Abstract
Objective:
To evaluate the utility of 18F-FDG-PET in predicting response to concomitant chemoradiation in locally-advanced esophageal cancer.
Summary Background Data:
Approximately 25% of esophageal cancer patients experience a pathologic complete response (pCR) to preoperative chemoradiation therapy. Computed tomography, endoscopy, and endoscopic ultrasound are unable to identify patients experiencing a pCR. Growing evidence supports the use of 18F-FDG-PET in the staging of esophageal cancer in its ability to detect occult metastatic and lymph nodal disease. The identification of patients with a pCR to chemoradiation could potentially spare those patients the morbidity associated with a resection.
Methods:
Eligibility criteria included T3-T4N0M0 or T1-T4N1M0 esophageal cancer. Patients underwent an initial 18F-FDG-PET before treatment and then repeated 4 to 6 weeks after chemoradiation, prior to the esophagectomy. Chemoradiation consisted of: cisplatinum, 5-fluorouracil, and radiation to a median dose of 50.4 Gy. Pathologic response was determined from a systematic review of the esophagectomy specimens.
Results:
Sixty-four patients have completed therapy to date. Response was as follows: pCR 27%, pathologic residual microscopic (pCRmicro) 14.5%, partial response 19%, and stable or progressive disease 39.5%. A pretreatment standardized uptake value (SUVmax1hour) ≥15 was associated with an observed 77.8% significant response (pCR + pCRmicro) compared with 24.2% for patients with a pretreatment SUVmax1hour <15 (P = 0.005). Significant response was observed in 71.4% of patients with a decrease in SUVmax1hour ≥10 compared with 33.3% when the SUVmax1hour decreased <10 (P = 0.004).
Conclusions:
Pretreatment and posttreatment 18F-FDG-PET can be useful for predicting significant response to chemoradiation in esophageal cancer. These data should be considered in evaluation of patients for esophagectomy after chemoradiation.
This prospective study evaluated the usefulness of 18F-FDG-PET in determining response to preoperative chemoradiation therapy in nonmetastatic locally advanced esophageal cancer. A high pretreatment SUVmax1hour or a large decrease in SUVmax1hour value after chemoradiation predicted for significant response to preoperative chemoradiation therapy.
Over the last 2 decades, concomitant chemoradiation followed by esophagectomy for locally advanced esophageal cancer has become a standard treatment option despite conflicting results from randomized trials.1–7 The prognosis of esophageal cancer following combined modality therapy remains poor, with long-term control rates of 25% to 35%.8 In modern studies, chemoradiation results in complete eradication of tumor in histologic specimens in 11.4% to 51% of patients with only microscopic residual in as many as 54%.5–7,9–12 When stratified by response to chemoradiation, investigators have identified a subgroup of patients with excellent long-term local control rates and improved long-term survival.1,5,10,13 For this reason, identifying responders to chemoradiation has prognostic value that may change clinical management.
Recently, 18F-FDG-PET was shown to improve sensitivity, specificity, and accuracy over computerized tomography (CT) and endoscopic ultrasound (EUS) in the initial staging of esophageal cancer patients.14–17 This improvement has led to the avoidance of unnecessary resections in patients with occult metastatic disease missed by CT or EUS. Similarly, CT and EUS have been shown to be poor predictors of response to chemoradiation with low accuracy in the post-chemoradiation setting.18 In head-to-head comparison, 18F-FDG-PET was shown to be superior to CT and EUS in determining post-chemoradiation stage.19 Several preliminary studies using 18F-FDG-PET in the post-chemoradiation setting are now available demonstrating strong associations of a 18F-FDG-PET-based response to histologic response and improved overall survival.20–25 To further delineate the utility of 18F-FDG-PET in detecting response to chemoradiation in esophageal cancer, we conducted a prospective trial to correlate pre-chemoradiation 18F-FDG-PET, with post-chemoradiation 18F-FDG-PET, and pathologic tumor response assessed in esophagectomy specimens. In addition, we evaluated the test characteristics of 18F-FDG-PET in determining post-chemoradiation restaging.
MATERIALS AND METHODS
From January 4, 2000 to August 24, 2004, 64 consecutive patients, with potentially curable, locally advanced esophageal cancer were either enrolled into a prospective phase II trial or formally monitored off-study. Institutional review board approval was obtained for both treatment groups. All patients underwent physical examination, chest and abdominal CT, endoscopy, baseline blood testing, and at least one 18F-FDG-PET scan. Bone scan was performed only if clinically indicated. The clinical tumor-node-metastasis system stage was defined according to the 2002 (version 6.0), American Joint Committee on Cancer staging system. EUS was performed in 52 (81.3%) patients and EGD in 12 (18.7%). A total of 57 patients were staged with 18F-FDG-PET before chemoradiation and 50 patients were staged with 18F-FDG-PET after chemoradiation. Pre- and post-chemoradiation 18F-FDG-PET imaging was available for 46 patients.
