Abstract
Objective:
To evaluate the diagnostic accuracy of esophageal and cardia adenocarcinoma in the Swedish Cancer Register.
Summary Background Data:
Based on cancer registers, a rising incidence of esophageal and cardia adenocarcinoma has been reported in several populations, but possible influence of differences in tumor classification has not been evaluated.
Methods:
In a nationwide study in 1995 through 1997, all Swedish patients, born in Sweden and younger than 80 years with esophageal or cardia adenocarcinoma and half of all patients with esophageal squamous cell carcinoma, were prospectively, uniformly, and thoroughly classified. This study classification was compared with the tumor classification in the Swedish Cancer Register, which is based on routine clinical practice.
Results:
The overall completeness of the Cancer Register was high (98.3%), whereas the site-specific completeness of the Register was 63% for esophageal adenocarcinoma, 74% for cardia adenocarcinoma, and 91% for esophageal squamous cell carcinoma. The incidence of esophageal adenocarcinomas was 16% higher in the study classification compared with that of the Register during the study period, whereas the incidence of cardia adenocarcinoma was 2% lower in the study classification.
Conclusions:
There is a diagnostic mismatch between esophageal and cardia adenocarcinoma in the clinical setting and, therefore, also in Cancer Registers. In etiologic and therapeutic research, this problem needs consideration, since these tumors have distinct risk factor profiles and could be subjected to different treatment strategies. The increasing incidence rate of esophageal adenocarcinoma in Sweden is unlikely to be explained by such differences in tumor classification, however.
Cancer registers form the basis for etiological and therapeutical research. The accuracy in classifying adenocarcinomas in the esophagus and the gastric cardia in the Swedish Cancer Register, based on routine clinical practice, was evaluated. A considerable diagnostic mismatch between the adenocarcinomas was found in the clinical setting, and consequently also in the Register.
Population-based register studies in several Western countries, including Sweden, have shown increasing incidence rates of esophageal adenocarcinoma, in particular, and also, but to a more moderate extent, of gastric cardia adenocarcinoma, in recent decades.1–10 Investigations in the last years have pointed to a continuous rise in the incidence of esophageal adenocarcinoma, which has been especially rapid in the United Kingdom and in the United States, where this tumor has shown an increase exceeding that of any other malignancy among white males.1,8 Adenocarcinomas of the esophagus and gastric cardia share some epidemiologic features, including incidence trends and sex distribution,9 but recent data suggest that these tumors have different risk factor profiles, 11–16 In addition, different subsites of these adenocarcinomas might be suitable for separate therapeutic strategies.17 However, no morphologic differences that distinguish between the different adenocarcinomas in the vicinity of the gastroesophageal junction have been identified. Thus, clinicians and pathologists are left to rely on estimates of anatomic features of the tumor. During endoscopies or surgery, it is often difficult to make a precise locational definition of tumors situated near the gastroesophageal junction; furthermore, there is no consensus on how to classify these tumors. Hence, there are reasons to suspect that mismatch in classification between these tumors is a problem in routine clinical practice. Since the diagnoses recorded in cancer registers are mostly based on hospital records, any level of disagreement of tumor classification in the clinical setting could distort etiologic or therapeutic research founded on these registers or have influence on the incidence rates reported from them. In an evaluation of the classification of gastric cancer in the Swedish Cancer Register, 15% of cardia cancer cases were classified as noncardia gastric cancer and the completeness was only 69%.18 The conclusion drawn from that study was that the observed increase in the incidence of cardia adenocarcinoma in the Swedish Cancer Register might be explained by disparities in tumor classification. To our knowledge, no similar study for assessment of the classification of esophageal cancer has been conducted.
With the purpose of evaluating the diagnostic accuracy of esophageal and cardia adenocarcinoma and esophageal squamous cell carcinoma in the routine clinical setting and to elucidate the question whether differences in tumor classification could affect the reported incidence rates, we compared a thorough and uniform prospective classification of a nationwide patient material in Sweden,11 with the classification recorded in the Swedish Cancer Register.
