized to the surgical resection group.
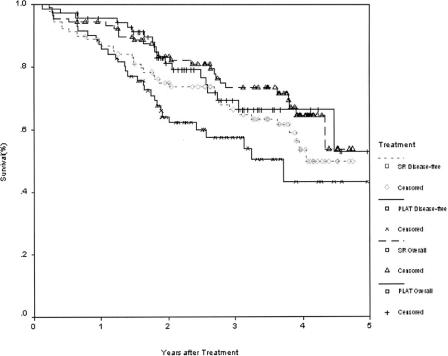
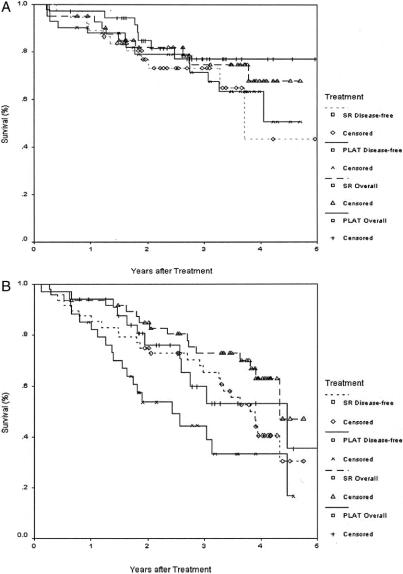
The 1-, 2-, 3-, and 4-year overall survival rates for the PLAT and the surgical resection groups were 95.8%, 82.1%, 71.4%, 67.9% and 93.3%, 82.3%, 73.4%, 64.0%, respectively. The corresponding disease-free survival rates for the 2 groups were 85.9%, 69.3%, 64.1%, 46.4% and 86.6%, 76.8%, 69%, 51.6%, respectively. There was no significant difference between the 2 groups in the overall survival and disease-free survival rates (Fig. 4). Also, there was no significant difference in the overall and disease-free survival rates between the 2 groups by analyzing tumors smaller than 3 cm, and between 3.1 and 5 cm, respectively (Fig. 5).
FIGURE 4. Overall and disease-free survivals for patients treated with percutaneous local ablative therapy (PLAT, n = 71) or surgical resection (SR, n = 90).
FIGURE 5. Overall and disease-free survivals with percutaneous local ablative therapy (PLAT, n = 71) and surgical resection (SR, n = 90) for tumor less than 3 cm (A), and between 3.1 cm and 5 cm (B).
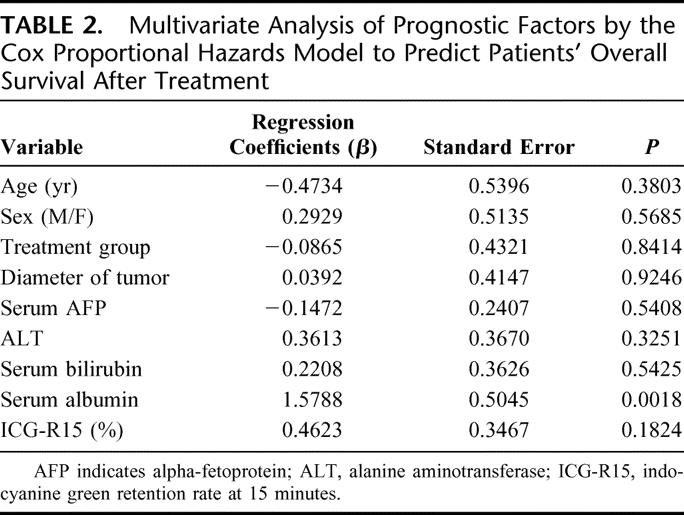
A multivariate Cox proportional hazards regression analysis was performed to assess the relative prognostic importance of the variables listed in Table 2 in predicting the overall survival after treatment. Serum albumin was found to be the only significant prognostic factor.
TABLE 2. Multivariate Analysis of Prognostic Factors by the Cox Proportional Hazards Model to Predict Patients' Overall Survival After Treatment
Treatment Mortality and Morbidity
One patient who received liver resection died within the same hospital admission, making a mortality rate of 1.1%. The in-hospital mortality for the RFA group was 0%. The 30-day and 60-day mortality rates for the surgical resection group were 1.1% and 1.1%, respectively. The corresponding figures for the PLAT group were 0% and 0%, respectively.
Major complications happened significantly more often after surgical resection than PLAT (50 of 90 versus 3 of 71, P < 0.05). Significant postoperative morbidity included liver failure (n = 2), gastrointestinal bleeding (n = 2), moderate/severe ascites (n = 27), and persistent jaundice for more than 30 days after surgery (n = 19). There were only 3 patients with a mild burn in the skin at the back at the site where the electrode pads were pasted after PLAT. There was no PLAT procedure-related hemorrhage, infection, or needle-tract seeding. Post PLAT fever of over 38.5°C was observed in 8 patients.