Treatment
Radiation Therapy
All patients received preoperative chemoradiation to a median radiation dose of 50.4 Gy concurrent with chemotherapy. Radiation was delivered in 1 of 2 fashions. Standard radiation was performed in 42 patients (66%) with 1.8- to 2-Gy fractions to an initial dose of 39.6 Gy followed by an off-cord boost for a total dose of 45 to 54 Gy. A total of 22 patients (34%) were enrolled on an institutional phase II trial and were treated with a hyperfractionated radiation schedule using a concomitant boost technique. During week 1 of the chemotherapy, patients received 1.6 Gy each am to a large AP:PA field followed by 1.6 Gy treatments each pm to an off-cord oblique boost volume. During weeks 2 to 4, patients received 1.8 Gy once-daily to a large AP:PA field and no chemotherapy. During week 5, patients received chemotherapy and 1.6 Gy delivered twice-daily to an off-cord oblique boost volume. The total radiation dose delivered over the 5 weeks was 59 Gy.
Chemotherapy
Patients enrolled in the phase II trial received cisplatinum and 5-fluorouracil (5-FU)-based chemotherapy. The chemotherapy dosage was as follows: cisplatinum: 100 mg/m2 on day 1 of radiation therapy along with 5-FU given at 1000 mg/m2 per day by continuous infusion for 96 hours. The 5-FU was started immediately after completion of the cisplatinum infusion on days 1 to 4 and a second course of 5-FU was given during week 5 (days 29–32) concurrent with the radiation therapy. For patients not formally enrolled into the phase II trial, the chemotherapy regimens were as follows: cisplatin and 5-FU in 38 patients, carboplatinum and 5-FU in 8 patients, paclitaxel and carboplatinum in 3 patients, 5-FU as a single agent in 2 patients, and mitomycin C with 5-FU in 2 additional patients. Detailed chemotherapy records were not available in 9 patients who received concurrent chemoradiation. Chemotherapy was declined by 2 patients.
Surgery
Exploratory laparotomy was performed 4 to 6 weeks after chemoradiation in patients eligible for esophagectomy. Esophagectomy was not performed in 16 patients for the following reasons: 1 patient had undergone previous partial esophagectomy 12 years prior, 1 patient died during chemoradiation, 3 patients were upstaged to M1 after chemoradiation, 2 refused esophagectomy, and 9 were not offered esophagectomy based on medical comorbidities. Forty-eight patients underwent exploratory laparotomy with completion esophagectomy in 44. One patient undergoing esophagectomy who declined chemoradiation was excluded from this analysis. Of the 43 patients undergoing chemoradiation followed by esophagectomy, R0 resections were achieved in 38 (86.4%), R1 resections were performed in 4 (10.6%) with positive radial margins, and 1 had unknown margins. Unresectable disease was found in 4 patients at exploratory laparotomy due to matted celiac nodes (1), liver metastases (2), or peritoneal carcinomatosis (1). Total gastrectomy was performed in 2 patients for disease that was widely resectable via laparotomy alone. Esophagectomy was performed by transhiatal technique (21) or transthoracic technique (21) at the discretion of the treating surgeon.
Scoring of Response to Chemoradiation
All pathologic specimens were reviewed by a single pathologist who was blinded to the results of the 18F-FDG-PET. Response was scored in 5 categories: pathologic complete response (pCR), pathologic microscopic residual disease (pCRmicro), pathologic partial response (pPR), pathologic stable disease (pSD), or pathologic progressive disease (pPD). pCR was defined as no tumor in the pathology specimen and pCRmicro was defined as residual microscopic disease in the specimen with questionable viability. Partial response, stable disease, and progressive disease were defined as a post-chemoradiation T or N stage less than the pre-chemoradiation T and N stage, a post-chemoradiation T or N stage equal to the pre-chemoradiation T and N stage, or a post-chemoradiation T or N stage greater than the pre-chemoradiation stage, respectively. For comparisons, patients experiencing a pCR or pCRmicro were termed significant responders and were compared with patients experiencing a PR, SD, or PD after chemoradiation. Patients with metastatic disease at any site were considered to have PD regardless of the response in the radiation field.
Positron Emission Tomography
Pretreatment and post-treatment 18F-FDG-PET scans were performed. A GE Advance PET scanner was used in all patients with a standard resolution of 6 to 8 mm. Blood glucose was determined prior to injection of 18F-FDG. Patients with blood glucose <150 mg/dL proceeded with radiopharmaceutical injection and imaging. Patients received 15 to 20 mCi 18F-FDG after 4 hours of fasting. Emission images were obtained from the chin to the pelvis. Attenuation correction was performed using transmission scans. Imaging was performed 1 hour post-radiopharmaceutical injection. 18F-FDG-PET images were interpreted by an experienced nuclear radiologist and correlated with CT. The qualitative criteria for malignancy included discrete areas of increased F-FDG activity greater than the blood pool activity. Quantitative and qualitative analysis of PET images was performed by one nuclear medicine physician. Regions of interest were placed over the qualitatively visualized area of maximum F-FDG activity within the tumor. Similar regions of interest were used on pre- and post-therapy scans. 18F-FDG-PET studies that demonstrated a diffuse area of increased activity were scored as inflammation. Quantitative analysis was performed using standardized uptake values and calculated as the maximum value 1 hour post-radiopharmaceutical injection (SUVmax1hour). The SUVmax1hour is the amount of radioactivity in the region corrected for decay, to the time of injection, normalized by the patient's lean body mass and the injected dose.
