Abstract
Objective:
To construct risk indices predicting adverse outcomes following surgery for small bowel obstruction (SBO).
Methods:
The VA National Surgical Quality Improvement Program contains prospectively collected data on more than 1 million patients. Patients undergoing adhesiolysis only or small bowel resection for SBO from 1991 to 2002 were selected. Independent variables included 68 presurgical and 12 intraoperative risk factors; dependent variables were 21 adverse outcomes including death. Stepwise logistic regression was used to construct models predicting 30-day morbidity and mortality and to derive risk index values. Patients were then divided into risk classes.
Results:
Of the 2002 patients, 1650 underwent adhesiolysis only and 352 underwent small bowel resection. Thirty-seven percent undergoing adhesiolysis only and 47% undergoing small bowel resection had more than 1 complication (P < 0.001). The overall 30-day mortality was 7.7% and did not differ significantly between the groups. Odds of death were highest for dirty or infected wounds, ASA class 4 or 5, age >80 years, and dyspnea at rest. Morbidity ranged from 22%, among patients with 0 to 7 risk points, to 62% for those with >19 risk points. Mortality ranged from 2% among patients with 0 to 12 risk points to 28% for those with >31 risk points.
Conclusions:
Morbidity and mortality after surgery for SBO in VA hospitals are comparable with those in other large series. The morbidity rate, but not the mortality rate, is significantly higher in patients requiring small bowel resection compared with those requiring adhesiolysis only (P < 0.001). The risk indices presented provide an easy-to-use tool for clinicians to predict outcomes for patients undergoing surgery for SBO.
This study examines the risk factors that predict morbidity and mortality following the surgical treatment of small bowel obstruction in an adult population from Department of Veterans Affairs (VA) Medical Centers. Morbidity and mortality rates in VA hospitals are comparable with those reported in other large series.
Mechanical small bowel obstruction (SBO) is a frequent indication for hospital admission. It is associated with significant morbidity and mortality and financial burden. The most common cause of SBO in medically underserved countries is hernia, but up to 70% of cases in the United States are due to adhesions.1 All patients who have had an operation in which the peritoneal cavity has been entered have a subsequent lifetime risk of obstruction secondary to adhesions. In an autopsy study of 752 cadavers, adhesions were found in 67% of those who had undergone a previous operation but in only 28% of cadavers with no previous operation.2 In a prospective analysis of 210 patients undergoing a laparotomy who had previously had one or more abdominal operations, 93% of patients had adhesions, compared with 10% of 115 patients who had asymptomatic adhesions at first-time laparotomy.3
The management of patients with acute SBO remains controversial. There are no uniform strategies regarding indications for or timing of operation. The decision to operate is at the discretion of the individual surgeon, but not based on high-quality evidence. Some surgeons support immediate operative management in almost all cases.4–7 However, studies on the natural history of adhesive obstructions have shown that more than 50% resolve with a conservative, nonoperative approach.8,9 Other surgeons use an initial nonoperative trial period of bowel decompression in stable patients without suspicion of bowel strangulation.10–14 As a result, the operative rates for SBO have been shown to vary widely, from 27% to 66%.15,16
The morbidity and mortality associated with operative management of SBO are recognized, but the responsible presurgical and intraoperative risk factors have not been identified. The Department of Veterans Affairs (VA) National Surgical Quality Improvement Program (NSQIP) was designed to overcome some of the limitations of retrospective analyses by prospectively gathering reliable, valid data about putative patient risk factors and outcomes of surgery in the VA healthcare system. These data are then used to construct mathematical models that report comparative risk-adjusted surgical morbidity and mortality rates. The initial National Veterans Affairs Surgical Risk Study that was conducted from October 1, 1991, through December 31, 1993, included 44 Veterans Affairs Medical Centers (VAMCs). The program was expanded on January 1, 1994, and now prospectively gathers data on surgical procedures performed at 123 VAMCs. There are more than 1 million entries to date. Each of these cases was selected according to defined criteria, assessed for 68 presurgical and 12 intraoperative variables judged likely to be predictors of complications and death, and monitored after surgery for 30-day mortality and for 21 specific and well-defined adverse outcomes. The reproducibility and accuracy of data collection have been demonstrated elsewhere.17–19 These data permit the construction of risk-prediction models using well-accepted statistical techniques.20 The present study uses these techniques to assess risk factors for morbidity and mortality following surgical treatment of SBO.
PATIENTS AND METHODS
Details of the NSQIP have been described previously.17 It is an ongoing observational study in which trained, dedicated nurses prospectively collect preoperative, intraoperative, and postoperative information on patients undergoing major cardiac and noncardiac surgery under general, spinal, or epidural anesthesia for operations known to have significant complication rates at 123 participating VAMCs. In the 119 VAMCs that perform fewer than 140 eligible surgical procedures per month, all operations are entered into the study. In the 4 VAMCs that perform more than 140 eligible surgical procedures each month, the first 36 eligible operations are entered into the study in each consecutive 8-day period, beginning with a different day each period.