METHODS
Design
As part of a nationwide, population-based, case-control study of risk factors for adenocarcinoma of the esophagus and gastric cardia in Sweden, all newly identified cases of adenocarcinoma of the esophagus or gastric cardia and half the newly diagnosed cases of esophageal squamous cell carcinoma (patients born on even-numbered days) were prospectively and uniformly classified.11 The study was conducted during the period December 1, 1994 through December 31, 1997, and encompassed the entire population of Sweden younger than 80 years, born in Sweden, and still living there during the study period. A comprehensive organization for rapid ascertainment of cases, with contact persons at all 195 relevant hospital departments in Sweden, as well as continuous collaboration with all 6 regional tumor registries, ensured that every case throughout the country was identified shortly after diagnosis. Tumors that were first discovered at autopsy were not included.
Study Classification
To optimize the classification of tumor site and histologic type for research purposes, uniform routines for the documentation of the tumors of the patients included in the study were introduced at all participating departments. For a case to be classified as a cancer of the gastric cardia, the tumor had to have its center within 2 cm proximal, or 3 cm distal, to the gastroesophageal junction. The gastroesophageal junction was defined as the point where the proximal longitudinal mucosal folds begin in the stomach. Squamous cell carcinomas were classified as esophageal even if the location was in the gastric cardia. The final diagnosis was based on the summary of the findings of 1) endoscopy, 2) surgery, 3) radiology, and 4) pathology.
Endoscopy
All patients were endoscopically examined at least once, and usually twice or more. For uniform endoscopic documentation of esophageal and cardia tumors for study purposes, a scheme for the measurement and biopsy sampling was introduced at all participating endoscopy units in Sweden. The distances between the gastroesophageal junction and the upper and lower limits of the tumor were measured. It was usually possible to pass the endoscopes beyond obstructing tumors, at least after balloon dilatation, but in patients where this was impossible or the gastroesophageal junction could not be identified, the borders of the tumor were measured as distances to the incisor teeth. At endoscopy, serial biopsy specimens were obtained every 2 cm starting from the proximal stomach, through the gastroesophageal junction, and in the esophagus, until normal-appearing squamous cell epithelium was reached. Additional specimens were obtained from the tumor as well as proximal, distal, and lateral closely to it.
Surgery
Surgeons gave standardized and detailed descriptions of the location of the tumor in the cases in which tumor resection or surgical exploration was performed. The size of the tumor, the upper border, lower border, and center of the tumor were described both in a separate study protocol and in a drawing in which the surgeon filled in the precise anatomic location of the cancer.
Radiology
Radiologic examination, including computerized tomography and endoscopic ultrasound, gave additional information with regard to tumor site.
Pathology
Pathologists used a study protocol to slice the surgical specimens in a uniform manner, a protocol that was regarded mandatory among Swedish pathologists examining the study tumors. Detailed descriptions of the location and histologic type were given in this protocol. To make uniformity possible and further reduce misclassification, all biopsy samples, surgical specimens, or both from 97% of the patients were finally rereviewed by a single, experienced, pathologist (A.L.).
In cases were the tumor classification was to any degree contradictory between the 4 diagnostic tools, a panel of investigators with experience in endoscopy, surgery, radiology, and pathology, agreed upon a final tumor classification.
Classification in the Swedish Cancer Register
In Sweden, both clinicians and pathologist are obliged to report every new case of cancer to one of the 6 regional cancer registers, which then report to the national Swedish Cancer Register.13 The clinicians report both tumors that were and were not verified by biopsy specimen. Cancer diagnoses based on death certificate only are not registered. The clinicians did not have access to the study protocols, why the diagnosis in the Cancer Register relied on the individual, clinical interpretation of tumor site, based on routine examinations. The tumors were coded according to the International Classification of Diseases for Oncology (ICD-O) version 2 and then converted to a modified version of the ICD-7. The specific site of each tumor was registered: the esophagus (upper, middle, distal, or unspecified parts), 150; gastric cardia, 151.1; and gastric corpus or pylorus, 151.0. The recording of histologic type relied on the report from the individual pathologist and was registered as: adenocarcinoma (096); squamous-cell carcinoma (146); and unspecified esophageal cancer (196).
Linkage Between Study Classification and Swedish Cancer Register
The National Registration Number is a 10-digit unique personal identifier assigned to all Swedish residents at birth. This Registration Number was documented in all patients in the study classification as well as in all patients in the Swedish Cancer Register, which enabled correct linkage of the tumor classification in the study with the Cancer Register in each individual patient of our study. Only Swedish residents born in Sweden were included in the study classification as well as those extracted from the Cancer Register.