Of the 44 patients undergoing esophagectomy following chemoradiation, 31 patients had both a pre-chemoradiation and post-chemoradiation 18F-FDG-PET imaging study, while 13 patients had a single 18F-FDG-PET scan: 10 patients of which had a pre-chemoradiation study and 3 of which had post-chemoradiation imaging. Definitive pathologic specimens for correlation with 18F-FDG-PET were available in 41 patients with pre-chemoradiation 18F-FDG-PET staging and 34 patients with post-chemoradiation 18F-FDG-PET staging. Based on the interpretation by the nuclear radiologist as either positive for cancer or negative for cancer, the test characteristics of the post-chemoradiation 18F-FDG-PET scan were calculated.
After treatment, all patients were evaluated with history, physical examination, chest and abdominal CT scans every 3 months for the first 2 years, then every 6 months for the next 3 years, and then at the discretion of the treating physician.
Stastitical Anlysis
Treatment factors that were analyzed included pCR and pCRmicro after chemoradiation versus macroscopic residual disease after chemoradiation. 18F-FDG-PET parameters that were analyzed included pretreatment SUVmax1hour ≥15 versus ≤15, magnitude decrease in SUVmax1hour following preoperative chemoradiation and percent decrease in SUVmax1hour following chemoradiation. Differences in pre- and post-chemoradiation SUVmax1hour were analyzed using a Student t test. χ2 and Fisher exact test were performed using Graphpad Prism version 4.00 for Windows, Graphpad software (San Diego, CA; www.graphpad.com).
RESULTS
Treatment Response
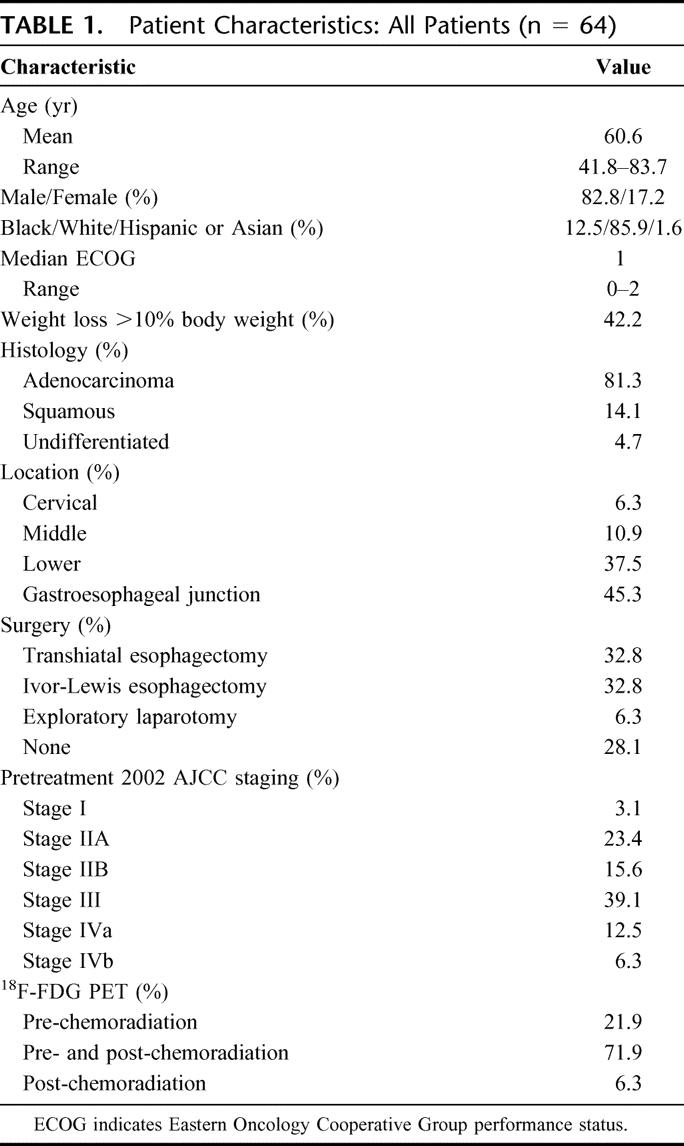
Patient characteristics are shown in Table 1. Of the 48 patients who underwent pathologic staging after chemoradiation, a pCR was found in13 patients (27.1%), a pCRmicro in was found in 7 patients (14.5%), a partial response was observed in 9 patients (18.75%), and either stable or progressive disease was found in 19 (39.5%) patients. Of the 29 patients (61.4%) experiencing significant clinical downstaging after chemoradiation, 20 were associated with having either no or microscopic residual disease (pCR or pCRmicro) in the esophagectomy specimen.
