Abstract
Objectives:
The present study examines the differences in gastrointestinal hormone production at 3 different reconstruction types after total gastrectomy.
Background Data:
Total gastrectomy causes significant weight loss, mainly due to a reduced caloric intake probably because of a lack of initiative to eat or early satiety during meals. Behind this phenomenon a disturbed gastrointestinal hormone production can be presumed.
Methods:
Patients participating in a randomized study were recruited for the clinical experiment. Seven patients with simple Roux-en-Y reconstruction, 11 with aboral pouch (AP) construction, and 10 with aboral pouch with preserved duodenal passage (APwPDP) reconstruction, as well as 6 healthy volunteers were examined. Blood samples were taken 5 minutes before and 15, 30, and 60 minutes after ingestion of a liquid test meal. Plasma concentrations for insulin, cholecystokinin, and somatostatin were determined by radioimmunoassay analysis.
Results:
Postprandial hyperglycemia was observed in patients after total gastrectomy most prominently in groups with duodenal exclusion (Roux-en-Y and AP) compared with healthy controls. Postprandial insulin curves reached significantly higher levels in all operated groups compared with controls, however, with no difference according to reconstruction type. Significantly higher cholecystokinin levels and higher integrated production of cholecystokinin were observed in Roux-en-Y and AP groups compared with APwPDP and control. Postprandial somatostatin levels were significantly different between the 4 groups, and highest levels and integrated secretions were reached in AP group, lowest in APwPDP and normal groups.
Conclusion:
A disturbed glucose homeostasis was observed in gastrectomized patients most prominently in the Roux-en-Y group. Also, cholecystokinin and somatostatin response differed significantly in favor of duodenal passage preservation after total gastrectomy. Cholecystokinin levels close to physiologic found at APwPDP reconstruction may contribute to a physiologic satiation in reconstructions with preserved duodenal passage after total gastrectomy.
Postprandial glucose, insulin, cholecystokinin, and somatostatin secretions were measured in 3 different reconstruction types: Roux-en-Y, aboral pouch, and aboral pouch with preserved duodenal passage, after total gastrectomy. Significantly higher cholecystokinin and somatostatin levels were found in patients with duodenal exclusion, which seemed reversible by reconstruction of the duodenal route.
Total gastrectomy is known to result in a significant weight loss in 40% to 90% of patients.1,2 The reason for this has long been examined. Although maldigestion and malabsorption of protein and fat resulting in steatorrhea are consistently reported,1–5 patients after total gastrectomy are able to keep in positive nitrogen balance.2,3 In an elegant experiment, Bradley et al3 showed that gastrectomized patients are physiologically capable of caloric intake sufficient to result in weight gain during an in hospital smorgasbord diet, while an accurate record was kept of their ad libitum intake. The same patients reached only 85% of recommended daily caloric allowances for the maintenance of ideal body weight after returning to their home environment. In view of the more than adequate caloric intake during hospitalization, neither limited capacity nor fear of dumping is an acceptable explanation. Lack of appetite, absence of hunger sensation, lack of personal initiative, or psychical disturbances could contribute to reduced intake, the authors concluded.3
The physiology of appetite and eating behavior has drawn increasing attention in the last decades. A number of peripheral and central markers involved in satiety and satiation have been investigated in healthy or obese, young or aged patients.6 Such investigations on gastrectomized subjects are limited in number and yielded inconsistent results.
In the present report, the most well-studied cholecystokinin (CCK), known to cause early satiety, insulin, examined in the long-term regulation of food intake, and somatostatin, involved in controversial roles in appetite of healthy individuals,6 but inevitably having a role in dumping syndrome,7 are examined in patients after total gastrectomy.
Total gastrectomy, by removing the hormone producing mucosa of the stomach and rearranging the gastrointestinal route for the passage of food, inevitably alters gastrointestinal hormone production. Different type of surgical reconstructions (pouch construction, exclusion or preservation of the duodenal passage) may result in a different magnitude of this disturbance. The altered production of gastrointestinal hormones may lead to an altered experience of hunger and satiety resulting in decreased caloric intake and reduced quality of life in patients after total gastrectomy.
