Abstract
Objective:
Because laparoscopic cholecystectomy (LC) is widely recognized as a “mild” or “mini-invasive” kind of surgery, in this prospective nonrandomized study, we investigated the effect of intestinal manipulation on intestinal permeability and endotoxemia, in patients undergoing elective cholecystectomy by comparing the laparoscopic with the laparotomic approach.
Summary Background Data:
The intestine is susceptible to operations at remote locations, and the barrier function is altered during intestinal manipulation, leading to bacterial or endotoxin translocation into the systemic circulation.
Methods:
Forty-three patients undergoing elective cholecystectomy were divided into either the laparotomic (n = 22) or laparoscopic (n = 21) approach. Intestinal permeability was measured preoperatively and at day 1 and day 3 after surgery using the lactulose/mannitol absorption test. Serial venous blood samples were taken at 0, 30, 60, 90, 120, and 180 minutes, and at 12, 24, and 48 hours after surgery, for endotoxin measurement using the chromogenic limulus amoebocyte lysate assay.
Results:
Intestinal permeability was significantly increased at day 1 [0.106 ± 0.005 (mean ± SEM)] in the laparotomic group compared with the preoperative level (0.019 ± 0.005, P < 0.05) and to the laparoscopic group at day 1 (0.019 ± 0.005, P < 0.05), which showed no change in comparison with the preoperative level. A significantly higher concentration of systemic endotoxin was detected intraoperatively in the laparotomic group of patients in comparison to the laparoscopic group (P < 0.05). There was a significant positive correlation between systemic endotoxemia and intestinal permeability (rs = 0.958; P = 0.001).
Conclusions:
An increase in intestinal permeability and a greater degree of systemic endotoxemia are observed during laparotomic cholecystectomy. This suggests that intestinal manipulation may impair gut mucosal barrier function and contribute to the systemic inflammatory response see in open cholecystectomy.
Intestinal manipulation may impair intestinal mucosal barrier function. We investigated the effect of intestinal manipulation on intestinal permeability and endotoxemia in patients undergoing elective cholecystectomy (laparotomic versus laparoscopic). An increase in intestinal permeability and a greater degree of systemic endotoxemia are observed during laparotomic cholecystectomy.
The intestinal epithelium undergoes a continuos renewal process and consists of cells at various stages of differentiation. The integrity of the intestinal epithelium is critical to health,1 and any damage to the cells can effect proliferation and differentiation, leading to altered cell population and functional changes in the intestine. The intestine acts as a barrier to the luminal contents, which include bacteria and endotoxins. The gut barrier is altered in certain pathologic conditions such as shock, trauma, or surgical stress, resulting in bacterial or endotoxin translocation from the gut lumen into the systemic circulation.2 This has been implicated in postoperative complications, such as systemic inflammatory response syndrome and multiorgan failure.3
Laparoscopic cholecystectomy (LC) is now considered the treatment of choice for symptomatic gallbladder stone disease.4–6 When compared with open cholecystectomy (OC), LC presents several advantage such as reduced postoperative pain, prompt postoperative bowel activity (6–24 hours after operation), reduced hospitalization (1–3 days), earlier return to work, better esthetic results, and reduced postoperative infections.6–11
Since LC is widely recognized as a “mild” or “mini-invasive” kind of surgery, in this prospective nonrandomized study we investigated the effect of intestinal manipulation on intestinal permeability and endotoxemia in patients undergoing elective cholecystectomy by comparing the laparoscopic with the laparotomic approach.