TABLE 1. Patient Characteristics: All Patients (n = 64)
Predictive Value of Pre-chemoradiation 18F-FDG-PET
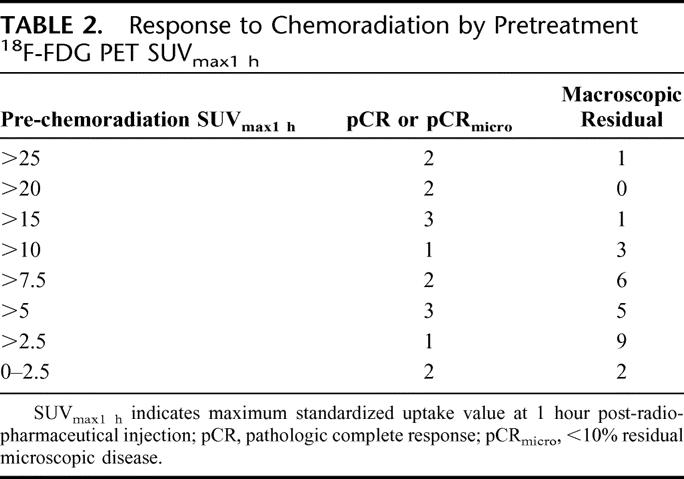
Of the 57 patients with pre-chemoradiation 18F-FDG-PET imaging, the mean SUVmax1hour was 9.9 (0–36.6) ± 1.0. Of the 49 patients with post-chemoradiation 18F-FDG-PET imaging, the mean SUVmax1hour was 4.4 (0–9.7) ± 0.4. There was a significant decrease between mean SUVmax1hour before and after chemoradiation (P = <0.001). The mean SUVmax1hour was higher in patients experiencing a pCR or pCRmicro when compared with patients with macroscopic disease after chemoradiation (13.4 ± 2.5 versus 7.1 ± 2.5, P = 0.02). A pre-chemoradiation 18F-FDG-PET SUVmax1hour ≥15 was associated with significant response to therapy with 7 of 9 patients (77.8%) experiencing a pCR or pCRmicro at the time of surgery. Patients with a pre-chemoradiation SUVmax1hour <15 however, were less likely to experience a significant response; only 9 of 34 patients or 26.4% were found to have a pCR or pCRmicro at the time of pathologic review (P = 0.005). A pre-chemoradiation SUVmax1hour ≥10 was associated with, but less likely to predict for, a significant response to treatment; only 10 of 15 patients (67%) demonstrated a significant response to treatment compared with 8 of 30 (26.7%) having a significant response with a pre-chemoradiation SUVmax1hour <10 (P = 0.043). Table 2 provides a complete report of the correlations between response to therapy and pre-chemoradiation SUVmax1hour.
TABLE 2. Response to Chemoradiation by Pretreatment 18F-FDG PET SUVmax1 h
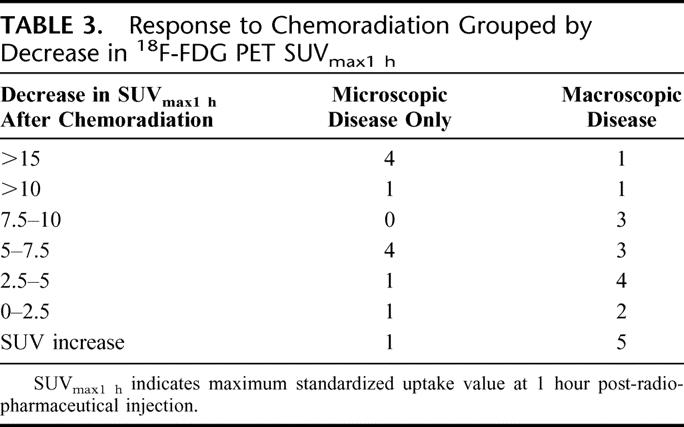
In the 31 evaluable patients with pre- and post-chemoradiation 18F-FDG-PET imaging and definitive pathology, the mean decrease in SUVmax1hour for patients with significant response (pCR or pCRmicro) to chemoradiation was 10.9 ± 2.9 whereas the mean difference in SUVmax1hour for the patients with macroscopic disease after chemoradiation was 4.8 ± 1.5 (P = 0.05). Pathologic response based upon magnitude of SUVmax1hour changes are shown in Table 3. In patients with a magnitude SUVmax1hour decrease of >5 units, 9 of 17 patients (52.9%) had a significant response to chemoradiation versus 3 of 14 patients (21.4%) with a SUVmax1hour decrease <5 (P = 0.155). For patients experiencing an SUVmax1hour decrease >10, which was limited to only 7 patients, 5 of those 7 patients (71.4%) experienced a significant response to chemoradiation versus only 7 of 21 patients (33.3%) with an SUVmax1hour decrease ≤10 (P = 0.004).