PATIENTS AND METHODS
Patients from the population of a prospective randomized trial comparing 3 postgastrectomy reconstruction types: simple Roux-en-Y reconstruction, aboral pouch (AP) construction, and aboral pouch with preserved duodenal passage (APwPDP) reconstruction - were recruited for gastrointestinal hormone measurements.8,9
The referred randomized trial included 98 patients followed for 24 months and resulted in a significant difference in lipid absorption, quality of life, and serum iron level and transferrin saturation in favor of duodenal passage preservation at 12 months postoperatively. AP without duodenal passage preservation resulted in only better quality of life and lipid absorption. Body weight, body mass index, serum proteins, immunoglobulins, and carbohydrate absorption did not differ between the groups. Part of the results have only been reported yet.8,9
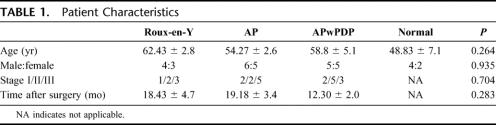
Seven patients with Roux-en-Y, 11 with AP, and 10 with APwPDP reconstruction gave their consents to hormone stimulation test. Six healthy volunteers served as control group. The average age of the patients was 56.32 years, and the male-to-female ratio was 19:15. Mean time elapsed after surgery was 16.54 months. Patient characteristics are shown in Table 1. The 3 different reconstruction types are demonstrated in Figure 1.
TABLE 1. Patient Characteristics
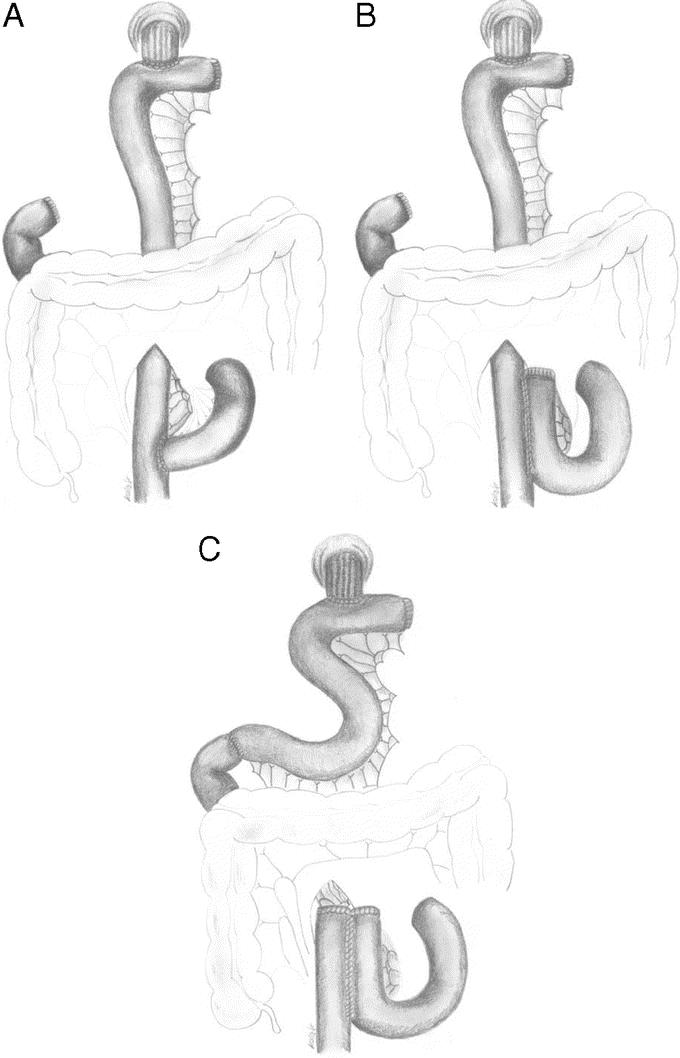
FIGURE 1. Examined reconstructions after total gastrectomy: simple Roux-en-Y (A), aboral pouch (B), and aboral pouch with preserved duodenal passage (C).
Operative Technique
In case of simple Roux-en-Y reconstruction after removal of the stomach and closure of the duodenal stump, the second jejunal loop is divided. The aboral end is pulled up to the esophagus and an end-to-side esophagojejunostomy is performed. The oral end is sewn into the pulled-up limb, under the mesocolon, 50 cm distal to the esophagojejunal anastomosis as an end-to-side jejunojejunostomy (Fig. 1A).
During the construction of an AP, the esophagojejunal anastomosis is formed the same way than in case of an Roux-en-Y reconstruction, but the jejunojejunostomy (50 cm distal to the esophagojejunostomy) is created as a 15-cm-long, antiperistaltic, side-to side anastomosis (Fig. 1B).
In case of AP with preserved duodenal passage, the same type of anastomosis is created for the esophagojejunal anastomosis as in case of Roux-en-Y reconstruction. Fifty centimeters distal to the esophagojejunostomy the Roux limb is divided preserving the continuity of its mesentery. The oral end at this division is anastomosed with the duodenal stump in an end-to-end fashion. The aboral end is closed, and the side of this loop is anastomosed to the Y limb in a side-to-side fashion to form the aboral pouch. The pouch is created as a 15-cm-long antiperistaltic side to side jejunojejunostomy under the mesocolon10 (Fig. 1C).
