Abstract
Objective:
To measure the clinical and economic impact of postoperative hospital-acquired pneumonia (HAP) and to identify risk factors for the development of HAP.
Summary Background Data:
Although postoperative HAP is recognized to be an major risk associated with surgery, little is known about the overall outcomes of patients whose hospital stay is complicated by HAP following surgery.
Methods:
We studied 618,495 patients who underwent an intra-abdominal operation from the National Inpatient Sample database over a 1-year period (January 2000 to December 2000) using CPT codes and discharge diagnoses identified by the Clinical Classification Software. Data collected included demographic characteristics, type of operation, in-hospital mortality, discharge disposition, length of stay, and hospital charges.
Results:
Of the 13,292 patients with HAP following intra-abdominal surgery, 1421 died prior to discharge (mortality = 10.7%) compared with 7217 deaths in the control group of patients without HAP following intra-abdominal surgery (mortality = 1.2%) (P < 0.001). HAP was independently associated with a 4.13-fold (95% confidence interval = 3.94–4.34) increase in risk to be discharged to a skilled nursing facility. The mean length of hospital stay for intra-abdominal patients who developed HAP was significantly greater compared with intra-abdominal surgery patients who did not develop HAP (17.10 days versus 6.07 days, P < 0.001). After adjusting for patient characteristics, HAP was independently associated with a 75% ($28,160.95; 95% confidence interval, $27,543.76–$28,778.13) mean increase in total hospital charges.
Conclusions:
Given the high incidence and significant impact of HAP on patient outcomes, early preventive strategies and interventions to reduce HAP should be a priority.
Hospital-acquired pneumonia occurs in approximately 10.7% of patients following intra-abdominal surgery and is associated with a 10-fold increase in in-hospital mortality, a 6-fold increase in discharge to skilled nursing facility, 55% increase in hospital length of stay, and a 68% increase in hospital charges.
Hospital-acquired pneumonia (HAP) is the second most common nosocomial infection and the leading cause of death among patients with hospital acquired infections, accounting for more than 50% of the deaths related to nosocomial infections.1–3 In the surgical population, mortality from postoperative HAP ranges from 19% to 45%, increasing to 65% in patients after septic intra-abdominal surgery.4–6 Hospital acquired infections are one of the foremost health care problems in the United States and have a significant impact on the overall patient hospitalization charges.2
Because rates of HAP vary widely and because interventions can reduce the rate of HAP, HAP has been identified as a measure of quality of care in inpatient populations.7 Patients having intra-abdominal surgery are particularly vulnerable to developing HAP.8,9 Despite the significance of HAP, little is known about the demographic risk factors or the economic and clinical outcomes of HAP, especially in intra-abdominal surgical patients. The purpose of this study was to evaluate patient and hospital characteristics that are associated with the development of HAP and explore the impact of HAP on clinical and economic outcomes in patients having intra-abdominal surgery.
MATERIALS AND METHODS
Patient Data
The 2000 Nationwide Inpatient Sample (NIS) is part of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality. This data set represents a sampling of 20% of all hospital inpatient stays from January 1, 2000 to December 31, 2000, from 994 hospitals in 28 states. The database is a weighted probability sample intended to provide national estimates of all U.S hospital admissions.
The NIS captures primary and secondary diagnoses and up to 15 Diagnostic Related Groups (DRG) or Clinical Classifications (CC) that use International Classification of Disease version 9 (ICD-9) codes to define comorbid disease, new-onset patient illness, procedures, and cost of care. To identify patients who underwent intra-abdominal surgery, we searched for diagnoses that spanned 142 Clinical Classification Software codes (CCS) that truncate thousands of ICD-9 codes into 280 categories by diagnosis (http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp). We collected data on patient age, sex, race, admission type, hospital length of stay (LOS), total hospital charges, mortality, primary diagnosis, and surgical procedure. This study was approved by our Institutional Review Board.