TABLE 3. Response to Chemoradiation Grouped by Decrease in 18F-FDG PET SUVmax1 h
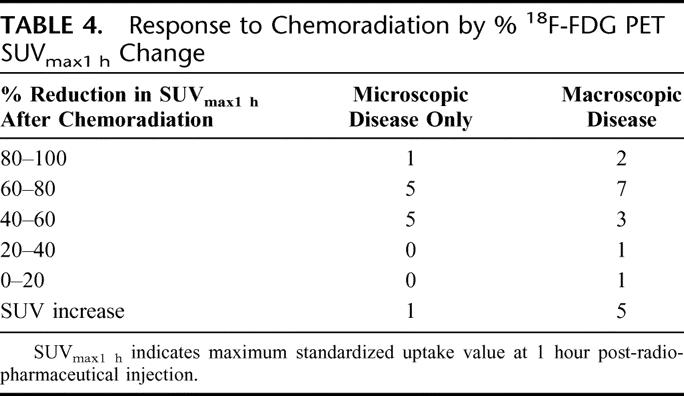
The median percent decrease in SUVmax1hour after chemoradiation was 52.8% (100% decrease to 84% increase). There was no difference in percent SUVmax1hour reduction between patients experiencing a significant response (pCR or pCRmicro) to therapy versus those patients having less of a response (PR, SD, or PD) after chemoradiation (P = 0.871). Pathologic response based on percent SUVmax1hour change is shown in Table 4. With a decrease in SUVmax1hour >40%, 11 of 23 (47.8%) experienced a significant response to compared with only 1 of 8 patients (12.5%) with a SUVmax1hour decrease ≤40% (P = 0.108).
TABLE 4. Response to Chemoradiation by % 18F-FDG PET SUVmax1 h Change
Analysis of 18F-FDG-PET Parameters and Response by Treatment Received
Forty-two patients received daily radiation to 45 to 54 Gy and 22 received accelerated hyperfractionated radiation with dose escalation to 59 Gy. The treatment duration was 5 weeks in both groups. There was a higher proportion of patients experiencing pCR or pCRmicro in the daily radiation treatment group versus the hyperfractionated group (55% versus 25%, P = 0.045). Overall, pretreatment and post-chemoradiation 18F-FDG-PET mean SUVmax1hour values, between treatment groups (daily versus twice daily radiation) were similar (11 versus 9.3, P = 0.45). Likewise, the magnitude change in the 18F-FDG-PET mean SUVmax1hour values between groups was similar (5.8 versus 8.1, P = 0.39). Also, the timing of 18F-FDG-PET was similar where pre- and post-chemoradiation 18F-FDG-PET were separated by a median of 2.5 months in both groups. In the hyperfractionated radiation group, patients experiencing a pCR or pCRmicro had a higher pretreatment SUVmax1hour when compared with nonresponders (17.3 versus 8.2, P = 0.022). In the once daily radiation group, there was a trend toward higher mean SUVmax1hour in patients experiencing pCR or pCRmicro (11.6 versus 6, P = 0.11).
Patterns of Failure in Patients Experiencing pCR and pCRmicro
Of the 20 patients with significant response in this series (pCR and pCRmicro), 8 patients are alive without disease, 7 patients have died without disease, and only 5 patients experienced disease recurrence (2 local and distant failure, 2 with distant failure only, 1 with out-of-radiation portal nodal failure). After excluding patients who died within 90 days of surgery, the median overall survival for patients experiencing a pCR or a pCRmicro was 36 months compared with 24 months in poorly responding patients (P = 0.4).
Sensitivity and Specificity for Post-chemoradiation 18F-FDG-PET
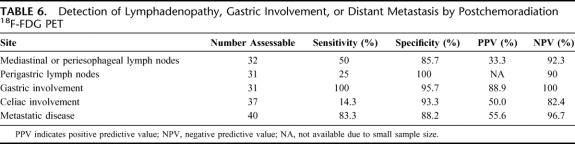
Definitive pathologic specimens were available in 41 patients with post-chemoradiation 18F-FDG-PET imaging. Figure 1 provides examples of pre- and post-chemoradiation imaging with 18F-FDG-PET. The best detection of primary disease was accomplished with an SUVmax1hour cutpoint of 4 with sensitivity, specificity, and positive and negative predictive values of 61.3%, 60.0%, 82.6%, and 33.3%, respectively. See Table 5 for the complete characteristics of post-chemoradiation 18F-FDG-PET. Table 6 demonstrates the test characteristics of 18F-FDG-PET in the detection of disease outside the primary tumor. Scans were interpreted as either positive or negative for this calculation. Sensitivity and specificity for the detection of metastatic disease were 83.3% and 88.2% with a positive and negative predictive value of 55.6% and 96.7%, respectively. Test characteristics for detecting lymphadenopathy in the mediastinum, perigastric, and celiac areas were associated with high specificity and negative predictive value ranging from 85.7% to 100% and 82.4% to 92.3%, respectively. Also, post-chemoradiation 18F-FDG-PET detected gastric invasion with high specificity, sensitivity, and predictive values.