Hormone Provocation Test
After an overnight fast (12–15 hours) a liquid test meal (500 kcal; 70 g carbohydrate, 36 g protein, 7 g fat) was administered at room temperature, in a sitting position. Blood samples were taken 5 minutes before, and 15, 30, and 60 minutes after ingestion of test meal. One sample from each patient at each time was sent for blood glucose analysis using the glucose oxidase method. Other sample, mixed with EDTA and aprotinin, was collected on ice, centrifuged at 4°C, and the plasma stored at −30°C for later hormone analysis.
Radioimmunoassays for Cholecystokinin, Insulin, and Somatostatin
Plasma CCK concentration was measured by a commercial CCK radioimmunoassay kit RK-069-04 (Phoenix Pharmaceuticals Inc. Belmont, CA). The CCK antibody was raised against CCK octapeptide 26–33 (nonsulfated). The sensitivity of the assay was 1 pg/tube. The CD50 for the calibration curve was 35.44 pg/tube.
Plasma insulin concentration was measured by a commercial insulin radioimmunoassay kit RK-400M (Institute of Isotopes, Budapest, Hungary). The insulin antibody was highly specific for human insulin with cross-reactivity to human proinsulin of 65%. The sensitivity of the radioimmunoassay was 5 μIU/mL, the interassay variance was 6.2%, and the intra-assay variance was 7.1%.
Plasma somatostatin was measured by a specific and sensitive radioimmunoassay developed at our department and described elsewhere in details.11
Statistics and Ethics
Experimental design was approved by the local ethics committee. All patients and healthy volunteers gave informed consent to the experiment. SPSS 11.5 software was applied to compare data. Statistical significance was analyzed by factorial analysis of variance (ANOVA) for the series of hormone values in time and one-way ANOVA for integrated secretions. Integrated secretions were calculated as the area under the hormone concentration curve. Parametric variables of patient characteristics were compared with one-way ANOVA, while for nonparametric variables the Kruskal-Wallis test was used. Results are expressed as mean ± SEM. Differences with a P value <0.05 were considered significant; P value is represented in most comparisons.
RESULTS
The 3 patient groups were homogeneous with regard to age, gender, stage of disease, and post surgical time (Table 1). All but 2 patients were operated on for gastric adenocarcinoma, 1 patient in AP group with gastrointestinal stromal tumor and 1 with a fibrosarcoma of the stomach. These 2 patients' disease stages are not shown in Table 1.
Glucose
Figure 2 demonstrates the blood glucose levels in the 4 groups during meal provocation test. Glucose curve for controls seems flat, while for the operated patients reach higher values. The curves look diabetoid, most prominently in Roux-en-Y patients. Factorial analysis of variance found significant difference between the curves for the 4 groups. Groups with duodenal exclusion (Roux-en-Y and AP) had significantly higher glucose levels compared with the normal control group.
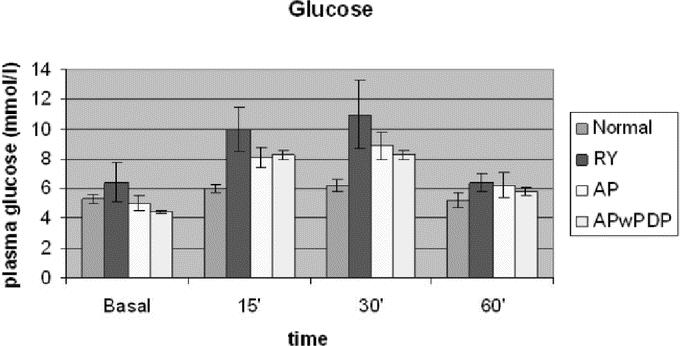
FIGURE 2. Basal and stimulated plasma glucose levels in Roux-en-Y, AP, and APwPDP patients and in normal controls. Curves differed significantly by factorial ANOVA (P = 0.002). Post hoc test showed Roux-en-Y versus normal (P = 0.025) and Roux-en-Y versus AP (P = 0.044).
Insulin
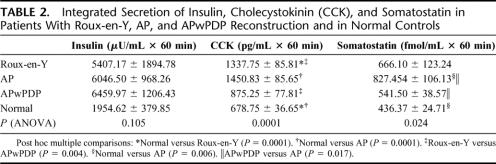
The insulin level increased to abnormally high values in all 3 gastrectomized groups in response to food stimulus, compared with healthy control (Fig. 3). The basal values did not differ between the 4 groups. The insulin curve ran highest in patients with preserved duodenal passage (APwPDP). Factorial ANOVA showed that the curves differed significantly according to the type of the groups. Post hoc comparisons showed significant difference between normal and AP and normal versus APwPDP groups (Fig. 3). The integrated secretion was higher in the operated groups than in controls; however, the difference did not reach significant level, probably because of the high standard deviation of insulin data (Table 2).