Study Population
The study included all adults, 18 years and older, who were admitted electively for intra-abdominal surgery without a primary diagnosis of pneumonia. We chose to focus on patients having intra-abdominal surgery because these patients are particularly vulnerable to developing HAP.8,9 After the intra-abdominal sample was selected, the discharge weight was applied to provide a national representative sample. The discharge weight was used for all analyses from this point forward and a separate discharge weight was used for any total hospitalization charge analysis that was provided as a variable in the NIS data set.
Outcome Variables
Primary Outcome Variables
The primary outcome variable was the presence of a discharge diagnosis of pneumonia. We included all patients with a secondary diagnosis of pneumonia after an intra-abdominal surgical procedure. We used the following CCS ICD-9 code to identify patients with HAP (code 122). This process was developed by Agency for Healthcare Research & Quality for researchers to aid in the analysis of large numbers of ICD-9 codes by truncating them from 12,000 diagnosis codes into clinically similar codes for statistical analyses (http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp).
Secondary Outcome Variables
The secondary outcome variables included in-hospital mortality, hospital LOS, total hospital charges, and patient disposition. Mortality in the data set indicates in-hospital death or death during the same admission as the surgical procedure. LOS was defined as the total number of hospital days from the date of admission to the date of discharge or death. Total hospital charges are expressed in year 2000 dollars. Patient disposition or discharge status, for patients who survived, was classified as home with or without home care, versus a skilled facility.
Independent Variables
We identified patient demographic characteristics and the type of surgical procedure performed. We identified patients’ age in years at the time of hospital admission, sex, and race. Race was defined as (1) white, (2) black, (3) Hispanic, (4) Asian or Pacific Islander, (5) Native American, and (6) other. Sex was defined as (1) female and (0) male.
Statistical Analysis
We performed descriptive analyses of the patient characteristics, types of surgical procedures, and outcomes associated with our primary outcome variable, the development of HAP. In preliminary exploratory analysis, the demographic, other independent variables and the dependent variables for patients with and without HAP were assessed separately: histograms, box plots, and proportions were used to characterize the distribution of the data.
Logistic regression was performed to evaluate the univariate and multivariate association between the independent variables and the development of HAP, for the impact of HAP on in-hospital mortality and for discharge disposition. Only patients who survived and were discharged were included in the discharge disposition analysis. We used linear regression to evaluate the univariate and multivariate association between the development of HAP and LOS and hospital charges. We used a forward logistic regression to determine what variables were included in these models and all subsequent models. Results are presented as odds ratio and 95% confidence intervals.
The results were reviewed to determine if assumptions of logistic regression were met. The goodness of fit determined how much of the variance resulted from the effect of the independent variables on the development of HAP. The Wald statistic was calculated to determine if any or all variables were significant predictors of HAP in the intra-abdominal surgical population.
Data were analyzed for multivariate outliers and the variables were assessed for multicollinearity. Collinearity diagnostics were completed and the tolerance criteria was 0.27, indicating that multicollinearity was not present. Prior to performing logistic regression analysis, data screening was completed to identify missing data and outliers. Less than 1% of the data in our sample were missing; therefore, the data set has been left in its original form, and no imputation of data was performed. We used SPSS version 11.2 software to perform all calculations (SPSS Inc., Chicago, IL).
