Abstract
Objective:
To evaluate the contribution of the liver to total circulatory reticuloendothelial system (RES) phagocytosis capacity in patients undergoing liver resection and to compare it with values in end-stage chronic liver disease.
Summary Background Data:
The mechanism whereby major liver resection is associated with a high incidence of infection is unknown. Significant impairment of RES phagocytosis has been described in liver failure, rendering such patients susceptible to infection; and we hypothesized that similar impairment might occur following major liver resection.
Methods:
A prospective study was conducted in which 99mTc-albumin microspheres blood clearance served as a parameter for RES phagocytosis and was studied together with indocyanine green blood clearance, actual liver volume measured by three-dimensional image analysis, and a clinical score of hepatic dysfunction in 17 patients undergoing liver resection and in 8 patients with end-stage chronic liver disease assessed for liver transplantation.
Results:
When expressed relative to volume unit of residual liver, microspheres clearance increased significantly in the immediate postoperative period (day 1) following major (0.009% versus 0.022% min−1mL−1, P < 0.001), but not minor liver resection. In contrast, the absolute rate of microsphere clearance decreased following major resection (15% min−1 versus 10% min−1, P < 0.001) and was comparable with the rate observed in end-stage chronic liver disease (9% min−1). This decrease in circulatory microspheres clearance after resection paralleled a decrease in indocyanine green clearance (R2 = 0.511, P = 0.006), and there was a trend for those with moderate liver dysfunction to have lower microspheres clearance rates (P = 0.068).
Conclusion:
Preservation of a minimum volume of functioning liver is a prerequisite for adequate RES phagocytosis capacity, and failure of this system may predispose patients undergoing major liver resection to infection as observed in clinical studies.
This study investigated whether the ability of the reticuloendothelial system to clear 99Tc-albumin microspheres from the circulation was compromised by major liver resection. Reticuloendothelial cell system clearance was increased per unit volume of residual liver; however, total clearance was significantly reduced and was comparable with that of patients with end-stage chronic liver disease.
The liver contains the largest number of fixed tissue macrophages (Kupffer cells) and as part of the reticuloendothelial cell system (RES) plays an important role in the body's innate immune defense.1,2 Bacterial infections such as catheter-related septicemia and spontaneous bacterial peritonitis are common in patients with acute and chronic liver failure supporting the assumption that the hepatic RES phagocytosis function is important to prevent the spread of microorganisms and its products into systemic circulation.3,4 Liver surgery is now performed with increasing safety, yet postoperative morbidity still occurs in up to 40% of patients undergoing major liver resection, with infection-related complications accounting for half of such complications.5 The risk of postoperative infection significantly increases with the extent of liver resection. It is about 2 times higher after standard and 4 times higher after extended liver resection compared with minor resection.6
There is preliminary evidence from animal models that major liver resection results in a measurable deficit in RES phagocytosis capacity, thereby rendering the body susceptible to infection.2,7,8 However, the effect of liver volume on the body's total RES phagocytosis capacity has never been studied in humans. The technique of radiolabeled albumin microspheres has previously been used to study RES phagocytosis capacity.9–11 Once injected into the bloodstream, microspheres undergo phagocytosis by the RES with approximately 90% to 95% of the injected dose being taken up by the liver.12 This technique has, however, never been applied to an intervention model under clinical condition where RES phagocytosis capacity may reach its limits. The aim of the present study was to investigate the response of total circulatory RES phagocytosis capacity to liver resection of various extents and to compare it with phagocytosis capacity in patients with end-stage chronic liver disease.
From these results, we wanted to determine the contribution of hepatic RES to body's total phagocytosis capacity and its relation to liver volume.
PATIENTS AND METHODS
Patients and Study Protocol
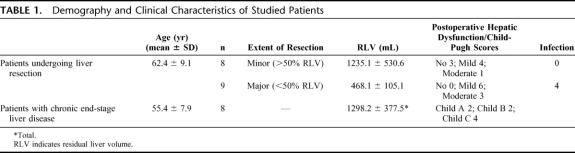
RES phagocytosis capacity was studied in 17 patients (10 male, 7 female), who were admitted to the Hepatobiliary Unit, Royal Infirmary Edinburgh for liver resection (Table 1). The indications for surgery were colorectal cancer liver metastases in 14 patients and hepatic metastases of gastrointestinal stroma cell tumor, hepatocellular carcinoma, and intrahepatic cholangiocarcinoma in 1 patient each. RES phagocytosis capacity was also studied in 8 patients (5 male, 3 female) with end-stage chronic liver disease admitted for liver transplantation assessment (Table 1). The cause of cirrhosis was alcoholic liver disease in 4 patients and primary biliary cirrhosis and cryptogenic cirrhosis in 2 patients each. The study was approved by the Lothian Research Ethics Committee (LREC/2002/1/31) and by the Administration of Radioactive Substances Advisory Committee (RPC 577-3256 17548). All patients gave written informed consent. Selection of patients for surgery and the technique of hepatic resection have been described previously.13 CT angio-portography was performed some weeks before admission in all patients undergoing liver resection as part of preoperative staging. Patients were admitted the day before surgery for RES phagocytosis and liver function studies and routine blood tests (Fig. 1). Preoperative total body scintigraphy was performed in the first 10 patients undergoing liver resection to study the body distribution of microspheres. All measurements except total body scintigraphy were repeated on the day after surgery (Fig. 1). None of the patients undergoing liver resection had any background chronic liver disease, and all had normal preoperative synthetic liver function. In patients with end-stage chronic liver disease, measurements were performed only once at the time of admission for liver transplantation assessment and a contrast-enhanced CT scan without angio-portography was undertaken at that time.
