Abstract
Objective:
Report the midterm results of laparoscopic resection for hepatocellular in chronic liver disease (CLD).
Summary Background Data:
Surgical resection for hepatocellular carcinoma (HCC) in chronic liver disease (CLD) remains controversial because of high morbidity and recurrence rates. Laparoscopic resection of liver tumors has recently been developed and could reduce morbidity.
Methods:
From 1998 to 2003, patients with HCC and CLD were considered for laparoscopic liver resection. Inclusion criteria were chronic hepatitis or Child's A cirrhosis, solitary tumor ≤5 cm in size, and location in peripheral segments of the liver. Mortality, morbidity, recurrence rates, and survival were analyzed.
Results:
A total of 27 patients were included. Liver resections included anatomic resection in 17 cases and non anatomic resection in 10. Seven conversions to laparotomy (26%) occurred for moderate hemorrhage in 5 cases and technical difficulties in 2 cases. Mortality and morbidity rates were 0% and 33%, respectively. Postoperative ascites and encephalopathy occurred in 2 patients (7%) who both had undergone conversion to laparotomy. Mean surgical margin was 11 mm (range, 1–47 mm). After a mean follow-up of 2 years (range, 1.1–4.7), 8 patients (30%) developed intrahepatic tumor recurrence of which one died. Treatment of recurrence was possible in 4 patients (50%), including orthotopic liver transplantation, right hepatectomy, radiofrequency ablation, and chemoembolization in 1 case each. There were no adhesions in the 2 reoperated patients. Overall and disease-free 3-year survival rates were 93% and 64%, respectively.
Conclusion:
Our study shows that laparoscopic liver resection for HCC in selected patients is a safe procedure with very good midterm results. This approach could have an impact on the therapeutic strategy of HCC complicating CLD as a treatment with curative intent or as a bridge to liver transplantation.
Laparoscopic liver resection for peripheral hepatocellular carcinoma in selected patients was associated with no mortality and minimal morbidity. Overall and disease-free 3-year survival rates were 93% and 64%, respectively. This approach could have an impact on the therapeutic strategy of hepatocellular carcinoma complicating chronic liver disease as a treatment with curative intent or as a bridge to liver transplantation.
Hepatocellular carcinoma (HCC), the most common primary liver cancer, occurs in 90% of the cases in patients with chronic liver disease (CLD).1 In recent years, its incidence has increased in Western countries, mainly as a consequence of chronic hepatitis C virus infections.2–5 Screening for HCC has become an accepted part of the management of patients with CLD, especially with the advent of new therapeutic modalities, and small (ie, <3–4 cm in diameter) asymptomatic HCCs are now increasingly recognized.6,7 Therapeutic options with curative intent for small HCCs in patients with CLD include liver transplantation, hepatic resection, and percutaneous treatments (ie, ethanol injection and radiofrequency ablation). Liver transplantation is the best theoretical treatment of those patients by removing both the tumor and underlying CLD. However, it cannot be applied on a large scale because of the high prevalence of HCC and the limited access to liver transplantation due to donor shortage. Moreover, the results of liver transplantation are significantly hampered by tumor progression with increased waiting time leading to significant drop out from the waiting list.8,9 The 2 other options (ie, resection and percutaneous techniques) are therefore more applicable on a larger scale. Surgical resection is generally considered an efficient treatment, but its use, in the context of CLD, is limited because of significant morbidity, which could be at least in part, related to the laparotomy itself.
The aim of the present study was therefore to study the feasibility, safety, and oncologic efficiency of laparoscopic resection of HCC in patients with CLD.
PATIENTS AND METHODS
Patients
A prospective evaluation of laparoscopic liver resection was initiated in our center in 1996.10 The first patients had liver tumors developed on normal liver parenchyma and after 2 years the indications were extended to patients with HCC and CLD. Selection criteria for laparoscopic approach in HCC patients included the following: chronic hepatitis or Child's class A cirrhosis, esophageal varices ≤grade 2, platelet count ≥100 × 109/L, and solitary lesion of ≤5 cm in diameter accessible to the laparoscopic approach (ie, located in the peripheral segments of the liver)10,11 and treatable by limited resection (<3 segments).
Imaging assessment of the liver included CT, MRI, or both and Doppler ultrasonography. Most resections intended to be anatomic (ie, resection of 1 or several anatomic segments) to resect the portal territory of the tumor. Segmentectomies were defined by their numbers and performed according to external segmental borders and use of intraoperative ultrasound as previously described.12 However, in small lesions developed in patients with portal hypertension and hepatic dysmorphy, nonanatomic resections were performed. These consisted of resection of less than 1 segment including the tumor and an intended 1-cm tumor-free margin.
The surgical techniques for laparoscopic liver resection used have been described previously.10 Briefly, the patients were placed in the supine position with lower limbs apart, except for isolated resections of segment 6 where the left lateral decubitus position was used. The procedures were performed with CO2 pneumoperitoneum, and abdominal pressure was electronically maintained below 12 mm Hg. A 30° laparoscope was used. The liver was examined by vision and intraoperative ultrasonography to confirm the number and size of the lesions and define their relationship with the intrahepatic vascular structures. A tape was placed around the porta hepatis and passed through a rubber drain for use as a tourniquet for portal triad clamping. Intermittent clamping was applied, with 15-minute clamping and 5-minute release periods. Hepatic transection was performed with a harmonic scalpel (Ultracision, Ethicon, USA) and ultrasonic dissector (Dissectron, Satelec, France). Bipolar electrocoagulation was used for minor bleeding, and larger structures were secured with clips. Portal pedicles and major hepatic veins were divided by application of a linear stapler. The resected specimen was placed in a plastic bag and externalized, without fragmentation, through a separate incision, either along a previous appendectomy incision or a suprapubic horizontal incision. This incision was immediately closed and the abdomen reinflated. The surgical field was irrigated and checked for bleeding or bile leak, and residual fluid was removed by suction. Abdominal drainage was usually omitted.