MATERIALS AND METHODS
From February 2003 to July 2004, we studied, in a prospective nonrandomized study, 43 patients consecutively (29 women, 14 men; mean age, 51.3 years), all presenting with symptomatic gallbladder stone disease. The diagnosis was confirmed by ultrasound examination. Serologic tests (AST, ALT, bilirubin, serum protein) were in the normal range. Patients with a history of acute cholecystitis, acute pancreatitis, or acute or chronic inflammatory or malignant intestinal disease were excluded from the study. During hospitalization, the patients were not given antispastic drugs, steroids, or nonsteroidal anti-inflammatory drugs (NSAIDs). The patients were classified as grade I or II, according to the American Society of Anesthesiologists (ASA) grading system.12
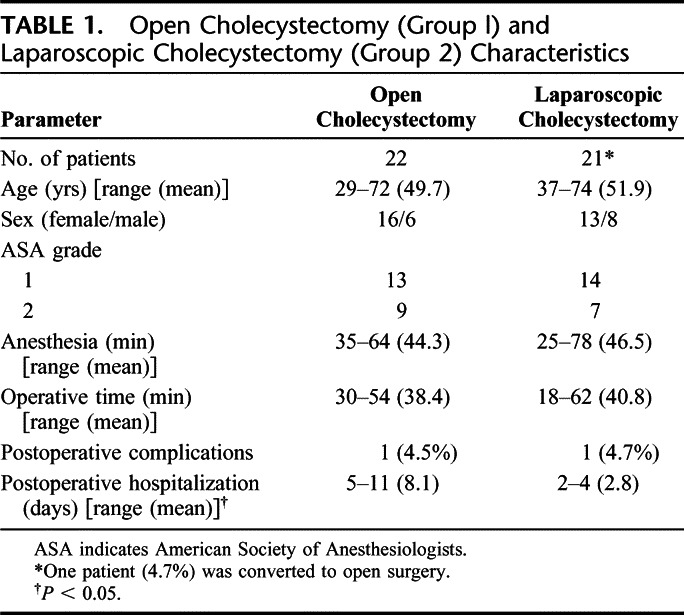
Altogether 22 patients (16 women, 6 men; mean age, 49.7 years) (group 1) (Table 1) underwent OC using a right subcostal incision. The remaining 21 patients (13 women, 8 men; mean age, 51.9 years) (group 2) (Table 1) underwent LC using the standard technique with 4 trocar incisions. All patients from group 1 were operated using the open technique by a surgical team different from ours, who prefers the OC procedure.
TABLE 1. Open Cholecystectomy (Group l) and Laparoscopic Cholecystectomy (Group 2) Characteristics
This study was approved by the Research Ethics Committee of the University of L'Aquila. All patients gave written consent. It was difficult to obtain patient agreement to randomization; so having 2 sources was the only way to obtain 2 groups. Including consecutive patients to the study hopefully reduced selection bias.
As shown in Table 1, age, sex, ASA grades, time of anesthesia, and operation were comparable in the 2 groups, but hospitalization was significantly shorter in group 2. There were no indications for intraoperative cholangiography in either group, nor did they receive blood transfusions. Anesthesia was obtained in both groups using the same procedure. Preanesthesia was done using atropine (0.01 mg/kg), plus promethazine (0.5 mg/kg), induction using sodium thiopental (5 mg/kg) and atracurium (0.5 mg/kg), and tracheal intubation and assisted ventilation using nitrogen dioxide (NO2)/oxygen 2:1. Removed gallbladders underwent histologic examination.
Blood Sampling
Serial venous blood samples were taken at 0, 30, 60, 90, 120, and 180 minutes, and at 12, 24, and 48 hours after surgery. Blood was collected in pyrogen-free tubes and centrifuged at 4°C and 2000 rpm for 10 minutes and aliquoted into sterile cryotubes (NUNC 36341, Intermed, Denmark) and stored at −80°C until analysis for subsequent determination of endotoxin.
Intestinal Permeability
Intestinal permeability was assessed preoperatively and at day 1 and day 3 after surgery using the lactulose/mannitol differential absorption test.13–15 This test is well validated in our department. It is nontoxic, noninvasive, simple to perform, relatively inexpensive, and reproducible. It has become accepted as a reliable method for assessing small intestinal permeability. A pretest sample of urine was collected after 6 hours of fasting for baseline urinary sugar measurement. After the pretest sample was obtained, patients were given 10 g of lactulose and 5 g of mannitol dissolved in 100 mL of water orally or via nasogastric tube. Urine was collected for 6 hours, aliquoted, and stored at −20°C until assayed. Urinary lactulose and mannitol concentrations were determined by an enzymatic technique.14,15 Mannitol excretion was corrected by subtraction of baseline values determined in the pretest samples and the lactulose/mannitol excretion ratios (L/M ratio) were calculated.