In the present study, all patients diagnosed with adhesive SBO (identified by ICD-9-CM code 560.81) from October 1991 through December 2002 were selected from the NSQIP database for analysis. Two distinct surgical procedures were used to treat patients with SBO, including CPT code 44005 (adhesiolysis) and CPT code 44120 (small bowel resection with anastomosis). Vital status was determined by the NSQIP study nurses and validated by matching social security numbers from the NSQIP data sets with records in the VA Beneficiary Identification and Records Locator Subsystem. This system has a sensitivity of greater than 94% for identification of death in veterans who have ever been treated at a VAMC.21,22
χ2 analysis was used to compare 30-day morbidity and mortality between patients undergoing adhesiolysis versus patients undergoing small bowel resection with anastomosis. Stepwise logistic regression analysis was used to construct models predicting 30-day morbidity (defined as one or more complications) and 30-day mortality. Independent variables examined included 68 presurgical clinical parameters (including demographics, comorbid conditions, and laboratory test values) and 12 intraoperative process-of-care variables (including emergency versus elective designation, anesthesia technique, ASA classification, wound classification, surgery performed by primary surgeon or first assistant, operation duration, intraoperative blood loss, intraoperative blood transfusion, CPT code for the index procedure, CPT codes for other procedures performed by the same surgical team, and CPT codes for concomitant procedures performed by a different surgical team) previously used by the NSQIP.17 The dependent variables were 21 specific adverse outcomes and 30-day mortality. Data regarding “redo” operations were excluded from the regression analyses as there are concerns regarding the reliability of this particular measure in the database. Cutoffs for laboratory tests, such as hematocrit, were NSQIP-defined and were based on normal curves.
The bivariate relationship between morbidity and mortality and each of the 78 candidate variables for entry into the logistic regression models was analyzed using the t test for continuous variables and the χ2 test for categorical variables. In the case of categorical variables with small cell sizes, the χ2 test was replaced by the Fisher exact test. Those variables that were significant at P < 0.20 were then entered into a stepwise logistic regression procedure. Standard measures of logistic regression model fit such as the c index and Hosmer-Lemeshow goodness-of-fit statistic were calculated.23
Risk indices for 30-day morbidity and 30-day mortality were calculated using the methods of Le Gall et al.24 Point values were assigned to each preoperative predictor by multiplying the β-coefficients from the logistic regression model by 10 and rounding off to the nearest integer. The point values represent the components of the risk index. The distribution of point totals was skewed, so a power transformation, ln (score +1), was used to decrease the skewness of the data. The transformed point totals for each patient were used in multiple logistic regression equations designed to convert the risk indices to the probabilities that each patient would experience the outcome of interest for the particular equation (either 30-day morbidity or 30-day mortality).
RESULTS
Patient Demographics
From the NSQIP database, 2002 patients with adhesive SBO were identified from October 1991 to December 2002. There were 1650 patients who underwent adhesiolysis and 352 who had a small bowel resection with anastomosis. Mean age ± SD was similar for the 2 groups (65 ± 13 for the adhesiolysis group and 66 ± 13 for the small bowel resection group). The majority of patients were male (95% per group).
Patient Preoperative Risk Factors
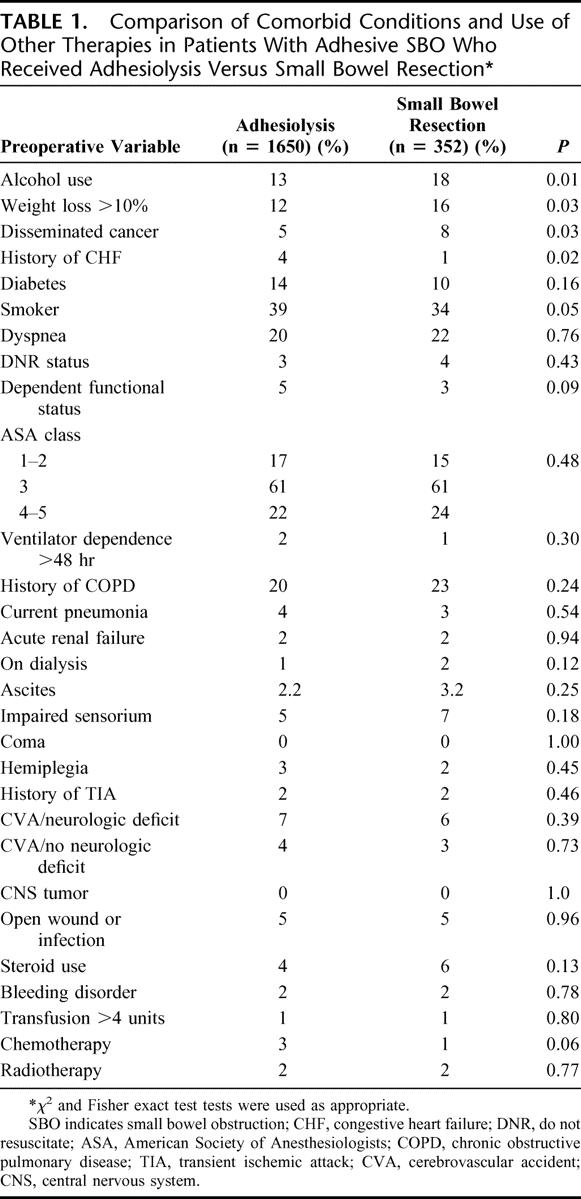
Preoperative comorbid conditions and use of other therapies (chemotherapy, radiation therapy, etc.) for both of the groups are listed in Table 1. There was a statistically significant difference between the 2 groups with respect to 4 comorbid conditions. The adhesiolysis group had a significantly lower frequency of 3 comorbid conditions compared with the small bowel resection group, including alcohol use (13% versus 18%), weight loss >10% (12% versus 16%), and disseminated cancer (5% versus 8%). The small bowel resection group had a lower frequency of congestive heart failure (CHF) (1%) compared with the adhesiolysis group (4%). There were no significant differences between the 2 groups with respect to any other comorbid conditions or use of other therapies.