Postoperative Management and Follow-up
Patients were transferred to the intensive care unit postoperatively for 24 hours and then transferred to the ward except when the clinical situation indicated otherwise. Postoperative care was identical to that of open liver resections in patients with CLD and included limited fluid administration, sodium restriction, perioperative antibioprophylaxis, proton pump inhibitors, and early mobilization. Liver function tests were monitored daily. Patients with chronic hepatitis B and active viral replication were treated by lamivudine, which was started preoperatively. Patients with chronic hepatitis C tentatively received interferon initiated 2 to 3 months after surgery. Follow-up included liver function tests, alpha-fetoprotein, and computed tomographic or magnetic resonance scan at 3 months and then every 6 months.
Studied Criteria
The study was undertaken in intention to treat without exclusion of patients converted to laparotomy. The studied criteria were duration of surgery and clamping, perioperative transfusions, pathologic margins, postoperative variations of liver tests, mortality, morbidity, hospital stay, and survival. Specific complications were those related to the liver resection procedure or the underlying liver disease and included: bile leak, operative site hemorrhage, ascites (defined as clinically detectable or as abdominal drainage output, when present, of 500 mL or more per day), hepatic encephalopathy, jaundice, and variceal bleeding. Other complications were recorded as nonspecific complications. Survival and tumor recurrence rates were estimated with actuarial methods.
RESULTS
From May 1996, to August 2003, 85 patients underwent laparoscopic liver resection. Among those and from 1998, 27 had HCC complicating CLD (32%). Patients' characteristics are summarized in Table 1. On a 0 to 4 scale of fibrosis, 20 patients had a score of 4 (cirrhosis) and 7 a score of 2 or 3.13 All but 2 patients had a lesion located in the anterolateral segments of the liver (ie, segments 2–6) (Fig. 1). Causes of liver disease were hepatitis B virus in 9 cases, hepatitis C virus in 8 cases, alcohol consumption in 8 cases, and other in 2 cases. The lesion was solitary in 26 patients. One patient had 2 lesions: one peripheral in the left lobe which was resected and one central in the right lobe which was treated by radiofrequency ablation during the same laparoscopic procedure.
TABLE 1. Patients Characteristics
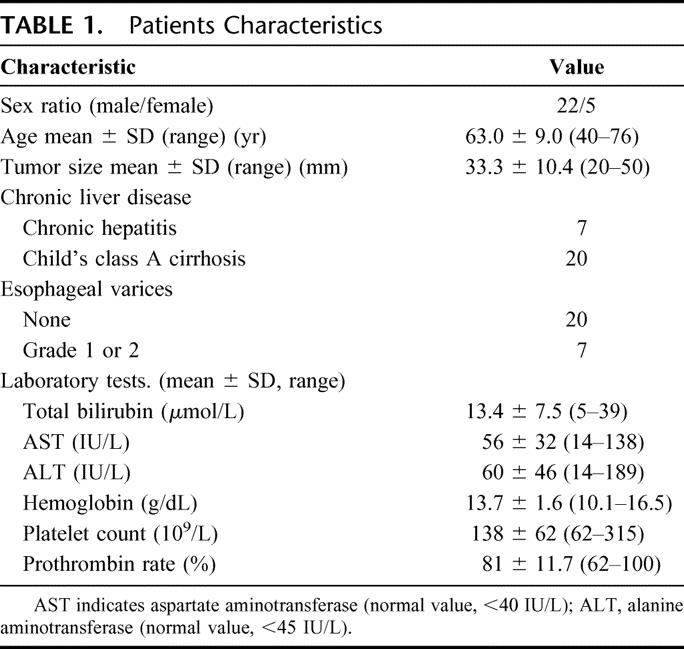
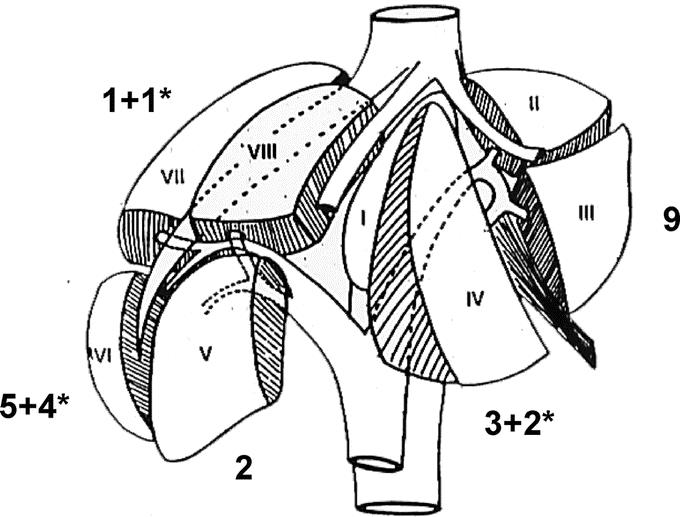
FIGURE 1. Tumor locations. *Conversion to laparotomy.