RESULTS
Study Population
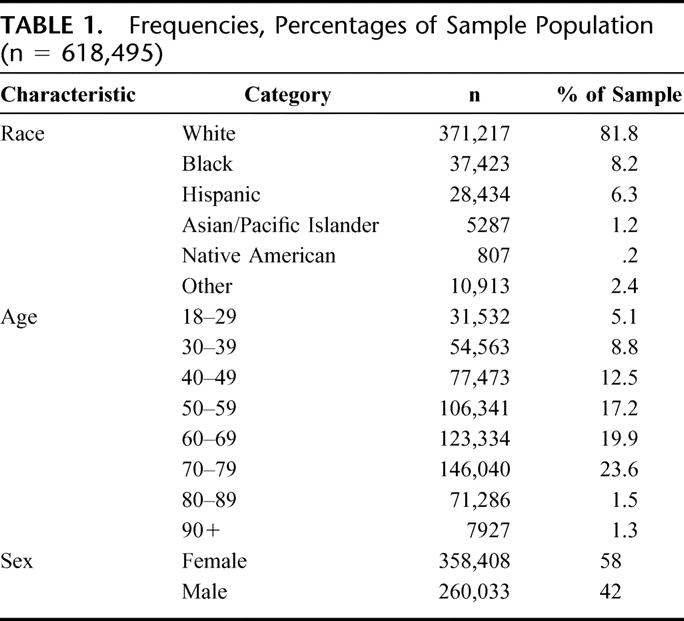
The 2000 NIS provided a 20% weighted sample consisting of 1,490,198 patient hospitalizations, of which 618,495 patients (42%) had an elective intra-abdominal operation (Fig. 1). The frequencies and distributions of the demographic characteristics for the study variables are presented in Table 1. The race/ethnicity variable of the patient sample was comprised primarily of White (81.8%), Black (8.2%), Hispanic (6.3%), and Other (2.4%). Other race included people of minority status and mixed races. The single largest group of patients by age was patients 70 to 79 years of age, comprising 23.6% of the sample, followed by patients 60 to 69 years of age comprising 19.9% of the sample. Fifty-eight percent of the patients were female and 42% were male.

FIGURE 1. Creation of the intra-abdominal surgical sample from the 2000 NIS.
TABLE 1. Frequencies, Percentages of Sample Population (n = 618,495)
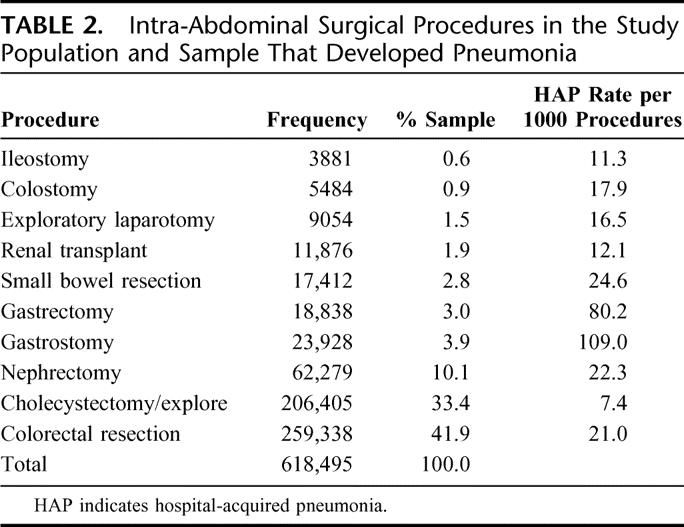
Among the sample of 618,495 who underwent an intra-abdominal operation, colorectal resection was the most common operation performed, accounting for 41.9% of the cases (Table 2). Open cholecystectomy was the second most common procedure, accounting for 33.4% of the sample and nephrectomy in 10.1% of patients. Other associated procedures included gastrostomy, permanent or temporary (3.9%), gastrectomy, partial or complete (3.0%), small bowel resections (2.8%), renal transplants (1.9%), and general exploratory laporotomy (1.5%). The incidence of HAP (per 1000 procedures) was greatest in patients undergoing a gastrostomy (109 cases), followed by gastrectomy (80 cases), small bowel resection (25 cases), nephrectomy (22 cases), colorectal resection (21 cases), colostomy (18 cases), exploratory laporotomy (16 cases), renal transplant (12 cases), ileostomy (11 cases), and cholecystectomy (7 cases).