TABLE 1. Comparison of Comorbid Conditions and Use of Other Therapies in Patients With Adhesive SBO Who Received Adhesiolysis Versus Small Bowel Resection
Preoperative Laboratory Tests
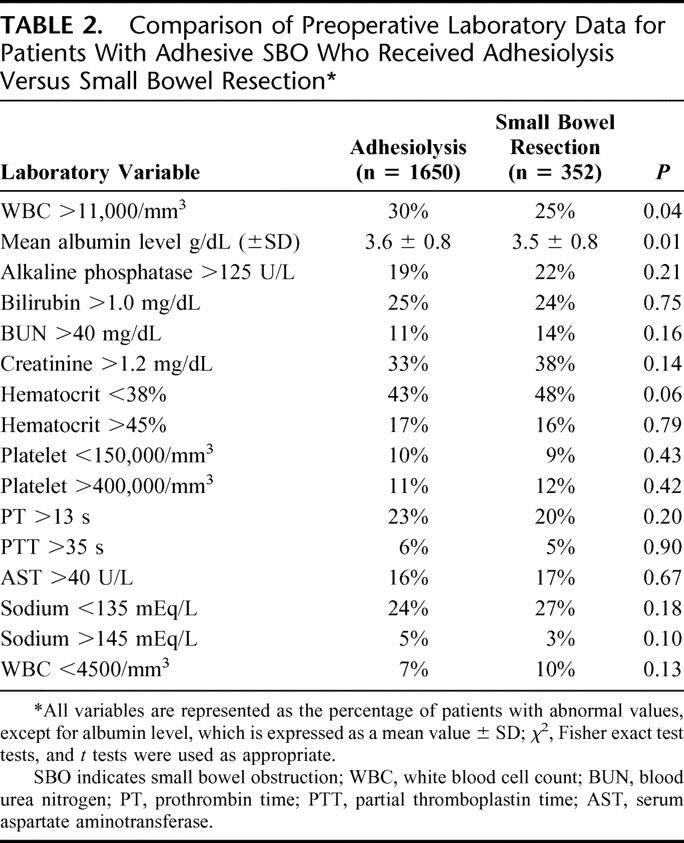
The preoperative laboratory values are shown in Table 2. The patients undergoing adhesiolysis had a significantly higher frequency of abnormal WBC count >11,000/mm3 (30%) compared with the small bowel resection group (25%). In contrast, the patients who had a small bowel resection had significantly lower mean serum albumin levels (3.5 ± 0.8 g/dL) compared with patients who had adhesiolysis (3.6 ± 0.8 g/dL). Although this difference is statistically significant, it is not clinically significant. There were no differences between groups for the remaining 14 preoperative tests (serum alkaline phosphatase >125 U/L, serum bilirubin >1.0 mg/dL, serum blood urea nitrogen >40 mg/dL, serum creatinine >1.2 mg/dL, hematocrit <38% or >45%, platelet count <150,000/mm3 or >400,000/mm3, prothrombin time >13 seconds, partial thromboplastin time >35 seconds, serum aspartate aminotransferase >40 U/L, serum sodium <135 mEq/L or >145 mEq/L, and WBC count <4500/mm3).
TABLE 2. Comparison of Preoperative Laboratory Data for Patients With Adhesive SBO Who Received Adhesiolysis Versus Small Bowel Resection
Operative Variables
Patients undergoing adhesiolysis were more likely to be treated emergently (61%) than patients undergoing small bowel resection (51%) (P < 0.01). By NSQIP criteria, emergency operations are defined as those performed as soon as possible and no later than 12 hours after the patient has been admitted to the hospital or after the onset of related preoperative symptomatology. All operations not coded as emergency were coded as elective. The 2 operations were performed by attending surgeons in an equivalent percentage of cases (17% for adhesiolysis and 19% for small bowel resection). The distribution of postgraduate year level of resident surgeons performing the operations differed significantly, but there was no clear trend and the differences by level between the procedures were small. Anesthetic technique, percent of operations performed on an inpatient basis, and disposition of the patients at discharge (home, acute care hospital, chronic care hospital) were similar between the 2 groups. There was a significant difference in the wound classification between the 2 groups (P < 0.0001): the adhesiolysis group had a higher percentage of wounds classified as clean and a lower percentage of clean/contaminated wounds (42% clean, 48% clean/contaminated, 9% contaminated, and 1% infected) compared with the small bowel resection group (16% clean, 66% clean/contaminated, 14% contaminated, and 4% infected). The mean operative time was significantly longer for the small bowel resection group (3.0 ± 1.9 hours) compared with the adhesiolysis group (2.0 ± 1.6 hours) (P < 0.0001). The mean number of units of blood transfused was also higher for the small bowel resection group (0.4 ± 1) compared with the adhesiolysis group (0.2 ± 1) (P < 0.01). χ2 analysis of the percentage of patients in each group who required a repeat operation for SBO over the 10-year period was evaluated. The adhesiolysis group had a 5.6% repeat rate and the bowel resection group had a 6.3% repeat rate (P = 0.60).