Statistical Analyses
The diagnosis given in the Swedish Cancer Register was compared with that given in our diagnostic efforts described above, ie, the study classification. The study classification was considered as gold standard in the statistical analyses. Hence, diagnoses in the Register were considered as being in agreement or in disagreement with the study classification. Completeness of the Register was defined as the number of cancer diagnoses in agreement divided by the number of cases in the study classification. Rate of false inclusion was defined as the number of esophageal cancers registered in disagreement in the Register divided by the total number of esophageal cancers in the Register. Positive predictive value was defined as the number of esophageal cancers registered in agreement divided by the total number of esophageal cancers in the Register.
Ethical Considerations
All regional ethics committees in Sweden approved the study, and individual written informed consent was obtained from the patients.
RESULTS
Distribution by Age, Sex, and Tumor Location in the Study Classification
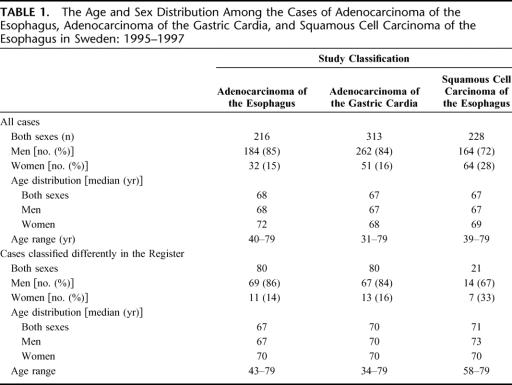
In total, we identified 757 cases of adenocarcinoma of the esophagus, squamous cell carcinoma of the esophagus, or adenocarcinoma of the gastric cardia in our prospective case recruitment, ie, the study classification, of which 529 (70%) were adenocarcinomas. Among the patients with esophageal cancer, 32% had adenocarcinoma, and the remaining 68% had squamous cell carcinoma. Among all adenocarcinomas, the gastric cardia (59%) was a more common site than the esophagus (41%). The age and sex distribution for the 3 studied tumors is presented in Table 1. The median age at diagnosis was between 67 and 68 years in each of the 3 tumor categories, and no important difference in age distribution was found between men and women. The male-to-female ratio was 6:1 for both adenocarcinoma of the esophagus and gastric cardia, and 3.5:1 for squamous cell carcinoma of the esophagus. The age and sex distribution among cases with diagnoses in disagreement between the 2 classifications was similar compared with all cases (Table 1) and with cases having their classification in agreement (data not shown).
TABLE 1. The Age and Sex Distribution Among the Cases of Adenocarcinoma of the Esophagus, Adenocarcinoma of the Gastric Cardia, and Squamous Cell Carcinoma of the Esophagus in Sweden: 1995–1997
Overall Completeness of the Swedish Cancer Register
Every cancer (100%) of the esophagus or cardia was histologically verified in the Swedish Cancer Register (as well as in the study classification) during the study period. Of the 757 patients identified in our study classification, 744 were also registered in the Cancer Register, rendering an overall completeness of 98.3% of the Register. There was, however, a considerable level of mismatch between the study classification and the Cancer Register with regard to the more precise tumor classification, and regarding adenocarcinomas in particular, as presented in Figures 1 to 3.
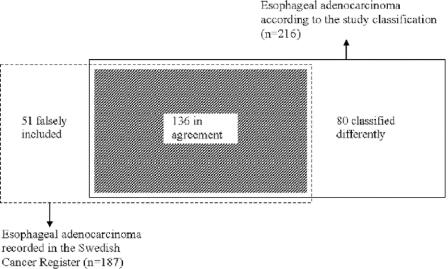
FIGURE 1. Venn diagram demonstrating concordance between incidence of esophageal adenocarcinoma registered in the Swedish Cancer Register, in 1995 through 1997, and that collected in the study classification during the same period.
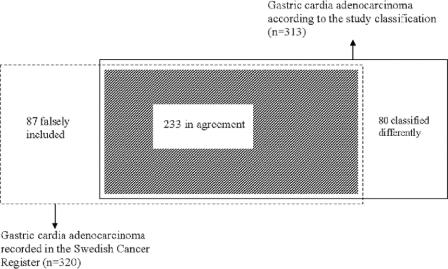
FIGURE 2. Venn diagram demonstrating concordance between incidence of gastric cardia adenocarcinoma registered in the Swedish Cancer Register, in 1995 through 1997, and that collected in the study classification during the same period.