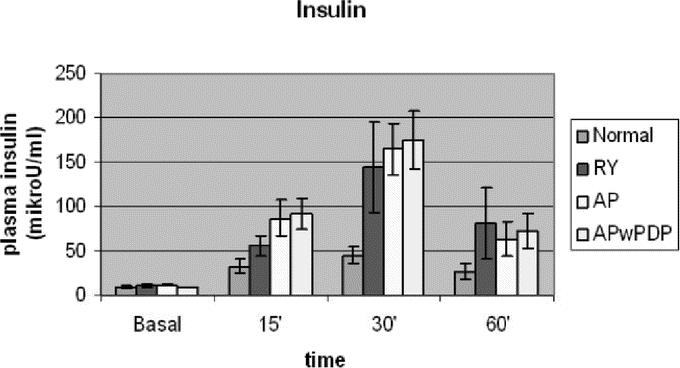
FIGURE 3. Basal and stimulated plasma insulin levels in Roux-en-Y, AP, and APwPDP patients and in normal controls. Curves differed significantly by factorial ANOVA (P = 0.005). Post hoc test showed normal versus AP (P = 0.002) and normal versus APwPDP (P = 0.001).
TABLE 2. Integrated Secretion of Insulin, Cholecystokinin (CCK), and Somatostatin in Patients With Roux-en-Y, AP, and APwPDP Reconstruction and in Normal Controls
Cholecystokinin
Figure 4 demonstrates CCK levels in response to test meal. Regarding this gastrointestinal hormone, the examined groups separated to a group with higher basal as well as stimulated values, incorporating patients with duodenal exclusion (Roux-en-Y and AP patients) and to a group of lower values, including APwPDP patients and the control group (Fig. 4). ANOVA analysis showed significant difference between the curves according to reconstruction type. Post hoc comparisons showed that all 4 groups differed significantly from each other except AP from Roux-en-Y. For integrated CCK secretion, Roux-en-Y and AP groups showed significantly higher amount of secretion compared with normal, while data for APwPDP did not differ from normal significantly (Table 2).
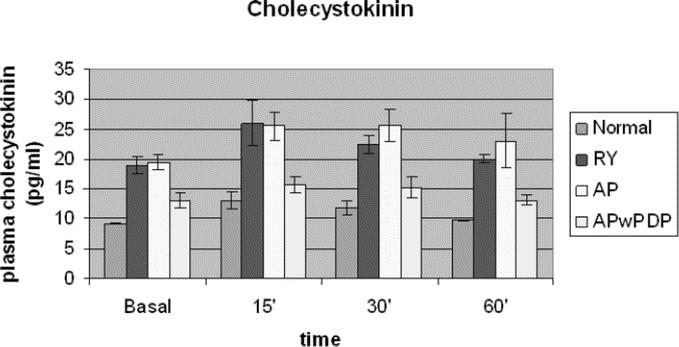
FIGURE 4. Basal and stimulated plasma cholecystokinin levels in Roux-en-Y, AP, and APwPDP patients and in normal controls. Curves differed significantly by factorial ANOVA (P < 0.001). Post hoc test showed normal versus Roux-en-Y (P < 0.001), normal versus AP (P < 0.001), normal versus APwPDP (P = 0.003), Roux-en-Y versus APwPDP (P < 0.001), and AP versus APwPDP (P < 0.001).
Somatostatin
The postprandial curves for somatostatin plasma levels (Fig. 5) differed even in shape between the groups. The control group reached peak value around 15 minutes and somatostatin level decreased from that point. In patients with duodenal exclusion (Roux-en-Y and AP), somatostatin level increased longer and further, almost twice as high as in normal controls, until around 30 minutes postprandially, then seemed to reach a plateau in AP patients, while starting to decrease in Roux-en-Y. In patients with APwPDP reconstruction, the peak and plateau were at the same time like in AP group; however, somatostatin level did not reach much higher values than in control patients. The data sets differed significantly regarding type of operation analyzed by factorial ANOVA. Post hoc tests showed significantly higher values for AP compared with normal and for AP compared with APwPDP; the rest of the groups did not differ significantly from each other. The integrated secretions also differed significantly with higher values for AP and lower for APwPDP and normal control groups (Table 2). Postprandial curve for Roux-en-Y group ran between the curves of AP and APwPDP, integrated secretion of somatostatin for Roux-en-Y patients were also between this 2 groups' results, but there were no significant differences of Roux-en-Y data from any other groups most likely because of the high standard deviation in this group.