Outcomes
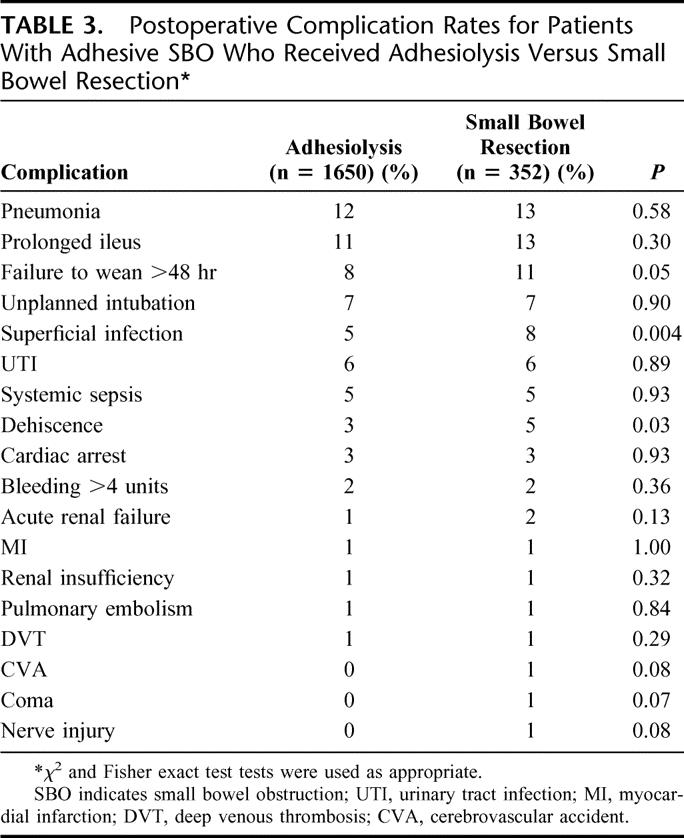
The 30-day mortality rate was similar for the small bowel resection (10%) and adhesiolysis (7%) groups (P = 0.13). The overall morbidity rate for the small bowel resection group was significantly higher (47%) compared with the adhesiolysis group (37%) (P < 0.001). The postoperative complications for each group are listed in Table 3. Pneumonia, prolonged ileus, failure to wean from the ventilator, unplanned intubation, superficial wound infection, urinary tract infection, systemic sepsis, and wound dehiscence were the most frequently reported complications, accounting for the majority of overall morbidity. Of these, 2 complications (superficial wound infection and wound dehiscence) occurred significantly more frequently in the small bowel resection group compared with the adhesiolysis group (P < 0.05). No complication occurred more frequently in the adhesiolysis group. Among those patients with prolonged ileus (defined as no return of bowel function [flatus, bowel movement] in 7 days), 8.3% in the adhesiolysis group (n = 181), and 4.7% in the bowel resection group (n = 43) required reoperation (P = 0.54).
TABLE 3. Postoperative Complication Rates for Patients With Adhesive SBO Who Received Adhesiolysis Versus Small Bowel Resection
Logistic Regression Models
For some of the less commonly used preoperative laboratory tests and preoperative risk factors, there were up to 32% missing values. To reduce the effect of missing values on the logistic regressions, those laboratory tests and preoperative risk factors with greater than 5% missing values were removed from further analysis.
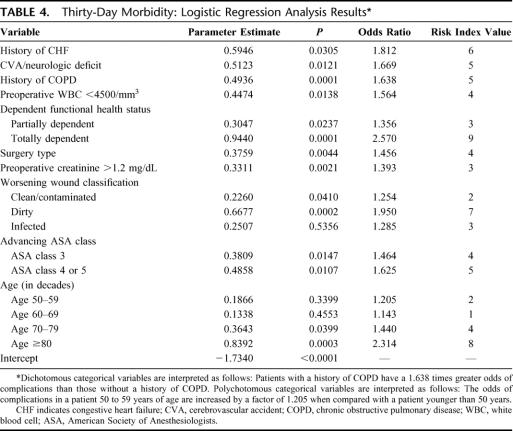
Using this cutoff, several preoperative variables (platelet count, serum albumin, serum alkaline phosphatase, serum bilirubin, prothrombin time, partial thromboplastin time, serum glutamic oxaloacetic transaminase, use of chemotherapy or radiotherapy, presence of sepsis, and presence of coma) were excluded from the logistic regression analyses, resulting in the list-wise elimination of only 8.2% of cases (164 of 2002). In the second stage of the model building process, polychotomous variables were reparameterized to permit calculation of risk index values for each level of these variables. In this second stage, only 6.5% of the original 2002 cases were eliminated from the model as only significant variables were used. Ten factors were predictive of postoperative 30-day morbidity in the logistic regression analysis (Table 4). History of CHF, history of cerebrovascular accident with neurologic deficit, history of chronic obstructive pulmonary disease, preoperative WBC count <4500/mm3, dependent functional health status, surgery type (small bowel resection versus adhesiolysis), preoperative creatinine >1.2 mg/dL, contaminated/infected wound classification, advanced ASA class, and advancing age (in decades) were all associated with increased 30-day morbidity (defined as one or more complications). Examination of the c index (0.681) and the Hosmer-Lemeshow goodness-of-fit statistic (P = 0.7868) for this model indicates an acceptable model fit.