Endotoxin Measurement
Endotoxin was quantified in duplicates using a modified chromogenic Limulus amoebocyte lysate assay (Quadratech, Epsom, U.K.). Test plasma samples and standard were diluted 1:10 in pyrogen-free water and heated to 75°C for 10 minutes to remove plasma inhibitors. The concentration of endotoxin in the sample was taken as the average of the duplicates calculated from a standard curve. The assay has a sensitivity of 8 pg/mL and is linear in the range 8 to 100 pg/mL. Each aliquot was assayed for endotoxin only once. If the assay gave poor duplicates or very high values, indicating possible contamination, a fresh aliquot of the same sample was retested.
Statistical Analysis
Results are expressed as mean ± SEM. Statistical analysis was performed using the Mann-Whitney U test or Spearman's rank-correlation coefficient (rs) where appropriate with significance taken at the 5% level.
RESULTS
The LC required almost the same operating time as the OC (Table 1). Hospitalization was shorter for LC (Table 1). In the laparoscopic group, conversion was required in 1 patient, leaving 20 patients for analysis.
Histologic examination showed in the open group acute cholecystitis in 6 patients and chronic cholecystitis in 16 patients. In the laparoscopic group, acute cholecystitis was present in 7 patients and chronic cholecystitis in 13 patients. There were no differences between the 2 groups concerning the severity of cholecystitis.
Intestinal Permeability
No difference was observed in the preoperative L/M ratios in the 2 groups of patients (Fig. 1). The L/M ratio was significantly increased in the open group at day 1 (0.106 ± 0.005) compared with the preoperative level (0.019 ± 0.005, P < 0.05). This increase was also significant compared with the day 1 level in the laparoscopic group (0.019 ± 0.005, P < 0.05), which stowed no increase in the mean L/M ratio from preoperative (0.020 ± 0.005) (Fig. 1). No correlation was found between the increased intestinal permeability and duration of surgery.
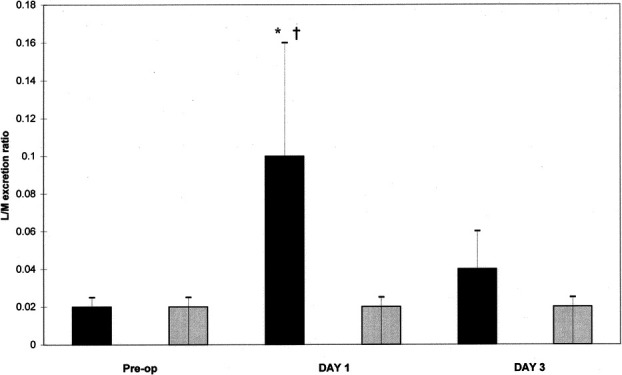
FIGURE 1. Intestinal permeability measured by lactulose/mannitol excretion ratio (L/M ratio). *P < 0.05 versus Preop. †P < 0.05 versus laparoscopy day 1. (▪), laparotomy group; ( ), laparoscopy group.
Endotoxin
The systemic endotoxin concentration rose significantly during the course of surgery but returned to near baseline by day 2 (Fig. 2). Systemic concentrations of endotoxin were higher in the open group at all intraoperative sampling times but reached significance only when the gallbladder was dissected from the underlying liver bed (open group, 25.76 ± 9.15 pg/mL versus laparoscopic group, 1.70 ± 1.69 pg/mL; P < 0.05) (Fig. 2). A significant correlation was observed between the maximum systemic endotoxin concentration and intestinal permeability measured at D1 (rs = 0.958; P = 0.001) (Fig. 3).
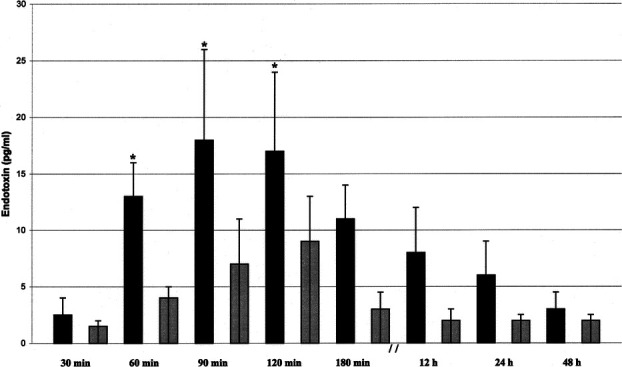
FIGURE 2. Systemic endotoxin concentration (mean ± SEM) *P < 0.05 versus 60, 90, and 120 minutes. (▪), laparotomy group; ( ), laparoscopy group.