TABLE 1. Demography and Clinical Characteristics of Studied Patients
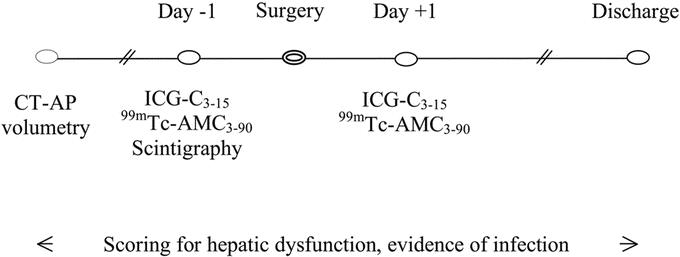
FIGURE 1. Study protocol for patients undergoing liver resection.
Production of 99mTc-Albumin Microspheres and Measurement of Blood Clearance (99mTc-AMC)
Albumin microspheres were produced according to a method described by Iio and Wagner with some modifications.10 Briefly, human albumin 4.5% (Scottish National Blood Transfusion Service, Edinburgh, UK) was diluted to a concentration of 3% with sodium chloride 0.9% for injection. The pH was adjusted to 10 by adding sodium hydroxide 1 mol/L, and the solution was heated in a water bath at 70°C under continuous stirring for 20 minutes. The temperature of the water bath was raised to 75°C. Samples were withdrawn for measurement of light absorbance at 525 nm at the beginning of the heating process and at 2-minute intervals. The bottle was removed from the water bath when the absorbance of the solution had increased by 0.025 ± 0.005 relative units from baseline and placed into another water bath at room temperature under continuous stirring. Hydrochloric acid 1 mol/L was added to adjust its pH to 7.5. Under aseptic conditions, 20-mL aliquots of the albumin microspheres suspension were filtered through sterile 0.22-μm Millex GS filters (Millipore UK Ltd, Watford, UK) into sterile vials and stored at 2°C to 8°C. On the day of measurement, the 99mTc-albumin microspheres injection for administration to the patient was prepared by adding 1 mL 99mTc-Nanocoll Injection (15 MBq/mL) (GE Healthcare, Amersham PLC, Little Chalfont, UK) to a vial containing 20-mL albumin microspheres suspension. A bolus of 99mTc-albumin microspheres was administered into the patient's forearm vein at a dose of 5 mg albumin/kg body weight (0.18 mL suspension/kg body weight).
Blood samples were taken before injection and at 3-minute intervals up to 30 minutes, at 10-minute intervals up to 60 minutes, and at 30-minute intervals up to 90 minutes after injection from the opposite arm. Sample tubes were weighed before and after blood collection to calculate the exact sample volume. Sample radioactivity was measured in a Cobra II Auto-Gamma counter (Perkin-Elmer Packard, Global Medical Instrumentation, Inc., Albertville, MN) immediately after blood collection, and values were expressed as counts per minute per sample weight (cpm/g). 99mTc-AMC was expressed as the percentage of radioactivity at each time point compared with radioactivity at 3 minutes. 99mTc-AMC was also related to actual liver volume and expressed as percent clearance per volume unit liver.
99mTc-Albumin Microspheres Body Distribution
The body distribution of 99mTc-albumin microspheres was visualized in patients undergoing liver resection using a GE Millennium VG gamma camera (GE Healthcare, UK) with an acquisition time of 12 minutes on a 256 × 256 matrix anterior-posterior view starting 30 minutes after microspheres injection. Preoperative scintigraphy showed that 95% of 99mTc-albumin is localized in the liver, whereas spleen, bone marrow, and lung together accounted for the residual 5% of radioactivity (Fig. 2).
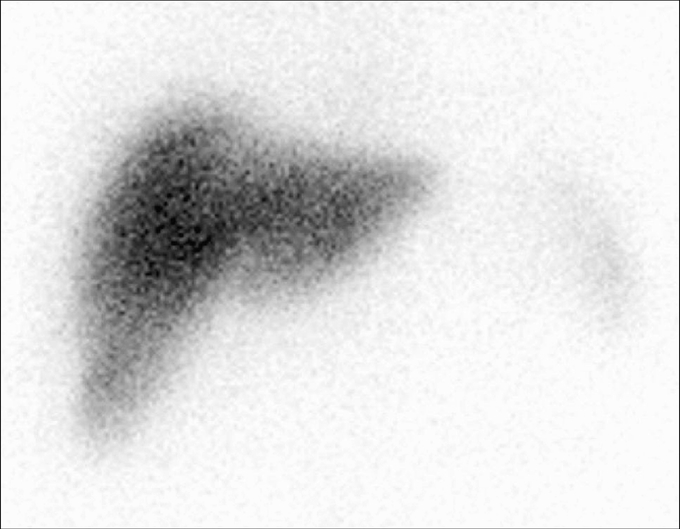
FIGURE 2. Preoperative 99mTc scintigraphy 30 minutes after albumin microspheres injection.