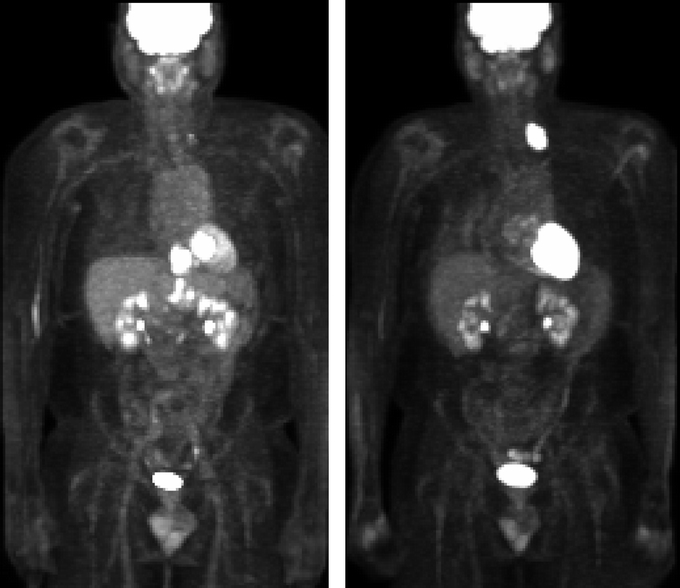
FIGURE 1. Demonstration of response and restaging by 18F-FDG-PET. Left, Pre-chemoradiation imaging is displayed, revealing an abnormality consistent with malignancy within the esophagus (SUV 24), retroperitoneum, and celiac nodal station. Right, Post-chemoradiation imaging is displayed revealing resolution of the abnormality within the esophagus, retroperitoneum, and celiac nodal station. Interval development of a left supraclavicular lymph node is noted.
TABLE 5. Test Characteristics of 18F-FDG PETmax1 h for Esophageal Cancer
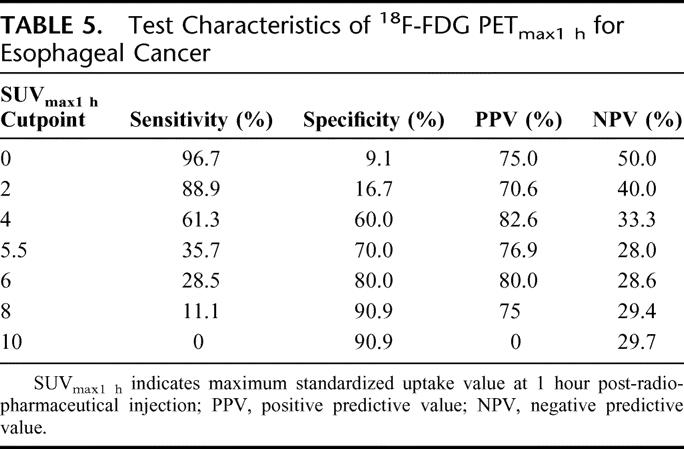
TABLE 6. Detection of Lymphadenopathy, Gastric Involvement, or Distant Metastasis by Postchemoradiation 18F-FDG PET
DISCUSSION
This prospective analysis reports the predictive power of pretreatment 18F-FDG-PET imaging for identifying patients likely to experience a significant pathologic tumor response following a course of preoperative chemoradiation. To our review, only a limited number of prospective studies have been published addressing the value of 18F-FDG-PET for determining response to chemoradiation in this clinical setting.20–24,26 An early trial reported by Weber et al describes an analysis of 40 esophageal cancer patients undergoing 18F-FDG-PET imaging prior to chemotherapy and then repeated 14 days after chemotherapy.21 The authors observed a strong correlation between 18F-FDG-PET tumor response and the clinical response to therapy: a 35% reduction in tumor SUVmax post-chemotherapy was associated with an improved response and survival. In a separate report by Flamen et al, 36 patients with carcinoma of the esophagus underwent 18F-FDG-PET imaging prior to receiving neoadjuvant therapy and then repeated prior to surgery.23 The investigators observed that an 80% or greater reduction in the tumor-to-liver uptake ratio between the 2 18F-FDG-PET studies was predictive for clinical response and improved survival. Downey et al, in a study of 39 patients with adenocarcinoma of the esophagus undergoing 18F-FDG-PET before and after chemoradiation, were also able to demonstrate an association between a large decrease in the post-chemoradiation tumor SUVmax with a significant response to treatment and survival.24 In a larger series from Swisher et al, a strong correlation was observed between patient survival and a post-chemoradiation 18F-FDG-PET SUVmax1hour <4.22 Interestingly, PET was not found to discriminate between complete response and microscopic residual disease as observed in our report. Further, the predictive values were poor for identifying residual esophageal disease, with a false negative rate of 18% and a false positive rate of 71%.