TABLE 2. Intra-Abdominal Surgical Procedures in the Study Population and Sample That Developed Pneumonia
Patient Characteristics and HAP
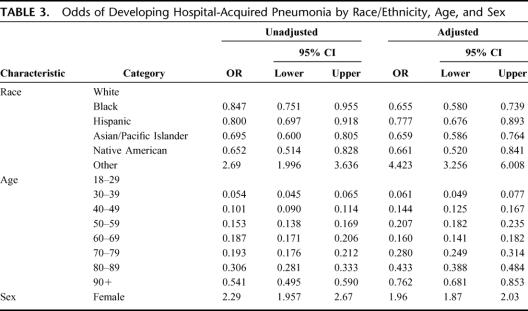
Overall, 10.7% (13,292 of 618,495) of all intra-abdominal surgical patients developed HAP in the postoperative period. In multivariate logistic regression analyses, race/ethnicity and sex were statistically reliable predictors for developing postoperative HAP, whereas age was not a predictor (Table 3). Women were twice as likely to develop HAP after intra-abdominal surgery compared with men (odds ratio [OR], 1.95; 95% confidence interval [CI], 1.87–2.03) and patient's whose race/ethnicity was identified as “Other” were 4.4 times more likely to develop HAP after intra-abdominal surgery compared with whites (OR, 4.42; 95% CI, 3.26–6.01). Although age greater than 70 years was associated with an increased odds ratio, age alone was not an independent/significant predictor of HAP.
TABLE 3. Odds of Developing Hospital-Acquired Pneumonia by Race/Ethnicity, Age, and Sex
Impact of HAP
In-Hospital Mortality
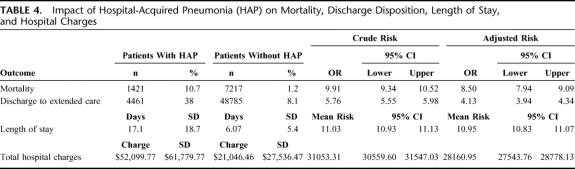
Of the 13,292 patients with HAP following intra-abdominal surgery, 1421 died (mortality = 10.7%) compared with 7217 deaths in the control group of patients without HAP following intra-abdominal surgery (mortality = 1.2%) (P < 0.001) (Table 4). In bivariate analysis, HAP was associated with a 10-fold increased risk for in-hospital mortality (95% CI, 9.34–10.52). After adjusting for patient characteristics, HAP was independently associated with a risk ratio of 8.5 (95% CI, 7.94–9.09) risk for in-hospital mortality.
TABLE 4. Impact of Hospital-Acquired Pneumonia (HAP) on Mortality, Discharge Disposition, Length of Stay, and Hospital Charges
Discharge Disposition
Of the 11,871 postoperative patients with HAP that survived, 7410 (62%) were discharged to home, 4461 (38%) to a skilled facility (Table 4). Of the patients discharged to home, 15% (n = 1763) required home health service. Overall, 52% of all patients with postoperative HAP required additional medical services beyond the tertiary care setting. In bivariate analysis, HAP was associated with a 5.76-fold (95% CI, 5.55–5.98) increased risk for discharge to a skilled nursing facility. After adjusting for patient characteristics, HAP was independently associated with a 4.13-fold (95% CI, 3.94–4.34) increased risk for discharge to a skilled nursing facility.
LOS and Hospital Charges
The mean length of hospital stay for intra-abdominal surgery patients who developed HAP was significantly greater compared with intra-abdominal surgery patients who did not develop HAP, 17.10 days (SD, 18.66 days) versus 6.07 days (SD, 5.37 days) (P < 0.001) (Table 4). In bivariate analysis, HAP was associated with a 55% increase in LOS, which represents an increased LOS by 11.03 days (95% CI, 10.93–11.13 days). After adjusting for patient characteristics, HAP remained independent.