Immunohistochemistry of Albumin Microspheres Phagocytosis by Mouse Hepatic Macrophages
Albumin microspheres uptake by the liver was initially studied by immunohistochemistry in Balb/C mice. Liver tissue was retrieved and fixed in 2% formaldehyde from control mice and from animals 30 minutes after injection of 100 μL unlabeled human albumin microspheres into the tail vein. To identify tissue macrophages in the liver, sections were probed with rat antimouse F4/80 macrophage-specific primary antibody (Serotec Ltd., Kiddlington, UK) 1:500. Sections were probed with mouse antihuman albumin monoclonal antibody clone HSA111 (Sigma-Aldrich Company Ltd., Dorset, UK) 1:500. The secondary antibody was goat antimouse IgG fluorescein isothiocyanate (FITC) conjugated F(ab′)2 fragments (Molecular Probes, Invitrogen, Paisley, UK) 1:5000. Nuclei were counterstained with Hoechst 33258 (Molecular Probes, Invitrogen, Paisley, UK). Slides were viewed using a Leica DM IRB inverted fluorescent microscope or bright field as appropriate. Merged images were performed under identical exposure conditions using Leica FW4000 v 1.0.2 software (Leica Microsystems Milton Keynes, UK). Immunohistochemistry confirmed that 30 minutes after peripheral injection albumin microspheres are found almost exclusively in the liver within hepatic macrophages (Fig. 3).
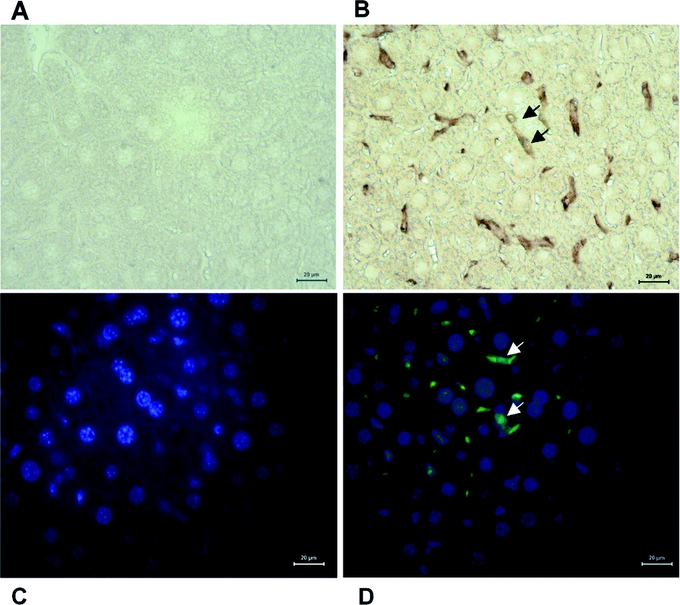
FIGURE 3. Immunohistochemistry studies of human albumin microspheres uptake by mouse liver. Liver section staining with nonspecific antibody (A) and macrophage specific F480 antibody (brown, arrows) (B). Liver section staining with anti human albumin fluorescein isothiocyanate (FITC) conjugated antibody (green) and Hoechst 33258 (blue staining of cell nuclei) showed no FITC fluorescence in a control mouse liver (C). Mouse liver section 30 minutes after microspheres injection demonstrates FITC (green) colored albumin microspheres within liver macrophages located between hepatocytes (D, white arrows).
Indocyanine Green Blood Clearance (ICG-C3–15)
Indocyanine green blood clearance was determined after peripheral venous bolus injection of 0.5 mg/kg body weight ICG. Blood samples were taken before and 3 and 15 minutes after injection and extinction was determined spectrophotometrically at 605 nm. The reduction in extinction from 3- to 15-minute sample was expressed in relative values (ICG-C3–15, %).
Intraoperative Measurement of Liver Blood Flow
Hepatic blood flow was measured in 6 patients undergoing major liver resection, following dissection of the common hepatic artery and main portal vein, using 6- to 8-mm and 12- to 14-mm handle ultrasonic flow probes, respectively (Transonic Systems, Kimal PLC, Uxbridge, UK). Traces and flow measurements were recorded on a dual-channel HT323 flowmeter (Transonic Systems). Measurements were taken before liver resection and at the end of the operation not less than 30 minutes after ligation of one or other main hepatic artery and portal vein branch.
Liver Volumetry
Liver volumetry was performed using a spiral multislice CT Toshiba Aquilion 16 scanner (Toshiba Medical Systems, CA) and Analyze 5.0 medical image analysis software (Analyze Direct, Inc; Biomedical Imaging Resource, Mayo Foundation, Rochester, MN) according to a previously described protocol.6,13 In patients undergoing surgery, total functional liver volume (TFLV) was calculated by subtracting tumor volume from total liver volume and residual liver volume (RLV) was measured after virtual resection. The extent of liver resection was grouped into major (RLV <50% of TFLV) and minor resection (RLV >50% of TFLV). In patients with end-stage chronic liver disease, total liver volume only was measured.