TABLE 4. Thirty-Day Morbidity: Logistic Regression Analysis Results
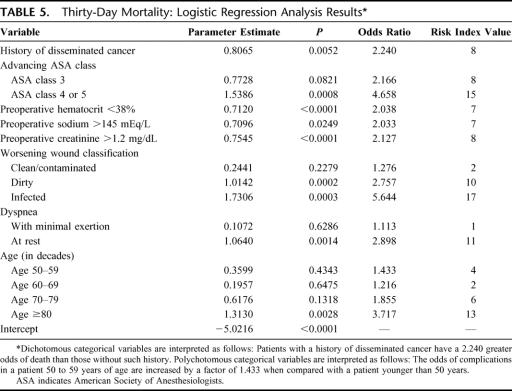
Eight factors were predictive of 30-day mortality in the logistic regression analysis (Table 5). Surgery type was not a significant predictor for mortality. History of disseminated cancer, advancing ASA class, preoperative hematocrit <38%, preoperative sodium >145 mEq/L, preoperative creatinine >1.2 mg/dL, contaminated/infected wound classification, dyspnea, and advancing age (in decades) were all associated with increased 30-day mortality. Examination of the c index (0.794) and the Hosmer-Lemeshow goodness-of-fit statistic (P = 0.6029) for this model indicates an excellent model fit.
TABLE 5. Thirty-Day Mortality: Logistic Regression Analysis Results
The point values assigned to each preoperative predictor used in calculating the 30-day morbidity and mortality risk index scores are shown in Tables 4 and 5. For 30-day morbidity, the variables with the highest risk index values included totally dependent functional health status, age >80 years, dirty wound status, and history of CHF. For 30-day mortality, infected wound status, ASA class 4 or 5, age >80 years, dirty wound status, and dyspnea at rest were the variables with the highest risk index values.
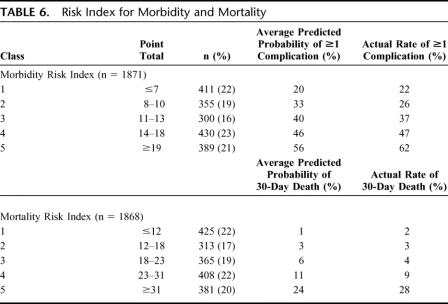
Patients were then categorized into 5 risk classes based on the predicted probability associated with various postoperative risk index scores. Table 6 shows the number of patients in each risk class, the average predicted probabilities of postoperative morbidity and mortality based on the models, and the actual postoperative morbidity and mortality rates. The risk indices predicted 30-day morbidity and mortality rates well across all risk classes. The lowest risk group (point total ≤7) was associated with predicted and actual morbidity rates of 20% and 22%, respectively. The highest risk group (point total ≥19) was associated with predicted and actual morbidity rates of 56% and 62%, respectively.
TABLE 6. Risk Index for Morbidity and Mortality
Although the range of the mortality risk index was broader than the morbidity risk index, the predicted probability of 30-day mortality was more accurate than the predicted probability of 30-day morbidity. The predicted and actual mortality rates never differed by more than 4% for any mortality class. The lowest risk group (point total ≤12) was associated with predicted and actual mortality rates of 1% and 2%, respectively. The highest risk group (point total ≥31) was associated with predicted and actual mortality rates of 24% and 28%, respectively.
DISCUSSION
Intestinal obstruction accounts for approximately 20% of all surgical admissions for acute abdomen in the United States, and the majority of these are SBO.25 The etiology of SBO is most commonly attributed to adhesions following previous elective abdominal and pelvic surgery. Hernias and neoplasia are the next 2 leading causes in wealthy countries, with smaller contributions from infections, inflammatory bowel diseases, intussusception, and gallstone ileus.26 Adhesive SBO often affects relatively young patients and can recur over the patient's lifetime. Many of these patients require operative intervention. By utilizing NSQIP data, we investigated the risk factors that contribute to the morbidity and mortality of adults requiring either adhesiolysis or small bowel resection for adhesive SBO.
A number of studies have focused on the influence of the original index operation on the course of the adhesive SBO, the response to conservative treatment, the need for surgical intervention, and recurrence.27–29 Matter et al29 found that adhesive SBO following a previous small bowel resection was more likely to be complete and require operative intervention than an adhesive SBO following surgery for appendectomy, colon resection, gastric resection or repair, or gynecologic resection. The interval between a patient's most recent laparotomy and initial admission for adhesions is widely distributed. In a series of >2000 patients followed for >10 years after abdominal surgery, Ellis30 noted that 1% of patients developed SBO secondary to adhesions within 1 year of operation. However, 20% of patients who ever developed SBO had the diagnosis more than 10 years later. The time interval to obstruction tended to be longer after appendectomy and herniorrhaphy than after colorectal and gynecologic procedures.
Although the rate is variable depending on the series being reported, up to 30% of patients presenting with adhesive SBO require operative intervention.9 The recurrence rate after initial admission and either nonoperative or operative treatment of adhesive SBO is also variable. Barkan et al31 found a 5-year recurrence rate of approximately 20% following operative and 40% following nonoperative treatment of initial episodes. Landercasper et al15 reported that 10% of patients having an operation and 17% of those not undergoing operation ultimately required an operative intervention for SBO with an average follow-up time of 4.4 years. The current data cannot account for the number of patients admitted to VA hospitals for nonoperative treatment of adhesive SBO. However, the rate of a second laparotomy for SBO in our cohort over a 10-year period was 5% to 6% in each group.