During the same period, we performed 56 open liver resections and 36 orthotopic liver transplantations for HCC. Therefore, laparoscopic liver resection accounted for 32% of resected HCCs and 23% of all surgically treated HCCs during the studied period. Open liver resections, most of them major resections (≥3 liver segments), were performed in patients with large HCC (mean size 72.5 ± 39.1 mm versus 33.3 ± 10.4 mm in laparoscopic patients, P < 0.05). Orthotopic liver transplantations were performed in patients with either more than one HCC nodule or small HCC and decompensated cirrhosis. These cases represented 18% of the transplantations performed during the same period. Percutaneous radiofrequency ablation was used in 82 patients with small HCC, either as a neoadjuvant treatment before liver transplantation or as the sole treatment, in patients with CLD and deeply located small HCC inaccessible to limited resection.
Intraoperative Results
Surgical procedures and results are summarized in Table 2. All but 1 resection were limited (<3 segments) and 1 patient underwent right hepatectomy. There were 17 anatomic (63%) and 10 nonanatomic resections (Figs. 2–6). Seven (26%) conversions to laparotomy occurred, all in resections of 1 segment or less. As shown in Figure 1, resections of segment 6 were more frequently converted, whereas there were no conversions in left lateral sectionectomies. The causes of conversion included moderate hemorrhage in 5 patients and technical difficulties in 2 cases. Conversions for hemorrhage were not associated with shock or need for highly urgent laparotomy. Technical difficulties included insufficient progression of the transection due to poor vision and doubt on surgical margin, in 1 case each both in tumors located in segment 4. Operative duration and cumulative clamping time were 240 ± 75 minutes (range, 150–360 minutes) and 54 ± 25 minutes (range, 15–117 minutes), respectively. In 6 patients, blood loss was ≥1000 mL and in the remnant it was 338 ± 182 mL (range, 100–800 mL). Three patients were transfused (15%), 1 intraoperatively and 2 in the postoperative period.
TABLE 2. Surgical Procedures and Results
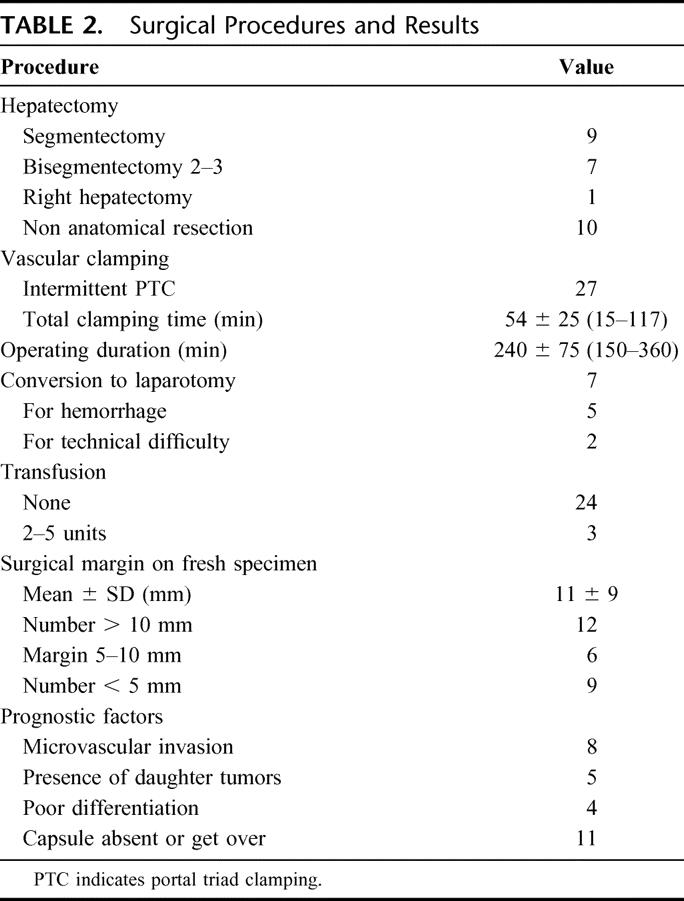
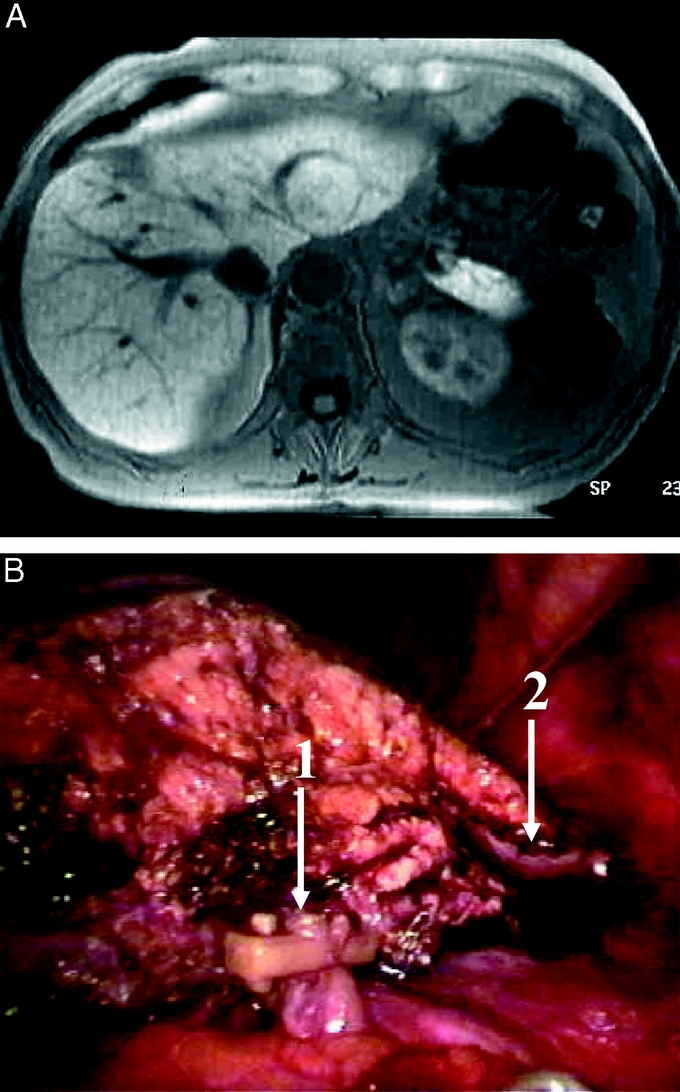
FIGURE 2. Left lateral sectionectomy for a 3-cm HCC in segment 2. Preoperative magnetic resonance (above) and operative view of completed resection (below). Arrow 1 shows a locked clip on a left arterial branch, and arrow 2 shows the stapled stump of the left hepatic vein.