Definition of Hepatic Dysfunction and Infection
The definition of postoperative hepatic dysfunction was based on serum concentration of total bilirubin μmol/L as follows: <20 = 0 points; 21–60 = 1 point; and >60 = 2 points; serum lactate mmol/L, <1.5 = 0 points; 1.6–3.5 = 1 point; and >3.5 = 2 points; prothrombin time seconds above normal <4 = 0 points; 4–6 = 1 point; and >6 = 2 points and signs of encephalopathy and categorized into 4 grades: none = 0 points; mild or moderate = 1 point; and severe = 2 points.6 Parameters were taken into account only when present for 2 consecutive observations within a 48-hour period. The severity of hepatic dysfunction was then graded using the sum of all scores as: 0 points = no dysfunction; 1–2 points = mild; 3–4 = moderate; and >4 severe hepatic dysfunction. In patients with chronic liver disease, the Child-Pugh classification was used to describe the severity of hepatic dysfunction.14 Clinically significant infections only were taken into account and defined as coincidence of positive microbial culture together with either local or general symptoms of inflammation. In patients undergoing liver resection, the presence of hepatic dysfunction and infection was noted from the time of admission until discharge.
Statistical Analysis
Statistical analysis was performed using SPSS 11.0 statistical analysis software (SPSS Inc., Chicago, IL). The area under the radioactivity clearance curve of 99mTc-albumin microspheres was calculated between 3 minutes and 30 minutes after microspheres injection and expressed as % clearance over time (AUC3–30).15 Normal distribution of 99mTc-AMC AUC3–30 and ICG-C3–15 was confirmed by Normal Q-Q plots and Kolmogorov-Smirnow statistics. Preoperative and postoperative values were compared using the paired-samples t test. Independent-samples t test was used to compare postoperative values between patients with different extent of liver resection and with values from patients with end-stage chronic liver disease. For patients with different severity of hepatic dysfunction, one-way analysis of variance was performed. Regression analysis was undertaken to evaluate the correlation between 99mTc-AMC AUC3–30, ICG-C3–15, and RLV.
RESULTS
Extent of Liver Resection, Residual Liver Volume, Hepatic Dysfunction and Infection
Minor liver resection (>50% RLV) was performed in 8 of 17 (47%) patients, leaving a mean ± SD RLV of 1236 ± 531 mL; whereas 9 (53%) patients underwent major resection (<50% RLV), leaving a mean ± SD RLV of 468 ± 105 mL. Moderate hepatic dysfunction developed in 1 of 8 (12.5%) patients after minor resection and in 3 of 9 (33.3%) patients after major resection (Table 1). None of the patients after minor resection but 4 of 9 (44%) patients after major resection developed postoperative infection (Table 1). The 4 patients with infection each had a culture proven chest infection; in addition, on the basis of positive blood cultures and appropriate other cultures, it was confirmed that 2 also had urine infections, 1 had a wound infection, and 1 had an intra-abdominal infected collection. The organisms grown included methicillin-resistant and nonresistant Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, and Bacteroides species. The mean ± SD TLV in patients with chronic liver disease was 1298 ± 378 mL. Two of these 8 (25%) patients had Child-Pugh grade A, 2 (25%) grade B, and 4 (50%) patients grade C chronic hepatic dysfunction (Table 1).
Liver Blood Flow Measurements
The mean ± SD total portal blood flow before liver resection was 925 ± 120 mL/min and after resection 828 ± 284 mL/min (P = 0.42, 2-tailed paired t test). The mean ± SD total hepatic artery flow before liver resection was 404 ± 252 mL/min compared with 216 ± 164 mL/min post resection, a significant reduction in total hepatic arterial flow (P < 0.02, 2-tailed paired t test). Taking account of the reduction liver volume associated with liver resection, mean ± SD portal blood flow per gram of liver tissue was 0.62 ± 0.08 mL/min before resection compared with 1.18 ± 0.41 mL/min, a significant increase in flow (P < 0.03, Wilcoxon Sign Rank test). By contrast, hepatic artery flow per gram of liver tissue remained unchanged before and after liver resection: 0.35 ± 0.18 mL/min compared with 0.33 ± 0.17 mL/min, respectively.
99mTc-Albumin Microspheres Clearance in Patients Undergoing Liver Resection
99mTc-AMC decreased both after minor and major resection compared with preoperative values (Fig. 4A, B). The mean (±SD) 99mTc-AMC AUC3–30 decreased from 13.7 ± 0.7% × minutes to 12.9 ± 1.1% × minutes after minor resection (paired-samples t test; P = 0.032) and from 14.7 ± 0.9% × minutes to 10.0 ± 1.0% × minutes after major resection (paired-samples t test; P < 0.001) (Fig. 4C). Postoperative 99mTc-AMC AUC3–30 significantly correlated with RLV (R2 = 0.690, P < 0.001) (Fig. 5). At the same time, a significant increase of 99mTc-AMC AUC3–30 per milliliter liver volume from 0.009% ± 0.002% × minutes/mL to 0.022% ± 0.005% × minutes/mL (P < 0.001) following major resection was found, whereas this was not the case after minor resection (P = 0.095) (Fig. 6). 99mTc-AMC AUC3–30 per volume unit of residual liver increased exponentially in patients with smaller RLV (R2 = 0.945, P < 0.001) (Fig. 7).