In contrast to our study, an elevated pretreatment 18F-FDG-PET SUVmax1hour has not been associated with significant response in any previous report. The reasoning for this remains unknown, but clear differences in the timing of 18F-FDG-PET, the length of treatment, and calculation of 18F-FDG-PET uptake intensity exist between available studies. In the report from Swisher et al, all patients underwent induction chemotherapy followed by concurrent chemoradiation.22 Thus, the interval between pretreatment 18F-FDG-PET and the completion of chemoradiation was 2 months longer than in our trial. Also, the SUV calculation in the series by Swisher et al did not correct for lean body mass, which was corrected in the present study. In the trial by Downey et al, the timing of 18F-FDG-PET was similar to ours and SUVs were calculated, but only 17 patients underwent esophagectomy and 18F-FDG-PET prediction of response was only suggested in their discussion.24 Finally, Flamen et al reported liver-to-tumor ratios and did not report SUVs.23
One limitation to the present study is the inclusion of 2 separate treatment cohorts (conventional versus hyperfractionated radiation therapy). One cohort received twice-daily hyperfractionated radiation therapy to 59 Gy concurrent with 5-FU and cisplatinum chemotherapy. The second cohort received conventional once-daily radiation to 45 to 54 Gy concurrent with chemotherapy at the discretion of the treating physician. There was no difference between timing of 18F-FDG-PET or treatment duration between groups. Also, there was no difference between pretreatment and post-chemoradiation 18F-FDG-PET mean SUVmax1hour values between treatment groups. Likewise, there was no difference in the magnitude change in the 18F-FDG-PET mean SUVmax1hour values between treatment groups. Interestingly, there was a higher response rate in the daily (conventional) radiation treatment group versus the hyperfractionated group. When analyzed by treatment received, the association of high pretreatment SUVmax1hour with a significant response remained statistically significant in the hyperfractionated radiation group and approached significance in the daily radiation group. Despite the higher likelihood of significant response in patients receiving once-daily radiation therapy, there were no 18F-FDG-PET-related differences identified that would signify any correlation of SUVmax1hour with treatment given.
In contrast to other series, we scored pathologic specimens with only microscopic residual cells to be significant responders. These patients were coupled with the completely responding patients to improve statistical power in this study. The survival advantage in those patients experiencing a complete response to preoperative therapy are well documented.25,27,28 Also, recent evidence indicates that patients with only microscopic residual disease after chemoradiation (those with less than 10% viable cells) experience excellent long-term disease-free and overall survival when compared with historical controls.25,27,28 In this series, microscopic disease only (pCRmicro) is equivalent to the term “less than 10% viable cells” used elsewhere.25,27,28 In comparison, our data support those earlier works. Further, while not statistically significant, the median survival of significant responders was 1 year longer than nonresponders. Also, in significant responders, there were only 4 out of 20 patients dead of disease, with 1 patient salvaged after an out of radiation-field nodal failure at last follow-up. In summary, the group of significant responders identified by 18F-FDG-PET in this series had excellent disease-free and overall survival.
In using 18F-FDG-PET-directed patient selection in esophageal cancer, the timing of 18F-FDG-PET is strongly debated. Some investigators have suggested that the survival advantage for chemoradiation responders is realized only in patients undergoing esophagectomy.6,12,29,30 Clearly, there is a subgroup of patients who experience long-term control after chemoradiation without surgical resection.31,32 A recent European trial of locally advanced squamous cell carcinoma of the esophagus has demonstrated no overall survival benefit to the addition of esophagectomy to chemoradiation therapy.33 On subgroup analysis, there was a significant survival detriment in nonresponders to chemoradiation, and survival was found to be improved for nonresponders undergoing R0 esophagectomy. It is not clear to us if the above results can be extrapolated to patients with adenocarcinoma of the esophagus. Our strategy would be to incorporate 18F-FDG-PET for identifying a patient subgroup with a high probability of response, based on a pretreatment 18F-FDG-PET. Following the results of this trial, patients with a low SUVmax1hour could be managed with surgery alone or chemoradiation followed by esophagectomy, where potential responders might be managed by chemoradiation alone. Only a prospective trial of 18F-FDG-PET-directed treatment can confirm the utility of such an approach stratifying treatment with 18F-FDG-PET.
This study provides support for the utility of 18F-FDG-PET to identify responders to chemoradiation. To determine if 18F-FDG-PET SUV and pCR/pCRmicro response in our study predicts for an improved overall survival will require additional long-term follow-up. Based upon our preliminary data and those studies previously discussed, we conclude that 18F-FDG-PET is a useful tool for identifying esophageal cancer patients likely to experience a significant response to preoperative chemoradiation. Clearly multi-institutional prospective studies are needed to determine the feasibility of 18F-FDG-PET for directing therapy for patients with locally advanced esophageal cancer.