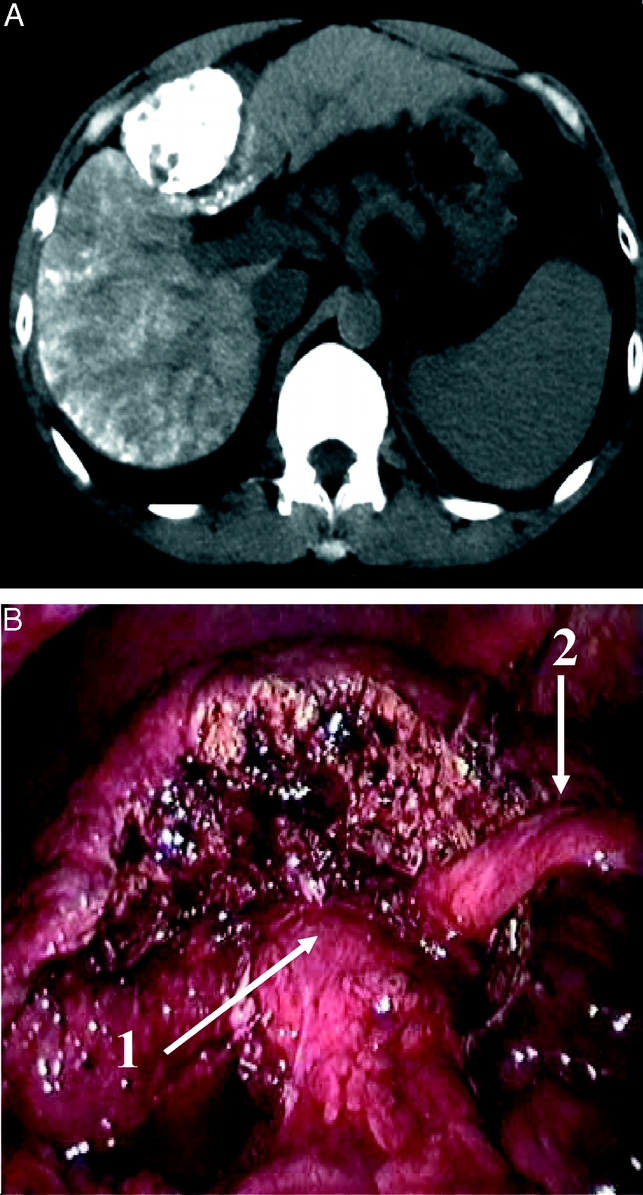
FIGURE 3. Resection of the anterior part of segment 4 (segment 4b) for a 4-cm HCC. Preoperative computed tomography showing the tumor in segment 4 enhanced by previous lipiodol injection (above) and operative view of completed resection (below). Arrow 1 shows the liver hilum, and arrow 2 shows the round ligament.
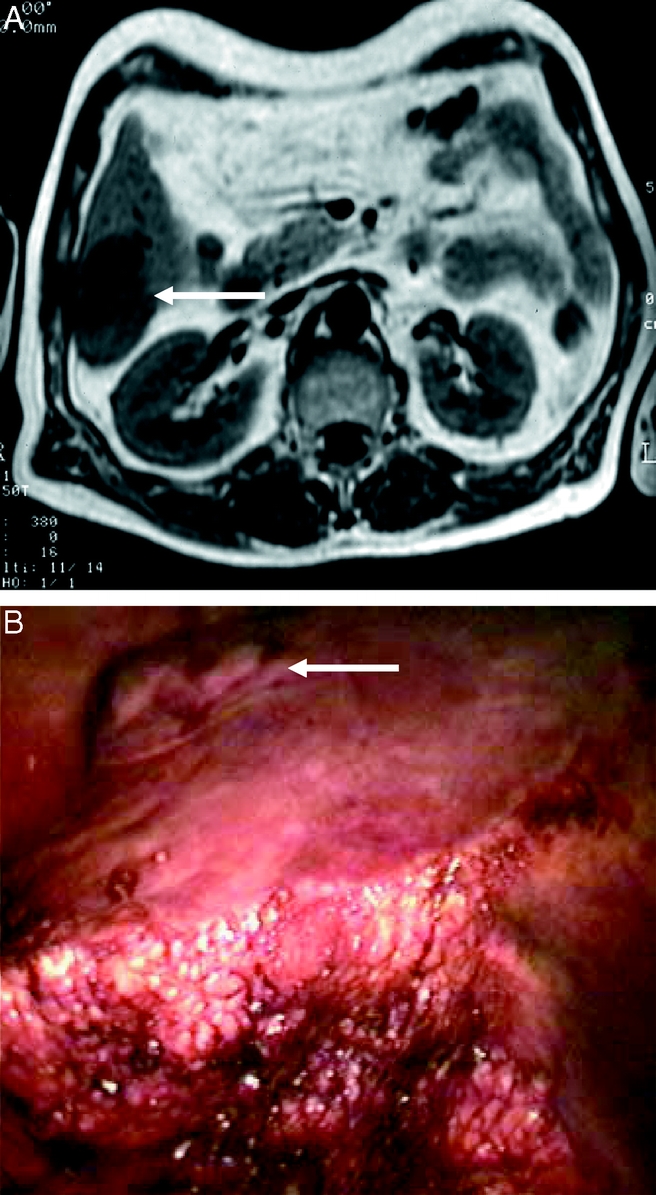
FIGURE 4. Resection of segment 6 for a 3-cm HCC (arrows show the tumor). Preoperative magnetic resonance (above) and operative view (below) showing the transection in progress using intermittent Pringle maneuver in a patient in left lateral decubitus position.