Mortality from SBO has declined from 50% to <3% over the past 100 years.15 Several advances in nonoperative management have contributed to this dramatic improvement. In 1933, Wangensteen and Paine32 reported an operative technique of advancing a long tube through the jejunum down to the point of obstruction. This relieved obstruction in 80% of patients without any further treatment. This technique was later accomplished through a nonoperative technique, utilizing a tube that was passed through the nose.33 The major deterrent to widespread adoption of this technique was the delay in passage of the tube from the stomach into the duodenum. This problem was overcome in 1978 when Douglas and Morrissey34 used upper gastrointestinal endoscopy to advance the tubes into the small bowel and thereby eliminate the delay in small bowel decompression. This approach is still used for selected patients. Gowen14 recently reported a success rate of 90% following a trial of long tube decompression for 48 to 72 hours.
One of the obvious uses of the NSQIP data is to compare the experience of the VAMC system with published reports from other institutions and systems. The overall 30-day mortality rate following surgery for SBO in our study was 7.3% for adhesiolysis and 9.7% for small bowel resection, which is higher than the published rates of <3% in the literature.7–9,12,15 This is likely due to the fact that the VA consists of older adults (mean age 65–66 years in this study) who are more likely to suffer from medical comorbidities compared with non-VA patients with SBO and also to the fact that many series report in-hospital mortality but not 30-day mortality. Likewise, a 37% morbidity rate following adhesiolysis and 47% morbidity rate following small bowel resection for SBO in VA patients is slightly higher than, but comparable to, rates reported in the recent literature from non-VA centers (18%–30% following adhesiolysis and 22%–40% following small bowel resection) when the above age and comorbidity factors are considered.7–9,12,15 Indeed, over 60% of the current VA population examined fell into the highest risk index classes,3–5 where the predicted morbidity rate is 40% to 56% and the predicted mortality rate is 6% to 24%.
The most common complications observed in this series in both adhesiolysis and small bowel resection groups following surgical treatment of SBO were pneumonia, prolonged ileus, failure to wean from the ventilator for >48 hours, unplanned intubation, superficial wound infection, urinary tract infection, systemic sepsis, and wound dehiscence. Prolonged ileus is the most common complication reported in many studies of surgery for SBO.3–5,7–9,12 The increased frequency of pneumonia, unplanned intubations, and failure to wean from the ventilator is likely secondary to the increased age of the VA patient population and increased prevalence of smoking compared with the non-VA population with SBO. However, as pointed out previously, the methods of data collection used by NSQIP are notably more reliable than in other reports.
The generation of 30-day morbidity and mortality risk models by stepwise logistic regression analysis provides information on the relative impact of significant risk factors as measured by the relevant odds ratios. The validity of some putative risk factors incorporated in the NSQIP study design have been confirmed by this study, whereas others failed to predict adverse events. Preoperative factors predictive of postoperative morbidity included history of CHF, cerebrovascular accident with neurologic deficit, history of chronic obstructive pulmonary disease, preoperative WBC <4500/mm3, dependent functional health status, preoperative creatinine >1.2 mg/dL, and advancing age (in decades). Intraoperatively, contaminated or infected wound classification and advancing ASA classification were also predictive of morbidity. Finally, performing a small bowel resection compared with adhesiolysis alone was also predictive of morbidity.
Preoperative factors that clearly impact the observed mortality rate include history of disseminated cancer, preoperative hematocrit <38%, preoperative sodium >145 mEq/L, preoperative creatinine >1.2 mg/dL, dyspnea, and advancing age (in decades). Intraoperative factors that predict mortality include advanced ASA class and contaminated or infected wound classification. Elevated WBC was not an independent prognostic risk factor for morbidity and mortality. The finding that elevated WBC occurred more frequently in cases of adhesiolysis than resection underscores the unreliable nature of leukocytosis in differentiating inflammation and infection. The lack of leukocytosis does not obviate the need for bowel resection in patients with SBO.
The potential risks of nonoperative management of SBO include bowel strangulation, necrosis, and subsequent peritoneal and systemic sepsis. There are no reliable clinical or laboratory signs of bowel strangulation. As a result, a primary goal in patients presenting with SBO is to diagnose whether strangulation is clearly present or when its presence cannot be reliably excluded. One limitation of the NSQIP data is that the data cannot account for the impact of nonoperative management approaches to SBO. The NSQIP was designed to collect data on a wide variety of major surgical procedures, and it has not been practical to include alternative nonsurgical therapy data in the database.
A second limitation is that the NSQIP data cannot differentiate those patients with preoperative imaging tests indicating complete versus partial obstruction or findings suggestive of bowel necrosis (ie, pneumatosis intestinalis). A third limitation is inability to construct separate regression models for the adhesiolysis and small bowel resection groups. The sample size of the NSQIP database as a whole, although large, permits only a limited estimation of the actual risk of adverse outcomes for some procedures.
A fourth limitation is that these data do not permit discrimination among the possible reasons for small bowel resection. That is, patients with gross perforation may account for proportionately more of the morbidity and mortality than patients who either undergo small bowel resection for nonperforated, but ischemic, small bowel or those whose bowel was damaged during adhesiolysis but was not otherwise abnormal. Similarly, the data in their present form do not permit identification of leaks or enterocutaneous fistulas because each sample from the NSQIP database can only be chosen based on a single diagnosis code (in this case, SBO). Other variables of interest that may impact treatment decisions and, therefore, outcomes but are not available in the NSQIP database include preoperative fever, physical findings (suspected peritonitis), and delay in initiation of operative therapy. Thus, we recognize the potential for selection bias.