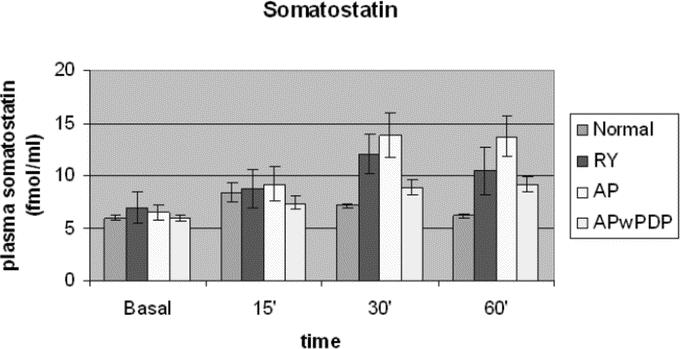
FIGURE 5. Basal and stimulated plasma somatostatin levels in Roux-en-Y, AP, and APwPDP patients and in normal controls. Curves differed significantly by factorial ANOVA (P = 0.001). Post hoc test showed normal versus AP (P = 0.001) and AP versus APwPDP (P = 0.027).
DISCUSSION
From a Medline search for gastrointestinal hormone measurements in patients undergone gastric surgery, no clear-cut conclusion could be drawn regarding basal and stimulated levels of the above measured hormones. Different studies measured endogenous production of hormones in response to either liquid or solid test meal, or to intraduodenal administration of nutrients. Others administered the hormones exogenously in a fasting or postprandial state and examined the reaction. Subjects have undergone either total or partial gastric resection, with regard, or regardless of the way of reconstruction or they had limited gastric surgery such as vagotomy, antrectomy, or pyloroplasty.4,5,12–20
Blood sugar regulation has long been described to be disturbed after gastrectomy, with early hyperglycemia and late hypoglycemia during oral glucose tolerance test.3 Regarding the type of reconstruction, Schwarz et al found significantly higher glucose levels in Roux-en-Y patients and in patients with pouch construction and duodenal exclusion, while in case of preservation of the duodenal route the pathologic glucose tolerance did not develop.12 Friess et al, though, in patients with total gastrectomy and preserved duodenal passage, found significantly higher glucose levels in the first 45 minutes after a liquid test meal.5 In our study, postprandial glucose curves ran significantly higher in patients with duodenal exclusion (Roux-en-Y and AP) compared with controls, supporting the hypothesis that duodenal exclusion disturbs glucose homeostasis more, than reconstructions with a preserved duodenal passage.
In contrast to the diabetoid postprandial glucose curves after gastric resection, it has also been described that gastrectomy improves glucose tolerance, ameliorates diabetes mellitus, and decreases insulin demands in diabetic patients.13 The elevated insulin levels and increased integrated insulin secretion after a test meal in gastrectomized patients found in the present study agree well with these clinical observations. Other studies in the literature on subjects after total gastrectomy regarding endogenous insulin production also found higher postprandial levels.5,12 Regarding duodenal passage preservation, Schwarz et al found the insulin level elevated only in jejunum interposition cases and not in cases where the duodenum was excluded. In our experiment, the postprandial insulin secretion draws an elevated curve for all operated groups regardless of the reconstruction type (Fig. 4). Insulin secretion is stimulated by the high blood glucose peak, as a result of the early and fast intestinal absorption of glucose, due to the rapid intestinal transit in gastrectomized state. In addition, enteroglucagon or glicentin, GLP-1, and GLP-2 also stimulates insulin release. Enteroglucagon is normally secreted by the distal intestinal mucosa if nutrients, especially glucose, reach as far as this point in the bowel. It stimulates insulin release to improve glucose metabolism. Enteroglucagon has been shown to be secreted by the small bowel at an increased rate after gastrectomy.14
CCK has been one of the most investigated gastrointestinal hormones in gastrectomized patients. In our experiment, the basal level was found to be raised in all 3 groups after total gastrectomy. The integrated postprandial secretion though were elevated only in reconstructions with duodenal exclusion (Roux-en-Y and AP), while in APwPDP reconstruction, where the duodenal route was preserved, the integrated postprandial secretion did not differ significantly from control.
Clinical studies investigating CCK secretion in patients after total gastrectomy4,5,12,15–18 found an equal or higher basal level of the hormone in gastrectomized patients compared with healthy controls. The postprandial level after gastrectomy was higher in most studies compared with normal,4,5,12,17,18 but lower in 2 studies.15,16 Some studies also calculated the integrated postprandial CCK secretion. It was found to be higher after total gastrectomy in 2 experiments4,12 and equal to normal in 1 experiment.18 In those studies, though, where CCK production in gastrectomized subjects were found to be the same or less inducible than in normal controls, a solid test meal was applied or fat load was given directly intraduodenally. CCK, by relaxing the fundus and increasing pyloric tone, has a well-established role in delaying gastric emptying,19 in this context, regulating its own release by a short circuit feedback mechanism. Nutrients entering the duodenum release CCK, which in turn inhibits gastric emptying. Consequently, nutrient flow ceases and CCK secretion decreases. If patients after total gastric resection are compared with healthy people, the difference is that operated patients do not have the target organ for this effect of CCK; therefore, CCK level remains elevated. The food entering the duodenum brings about CCK secretion, but there is no way for CCK to stop nutrient flow, which results in a higher CCK level. Experiments with a setting of intraduodenal administration of test food can show the differences more precisely in CCK-producing capacity between healthy and operated subjects, ie, CCK levels raise more slowly, but the overall integrated production is equal to normal.16,20 The obtained results, though, will not reflect everyday situation, nor will they help to reveal the background of decreased initiative of gastrectomized patients to eat.