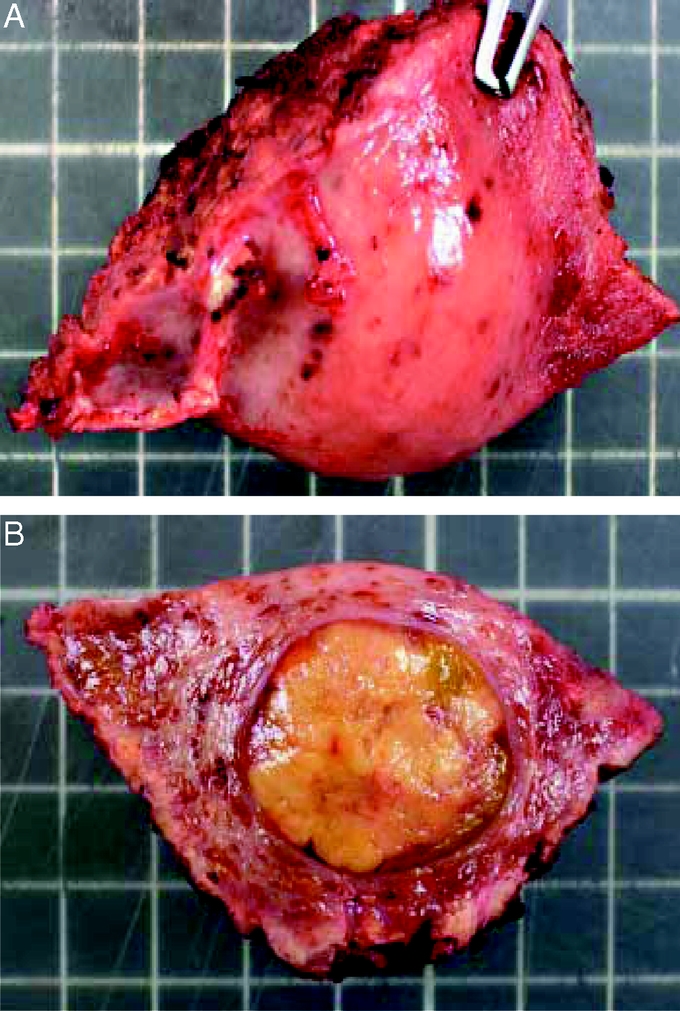
FIGURE 5. Specimen from non anatomic resection of segment 5 for a 2.5-cm encapsulated HCC. Surgical margin is 1 cm.
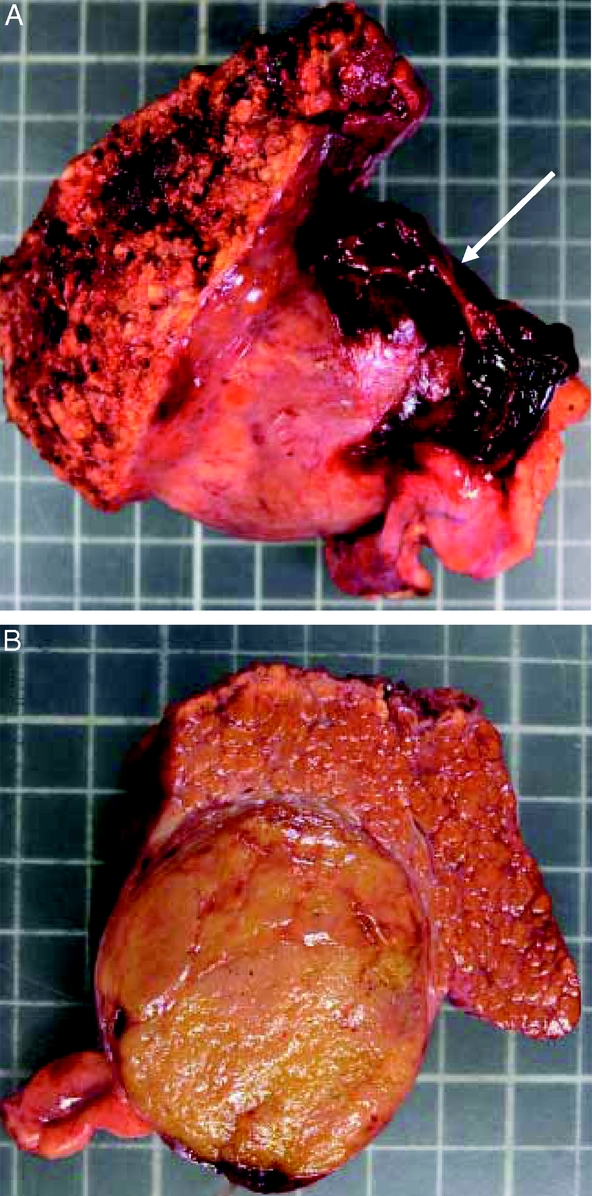
FIGURE 6. Specimen of resection of segment 3 for a 5-cm HCC. This tumor was previously ruptured as shown by residual hematoma (arrow). Surgical margin is 2 cm.