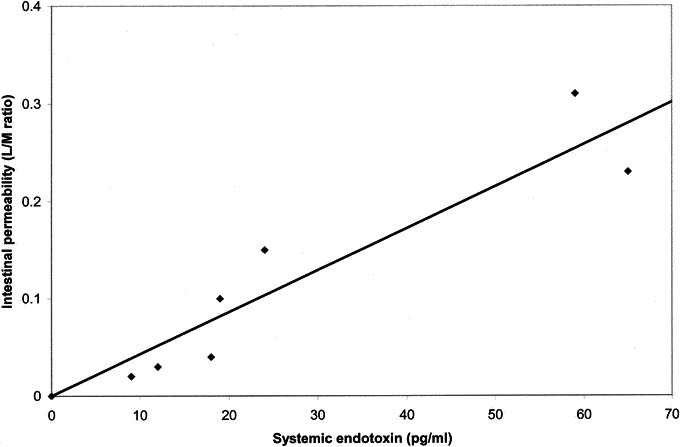
FIGURE 3. Correlation between systemic endotoxin concentration and intestinal permeability measured as lactulose/mannitol excretion ratio (L/M ratio) in the laparotomy group (rs = 0.958; P = 0.001).
Only 1 patient underwent conversion from laparoscopic to open procedure. This patient had intestinal permeability parameters (L/M excretion ratio: 0.105) and systemic endotoxin concentration (26.01 pg/mL) similar to the open group.
One patient from the open group developed pneumonia and 1 patient from the laparoscopic group developed cerebrovascular accident (Table 1). For these 2 patients, intestinal permeability parameters (L/M ratio: OC = 0.108; LC = 0.021) and systemic endotoxin concentration (OC = 26.31; LC = 1.65) were similar to the group of belonging.
DISCUSSION
The intestinal epithelium is a self-renewing monolayer arising from stem cells located at or near the base of the crypts.16,17 Anup et al,18 using a rat model, showed that laparotomy with mild intestinal handling can result in permeability alterations and oxidative stress in the enterocytes, mainly as a result of activation of xanthine oxidase, and the damage is reversible with time.19
In our study, a change of intestinal permeability was demonstrated only in patients undergoing open cholecystectomy, with no change occurring in the laparoscopic group. Since the 2 groups were similar, the major factor that differed between the 2 groups of patients was the effect of the surgical approach on intestinal manipulation. There was no difference in the morbidity and mortality in the 2 groups (Table 1). This may reflect the small sample size in this study. In the laparotomic technique, the peritoneum is manipulated, the small intestine handled, and the mesentery placed under traction, while in the laparoscopic approach these are absent or minimized. These factors, which are integral parts of the laparotomic approach, may lead to the production of vasoactive agents that can cause both local and systemic hemodynamic changes.20,21 There is evidence to support the role of prostaglandins in causing these hemodynamic responses, even though the exact mechanism leading to their generation is not fully understood.22
The mucosa of the intestine and the endothelium of blood vessels contain enzymes capable of synthesizing prostaglandins, production of which may be initiated by neural, ischemic, toxic, or mechanical stimuli.23 The generation of these vasoactive prostaglandins can therefore induce splanchnic ischemia with subsequent disruption of mucosal integrity and increased intestinal permeability. Therefore, intestinal manipulation may lead to the endotoxemia detected in the systemic circulation in patients undergoing cholecystectomy. The results of the present study concur with this suggestion, as endotoxin was found in the systemic circulation in the laparotomic group of patients. This implies that mere manipulation of the intestine may have a deleterious effect on intestinal mucosa. Regardless of the etiology of impaired mucosal barrier function, permeation of luminal contents across the bowel wall does occur and results in endotoxemia.24–29 Endotoxin is a potent stimulator of release of cytokines, such as interleukin-6 and tumor necrosis factor. These inflammatory mediators play an important role in the pathogenesis of systemic inflammatory response syndrome7,10 and multiple-organ dysfunction syndrome.30 However, the significance of the correlation between systemic endotoxin concentration and increased intestinal permeability remains unclear. In our study, the endotoxemia was detected mainly during the intraoperative period, but the increased intestinal permeability was observed only 24 hours after surgery. Although it would be easy to suggest that the endotoxemia was due to an increase in bowel permeability, one may develop independently of the other. Indeed, endotoxemia is known to reduce splanchnic blood flow and cause disruption of mucosal barrier function.31
Furthermore, exposing the peritoneal cavity to the atmosphere may in itself lead to endotoxemia.32
The findings of this study suggest that intestinal manipulation during the initial abdominal exploration can lead to systemic endotoxemia. This agrees with the hypothesis that intestinal manipulation may impair intestinal mucosal barrier function and contribute to the systemic inflammatory response seen in OC.10 The recent introduction of LC may reduce intestinal mucosal dysfunction and merit further investigations.