Footnotes
Supported in part by a grant to EAL, from the National Cancer Institute (1R21 CA 89410-01).
Reprints: Edward A. Levine, MD, Surgical Oncology, Wake Forest University, Medical Center Blvd., Winston-Salem, NC 27157. E-mail: elevine@wfubmc.edu.
REFERENCES
- 1.Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long-term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–2174. [PubMed] [Google Scholar]
- 2.Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology. 1994;41:391–393. [PubMed] [Google Scholar]
- 3.Burmeister BH, Smithers BM, Fitzgerald L, et al. A randomized phase III trial of preoperative chemoradiation followed by surgery (CR-S) versus surgery alone (S) for localized resectable cancer of the esophagus. Prog Proc Am Soc Clin Oncol. 2002;21:130a. [Google Scholar]
- 4.Le PE, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779–1784. [DOI] [PubMed] [Google Scholar]
- 5.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. [DOI] [PubMed] [Google Scholar]
- 6.Vogel SB, Mendenhall WM, Sombeck MD, et al. Downstaging of esophageal cancer after preoperative radiation and chemotherapy. Ann Surg. 1995;221:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. [DOI] [PubMed] [Google Scholar]
- 8.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. [DOI] [PubMed] [Google Scholar]
- 9.Bates BA, Detterbeck FC, Bernard SA, et al. Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol. 1996;14:156–163. [DOI] [PubMed] [Google Scholar]
- 10.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. [DOI] [PubMed] [Google Scholar]
- 11.Stahl M, Wilke H, Fink U, et al. Combined preoperative chemotherapy and radiotherapy in patients with locally advanced esophageal cancer: interim analysis of a phase II trial. J Clin Oncol. 1996;14:829–837. [DOI] [PubMed] [Google Scholar]
- 12.Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol. 1993;11:1118–1123. [DOI] [PubMed] [Google Scholar]
- 13.Swisher SG, Ajani JA, Komaki R, et al. Long-term outcome of phase II trial evaluating chemotherapy, chemoradiotherapy, and surgery for locoregionally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2003;57:120–127. [DOI] [PubMed] [Google Scholar]
- 14.Block MI, Patterson GA, Sundaresan RS, et al. Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg. 1997;64:770–776. [DOI] [PubMed] [Google Scholar]
- 15.Flamen P, Lerut A, Van CE, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202–3210. [DOI] [PubMed] [Google Scholar]
- 16.Flanagan FL, Dehdashti F, Siegel BA, et al. Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol. 1997;168:417–424. [DOI] [PubMed] [Google Scholar]
- 17.Luketich JD, Friedman DM, Weigel TL, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg. 1999;68:1133–1136. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal B, Swisher S, Ajani J, et al. Endoscopic ultrasound after preoperative chemoradiation can help identify patients who benefit maximally after surgical esophageal resection. Am J Gastroenterol. 2004;99:1258–1266. [DOI] [PubMed] [Google Scholar]
- 19.Swisher SG, Maish M, Erasmus JJ, et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152–1160. [DOI] [PubMed] [Google Scholar]
- 20.Wieder HA, Brucher BL, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–908. [DOI] [PubMed] [Google Scholar]
- 21.Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058–3065. [DOI] [PubMed] [Google Scholar]
- 22.Swisher SG, Erasmus J, Maish M, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776–1785. [DOI] [PubMed] [Google Scholar]
- 23.Flamen P, Van CE, Lerut A, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13:361–368. [DOI] [PubMed] [Google Scholar]
- 24.Downey RJ, Akhurst T, Ilson D, et al. Whole body 18F-FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol. 2003;21:428–432. [DOI] [PubMed] [Google Scholar]
- 25.Swisher SG, Ajani JA, Komaki R, et al. Long-term outcome of phase II trial evaluating chemotherapy, chemoradiotherapy, and surgery for locoregionally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2003;57:120–127. [DOI] [PubMed] [Google Scholar]
- 26.Brucher BL, Weber W, Bauer M, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg. 2001;233:300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. Ann Thorac Surg. 2001;72:1918–1924; discussion 1924–1925. [DOI] [PubMed]
- 28.Swisher SG, Hofstetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg. 2005;241:810–817; discussion 817–820. [DOI] [PMC free article] [PubMed]
- 29.Kitamura M, Sumiyoshi K, Sonoda K, et al. The clinical and histopathological contributing factors influencing the effectiveness of preoperative hyperthermo-chemo-radiotherapy for the patients with esophageal cancer. Hepatogastroenterology. 1997;44:175–180. [PubMed] [Google Scholar]
- 30.Triboulet JP, Amrouni H, Guillem P, et al. Long-term results of esophageal epidermoid cancers in complete remission after preoperative chemo-radiotherapy. Ann Chir. 1998;52:503–508. [PubMed] [Google Scholar]
- 31.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. [DOI] [PubMed] [Google Scholar]
- 32.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. [DOI] [PubMed] [Google Scholar]
- 33.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. [DOI] [PubMed] [Google Scholar]