Postoperative Results
There were no deaths. Postoperative profiles of serum bilirubin, aspartate aminotransferase, and prothrombin time are shown in Figure 7. Nine (33%) patients developed 11 complications. Two (7%) patients developed specific complications, including ascites and grade 1 encephalopathy occurred. These 2 patients had been converted to laparotomy. Three of the 20 nonconverted patients (15%) developed mild transient jaundice (≤70 μmol/L, normal value < 21 μmol/L), including 1 patient who underwent right hepatectomy, but none developed ascites, coagulopathy, or encephalopathy. There was no variceal bleeding and no bile leaks. Six (22%) patients developed nonspecific complications. One converted patient developed an incisional abscess. Five unconverted patients developed nonspecific complications, including incisional hernia on 12-mm port orifice (1 case, requiring reoperation at day 8), respiratory complication (3 cases: 2 pneumonias and 1 decompensated chronic obstructive disease), systemic infection (1 case). Hospital stay was 15.2 ± 17.5 days (median, 9 days; range, 4–76 days). Hospital stay in uncomplicated patients was 7.8 ± 2.6 days (median, 7 days; range, 4–13 days).
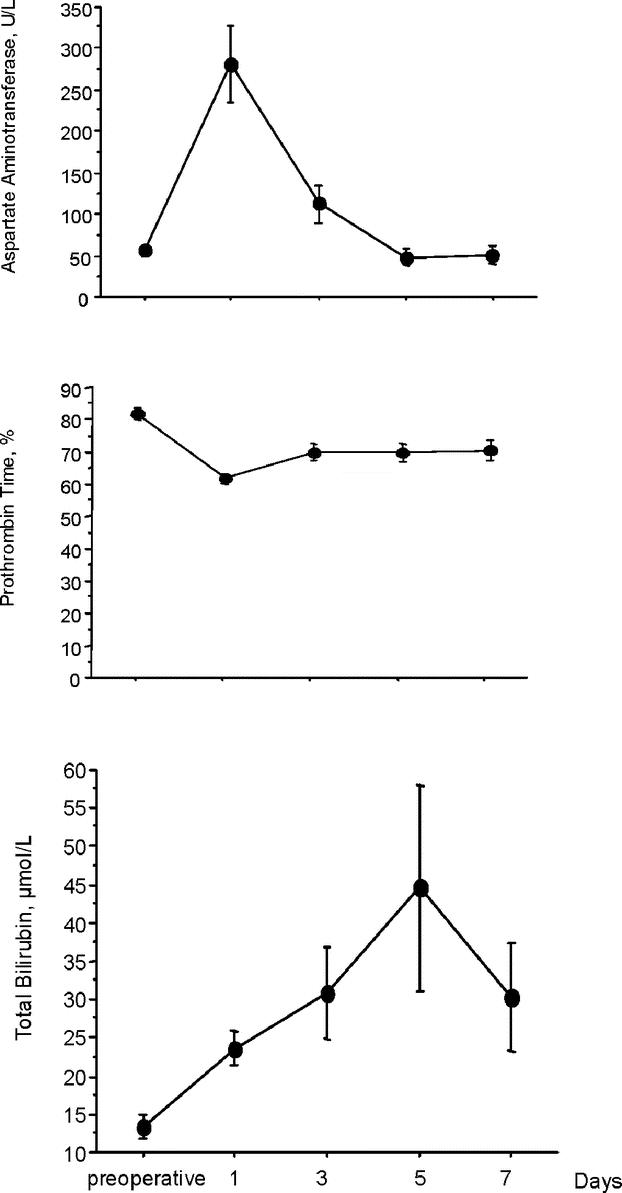
FIGURE 7. Postoperative change in prothrombin rate, total bilirubin level, and aspartate aminotransferase level.
Survival and Recurrence
After a mean follow-up of 2 years (range 1.1–4.7 years), 8 patients (30%) developed tumor recurrence in the liver. Three had local recurrence (ie, in the same segment): 2 after nonanatomic resection and 1 after segmentectomy. The 5 other recurrences occurred in another liver segment. In addition, distant metastases occurred in 1 patient. Treatment of recurrence was possible in 4 patients (50%), including orthotopic liver transplantation, right hepatectomy, radiofrequency ablation, and chemoembolization in 1 case each. Orthotopic liver transplantation and repeat hepatectomy were performed in 2 patients who initially had an unconverted laparoscopic procedure, and no adhesions were found at laparotomy.
Two patients died: 1 from unrelated cause (leukemia) and 1 from hepatic and bone recurrence. None of the patients developed chronic decompensation of liver disease. Overall and disease-free 3-year survival rates were 93% and 64%, respectively (Fig. 8).
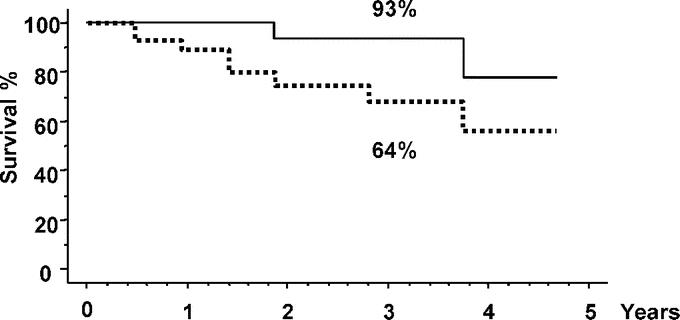
FIGURE 8. Actuarial overall (continuous line) and disease-free survival (dotted line).