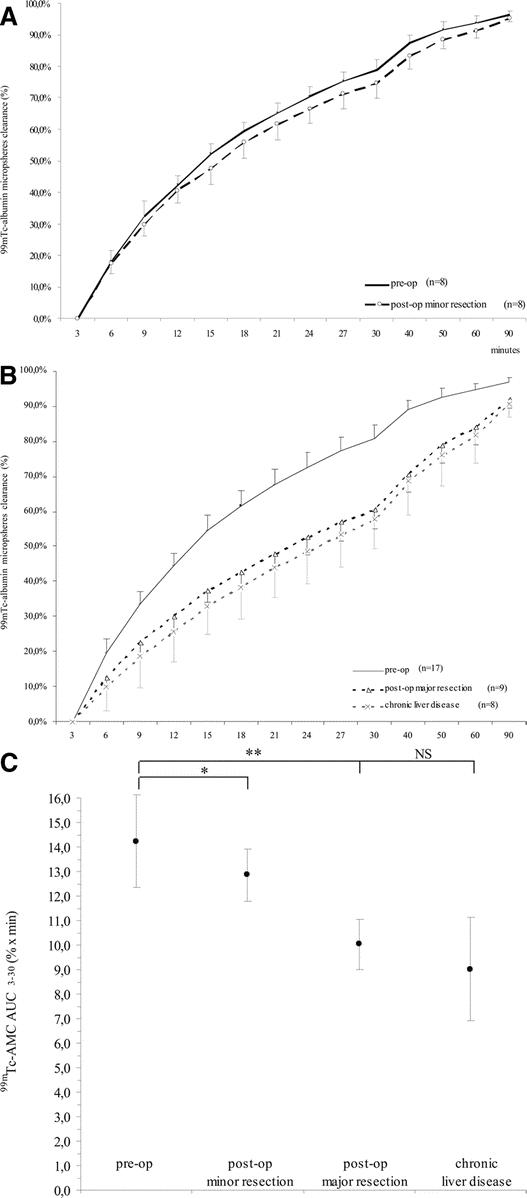
FIGURE 4. Preoperative and postoperative 99mTc-albumin microspheres clearance (99mTc-AMC) in patients undergoing liver resection and in patients with end-stage chronic liver disease. Preoperative and postoperative 99mTc-AMC in patients undergoing minor liver resection (A) and in patients undergoing major liver resection (B) compared with patients with end-stage chronic liver disease. C, Preoperative and postoperative 99mTc-AMC AUC3–30 values (% × min) in patients undergoing liver resection compared with patients with end-stage chronic liver disease. Paired samples t test: *P = 0.032 and **P < 0.001; independent samples t test: P = not significant.
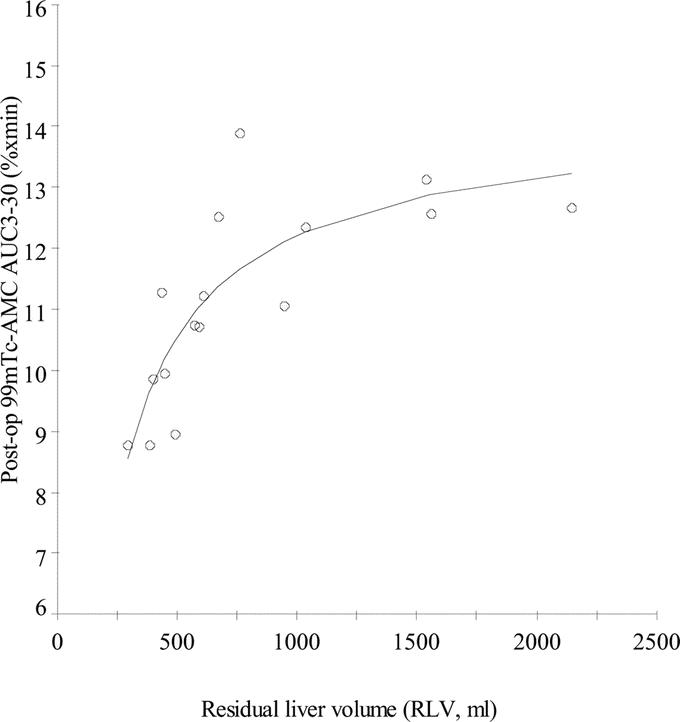
FIGURE 5. S-shape correlation analysis between postoperative 99mTc-AMC AUC3–30 and RLV (n = 16, R2 = 0.690, P < 0.001).
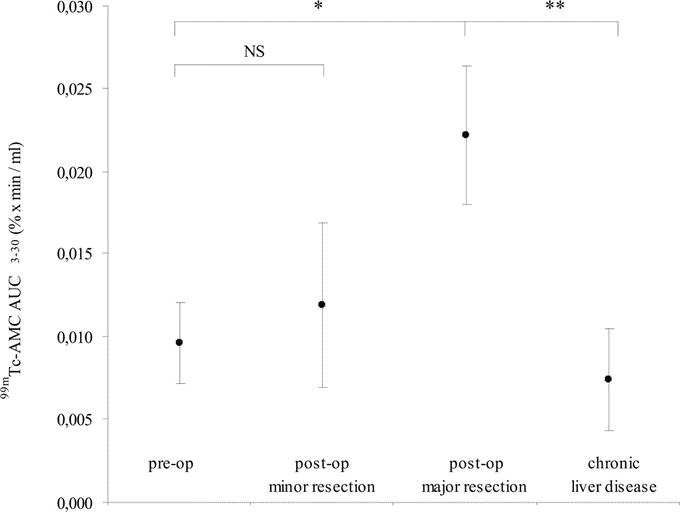
FIGURE 6. Preoperative and postoperative 99mTc-AMC AUC3–30 per milliliter liver volume in patients undergoing liver resection and in patients with end-stage chronic liver disease. Paired samples t test: *P < 0.001; independent samples t test: **P < 0.001.