REFERENCES
- 1.Hermiston ML, Gordon JL. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JC, Christo NV, Meakins JL. The gastrointestinal tract: the ‘undrained abscess' of multiple-organ failure. Ann Surg. 1993;218:111. doi: 10.1097/00000658-199308000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131:241–244. doi: 10.1067/msy.2002.116408. [DOI] [PubMed] [Google Scholar]
- 4.Flum DR. Laparoscopic cholecystectomy safer than open? Administrative data gone astray. Am J Gastroenterol. 2004;99:1399. doi: 10.1111/j.1572-0241.2004.20229.x. [DOI] [PubMed] [Google Scholar]
- 5.Bittner R. The standard of laparoscopic cholecystectomy. Langenbecks Arch Surg. 2004;389:157–163. doi: 10.1007/s00423-004-0471-1. [DOI] [PubMed] [Google Scholar]
- 6.Fathy O, Zeid MA, Abdallah T. et al. Laparoscopic cholecystectomy: a report on 2000 cases. Hepatogastroenterology. 2003;50:967–971. [PubMed] [Google Scholar]
- 7.Schietroma M, Carlei F, Mownah A. et al. Changes in the blood coagulation, fibrinolysis, and cytokine profile during laparoscopic and open cholecystectomy. Surg Endosc. 2004;18:1090–1096. doi: 10.1007/s00464-003-8819-0. [DOI] [PubMed] [Google Scholar]
- 8.Schietroma M, Carlei F, Rossi M. et al. Neutrophil-elastase in patients undergoing open versus laparoscopic cholecystectomy. Surgery. 200;130:898. doi: 10.1067/msy.2001.117374. [DOI] [PubMed] [Google Scholar]
- 9.Shamiyeh A, Wayand W. Laparoscopic cholecystectomy: early and late complication and their treatment. Langenbecks Arch Surg. 2004;389:164–171. doi: 10.1007/s00423-004-0470-2. [DOI] [PubMed] [Google Scholar]
- 10.Schietroma M, Carlei F, Lezoche E. et al. Evaluation of immune response in patients after open or laparoscopic cholecystectomy. Hepatogastroenterology. 2001;48:642–646. [PubMed] [Google Scholar]
- 11.Carlei F, Schietroma M, Cianca G. et al. Effects of laparoscopic and conventional (open) cholecystectomy on human leukocyte antigen-DR expression in peripheral blood monocytes: correlations with immunologic status. World J Surg. 1999;23:18–22. doi: 10.1007/s002689900559. [DOI] [PubMed] [Google Scholar]
- 12.American Society of Anesthesiologists. New classification of physiology status. Anesthesiologists. 1963;24:111. [Google Scholar]
- 13.Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107:411–416. [PubMed] [Google Scholar]
- 14.Behrens RH, Doherty H, Elia M. et al. A simple enzymatic method for the assay of urinary lactulose. Clin Chim Acta. 1984;137:361–367. doi: 10.1016/0009-8981(84)90125-6. [DOI] [PubMed] [Google Scholar]
- 15.Cocran AC, Page IH. A method for the determination of mannitol in plasma and urine. J Biol Chem. 1947;170:165–171. [Google Scholar]
- 16.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Intern J Exp Pathol. 1997;78:219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmy T, Anup R, Prabhu R. et al. Effect of surgical manipulation of the rat intestine on enterocyte populations. Surgery. 2001;130:479–488. doi: 10.1067/msy.2001.115832. [DOI] [PubMed] [Google Scholar]