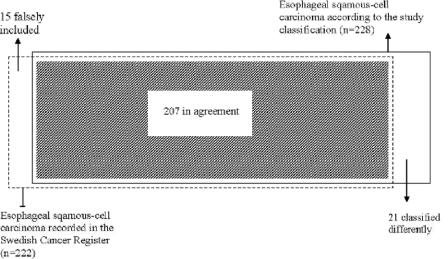
FIGURE 3. Venn diagram demonstrating concordance between incidence of esophageal squamous cell carcinoma registered in the Swedish Cancer Register, in 1995 through 1997, and that collected in the study classification during the same period.
Completeness of Esophageal Adenocarcinoma Classification
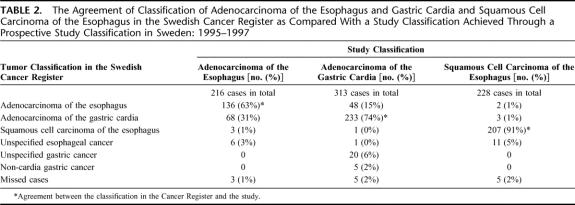
In total, 216 cases of esophageal adenocarcinoma were identified in the study classification, while in the Cancer Register, 187 esophageal adenocarcinomas were recorded, rendering a 16% higher incidence in the study classification. The agreement between the study classification and the Cancer Register is presented in the Venn diagram in Figure 1. Agreement on both site and histologic type classification was found in 136 of the esophageal adenocarcinomas, rendering a completeness of the Cancer Register of 63% (136 of 216). There were 80 patients with esophageal adenocarcinoma according to the study classification that were classified differently in the Register (Table 1). The positive predictive value of a diagnosis of esophageal adenocarcinoma in the Cancer Register was 73% (136 of 187), hence, the rate of false inclusion was 27% (51 of 187). There were no differences between patients classified in agreement and patients classified in disagreement with regard to sex or age (Table 1). A majority of the cases classified in disagreement (n = 68) had a false diagnosis of gastric cardia adenocarcinoma (Table 2). Only 9 patients with esophageal adenocarcinoma according to the study classification were classified as esophageal squamous cell carcinoma or unspecified esophageal tumor in the Register (Table 2).
TABLE 2. The Agreement of Classification of Adenocarcinoma of the Esophagus and Gastric Cardia and Squamous Cell Carcinoma of the Esophagus in the Swedish Cancer Register as Compared With a Study Classification Achieved Through a Prospective Study Classification in Sweden: 1995–1997
Completeness of Gastric Cardia Adenocarcinoma Classification
A total of 313 patients with a diagnosis of cardia adenocarcinoma were observed in the study classification, while 320 patients were registered in the Cancer Register, which indicates a 2% lower incidence in the study classification. The agreement in classification of cardia cancer is presented in Figure 2. In the Cancer Register, the diagnoses in 233 patients were in agreement with the study classification rendering a completeness of 74% (233 of 313), while 80 patients with gastric cardia adenocarcinoma in the study classification were classified differently in the Register. The positive predictive value for cardia adenocarcinoma in the Cancer Register was 73% (233 of 320). Among cases with diagnoses in disagreement, no difference between sexes was found, but they were on average somewhat older than cases with diagnoses in agreement (Table 1). Disparate classification as adenocarcinoma of the esophagus (48 cases), unspecified gastric cancer (20 cases), or noncardia gastric cancer (5 cases) in the Register dominated, while classification as esophageal squamous cell carcinoma or unspecified esophageal cancer was rare (Table 2).
Completeness of Esophageal Squamous Cell Carcinoma Classification
There were 228 patients with esophageal squamous cell carcinoma in the study classification, representing all eligible cases occurring in the study base born on even dates. The corresponding number in the Cancer Register was 222, rendering a 3% higher incidence in the study classification. In Figure 3, the differences between the Cancer Register and the study classification are presented. Compared with the adenocarcinomas, disparate classification of squamous cell carcinoma was rare, the completeness of the Register was 91% (207 of 228). The patients classified in agreement were 207, rendering a positive predictive value of 93% (207 of 222) in the Cancer Register. Among patients with disagreeing classifications, women and older persons were somewhat overrepresented (Table 1). Among the 21 patients with a different classification in the Register, 16 were classified as adenocarcinomas or esophageal cancer of unspecified histologic type (Table 2).