In our study, different reconstruction types were compared after total gastrectomy to examine the significance of duodenal exclusion or preservation and pouch construction. Of the above cited experiments, Schwarz et al examined CCK secretion in response to a 400-kcal liquid test meal in patients after total gastrectomy with Roux-en-Y reconstruction, with a 10- or a 20-cm-long oral pouch and Roux-en-Y reconstruction and with jejunum interposition and either 10- or 20-cm-long oral pouches. The peak concentrations of CCK as well as the integrated postprandial CCK secretions were significantly higher in all operated groups compared with healthy controls, but there were no significant differences between the operated groups.12 In the present study, patients after total gastrectomy with preserved duodenal passage had about the same postprandial secretions than normal, but patients with duodenal exclusion (Roux-en-Y and AP) had significantly higher postprandial CCK production.
The disturbed feedback of gastric emptying on CCK secretion can explain higher CCK levels after gastrectomy in general, but the normal levels in case of duodenal passage preservation must have a different explanation. The exocrine pancreatic function is described to be insufficient after gastrectomy,5 maybe partly because of the vagal denervation of the pancreas,20 but more importantly because of a pancreatico-cibal asynchrony,3 ie, a disturbed speed and order of food passage and endocrine and exocrine secretions along the gut. There is a feedback regulation of pancreatic secretion by intraluminal proteases described in humans.21 The same feedback is certainly mediated by CCK in dogs, but in humans the role of CCK is less clear; rather, it seems to act together with the cholinergic system as a neuromodulator.22 If this feedback operates, at least partly, with CCK in humans, the exocrine pancreatic insufficiency,5 caused by the pancreatico-cibal asynchrony, may also add to the elevated level of CCK in case of duodenal exclusion and might explain the normal levels when duodenal passage is preserved. This is supported by our earlier finding of a significantly better lipid absorption, measured by the Lipiodol test in patients with preserved duodenal passage (APwPDP) compared with Roux-en-Y.9
CCK has extensively been examined in connection with appetite and satiety.6 Experiments on healthy individuals proved a dose-dependent suppressing effect of CCK on appetite.23 Studies with young and elderly healthy individuals revealed an elevated basal and postprandial level of CCK in the elderly, probably as a reason for the observed reduced food intake in this group.24 This so-called anorexia of aging is accompanied by a slowing of gastric emptying, which can also be attributable to the higher CCK level. Our results of a more physiologic CCK response in reconstruction preserving the duodenal route support the application of duodenal passage preservation for postgastrectomy reconstruction, to avoid the probable satiating effect of CCK. This probable difference in appetite did not result in higher body weight in patients with duodenal passage preservation, as reported in our randomized trial; however, it may contribute to the better quality of life found in this group in the first postoperative years.9 The direct effect of CCK on appetite in gastrectomized subjects, where gastric fullness cannot attribute to the CCK's effect to reach satiety,25 remains to be elucidated.
Somatostatin in connection with gastrectomy was mainly examined as a potential cure for dumping syndrome.7,26 Given exogenously, somatostatin (or its long-acting analogue octreotide) proved to be able to alleviate symptoms of dumping, reduce pulse rate, and abolish late hypoglycemia. However, in chronic use, intractable abdominal cramps and diarrhea can develop,27 the inhibition of insulin release by somatostatin leads to hyperglycemia and diabetes,28 and the inhibition of pancreatic exocrine secretion may worsen malabsorption and dumping.27 Those few studies that examined endogenous production of somatostatin revealed that patients who are doing well after gastrectomy tends to have higher basal levels of this hormone, while those who have dumping symptoms and manometric proof of abnormal bowel motility have lower levels of somatostatin.29 No study has examined endogenous somatostatin production in response to test meal in gastrectomized patients. In our study, somatostatin levels in patients after gastrectomy were not different from normal in the fasting state. In the first 15 minutes, it raised slightly in all 4 groups. Afterward it returned to basal level in healthy controls, remained slightly raised in patients with preserved duodenal passage, while increased in cases of duodenal exclusion. Sixty minutes were not enough to detect it return to basal level in the operated groups, but certainly it increased significantly higher in patients with duodenal exclusion (Roux-en-Y and AP). Secretion of somatostatin is stimulated by most of the factors that stimulate insulin production, such as enteral glucose and amino acid load. Cholecystokinin as well as insulin also increase somatostatin secretion. In response, somatostatin acts as a generally inhibiting gastrointestinal hormone, decreasing the secretion of most gastrointestinal hormones. Thus, the observed differences between normal and duodenal exclusion patients may only represent a response to the higher levels of other hormones such as insulin and CCK in patients with duodenal exclusion.