- 18.Anup R, Susama P, Balasubramanian KA. The role of xanthine oxidase in small bowel mucosal dysfunction after surgical stress. Br J Surg. 2000;87:1094–1101. doi: 10.1046/j.1365-2168.2000.01469.x. [DOI] [PubMed] [Google Scholar]
- 19.Anup R, Aparna V, Pulimood A. et al. Surgical stress and the small intestine: role of oxygen free radicals. Surgery. 1999;125:560. [PubMed] [Google Scholar]
- 20.Kalff JC, Türler A, Schwarz NT. et al. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. 2003;237:301–315. doi: 10.1097/01.SLA.0000055742.79045.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soybel DI, Zinner MJ. Ileus and the macrophage. Ann Surg. 2003;237:316–318. doi: 10.1097/00000658-200303000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seltzer JL, Goldberg ME, Larijani GE. et al. Prostacyclin mediation of vasodilation following mesenteric traction. Anesthesiology. 1988;68:514–518. doi: 10.1097/00000542-198804000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Hudson JC, Wurm WH, O'Donnel TF. et al. Hemodynamics and prostacyclin release in the early phases of aortic surgery: comparison of transabdominal and retroperitoneal approaches. J Vasc Surg. 1988;7:190–198. [PubMed] [Google Scholar]
- 24.Roumen RMH, Frieling JTM, van Tits HWHJ. et al. Endotoxaemia after major vascular operations. J Vasc Surg. 1993;18:853–857. [PubMed] [Google Scholar]
- 25.Soong CV, Blair PHB, Halliday MI. et al. Bowel ischaemia and organ impairment in elective abdominal aortic aneurysm surgery. Br J Surg. 1994;81:965–968. doi: 10.1002/bjs.1800810712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welch M, Douglas JT, Smyth JV. et al. Systemic endotoxaemia and fibrinolysis during aortic surgery. Eur J Vasc Endovasc Surg. 1995;9:228–232. doi: 10.1016/s1078-5884(05)80095-4. [DOI] [PubMed] [Google Scholar]
- 27.Soong CV, Blair PHB, Halliday MI. et al. Endotoxaemia, the generation of the cytokines and their relationship to intramucosal acidosis of the sigmoid colon in elective abdominal aortic aneurysm repair. Eur J Vasc Surg. 1994;81:965–968. doi: 10.1016/s0950-821x(05)80366-4. [DOI] [PubMed] [Google Scholar]
- 28.Cabie A, Farkas JC, Fitting C. et al. High levels of portal TNF-α during abdominal aortic surgery in man. Cytokine. 1993;5:448–453. doi: 10.1016/1043-4666(93)90034-3. [DOI] [PubMed] [Google Scholar]
- 29.Baigrie BJ, Lamont PM, Whiting S. et al. Portal endotoxin and cytokine response during abdominal aortic surgery. Am J Surg. 1993;166:248–251. doi: 10.1016/s0002-9610(05)80967-5. [DOI] [PubMed] [Google Scholar]
- 30.Deitch EA. Multiple-organ failure: pathophysiology and potential future therapy. Ann Surg. 1992;216:117. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deitch EA, Berg R, Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch Surg. 1987;122:18–19. doi: 10.1001/archsurg.1987.01400140067008. [DOI] [PubMed] [Google Scholar]
- 32.Watson RW, Redmond HP, McCarthy J. et al. Exposure of the peritoneal cavity to air regulates early inflammatory responses to surgery in a murine model. Br J Surg. 1995;82:1060–1065. doi: 10.1002/bjs.1800820820. [DOI] [PubMed] [Google Scholar]