DISCUSSION
Our study identifies a disparity in the tumor classification of adenocarcinomas of the esophagus and gastric cardia between our study classification and the classification in the Swedish Cancer Register, based on the routine clinical setting. Compared with the adenocarcinomas, the level of agreement of classification of squamous cell carcinoma in the esophagus was high.
To evaluate the accuracy and degree of misclassification of diagnostic tests or methods, there is a need for a gold standard, representing an ideal truth, for comparison. We compared the tumor classification made in routine clinical practice with our thorough, uniform, near-complete, and prospective study classification. Yet, a problem with our study is the uncertainty of the accuracy of our study classification. The anatomy may be deranged, and it may be difficult to identify the gastroesophageal junction or to obtain valid measures of distances from this junction. Furthermore, there are no natural lines of division between adenocarcinoma of the esophagus, gastric cardia, and gastric fundus and there is no consensus on the tumor classification.19–21 Cancer Registers are usually based on hospital records, which currently use ICD-O subsite classification.21 ICD-O has limitations when classifying tumors in the proximity of the gastroesophageal junction, for example, by using 2 alternate subsite classifications for esophageal carcinoma and lack of definition for distal border of the gastric cardia,21 resulting in a misclassification of up to 15% of carcinomas in the proximity of the gastroesophageal junction.22 In the present study, however, we did an extensive nationwide effort to obtain the best possible classification for research purposes. We used the limits for cardia adenocarcinoma 3 cm below and 2 cm above the gastroesophageal junction, which is an arbitrary definition that cannot be viewed as better than other definitions. It is, however, distinct and we followed this definition strictly when classifying all tumors. On the other hand, the classification in the Cancer Register emanates from diagnoses set by a large number of clinicians and pathologists, experienced and inexperienced, throughout the nation. Therefore, there are good reasons to assume that our study classification was more accurate and standardized than the diagnoses in the Cancer Register. The use of more conservative measures of the gastric cardia could to a limited extent influence our results. In another study regarding cardia cancer classification from our department, the limits 2 cm below and 1 cm above the gastroesophageal border were used.18 Such a definition in the present study would increase the subsite completeness of adenocarcinomas in both the esophagus and cardia in the Cancer Register but also render a higher rate of classifying esophageal adenocarcinoma as cardia adenocarcinoma in the Register. Our definition of the borders of the gastric cardia meant that a few squamous cell carcinomas were found within these borders. We do not suggest that these tumors arise from the gastric cardia mucosa but originate from squamous cell epithelium of the most distal part of the esophagus, why these tumors were, in accordance with the predetermined study protocol, defined as located in the esophagus. Since these tumors were few (<5%), they represent an acceptable size of margin of error.
Despite our finding of a considerable disagreement of tumor classification among adenocarcinomas located near the gastroesophageal junction, the reported incidence rates seem to have been close to the true rates in Sweden, mainly due to high rates of falsely included cases. Our results indicate that the true incidence of esophageal adenocarcinoma might be 16% higher than recorded in the Swedish Cancer Register, while the true incidence of both cardia cancer and esophageal squamous cell carcinoma is similar to the rates in the Cancer Register.
Several factors involving the tumor classification could have a bearing on reports of increasing incidence rates of adenocarcinomas in the gastroesophageal junction. Apart from the last decade's great diagnostic progress in endoscopy and radiologic imaging, the recent attention regarding these tumors could have an influence on the clinicians’ and pathologists’ diagnostic awareness. Moreover, the lack of consensus regarding the borders of these tumors renders disparity in the classification.21–23 We estimated the influence of the level of disagreement of classification found in the present study on the incidence trends in Sweden. According to the completeness of the Cancer Register and the rate of false inclusion, the true incidence rate of esophageal adenocarcinoma can be 59% (1/completeness) higher or 27% (rate of false inclusion) lower than the reported rates from the Register. According to the data from the Register, the male incidence rate of esophageal adenocarcinoma was 0.5 of 100,000 person-years during 1964 to 1969, while this rate increased to 2.0 of 100,000 person-years during 1995 to 1999. Even under the extreme assumptions, ie, if in the early period the frequency of missed cases of esophageal adenocarcinoma was as high as 37% with no false inclusion, and in the latest period the false inclusion rate was as high as 27% with no missed cases, the estimated incidence rates will be 0.8 of 100,000 person-years during 1960 to 1964 and 1.5 of 100,000 person-years during 1995 to 1999. Thus, the observed increasing incidence of esophageal adenocarcinoma in Sweden should not be entirely explained by differences in tumor classification.