Post-Treatment Pain and Hospital Stay
While every patient had moderate/severe pain which required analgesics after surgical resection, only 16 patients required the administration of analgesics after PLAT (P < 0.05).
The hospital stay after surgery was significantly longer after surgical resection (19.70 ± 5.61) than PLAT (9.18 ± 3.06) (P < 0.05).
DISCUSSION
Partial hepatectomy is nowadays still considered the “gold standard” of treatment with an aim of providing a “cure” in patients with resectable HCC, good liver function, and good general condition. Recent improvements in perioperative management has made partial hepatectomy safe.18 The perioperative mortality should be approaching 0% in noncirrhotic liver resection, and it should be below 5% in cirrhotic liver resection. Unfortunately, a significant proportion of patients cannot be offered surgery at the time of diagnosis of HCC. HCC commonly arises from a liver with a background cirrhosis or chronic hepatitis (hepatitis B or C). Partial hepatectomy is often contraindicated because of the patient's compromised liver function and associated comorbidities. Another major drawback of partial hepatectomy is the high postoperative morbidity. Also, the need to excise a significant part of the liver together with the tumor, especially when the HCC is situated in the center of the liver, makes the search for a less invasive procedure with the ability to ablate the tumor completely an attractive alternative.
The first attempts in using PLAT to treat small HCC involved the use of ethanol injection. Nonrandomized studies have shown PEI to give a 3-year survival rate of 47% to 77%.7,8,10,19,20 Unfortunately, the rate of post-treatment recurrence within the liver exceeds 50% in 2 years.7,8 However, the safety and the minimal invasiveness of PEI have made the treatment popular for small HCC in many centers in the world, especially in situations in which surgical resection has high operative risks.
Attempts have been made to compare PEI with partial hepatectomy. As far as we know, there have not been any prospective randomized trials to compare the efficacy of any form of PLAT with surgical resection for an operable-stage HCC in terms of survival. A retrospective study comparing 39 patients treated with PEI and 58 patients with surgical resection for small HCC (<3 cm each and 3 or fewer in number) reported no difference in 1-, 3-, and 5-year recurrence-free survival and overall survival.21 In contrast, another large retrospective study of patients with HCC less than 5 cm in diameter enrolled in the Liver Cancer Study Group of Japan found that patients who received liver resection (n = 8010) had better survival than PEI (n = 4037) or transarterial chemoembolization (n = 841).22 However, these studies were not prospective randomized studies. One explanation for surgery's superiority in the Japanese study22 may be that the proportion of patients with associated cirrhosis was probably lower in the surgical resection group than in the nonsurgically treated group, even when the same clinical stage of the tumor was compared. Thus, metachronous multicentric carcinogenesis and development of hepatic failure would have occurred less frequently in the surgical resection group.6 On the other hand, surgical resection may genuinely be better than PEI in the treatment of small HCC. Most surgeons will perform concomitant resection of the whole Couinaud segment containing the tumor, based on the observation that HCC can invade the tributaries of the portal branches and shed tumor emboli into the neighboring branches of the same liver segment.23 Thus, clearance of the tumor and potential sites of microscopic disease within the liver segment (segment-based anatomic liver resection) will be more complete and more effective for treating small HCC than nonanatomic PEI.6,23 Thus, the question as to whether surgical resection is better than PEI cannot be resolved without the use of a prospective randomized trial.
When nonrandomized9–13 and randomized14–16 comparative studies showed RFA to be a better treatment than PEI, and the survival results of RFA were close to the results achieved with surgical resection, a plea was made that a prospective randomized study be conducted between RFA and surgical resection on patients with resectable and small HCC.6 We believe this study is the first prospective randomized study on this topic.
Our study showed that the overall and disease-free survivals were the same for patients with a solitary HCC of 5 cm or smaller in diameter treated with either PLAT or surgical resection. However, PLAT has the advantage over surgical resection in causing less post-treatment complications, less pain, and a shorter hospital stay. Our study also showed that for PLAT to be an effective treatment of small solitary HCC, the initial treatment of RFA has to be followed by assessment of viable residual tumor with CT 4 weeks after the treatment. If viable residual tumor is present, another session of RFA or PEI has to be given.