DISCUSSION
This is the first series of laparoscopic liver resection for HCC in CLD studying both short-term and survival results. It should be emphasized that considering laparoscopic liver resection requires 2 conditions: 1) indications must be accurate in terms of tumor location and assessment of underlying liver function; and 2) specific expertise and training, in both hepatic and laparoscopic surgery, as well as access to adequate technology must be assured.
The present series shows the feasibility and safety of the procedure in selected patients and gives an insight to the efficiency, value, and place of this procedure in the management of small HCC relatively to other therapeutic modalities.
The feasibility of the procedure in our selected patients was 74%, hampered by a 26% conversion rate, which compares to those reported for other complex laparoscopic procedures such as colectomy.14 Five of the 7 conversions were for hemorrhage. These were mild bleedings which, however, disturbed the progression of the procedure. No emergency conversion was required. Other causes of conversion were insufficient progression of the procedure due to lengthy transection and doubtful surgical margin in 1 case each. Hemorrhage and insufficient progression reflect the fact that the technique of laparoscopic hepatic transection is not a completely solved problem, especially in cirrhotic livers, but the technique continues to improve. It can be noted that there were no conversions in left lateral sectionectomies and that most conversions occurred in resections of segment 6, a peripheral but posterior segment. The use of the left lateral decubitus position and more experience in transection technique improved this problem. We showed in previous studies that operative durations of laparoscopic liver resections were significantly longer than those of matched open counterparts.15,16 This was confirmed in the present study with a 4-hour mean operative time which, however, improved with the learning curve. Longer operative durations were also found with other complex laparoscopic procedures, such as colectomies,14,17 and it seems, at least in part, inherent to the laparoscopic approach. Technical refinements and experience should help improving this aspect in the future.
The safety of the procedure is attested by the absence of mortality and the low specific morbidity. Indeed, there was only 1 case each of postoperative ascites and encephalopathy, which both occurred in converted patients. In unconverted patients, there were no postoperative ascites or liver failure. These findings are important since ascites and liver failure are the main complications of liver resection, even minor ones, in cirrhotic patients.18,19 There were no other specific complications such as bile leak or postoperative bleeding, which is another indicator of the safety of the procedures. The rate of nonspecific complications (pulmonary, infectious) was in the usual range for patients with that grade of comorbidity. Of note is 1 case of early incisional hernia requiring reoperation. This occurred on a 12-mm port site and emphasizes the need for careful fascia closure of port sites of 10 mm or more.
The efficacy of the procedure is attested by good recurrence-free and overall survival rates. After initial concerns about the use of laparoscopy in patients with malignancy, it is now established that such patients can undergo laparoscopic surgery without increased risk of tumor dissemination provided that adequate surgical technique is used.14,17 Our study supports the same results in patients with HCC. After a mean follow-up of 2 years, the recurrence rate was 30% and 3-year overall and disease-free survival rates were 93% and 64%, respectively, which compare favorably with previous reports of open resections of small HCC in CLD.6,20–24
The principle of hepatectomy for small HCC in patients with CLD is to perform a parenchymal-preserving procedure because of the underlying liver disease and impaired liver regeneration. This is achieved by resection of the tumor with a sufficient margin of non tumorous liver. For this matter, anatomic resection (ie, resection of 1 or more anatomic segments) rather than nonanatomic resection (so-called wedge resection) has been advocated by some authors, to resect the portal territory of the tumors, the portal system being the theoretical pathway for intrahepatic tumor spread.19 We performed anatomic resection every time it was possible, but in 10 of 27 cases (37%), mainly because of liver dysmorphy and portal hypertension due to cirrhosis, we elected to perform a nonanatomic resection. It should be emphasized that we would also have done a nonanatomic resection in those patients by open approach. Local recurrence in the same segment occurred in 3 patients, of whom 2 had nonanatomic resection. Intrahepatic recurrence occurred in 30% of the patients, half of which could be treated including orthotopic liver transplantation and open hepatectomy in 1 case each. Interestingly, no adhesions were found at laparotomy in those 2 patients, which is a major advantage of the initial laparoscopic procedure.
The advantages of laparoscopy over laparotomy include reduced invasiveness, less postoperative pain, and quicker rehabilitation. However, these early outcome measures, which are very valuable, should not lead to overlook longer-term advantages related to the avoidance of long abdominal incisions. Besides cosmetic issues, of limited value in patients with malignancy, these advantages include preservation of abdominal collateral venous circulation, which may participate in the absence of postoperative ascites, and avoidance of peritoneal adhesions, valuable in these patients who may require repeat operations for tumor recurrence. It should also be noted that none of the patients developed chronic decompensation of liver disease during the studied period. So far, we have limited laparoscopic liver resection to patients with compensated cirrhosis because preservation of liver function was the main goal of the procedure. We consider that patients with small HCC and decompensated cirrhosis are candidates for direct liver transplantation.