Our study shows that disparities in the classification of tumors in the gastroesophageal region were mostly due to discrepancies in the anatomic subsite classification, while the agreement of histologic diagnosis was good. The disagreement of classification between adenocarcinomas located in the gastric cardia or esophagus seems to be present in both directions. This observation should not be compelled to Swedish circumstances only but be a general problem. Disparities in classification of esophageal and cardia adenocarcinomas could be of importance mainly for 2 reasons. First, it could influence the results of any research, eg, etiologic or therapeutic, of esophageal and cardia adenocarcinoma that are based on the tumor classification of cancer registers. Second, because studies of the incidence of esophageal and cardia cancer, including Swedish studies, used data from the population-based cancer registers,1–10 differences in tumor classification among clinicians could distort the results.
Some investigators argue that adenocarcinomas in the gastroesophageal region do not differ, ie, between a location in the esophagus or gastric cardia, but represent the same disease and with regard to the difficulties in specifying their location, they should therefore be classified as one entity.19,24,25 Others stress marked differences in sex distribution, association with Barrett esophagus, tumor grading, tumor growth pattern and stage distribution, and consequently advocate separate classes.17 Population-based data support differences between the subsite classes with regard to geographic and ethnic distribution26–28 and incidence trends.9,29 In addition, recent studies,11,12,14–16,26,30,31 including results from the present case-control study,11,12,30,31 have revealed distinct differences in the risk factor profiles of esophageal adenocarcinoma and gastric cardia adenocarcinoma. Possibly, true adenocarcinoma of the gastric cardia, originating from the cardia-specific epithelium, is rare, and the adenocarcinomas classified as of cardia origin are a mixture of the 2 separate entities of esophageal and gastric adenocarcinoma. Further research that uses separate entities is required to confirm or refute these apparent discrepancies in epidemiology and risk factors of these tumors. In addition, for a surgeon, patients with tumors in the vicinity of the gastroesophageal junction represent a specific challenge. Consensus in the optimal surgical approach to treat these patients is lacking and the patient could be subjected to different types of resections. Again, further research of the therapeutic effects should use separate classification of the adenocarcinomas near the gastroesophageal junction. The most reasonable step forward is by accepting a uniform method of classifying the anatomic subsites of these tumors, a classification that carries weight and support in the clinical setting20 and evaluates any evolving differences.
CONCLUSION
Our study reveals a substantial degree of mismatch between adenocarcinoma of the esophagus and gastric cardia. In etiologic and therapeutic research, this problem is of importance and needs careful consideration because these 2 tumors may have distinct risk factor profiles and could be subjected to different treatment strategies. The previously reported increasing incidence rate of esophageal adenocarcinoma does not seem to be explained by the level of disagreement of tumor classification in Sweden, however.
ACKNOWLEDGMENTS
The authors thank Leila Nyrén for coordination of the study and for the collaboration with Lotti Barlow at the Swedish Cancer Register.
Footnotes
Supported by the Swedish Cancer Society.
Reprints: Mats Lindblad, MD, PhD, Department of Surgery, P9:03, Karolinska University Hospital, Solna, SE-171 76 Stockholm, Sweden. E-mail: mats.lindblad@karolinska.se.