Hospitalization Charges
The mean total hospitalization charges for intra-abdominal surgery patients who developed HAP were significantly higher (mean, $52,099.77; SD, $61,779.77) when compared with intra-abdominal patients who did not develop HAP (mean, $21,046.46; SD, $27,536.47) (t, 13386 = 57.8, P = 0.000). In bivariate analyses, HAP was associated with a 68% ($31,053.31; 95% CI, $300559.60–$31547.03) unadjusted mean increase in total hospital charges. After adjusting for patient characteristics, HAP was independently associated with a 75% ($28,160.95; 95% CI, $27,543.76–$28,778.13) mean increase in total hospital charges.
DISCUSSION
In this study, we found a 10.7% incidence of HAP in the intra-abdominal surgical patients sampled. Moreover, patients who develop HAP had a 10-fold increase for in-hospital mortality, 6-fold increase in risk for discharge to a skilled nursing facility, a 55% increase in hospital LOS, and a 68% increase in total hospitalization charges. Given the incidence and impact of HAP and given that interventions are available to reduce HAP; efforts to reduce HAP should be a research priority.
To our knowledge, this is the first study to evaluate the incidence of HAP in an intra-abdominal surgery population using a large patient population. Previous estimates of 1.3% to 17.5% varied depending on the diagnosis criteria used and the type of surgery performed.8,10 Our results are within this published range and focus on intra-abdominal operations only.
Our study identified some important findings and implications for future research. First, we found female patients were 2 times more likely than male patients to develop HAP. While Konrad et al found that men were more likely than women to develop HAP in the ICU setting,11 there were no other published studies found that identifies sex/gender as a risk factor for HAP. Given these findings, further research is needed to better understand the influence of sex on HAP and whether the greater incidence in women is confounded by an increased incidence of frailty in women or comorbid disease. Although the odds ratio was increased for patients 70 years of age and older, it was not a significant predictor in HAP. Our results differ from previous researchers who found that patients age 60 years and older was an independent predictor of HAP compared with patients less than 60 years of age.12–14
Second, HAP was associated with certain intra-abdominal operations. Gastrostomy tube placement ranked among the highest that likely represents the commonly associated comorbidity of mental status deficits and immobility, known risk factors for aspiration. By contrast, we observed that procedures that are most commonly performed in younger, healthier patients (ie, elective cholecystectomy) had very low rates of HAP. Accordingly, we maintain that the risk for HAP and possible mortality should be considered when discussing the risk benefit ratio of select procedures with patients and their families. For intra-abdominal surgery patients who developed HAP, the mortality rate was 10.7% compared with 1.2% for the intra-abdominal surgery population who did not develop HAP. The literature that discusses mortality and HAP has found mortality rates in the United States to be 24% to 50% and, in rare circumstances, up to 76% when caused by certain high-risk pathogens.15,16
Third, 38% of patients with HAP will be discharged to a skilled nursing facility. Prior studies did not evaluate the association between HAP and discharge disposition. This finding has important implications for a patient's quality of life, functional status for hospital discharge planning, and for overall costs of care. As such, our estimates for the total charges for HAP underestimate the total costs to insurers, including the federal government, who are often responsible for these costs. Independent of the postdischarge charges, patients with HAP stayed 11 days longer than patients without HAP and charged on average $31,000 more. Strategies to reduce these combined charges should be a priority for healthcare insurers. Our findings regarding the incidence and impact of HAP are important given that therapies are available to prevent HAP and ventilator-associated pneumonia.1,14,17–20
Finally, the racial groups most at risk for developing HAP after intra-abdominal surgery were those categorized as “other.” This group of patients represented 2.4% of the sample by race. The group was 4.4 times more likely to develop HAP when compared with the reference group of white patients. This group represents racial groups not collected in some states and many that represent those of mixed race. Future research is needed to more concisely classify these patients and identify why their risk for HAP is so high. Our literature review produced only one study that evaluated race as a factor in the development in HAP. Kollef identified 43 patients with HAP, 38 white, 4, black, and one as “other” but found that race was not a significant predictor of HAP.14 The Institute of Medicine report revealed evidence that racial and ethnic disparities in health care are consistent across a range of illnesses and throughout many healthcare services.21 The results of this study reveal that there are populations more vulnerable to HAP but not specifically a single racial group.