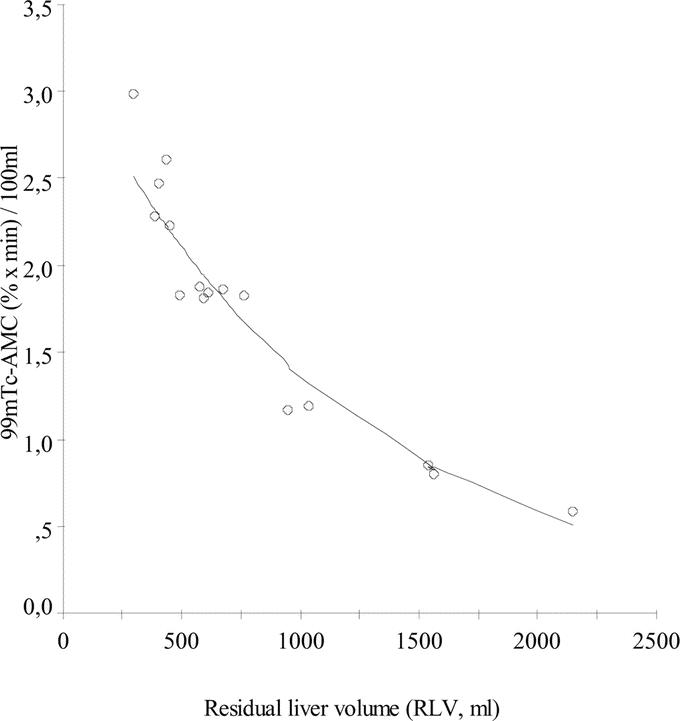
FIGURE 7. Exponential correlation analysis between relative-to-volume 99mTc-AMC AUC3–30 and RLV (n = 16, R2 = 0.945, P < 0.001).
99mTc-Albumin Microspheres Clearance in Patients With End-Stage Chronic Liver Disease
99mTc-AMC AUC3–30 was 9.02% ± 2.10% × minutes in patients with end-stage chronic liver disease. This was significantly less compared with preoperative values of patients undergoing liver resection (P < 0.001) (Fig. 4B, C). However, when 99mTc-AMC AUC3–30 in end-stage chronic liver disease was compared with values after major resection, no significant difference was found (P = 0.214) (Fig. 4C).
Relation Between 99mTc-Albumin Microspheres Clearance and Hepatocellular Function
Preoperative ICG-C3–15 was 90.1% ± 5.1% in patients undergoing liver resection compared with 27.2% ± 7.9% in end-stage chronic liver disease. ICG-C3–15 decreased to 73.1% ± 9.2% after major resection (paired samples t test; P = 0.003) but remained unchanged at 90.2% ± 6.0% after minor resection. A significant linear correlation between 99mTc-AMC AUC3–30 and ICG-C3–15 in patients who underwent resection was found (R2 = 0.511; P = 0.006). Postoperative 99mTc-AMC AUC3–30 was 11.8% ± 1.7% × min in patients with no or mild postoperative hepatic dysfunction compared with 10.0% ± 1.4% × min in patients with moderate hepatic dysfunction (independent samples t test; P = 0.068). Postoperative 99mTc-AMC AUC3–30 was 12.2% ± 2.3% × min in patients who developed infection compared with 10.5% ± 1.2% × min for those who did not (independent samples t test; P = 0.23).
DISCUSSION
In the present study, major liver resection was used as a model to investigate the effect of reduced RES volume on the ability of the body to deal with a microbial challenge. 99mTc labeled albumin microspheres were used to simulate bacterial particles and their clearance from the circulation served as a surrogate marker of RES phagocytosis capacity. Microbial pathogens and albumin microspheres are taken up by identical receptors on the surface of hepatic macrophages, such as macrophage mannose receptors, macrophage scavenger receptors, complement receptors, and Fc immunoglobulin receptors, thus estimating the clearance capacity of microbial pathogens based on microspheres clearance seems reliable.16–19
It was shown in the lymphatic translocation model of endotoxemia that the liver plays an important role in ensuring clearance of bacteria and their products from systemic circulation.20 In the perioperative setting of liver resection, gut bacterial translocation increases the demands on the hepatic RES to keep the systemic circulation relatively sterile.21,22 Our data support the hypothesis that the liver is a major contributor to total body RES phagocytosis capacity and that this is significantly impaired by major liver resection.
The S-shape correlation between total microspheres clearance and RLV highlights the large capacity of the body to cope with a significant reduction in hepatic RES volume following resection. Total microspheres clearance remains relatively stable for a large range of volumes of resected liver. Similarly, microspheres clearance relative to unit volume of residual liver increased exponentially, suggesting the presence of adaptive response mechanisms to compensate for the loss of hepatic RES. However, a sudden breakdown of total circulatory phagocytosis capacity was noted below which microspheres clearance dropped to values comparable with those seen in patients with end-stage chronic liver disease. By extrapolation of absolute and relative clearance curves in relation to liver volume, it becomes clear that adaptive response mechanisms are able to remain microspheres clearance stable for an approximate minimal RLV of 600 mL. Below this critical RLV, total microspheres clearance continuously declines despite an infinitely increasing relative clearance per volume unit residual liver. It would be important to know whether the maintenance of circulatory RES phagocytosis capacity depends on intrahepatic or extrahepatic pathways and whether any of these can be stimulated further to preserve clearance of pathogens in patients undergoing major liver resection.