REFERENCES
- 1.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 2.Hansson LE, Sparen P, Nyren O. Increasing incidence of both major histological types of esophageal carcinomas among men in Sweden. Int J Cancer. 1993;54:402–407. [DOI] [PubMed] [Google Scholar]
- 3.Hansson LE, Sparen P, Nyren O. Increasing incidence of carcinoma of the gastric cardia in Sweden from 1970 to 1985. Br J Surg. 1993;80:374–377. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong RW, Borman B. Trends in incidence rates of adenocarcinoma of the oesophagus and gastric cardia in New Zealand, 1978–1992. Int J Epidemiol. 1996;25:941–947. [DOI] [PubMed] [Google Scholar]
- 5.Hansen S, Wiig JN, Giercksky KE, et al. Esophageal and gastric carcinoma in Norway 1958–1992: incidence time trend variability according to morphological subtypes and organ subsites. Int J Cancer. 1997;71:340–344. [DOI] [PubMed] [Google Scholar]
- 6.Moller H, Jensen OM. Trends in the occurrence of esophageal, cardial and stomach cancer in Denmark 1943–1982: Neoplasm statistics No. 19. Ugeskr Laeger. 1987;149:1904–1909. [PubMed] [Google Scholar]
- 7.Powell J, McConkey CC. The rising trend in oesophageal adenocarcinoma and gastric cardia. Eur J Cancer Prev. 1992;1:265–269. [DOI] [PubMed] [Google Scholar]
- 8.Bollschweiler E, Wolfgarten E, Gutschow C, et al. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–555. [DOI] [PubMed] [Google Scholar]
- 9.Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 10.Walther C, Zilling T, Perfekt R, Moller T. Increasing prevalence of adenocarcinoma of the oesophagus and gastro-oesophageal junction: a study of the Swedish population between 1970 and 1997. Eur J Surg. 2001;167:748–757. [DOI] [PubMed] [Google Scholar]
- 11.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. [DOI] [PubMed] [Google Scholar]
- 12.Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–890. [DOI] [PubMed] [Google Scholar]
- 13.Centre for Epidemiology, National Board of Health and Welfare. Cancer Incidence in Sweden 1997: Statistics—Health and Diseases. Sweden: Centre for Epidemiology, National Board of Health and Welfare, 1999:8. [Google Scholar]
- 14.Farrow DC, Vaughan TL, Sweeney C, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–238. [DOI] [PubMed] [Google Scholar]
- 15.Gammon MD, Schoenberg JB, Ahsan H, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–1284. [DOI] [PubMed] [Google Scholar]
- 16.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer Causes Control. 2001;12:721–732. [DOI] [PubMed] [Google Scholar]
- 17.Siewert JR, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekstrom AM, Signorello LB, Hansson LE, et al. Evaluating gastric cancer misclassification: a potential explanation for the rise in cardia cancer incidence. J Natl Cancer Inst. 1999;91:786–790. [DOI] [PubMed] [Google Scholar]
- 19.Dolan K, Morris AI, Gosney JR, et al. Three different subsite classification systems for carcinomas in the proximity of the GEJ, but is it all one disease? J Gastroenterol Hepatol. 2004;19:24–30. [DOI] [PubMed] [Google Scholar]
- 20.Siewert JR, Stein HJ. Carcinoma of the cardia. Carcinoma of the gastroesophageal junction: classification, pathology and extent of resection. Dis Esophagus. 1996;9:173–182. [Google Scholar]
- 21.Percy C, Holten VV, Muir C. International Classification of Diseases for Oncology, 2nd ed. Geneva: World Health Organization, 1990. [Google Scholar]
- 22.Dolan K, Sutton R, Walker SJ, et al. New classification of oesophageal and gastric carcinomas derived from changing patterns in epidemiology. Br J Cancer. 1999;80:834–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siewert JR, Holscher AH, Becker K, et al. Cardia cancer: attempt at a therapeutically relevant classification. Chirurg. 1987;58:25–32. [PubMed] [Google Scholar]
- 24.Sihvo EI, Salminen JT, Ramo OJ, et al. The epidemiology of oesophageal adenocarcinoma: has the cancer of gastric cardia an influence on the rising incidence of oesophageal adenocarcinoma? Scand J Gastroenterol. 2000;35:1082–1086. [DOI] [PubMed] [Google Scholar]
- 25.Wijnhoven BP, Siersema PD, Hop WC, et al. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity: Rotterdam Oesophageal Tumour Study Group. Br J Surg. 1999;86:529–535. [DOI] [PubMed] [Google Scholar]
- 26.Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30:1415–1425. [DOI] [PubMed] [Google Scholar]
- 27.Kubo A, Corley DA. Marked regional variation in adenocarcinomas of the esophagus and the gastric cardia in the United States. Cancer. 2002;95:2096–2102. [DOI] [PubMed] [Google Scholar]
- 28.Kubo A, Corley DA. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol. 2004;99:582–588. [DOI] [PubMed] [Google Scholar]
- 29.Corley DA, Kubo A. Influence of site classification on cancer incidence rates: an analysis of gastric cardia carcinomas. J Natl Cancer Inst. 2004;96:1383–1387. [DOI] [PubMed] [Google Scholar]
- 30.Lagergren J, Bergstrom R, Lindgren A, et al. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer. 2000;85:340–346. [PubMed] [Google Scholar]
- 31.Ye W, Held M, Lagergren J, et al. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst. 2004;96:388–396. [DOI] [PubMed] [Google Scholar]