A fifth limitation is the fact that there are occasional inaccuracies in data collection (for example, the misclassification of 16% of bowel resection cases as “clean”). The NSQIP recognizes this and efforts are underway to educate operating room personnel regarding this point.
A general limitation of doing research in the VA system is that the population consists of predominantly older males who often have multiple comorbidities and a relatively low socioeconomic status as compared with the population at large. However, as the NSQIP database has grown, the statistical power of the models derived has increased. One of the great strengths of the NSQIP is that it is a current, constantly enlarging database with the ability to keep pace with frequent changes and advancements in surgical technique and patient care. The prospective nature of the data collection and use of sophisticated statistical modeling will allow us to better predict the ability of our patients to tolerate proposed operations and generate new hypotheses for improved preoperative and intraoperative care. The NSQIP methodology is currently being introduced into non-VAMC hospitals. Future reports should reveal whether conclusions reached using VA NSQIP data are generalizable to other hospital populations.
A sixth and final criticism of the NSQIP database has been that the 123 VAMCs contributing to the database vary widely in size and in the volume of operations performed annually. For example, only 4 of the VAMCs perform >140 major surgical cases per month. There has been an assumption by some that better surgical outcomes are achieved in hospitals with larger surgical volumes.35–38 However, Khuri et al39 recently analyzed the relationship between surgical volume and outcome in 8 commonly performed operations of intermediate complexity in the VAMCs. They found that there were no statistically significant associations between procedure or specialty volume and 30-day mortality rate for any of the operations analyzed. While these findings need to be validated by others outside the VAMC system, it provides support for the contention that surgical volume at individual VAMCs is highly unlikely to be an accurate measure of the quality of surgery performed.
The morbidity and mortality models presented here provide an insight into the relative significance of various preoperative and intraoperative events. The odds ratios and the risk index values are different ways of estimating the increased risk of 30-day morbidity and mortality for each individual preoperative and intraoperative variable. However, the advantage of the postoperative risk indices is the ability to easily sum the point values and readily determine the likelihood of 30-day morbidity or mortality (Table 6). This is a handy tool for clinicians. The calculated postoperative risk indices for 30-day morbidity and mortality may be useful in targeting perioperative testing and supportive care to high-risk patients. Future prospective studies are needed to test the generalizability of this newly developed risk index to other samples. While it is true that some patient risk factors are not modifiable, such as steroid use, health status, disseminated cancer, and chronic obstructive pulmonary disease, many of the preoperative and intraoperative factors identified by the NSQIP may be altered. Preoperative fluid resuscitation in patients with high serum creatinine levels, platelet and fresh frozen plasma transfusions for patients with low platelet levels or prolonged prothrombin times, and prevention of intraoperative wound contamination are some of the factors identified as important in preventing morbidity and mortality. We anticipate that analyses such as those performed in the present study will provide hypotheses on which to base future trials designed to decrease the risk of adverse outcomes.
Footnotes
Supported by the Veterans Health Administration of the U.S. Department of Veterans Affairs, Washington, DC.
Reprints: Walter E. Longo, MD, Department of Surgery, Yale University Medical Center, 330 Cedar St., New Haven, CT 06520. E-mail: walter.longo@yale.edu.
REFERENCES
- 1.Pickleman J. Small bowel obstruction. In: Zinner MJ, ed. Maingot's Abdominal Operations, 10th ed. London: Prentice Hall, 1997:1159–1172. [Google Scholar]
- 2.Weibel MA, Majno G. Peritoneal adhesions and their relation to abdominal surgery: a postmortem study. Am J Surg. 1973;126:345–353. [DOI] [PubMed] [Google Scholar]
- 3.Menzies D, Ellis H. Intestinal obstruction from adhesions: how big is the problem? Ann R Coll Surg Engl. 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- 4.Zadeh BJ, Davis JM, Canizaro PC. Small bowel obstruction in the elderly. Am Surg. 1985;51:470–473. [PubMed] [Google Scholar]
- 5.Fabri PJ, Rosemurgy A. Reoperation for small intestinal obstruction. Surg Clin North Am. 1991;71:131–146. [DOI] [PubMed] [Google Scholar]
- 6.Shatila AH, Chamberlain BF, Webb WR. Current status of diagnosis and management of strangulation obstruction of the small bowel. Am J Surg. 1976;132:299–303. [DOI] [PubMed] [Google Scholar]
- 7.Davidson AT. Early operation in the treatment of small bowel obstruction. J Natl Med Assoc. 1981;73:245–246. [PMC free article] [PubMed] [Google Scholar]
- 8.Cox MR, Gunn IF, Eastman MC, et al. The safety and duration of nonoperative treatment for adhesive small bowel obstruction. Aust NZ J Surg. 1993;63:367–371. [DOI] [PubMed] [Google Scholar]