An important issue is how laparoscopic liver resection fits in the algorithm of management of small isolated HCC in patients with compensated cirrhosis. Our approach is that laparoscopic resection does not compete with other therapeutic modalities (ie, liver transplantation and percutaneous ablation) but provides additional possibilities. Liver transplantation is theoretically the best treatment of small HCC on CLD because it cures both the tumor and the underlying liver disease, thus preventing the development new HCC foci.1 However, unless some priority is given to HCC patients,25 organ shortage leads to increased waiting times, tumor progression, and dropout from the waiting list, which may be higher than 20% for waiting times >1 year.9 This leads most authors to recommend the use of neoadjuvant therapies aimed at controlling tumor progression during the waiting time. In isolated tumors, percutaneous radiofrequency ablation is the most often used one.1,26,27 The efficiency of radiofrequency ablation in patients awaiting transplant was recently assessed on total hepatectomy specimens showing a 55% complete necrosis rate (63% in tumors <3 cm).28 However, this approach has a few drawbacks: in patients with long waiting times, the risk of recurrence is high and pathologic examination of the tumor is not possible. In addition, percutaneous radiofrequency ablation is more hazardous in subcapsular lesions because of the risk of tumor seeding, which may be higher than 10% in these subcapsular locations.8 By contrast, such small subcapsular HCCs are good candidates for laparoscopic liver resection with limited sacrifice of functional parenchyma as neoadjuvant treatment before liver transplantation. An additional advantage of liver resection as a bridge to transplantation is the possibility of full pathologic study of the specimen, which permits the assessment of prognostic factors of HCC such as microvascular invasion, satellite nodules, tumor differentiation, and molecular biology markers of recurrence all of which may participate in the transplant decision-making.29–32 It should also be noted that not all patients with small HCC in CLD are candidates for liver transplantation because of age, persistent alcohol consumption, or associated diseases, and these were most of our patients. Here again, the same complementary indications of radiofrequency ablation for deep lesions and laparoscopic resection for peripheral lesions apply. In addition, the possibility of cure by resection may be improved by the use of adjuvant therapies in patients in whom HCC is associated with chronic hepatitis B or C, where lamivudine and interferon, respectively, may reduce the recurrence rates as suggested in recent promising studies.33,34
Our study shows that laparoscopic liver resection for small peripheral HCC in patients with CLD and preserved liver function is a safe procedure with good midterm results. It suggests that laparoscopic liver resection represents an acceptable alternative to open resection in this setting and could have an impact on the therapeutic strategy of HCC complicating CLD, as a treatment with curative intent or as a bridge to liver transplantation.
Footnotes
Reprints: Daniel Cherqui, MD, Department of Digestive Surgery, Hôpital Henri Mondor, 51, avenue De Lattre de Tassigny 94010 Créteil, France. E-mail: daniel.cherqui@hmn.ap-hop-paris.fr.
REFERENCES
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Robinson SD, Foster GR, Arora S, et al. Increase in primary liver cancer in the UK, 1979–94. Lancet. 1997;350:1142–1143. [DOI] [PubMed] [Google Scholar]
- 3.Deuffic S, Poynard T, Buffat L, et al. Trends in primary liver cancer. Lancet. 1998;351:214–215. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto J, Okada S, Shimada K, et al. Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology. 2001;34(4 Pt 1):707–713. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. [DOI] [PubMed] [Google Scholar]
- 9.Yao FY, Bass NM, Nikolai B, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873–883. [DOI] [PubMed] [Google Scholar]
- 10.Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katkhouda N, Hurwitz M, Gugenheim J, et al. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg. 1999;229:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chouillard E, Cherqui D, Tayar C, et al. Anatomical bi- and trisegmentectomies as alternatives to extensive liver resections. Ann Surg. 2003;238:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20(1 Pt 1):15–20. [PubMed] [Google Scholar]
- 14.The clinical outcomes of surgical therapy study group: a comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. [DOI] [PubMed] [Google Scholar]
- 15.Laurent A, Cherqui D, Lesurtel M, et al. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg. 2003;138:763–769. [DOI] [PubMed] [Google Scholar]
- 16.Lesurtel M, Cherqui D, Laurent A, et al. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196:236–242. [DOI] [PubMed] [Google Scholar]
- 17.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. [DOI] [PubMed] [Google Scholar]
- 18.Lang BH, Poon RT, Fan ST, et al. Perioperative and long-term outcome of major hepatic resection for small solitary hepatocellular carcinoma in patients with cirrhosis. Arch Surg. 2003;138:1207–1213. [DOI] [PubMed] [Google Scholar]
- 19.Regimbeau JM, Kianmanesh R, Farges O, et al. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131:311–317. [DOI] [PubMed] [Google Scholar]
- 20.Belghiti J, Panis Y, Farges O, et al. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CS, Sheu JC, Wang M, et al. Long-term outcome after surgery for asymptomatic small hepatocellular carcinoma. Br J Surg. 1996;83:330–223. [DOI] [PubMed] [Google Scholar]
- 22.Nagashima I, Hamada C, Naruse K, et al. Surgical resection for small hepatocellular carcinoma. Surgery. 1996;119:40–45. [DOI] [PubMed] [Google Scholar]
- 23.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bigourdan JM, Jaeck D, Meyer N, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl. 2003;9:513–520. [DOI] [PubMed] [Google Scholar]
- 25.Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(5 suppl 1):261–267. [DOI] [PubMed] [Google Scholar]
- 26.Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914–921. [DOI] [PubMed] [Google Scholar]
- 27.Livraghi T, Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. [DOI] [PubMed] [Google Scholar]
- 28.Mazzaferro V, Battiston C, Perrone S, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cillo U, Vitale A, Bassanello M, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plessier A, Codes L, Consigny Y, et al. Underestimation of the influence of satellite nodules as a risk factor for post-transplantation recurrence in patients with small hepatocellular carcinoma. Liver Transpl. 2004;10(2 suppl 1):86–90. [DOI] [PubMed] [Google Scholar]
- 31.Iizuka N, Oka M, Yamada-Okabe H, et al. Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet. 2003;361:923–929. [DOI] [PubMed] [Google Scholar]
- 32.Sala M, Fuster J, Llovet JM, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl. 2004;10:1294–1300. [DOI] [PubMed] [Google Scholar]
- 33.Shiratori Y, Shiina S, Teratani T, et al. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299–306. [DOI] [PubMed] [Google Scholar]
- 34.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. [DOI] [PubMed] [Google Scholar]