Liver resection initiates a complex cascade of pro-inflammatory response in the liver, including the expression of cell membrane-bound proteins such as adhesion molecules and phagocytosis receptors as well as the release of cytokines and chemoattractant factors.23,24 Pro-inflammatory stimuli increase the phagocytosis capacity of the individual cell and contribute to the recruitment of peripheral blood leukocytes into the liver.8,25–27 In addition to intrahepatic changes following resection, there is a significant increase in phagocytosis capacity at extrahepatic sites such as spleen, bone marrow, and lung.7,8
The liver synthesizes a large quantity of proteins that provide recognition sites for the innate immune system and are involved in opsonization and neutralization of potentially harmful microorganisms.28–30 An increased production of such proteins is seen in response to surgery, but synthesis is impaired in elderly patients and in patients with liver dysfunction. In addition, liver-specific acute phase protein synthesis is decreased following major resection.28,31,32 Substitution of circulating proteins of the humeral innate immune defense by recombinant products was successful in patients with hereditary deficiency to prevent infection.33,34 The extent to which deficiencies in these proteins resulting from liver surgery contributes to impairment of innate immunity has not been investigated.
We have shown previously that after liver resection a high proportion of patients with postoperative severe hepatic dysfunction developed infections compared with patients with mild hepatic dysfunction.6 In the present study, we found a strong correlation between clearance of albumin microspheres and ICG, supporting the assertion that hepatocellular function and RES phagocytosis are related if only in terms of liver volume. It is known from ICG clearance studies that hepatic clearance is not only a matter of hepatocellular function but depends on liver blood flow.35,36 Analysis of blood flow changes immediately after liver resection showed portal blood flow per volume unit residual liver increased, and hepatic artery flow per volume unit remained constant. These changes are likely to contribute to the adaptive response to liver resection and are important to maintain hepatic clearance and metabolism.37
Logic dictates that liver resection should preserve a minimum amount of functioning RLV likely to contribute to both synthetic liver function and total circulatory phagocytosis capacity, thereby minimizing postoperative liver dysfunction and sepsis. Whether strategies supporting the innate immune response following major liver resection would have benefit in terms of reducing postoperative infection remains to be established.
Footnotes
The study was performed in collaboration with the Wellcome Trust Clinical Research Facility and Image Analysis Core (Edinburgh) and was supported by grants from the Chief Science Office Scotland and Tenovus Scotland. Dr. Schindl was supported by the Austrian Science Fund (grant no. J2147), and Dr. Wigmore was supported by the Wellcome Trust (grant no. 065029).
Reprints: Stephen J. Wigmore, MD, Tissue Injury & Repair Group, MRC Centre for Inflammation Research, Medical School building (6th floor), University of Edinburgh, Teviot Place, Edinburgh EH8 9AG, United Kingdom. E-mail: s.wigmore@ed.ac.uk.
REFERENCES
- 1.Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343:338–344. [DOI] [PubMed] [Google Scholar]
- 2.Hamazaki K, Sato S, Yunoki M, et al. Kupffer cell function in chronic liver injury and after partial hepatectomy. Res Exp Med (Berl). 1994;194:237–246. [DOI] [PubMed] [Google Scholar]
- 3.Navasa M, Rodes J. Bacterial infections in cirrhosis. Liver Int. 2004;24:277–280. [DOI] [PubMed] [Google Scholar]
- 4.Wade J, Rolando N, Philpott-Howard J, et al. Timing and aetiology of bacterial infections in a liver intensive care unit. J Hosp Infect. 2003;53:144–146. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406 ; discussion 406–397. [DOI] [PMC free article] [PubMed]
- 6.Schindl MJ, Redhead DN, Fearon KCH, et al. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Andersson R, Ding J, et al. Reticuloendothelial system function following acute liver failure induced by 90% hepatectomy in the rat. HPB Surg. 1993;6:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arii S, Shibagaki M, Takahashi S, et al. Changes in the reticuloendothelial phagocytic function after partial hepatectomy. J Lab Clin Med. 1985;105:668–672. [PubMed] [Google Scholar]
- 9.Huet PM, Marleau D, Lavoie P, et al. Extraction of 125I-albumin microaggregates from portal blood: an index of functional portal blood supply in cirrhotics. Gastroenterology. 1976;70:74–81. [PubMed] [Google Scholar]