The whole problem of postgastrectomy symptoms might be attributed to the accelerated intestinal transit. The rapid transit results in accelerated glucose absorption bringing about higher output of insulin. Accelerated transport of peptides and lipids gives an abnormally large stimulus to CCK production, magnified by the brake in the feedback regulation. All ends in abnormally high gastrointestinal hormone levels, which brings about increased production of somatostatin. And somatostatin will arrest, as needed, this cascade of GI hormone production but additionally reduces gut motility and digestive juice production. The whole phenomenon probably becomes less significant with time due to the intestinal adaptation.18 This hypothesis, though, needs further evaluation.
CONCLUSION
Our experiment supports a diabetoid blood glucose profile in the first postprandial hour in patients after gastrectomy and routine Roux-en-Y reconstruction, with higher insulin concentrations, elevated cholecystokinin levels, and an increasing somatostatin release after 15 to 30 minutes postprandially. The creation of a pouch seems not to improve much on this disturbed regulation. Duodenal passage preservation, however, helps to moderate the postprandial cholecystokinin elevation and results in a less steep postprandial plasma somatostatin curve, probably reflecting a decreased need for arresting the abnormally high output of other gastrointestinal hormones in these patients.
Our earlier data proved better quality of life in the first postoperative year for AP compared with Roux-en-Y patients, and for APwPDP compared with both AP and Roux-en-Y.9 Long-term data have not been reported yet. This better life quality may at least partly come from the differences in gastrointestinal hormone profile.
Weather these differences in gastrointestinal hormone production in favor of duodenal passage preservation result in less compromise in appetite and hunger sensation and are able to contribute to a less reduced caloric intake in patients after gastrectomy needs further evaluation. Furthermore, recently discovered hormones involved in appetite and meal size regulation, such as ghrelin and leptins, need to be examined in gastrectomized patients.
Footnotes
Supported by the Hungarian Research Grant OTKA T-42726. Dr. Németh's was supported by the Hungarian Academy of Sciences and Hungarian Research Grants OTKA T-43467 and ETT 03597/2003.
Reprints: Katalin Kalmár, MD, University of Pécs.
REFERENCES
- 1.Adams JF. The clinical and metabolic consequences of total gastrectomy: I. Morbidity, weight and nutrition. Scand J Gastroenterol. 1967;2:137–149. [DOI] [PubMed] [Google Scholar]
- 2.Kelly WD, MacLean LD, Perry JF, et al. A study of patients following total and near total gastrectomy. Surgery. 1953;35:964–982. [PubMed] [Google Scholar]
- 3.Bradley EL III, Isaacs J, Hersh T, et al. Nutritional consequences of total gastrectomy. Ann Surg. 1975;182:415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs M, Köhler H, Schafmayer A, et al. Influence of partial and total gastrectomy on plasma cholecystokinin and neurotensin and on the exocrine pancreatic function. Zentralbl Chir. 1995;120:472–477. [PubMed] [Google Scholar]
- 5.Friess H, Böhm J, Müller MW, et al. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol. 1996;91:341–347. [PubMed] [Google Scholar]
- 6.De Graaf C, Blom WAM, Smeets PAM, et al. Biomarkers of satiation and satiety. Am J Clin Nutr. 2004;79:946–961. [DOI] [PubMed] [Google Scholar]
- 7.Primrose JN, Johnston D. Somatostatin analogue SMS 201-995 (octreotide) as a possible solution to the dumping syndrome after gastrectomy or vagotomy. Br J Surg. 1989;76:140–144. [DOI] [PubMed] [Google Scholar]
- 8.Kalmár K, Cseke L, Zámbó K, et al. Comparison of quality of life and nutritional parameters after total gastrectomy and a new type of pouch construction with simple Roux-en-Y reconstruction. Dig Dis Sci. 2001;46:1791–1796. [DOI] [PubMed] [Google Scholar]
- 9.Kalmár K, Káposztás Zs, Cseke L, et al. Effect of pouch construction and preservation of the duodenal passage ont he nutritional and motility parameters and quality of life after total gastrectomy. Eur J Surg Oncol. 2004;30:171. [Google Scholar]