- 9.Miller G, Boman J, Shrier I, et al. Natural history of patients with adhesive small bowel obstruction. Br J Surg. 2000;87:1240–1247. [DOI] [PubMed] [Google Scholar]
- 10.Assalia A, Schein M, Kopelman D, et al. The therapeutic effect of oral Gastrografin in adhesive, partial small bowel obstruction: a prospective randomized trial. Surgery. 1994;115:433–437. [PubMed] [Google Scholar]
- 11.Peetz DJ, Gamelli RL, Pilcher DB. Intestinal intubation in acute mechanical small bowel obstruction. Arch Surg. 1982;117:334–336. [DOI] [PubMed] [Google Scholar]
- 12.Stewardson RH, Bombeck CT, Nyhus LM. Critical operative management of small bowel obstruction. Ann Surg. 1978;187:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brolin RE. Partial small bowel obstruction. Surgery. 1984;95:145–149. [PubMed] [Google Scholar]
- 14.Gowen GF. Long tube decompression is successful in 90% of patients with adhesive small bowel obstruction. Am J Surg. 2003;185:512–515. [DOI] [PubMed] [Google Scholar]
- 15.Landercasper J, Cogbill TH, Merry WH, et al. Long-term outcome after hospitalization for small-bowel obstruction. Arch Surg. 1993;128:765–771. [DOI] [PubMed] [Google Scholar]
- 16.Hofstetter SR. Acute adhesive obstruction of the small intestine. Surg Gynecol Obstet. 1981;152:141–144. [PubMed] [Google Scholar]
- 17.Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180:519–531. [PubMed] [Google Scholar]
- 18.Khuri SF, Daley J, Henderson W, et al. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:315–327. [PubMed] [Google Scholar]
- 19.Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:328–340. [PubMed] [Google Scholar]
- 20.Khuri SF, Daley J, Henderson W, et al. The Department of Veterans Affairs' NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program 22 for the measurement and enhancement of the quality of surgical care. Ann Surg. 1998;228:491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page WF, Braun MM, Caporaso NE. Ascertainment of mortality in the US veteran population: World War II veteran twins. Mil Med. 1995;160:351–355. [PubMed] [Google Scholar]
- 22.Fisher SG, Weber L, Goldberg J, et al. Mortality ascertainment in the veteran population: alternatives to the National Death Index. Am J Epidemiol. 1995;141:242–250. [DOI] [PubMed] [Google Scholar]
- 23.Hosmer DW, Hosmer T, Le Cessie S, et al. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;15:965–980. [DOI] [PubMed] [Google Scholar]
- 24.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. [DOI] [PubMed] [Google Scholar]
- 25.Welch JP. General consideration and mortality in bowel obstruction. In: Welch JP, ed. Bowel Obstruction: Differential Diagnosis and Clinical Management. Philadelphia: Saunders, 1990:59–95. [Google Scholar]
- 26.Mucha P. Small intestinal obstruction. Surg Clin North Am. 1987;67:597–620. [DOI] [PubMed] [Google Scholar]
- 27.Ellis CN, Boggs HW, Slagle GW, et al. Small bowel obstruction after colon resection for benign and malignant diseases. Dis Colon Rectum. 1991;34:367–371. [DOI] [PubMed] [Google Scholar]
- 28.Meagher AP, Moller C, Hoffmann DC. Non-operative treatment of small bowel obstruction following appendectomy or operation on the ovary or tube. Br J Surg. 1993;80:1310–1311. [DOI] [PubMed] [Google Scholar]
- 29.Matter I, Khalemsky L, Abrahamson J, et al. Does the index operation influence the course and outcome of adhesive intestinal obstruction? Eur J Surg. 1997;163:767–772. [PubMed] [Google Scholar]
- 30.Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg Suppl. 1997;557:5–9. [PubMed] [Google Scholar]
- 31.Barkan H, Webster S, Ozeran S. Factors predicting the recurrence of adhesive small bowel obstruction. Am J Surg. 1995;170:361–365. [DOI] [PubMed] [Google Scholar]
- 32.Wangensteen OH, Paine JR. Treatment of intestinal obstruction by suction with a duodenal tube. JAMA. 1933;101:1532–1539. [Google Scholar]
- 33.Abbott WO, Johnston CG. Intubation studies of the human small intestine. Surg Gynecol Obstet. 1938;66:691–698. [Google Scholar]
- 34.Douglas DD, Morrissey JF. A new technique for rapid endoscopic assisted intubation of the small intestine. Arch Surg. 1978;113:196–198. [DOI] [PubMed] [Google Scholar]
- 35.Hannan EL, Kilburn H, Bernard H, et al. Coronary artery bypass surgery: the relationship between in-hospital mortality rate and surgical volume after controlling for clinical risk factors. Med Care. 1991;29:1094–1097. [PubMed] [Google Scholar]
- 36.Wen SW, Simunovic M, Williams JI, et al. Hospital volume, calendar age, and short term outcomes in patients undergoing repair of abdominal aortic aneurysms: the Ontario experience, 1988–1992. J Epidemiol Community Health. 1996;50:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor HD, Dennis DA, Crane HS. Relationship between mortality rates and hospital volume for Medicare patients undergoing major orthopaedic surgery of the hip, knee, spine, and femur. J Arthroplasty. 1997;12:235–242. [DOI] [PubMed] [Google Scholar]
- 38.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 39.Khuri SF, Daley J, Henderson W, et al. Relation of surgical volume to outcome in eight common operations: results from the VA National Surgical Quality Improvement Program. Ann Surg. 1999;230:414–432. [DOI] [PMC free article] [PubMed] [Google Scholar]