- 10.Iio M, Wagner HN Jr, Scheffel U, et al. Studies of the reticuloendothelial system (RES): I. Measurement of the phagocytic capacity of the RES in man and dog. J Clin Invest. 1963;42:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner HN Jr, Iio M, Hornick RB. Studies of the reticuloendothelial system (RES): II. Changes in the phagocytic capacity of the RES in patients with certain infections. J Clin Invest. 1963;42:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolognesi M, Merkel C, Gatta A. The use of human albumin millimicrospheres tagged with 99mTc in the evaluation of the removal capacity of the reticuloendothelial system. Eur J Nucl Med. 1987;13:254–257. [DOI] [PubMed] [Google Scholar]
- 13.Wigmore SJ, Redhead DN, Yan XJ, et al. Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg. 2001;233:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. [DOI] [PubMed] [Google Scholar]
- 15.Matthews JN, Altman DG, Campbell MJ, et al. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opanasopit P, Shirashi K, Nishikawa M, et al. In vivo recognition of mannosylated proteins by hepatic mannose receptors and mannan-binding protein. Am J Physiol Gastrointest Liver Physiol. 2001;280:G879–889. [DOI] [PubMed] [Google Scholar]
- 17.Kindberg GM, Tolleshaug H, Skotland T. Uptake and degradation of radioactively labelled albumin microspheres as markers for Kupffer cell phagocytosis. Cell Tissue Res. 2000;300:397–400. [DOI] [PubMed] [Google Scholar]
- 18.van der Laan LJ, Dopp EA, Haworth R, et al. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J Immunol. 1999;162:939–947. [PubMed] [Google Scholar]
- 19.Fraser IP, Koziel H, Ezekowitz RA. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin Immunol. 1998;10:363–372. [DOI] [PubMed] [Google Scholar]
- 20.Xu DZ, Lu Q, Adams CA, et al. Trauma-hemorrhagic shock-induced up-regulation of endothelial cell adhesion molecules is blunted by mesenteric lymph duct ligation. Crit Care Med. 2004;32:760–765. [DOI] [PubMed] [Google Scholar]
- 21.Wang XD, Soltesz V, Andersson R, et al. Bacterial translocation in acute liver failure induced by 90 per cent hepatectomy in the rat. Br J Surg. 1993;80:66–71. [DOI] [PubMed] [Google Scholar]
- 22.van Leeuwen PA, Hong RW, Rounds JD, et al. Hepatic failure and coma after liver resection is reversed by manipulation of gut contents: the role of endotoxin. Surgery. 1991;110:169–174. [DOI] [PubMed] [Google Scholar]
- 23.Selzner N, Selzner M, Odermatt B, et al. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. [DOI] [PubMed] [Google Scholar]
- 24.Decker K. The response of liver macrophages to inflammatory stimulation. Keio J Med. 1998;47:1–9. [DOI] [PubMed] [Google Scholar]
- 25.Dini L, Lentini A, Massimi M, et al. Modulation of the expression of galactose-specific receptors on Kupffer cells after partial hepatectomy and zymosan stimulation. Liver. 1993;13:25–30. [DOI] [PubMed] [Google Scholar]
- 26.Arii S. Biological significance and prognostic role of opsonic activity for Kupffer cell phagocytosis in experimental liver injuries and partially hepatectomized patients. Nippon Geka Hokan. 1986;55:653–661. [PubMed] [Google Scholar]
- 27.Bouwens L, Baekeland M, Wisse E. Importance of local proliferation in the expanding Kupffer cell population of rat liver after zymosan stimulation and partial hepatectomy. Hepatology. 1984;4:213–219. [DOI] [PubMed] [Google Scholar]
- 28.Lan AK, Luk HN, Goto S, et al. Stress response to hepatectomy in patients with a healthy or a diseased liver. World J Surg. 2003;27:761–764. [DOI] [PubMed] [Google Scholar]
- 29.Furumoto K, Ogawara K, Nagayama S, et al. Important role of serum proteins associated on the surface of particles in their hepatic disposition. J Control Release. 2002;83:89–96. [DOI] [PubMed] [Google Scholar]
- 30.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. [DOI] [PubMed] [Google Scholar]
- 31.Suttner SW, Surder C, Lang K, et al. Does age affect liver function and the hepatic acute phase response after major abdominal surgery? Intensive Care Med. 2001;27:1762–1769. [DOI] [PubMed] [Google Scholar]
- 32.Cornell RP. Acute phase responses after acute liver injury by partial hepatectomy in rats as indicators of cytokine release. Hepatology. 1990;11:923–931. [DOI] [PubMed] [Google Scholar]
- 33.Summerfield JA. Clinical potential of mannose-binding lectin-replacement therapy. Biochem Soc Trans. 2003;31:770–773. [DOI] [PubMed] [Google Scholar]
- 34.Jensenius JC, Jensen PH, McGuire K, et al. Recombinant mannan-binding lectin (MBL) for therapy. Biochem Soc Trans. 2003;31:763–767. [DOI] [PubMed] [Google Scholar]
- 35.El-Desoky A, Seifalian AM, Cope M, et al. Experimental study of liver dysfunction evaluated by direct indocyanine green clearance using near infrared spectroscopy. Br J Surg. 1999;86:1005–1011. [DOI] [PubMed] [Google Scholar]
- 36.Skak C, Keiding S. Methodological problems in the use of indocyanine green to estimate hepatic blood flow and ICG clearance in man. Liver. 1987;7:155–162. [DOI] [PubMed] [Google Scholar]
- 37.Baer HU, Guastella T, Wheatley AM, et al. Acute effects of partial hepatectomy on liver blood flow in the jaundiced rat. J Hepatol. 1993;19:377–382. [DOI] [PubMed] [Google Scholar]