This study can be criticized on the following aspects: first, post randomization exclusion occurred in 19 of 90 patients randomized to receive PLAT. The reason for the exclusion was because these patients withdrew their consent for PLAT. It is still a strong belief by many doctors and patients in China that liver resection is a more definitive treatment to cure cancer. All these patients who withdrew their consent opted for surgical resection instead. We do not think that these exclusions would have affected the results of this study. Second, the mean follow-up for our patients in the study was relatively short, and it was just beyond 2 years. Judging from the overall and disease-free survival curves, we do not think that there will be a significant difference in survivals given a longer period of follow-up. Finally, the sample size of the study was relatively small. However, we do not think that a larger sample size would have affected the results of this study as the survival curves for the 2 treatment groups were almost exactly the same.
CONCLUSION
This prospective randomized trial showed PLAT to give the same overall and disease-free survivals as surgical resection for patients with solitary and small HCC. PLAT has the advantage over liver resection in giving a better short-term postoperative results because PLAT is a less invasive procedure.
Footnotes
Supported by the grant of Sciences and Technology Committee of Guangdo Province, China, 2002.
Reprints: Min-Shan Chen, MD, PhD, Department of Hepatobiliary Surgery, Cancer Centre of Sun Yat-Sen University, 651 Dongfeng Road East, Guangzhou 510060, P.R. China. E-mail: Cms64@21cn.com.
REFERENCES
- 1.Bosch X, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Robinson SD, Foster GR, Arora S, et al. Increase in primary liver cancer in the UK 1979–94. Lancet. 1997;350:1142–1143. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. [DOI] [PubMed] [Google Scholar]
- 4.Liver Cancer Study Group of Japan. Primary liver cancers in Japan. Cancer. 1980;45:2663–2669.6155197 [Google Scholar]
- 5.Lai EC, Fan ST, Lo CM, et al. Hepatic resection for hepatocellular carcinoma: an audit of 343 patients. Ann Surg. 1995;221:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau WY, Leung TW, Yu SC, et al. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castells A, Bruix J, Bru C, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993;18:1121–1126. [PubMed] [Google Scholar]
- 8.Livraghi T, Bolondi L, Lazzaroni S, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis: a study on 207 patients. Cancer. 1992;69:925–929. [DOI] [PubMed] [Google Scholar]
- 9.Curley SA, Izzo F, Ellis LM, et al. Radiofrequency ablation of hepatocellular carcinoma in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015–1022. [DOI] [PubMed] [Google Scholar]
- 11.Wood TF, Rose DM, Chung M, et al. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations and complications. Ann Surg Oncol. 2000;7:593–600. [DOI] [PubMed] [Google Scholar]
- 12.Nicoli N, Casari A, Marchiori L, et al. Intraoperative and percutaneous radiofrequency thermal ablation in the treatment of hepatocellular carcinoma. Chir Ital. 2000;52:29–40. [PubMed] [Google Scholar]
- 13.Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914–921. [DOI] [PubMed] [Google Scholar]
- 14.Lencioni RA, Cioni D, Donati F, et al. Percutaneous treatment of small hepatocellular carcinoma in cirrhosis: radiofrequency thermal ablation vs ethanol injectionā prospective, randomized trial (final report). Radiology. 1999;213(suppl):123. [Google Scholar]
- 15.Shiina S, Teratani T, Obi S, et al. Prospective randomized controlled trial comparing percutaneous radio-frequency ablation and percutaneous ethanol injection therapy for small hepatocellular carcinoma. Gastroenterology. 2000;118(suppl):959. [Google Scholar]
- 16.Olschewski M, Lencioni R, Allgaier H, et al. A randomized comparison of radiofrequency thermal ablation and percutaneous ethanol injection for the treatment of small hepatocellular carcinoma [Abstract]. Proc Am Soc Clin Oncol. 2001:500. [Google Scholar]
- 17.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona 2000 EASL Conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. [DOI] [PubMed] [Google Scholar]
- 18.Lau WY. Management of hepatocellular carcinoma. J R Coll Surg Edinb. 2002;47:389–399. [PubMed] [Google Scholar]
- 19.Shiina S, Tagawa N, Niwa Y, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;60:1023–1028. [DOI] [PubMed] [Google Scholar]
- 20.Isobe H, Sakai H, Imari Y, et al. Intratumor ethanol injection therapy for solitary minute hepatocellular carcinoma: a study of 37 patients. J Clin Gastroenterol. 1994;18:122–126. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto J, Okada S, Shimada K, et al. Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology. 2001;34:707–713. [DOI] [PubMed] [Google Scholar]
- 22.Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and non-surgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224–1229. [DOI] [PubMed] [Google Scholar]
- 23.Shi M, Zhang CQ, Zhang YQ, et al. Micrometastasis of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–381. [DOI] [PubMed] [Google Scholar]