- 10.Horváth ÖP, Kalmár K, Cseke L. Aboral pouch with preserved duodenal passage: new reconstruction method after total gastrectomy. Dig Surg. 2002;19:261–264. [DOI] [PubMed] [Google Scholar]
- 11.Németh J, Helyes Zs, Görcs T, et al. Development of somatostatin radioimmunoassay for the measurement of plasma and tissue contents of hormone. Acta Physiol Hung. 1996;84:313–315. [PubMed] [Google Scholar]
- 12.Schwarz A, Büchler M, Usinger K, et al. Importance of the duodenal passage and pouch volume after total gastrectomy and reconstruction with the Ulm pouch: prospective randomized clinical study. World J Surg. 1996;20:60–66. [DOI] [PubMed] [Google Scholar]
- 13.Friedman MN, Sancetta AJ, Magovern GJ. The amelioration of diabetes mellitus following subtotal gastrectomy. Surg Gynecol Obstet. 1955;100:201–204. [PubMed] [Google Scholar]
- 14.Naito H, Ohneda A, Kojima R, et al. Plasma glicentin in diabetic and gastrectomized patients. Regul Pept. 1999;79:55–61. [DOI] [PubMed] [Google Scholar]
- 15.Bergh C, Sjostedt S, Hellers G, et al. Meal size, satiety and cholecystokinin in gastrectomized humans. Physiol Behav. 2003;78:143–147. [DOI] [PubMed] [Google Scholar]
- 16.Klein P, Reingruber B. The Longmire gastrectomy in the animal model: postoperative changes in fat resorption and the hormones cholecystokinin and secretin. Langenbecks Arch Chir. 1994;379:271–279. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Fuchigami A, Hosotani R, et al. Release of cholecystokinin and gallbladder contraction before and after gastrectomy. Ann Surg. 1987;205:27–32. [PMC free article] [PubMed] [Google Scholar]
- 18.Satake K, Takeuchi T, Watanabe S, et al. Postprandial plasma cholecystokinin response in patients after gastrectomy and pancreatoduodenectomy. Am J Gastroenterol. 1986;81:1038–1042. [PubMed] [Google Scholar]
- 19.Liddle R A, Morita ET, Conrad CK, et al. Regulation of gastric emptying in humans by cholecystokinin. J Clin Invest. 1986;77:992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masclee AAM, Jansen JBMJ, Driessen WMM, et al. Delayed plasma cholecystokinin and gallbladder responses to intestinal fat in patients with Billroth I and Billroth II gastrectomy. Surgery. 1989;106:502–508. [PubMed] [Google Scholar]
- 21.Schick RR, Schusdziarra V, Mössner J. Effect of CCK on food intake in man: physiological or pharmacological effect? Z Gastroenterol. 1991;29:53–58. [PubMed] [Google Scholar]
- 22.MacIntosh CG, Andrews JM, Jones KL, et al. Effects of age on concentrations of plasma cholecystokinin, glucagons-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am J Clin Nutr. 1999;69:999–1006. [DOI] [PubMed] [Google Scholar]
- 23.Kissilef HR, Carretta JC, Geliebter A, et al. Cholecystokinin and stomach distention combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285:992–998. [DOI] [PubMed] [Google Scholar]
- 24.Owyang C, Louie DS, Tatum D. Feedback regulation of pancreatic enzyme secretion: suppression of cholecystokinin release by trypsin. J Clin Invest. 1986;77:2042–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adler G, Beglinger C, Braun U, et al. Interaction of the cholinergic system and cholecystokinin in the regulation of endogenous and exogenous stimulation of pancreatic secretion in humans. Gastroenterology. 1991;100:537–543. [DOI] [PubMed] [Google Scholar]
- 26.Reasbeck PG, Van Rij AM. The effect of somatostatin on dumping after gastric surgery: a preliminary report. Surgery. 1985;99:462–468. [PubMed] [Google Scholar]
- 27.Thirbly RC. Somatostatin and postgastrectomy dumping. Gastroenterology. 1989;97:1344. [DOI] [PubMed] [Google Scholar]
- 28.Gray JL, Debas HT, Mulvihill SJ. Control of dumping symptoms by somatostatin analogue in patients after gastric surgery. Arch Surg. 1991;126:1231–1235, discussion 1235–1236. [DOI] [PubMed]
- 29.Tomita R, Fujisaki S, Tanjoh K, et al. Studies on gastrointestinal hormone and jejunal interdigestive migrating motor complex in patients with or without early dumping syndrome after total gastrectomy with Roux-en-Y reconstruction for early gastric cancer. Am J Surg. 2003;185:354–359. [DOI] [PubMed] [Google Scholar]