Study Limitations
There are 3 limitations to this study. First, the validity of the diagnosis of HAP in discharge data is based on hospital discharge records not on preset clinical criteria. We assumed that the diagnosis of pneumonia was valid since patients in the study group had elective intra-abdominal surgery, a group unlikely to be operated on in the setting of preexisting pneumonia. The diagnosis of pneumonia can be challenging, especially when clouded by other postoperative changes. Montravers et al completed one of the largest prospective surveillance studies of HAP in the surgical population.8 The study involved 66 teaching hospitals and 164 nonteaching hospitals and looked at clinical circumstances, bacterial type, and therapeutic features in surgical patients suspected of having HAP. The researchers found that, of the 837 cases of suspected HAP, only 261 were confirmed to have HAP, 392 had a possible HAP, and 184 of the cases had a low probability of having HAP.8 This affirmed an earlier position of the American Thoracic Society that pneumonia in surgical patients is difficult to diagnose.22
Although our results are a national representative sample, they are only generalizable to intra-abdominal surgery patients who underwent elective surgery. Further, as a secondary data analysis, this study was limited to the process for data collection and the use of variables collected by the NIS. As such, we do not have detailed clinical data regarding factors that may have contributed to HAP and cannot establish causal relationships between patient characteristics and the development of HAP. However, it would be exceedingly expensive to conduct a prospective cohort study in this large a sample of hospitals and patients. Finally, we could not differentiate between HAP and other types of pneumonias such as aspiration pneumonia or ventilator-associated pneumonia. Some of the patients labeled as HAP may have also had aspiration pneumonia or VAP. This would likely increase the mortality, and charges associated with HAP.
Despite these limitations, the data sample was a national probability sample and was representative of all patients in the United States with statistical power to identify patients with HAP. Because of the sample size, the results of the study more closely approximate the true incidence of HAP than smaller cohorts of patients. In addition, identifying the demographic risk factors for the development of HAP is clinically useful information.
CONCLUSION
HAP is common and occurs in 10.7% of patients following intra-abdominal surgery. Adult women who undergo an intra-abdominal surgical procedure at any age are at 2 times greater risk of developing HAP than men. Patients of mixed races and other racial minorities are 4.4 times more likely to develop HAP than white patients. After adjusting for patient characteristics, patients who develop HAP have a 9-fold increased risk of in-hospital mortality, a 4-fold risk for discharge to skilled nursing facility, an average 11-day increase in LOS, and a $28,000 increase in total hospital charges. A nosocomial infection like HAP has associated negative clinical and economic outcomes for all stakeholders. Patient disposition will contribute to overall expenditures as more than one half of all HAP patients require additional care through home health care, rehabilitation, or nursing homes. Postoperative pneumonia may be unique in surgical patients from generalized hospital pneumonia among nonsurgical patients. Surgical patients are often administered antibiotics for intra-abdominal issues, onset of pneumonia may be in nonventilated patients, or they may have short periods of mechanical ventilation. These potentially confounding variables make diagnostic and treatment guidelines developed in nonsurgical populations difficult to apply to surgical patients.22 Further study in needed to understand the optimal treatment of pneumonia in select surgical populations. Given the incidence and significant impact of HAP effective strategies in preventing and treating HAP should be a clinical and research priority.
Footnotes
Reprints: David A. Thompson, DNSc, MS, RN, Johns Hopkins University School of Medicine, Quality and Safety Research Group, 901 South Bond Street, Suite 318, Baltimore, MD 21231-3305. E-mail: dthomps1@jhmi.edu.
REFERENCES
- 1.Cook DJ, Kollef MH. Risk factors for ICU-acquired pneumonia. JAMA. 1998;279:1605–1606. [DOI] [PubMed] [Google Scholar]
- 2.Baker JJ, Lambert RL, Poulos KM, et al. Managing the cost of care: a predictive study to identify critical care patients at risk for nosocomial pneumonia. J Health Care Finance. 2000;26:73–82. [PubMed] [Google Scholar]
- 3.Baughman RP, Tapson V, McIvor A. The diagnosis and treatment challenges in nosocomial pneumonia. Diagn Microbiol Infect Dis. 1999;33:131–139. [DOI] [PubMed] [Google Scholar]
- 4.Ephgrave KS, Kleiman-Wexler R, Pfaller M, et al. Postoperative pneumonia: a prospective study of risk factors and morbidity. Surgery. 1993;114:815–819. [PubMed] [Google Scholar]
- 5.Fujita T, Sakurai K. Multivariate analysis of risk factors for postoperative pneumonia. Am J Surg. 1995;169:304–307. [DOI] [PubMed] [Google Scholar]
- 6.Richardson JD, DeCamp MM, Garrison RN, et al. Pulmonary infection complicating intra-abdominal sepsis: clinical and experimental observations. Ann Surg. 1982;195:732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AHRQ. AHRQ's Patient Safety Initiative: Building Foundations, Reducing Risk [Interim Report to the Senate Committee on Appropriations, 04-RG005]. Rockville, MD, AHRQ Publications, 2003. [Google Scholar]
- 8.Montravers P, Veber B, Auboyer C, et al. Diagnostic and therapeutic management of nosocomial pneumonia in surgical patients: results of the Eole study. Crit Care Med. 2002;30:368–375. [DOI] [PubMed] [Google Scholar]
- 9.Hall JC, Tarala RA, Hall JL, et al. A multivariate analysis of the risk of pulmonary complications after laparotomy. Chest. 1991;99:923–927. [DOI] [PubMed] [Google Scholar]
- 10.Delgado-Rodriguez M, Medina-Cuadros M, Martinez-Gallego G, et al. Usefulness of intrinsic surgical wound infection risk indices as predictors of postoperative pneumonia risk. J Hosp Infect. 1997;35:269–276. [DOI] [PubMed] [Google Scholar]
- 11.Konrad F, Wiedeck H, Kilian J, et al. Risk factors in nosocomial pneumonia in intensive care patients: a prospective study to identify high-risk patients. Anaesthesist. 1991;40:483–490. [PubMed] [Google Scholar]
- 12.Celis R, Torres A, Gatell JM, et al. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1998;93:318–324. [DOI] [PubMed] [Google Scholar]
- 13.Torres A, Aznar R, Gatell JM, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–528. [DOI] [PubMed] [Google Scholar]
- 14.Kollef MH. Ventilator-associated pneumonia: a multivariate analysis. JAMA. 1993;270:1965–1970. [PubMed] [Google Scholar]
- 15.Rello J, Rue M, Jubert P, et al. Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit Care Med. 1997;25:1862–1867. [DOI] [PubMed] [Google Scholar]
- 16.Pennington J. Nosocomial respiratory infection. In: Principles and Practice of Infectious Disease. St. Louis, MO: Churchill Livingston, 1990:2199–2205. [Google Scholar]
- 17.Berenholtz S, Milanovich S, Faircloth A, et al. Improving care for the ventilated patient. Jt Comm J Qual Saf. 2004;30:195–204. [DOI] [PubMed] [Google Scholar]
- 18.Cook D, Meade M, Hand L, et al. Toward understanding evidence uptake: semirecumbency for pneumonia prevention. Crit Care Med. 2002;30:1472–1477. [DOI] [PubMed] [Google Scholar]
- 19.Fagon JY, Chastre J, Hance AJ, et al. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. [DOI] [PubMed] [Google Scholar]
- 20.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. [DOI] [PubMed] [Google Scholar]
- 21.Berwick DM. A user's manual for the IOM's ‘Quality Chasm’ report. Health Aff (Millwood). 2002;21:80–90. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, American Thoracic Society, November 1995. Am J Respir Crit Care Med. 1996;153:1711–1725. [DOI] [PubMed] [Google Scholar]