Abstract
A bacterial isolate, designated strain SZ, was obtained from noncontaminated creek sediment microcosms based on its ability to derive energy from acetate oxidation coupled to tetrachloroethene (PCE)-to-cis-1,2-dichloroethene (cis-DCE) dechlorination (i.e., chlororespiration). Hydrogen and pyruvate served as alternate electron donors for strain SZ, and the range of electron acceptors included (reduced products are given in brackets) PCE and trichloroethene [cis-DCE], nitrate [ammonium], fumarate [succinate], Fe(III) [Fe(II)], malate [succinate], Mn(IV) [Mn(II)], U(VI) [U(IV)], and elemental sulfur [sulfide]. PCE and soluble Fe(III) (as ferric citrate) were reduced at rates of 56.5 and 164 nmol min−1 mg of protein−1, respectively, with acetate as the electron donor. Alternate electron acceptors, such as U(VI) and nitrate, did not inhibit PCE dechlorination and were consumed concomitantly. With PCE, Fe(III) (as ferric citrate), and nitrate as electron acceptors, H2 was consumed to threshold concentrations of 0.08 ± 0.03 nM, 0.16 ± 0.07 nM, and 0.5 ± 0.06 nM, respectively, and acetate was consumed to 3.0 ± 2.1 nM, 1.2 ± 0.5 nM, and 3.6 ± 0.25 nM, respectively. Apparently, electron acceptor-specific acetate consumption threshold concentrations exist, suggesting that similar to the hydrogen threshold model, the measurement of acetate threshold concentrations offers an additional diagnostic tool to delineate terminal electron-accepting processes in anaerobic subsurface environments. Genetic and phenotypic analyses classify strain SZ as the type strain of the new species, Geobacter lovleyi sp. nov., with Geobacter (formerly Trichlorobacter) thiogenes as the closest relative. Furthermore, the analysis of 16S rRNA gene sequences recovered from PCE-dechlorinating consortia and chloroethene-contaminated subsurface environments suggests that Geobacter lovleyi belongs to a distinct, dechlorinating clade within the metal-reducing Geobacter group. Substrate versatility, consumption of electron donors to low threshold concentrations, and simultaneous reduction of electron acceptors suggest that strain SZ-type organisms have desirable characteristics for bioremediation applications.
Environmental pollutants, such as chlorinated solvents, toxic metals, and radionuclides (e.g., uranium), are strictly regulated due to their negative effects on ecosystem function and human health. Tetrachloroethene (PCE) and trichloroethene (TCE) are frequently used as solvents and degreasing agents. As a result of extensive usage, improper disposal, and accidental spills, PCE and TCE became widely distributed, pervasive groundwater contaminants. Uranium was released in significant amounts from nuclear weapon complexes managed by the U.S. Department of Energy (DOE) during the Cold War arms race. It was estimated that more than 80% of DOE sites have radionuclide contamination and at least 27% have volatile organic hydrocarbons as cocontaminants (47).
The considerable knowledge that has accrued on the fate of specific contaminants and the bacteria involved in the transformation and degradation pathways has led to successful bioremediation field studies. For instance, acetate additions to stimulate dissimilatory metal-reducing organisms promoted reduction of soluble U(VI) to insoluble U(IV) and contributed to plume containment (2, 15, 21). Biostimulation and bioaugmentation approaches were also successfully implemented at chloroethene-contaminated sites (13, 29, 39). The majority of sites, however, contain multiple contaminants, and mixed-waste scenarios are a major challenge that successful remedies must address. Many U(VI)-impacted DOE sites have elevated concentrations of nitrate, sulfate, chlorinated solvents (e.g., PCE and TCE), and other chloroorganic pollutants, including polychlorinated biphenyls (47). Unfortunately, mixed-waste scenarios received little attention, and the effects of cocontaminants on desired biotransformation processes are unclear. Obviously, populations that consume toxic electron acceptors (i.e., oxidized radionuclides and chloroorganic compounds) under a range of redox conditions (e.g., in the presence of alternate, energetically favorable oxidants) are desirable at sites impacted by nitrate, uranium, and chlorinated solvents.
In this study, we report the isolation and characterization of a novel Geobacter species, strain SZ, capable of coupling the oxidation of acetate and H2 to the reduction of a variety of electron acceptors, including PCE, TCE, nitrate, and uranium.
MATERIALS AND METHODS
Isolation procedure and growth conditions.
Su-Zi Creek sediment was collected near a suburban residential area with no reported contamination in June 2002 near Seoul, South Korea. Sterile 50-ml plastic containers were filled completely with sediment, capped, and shipped to the laboratory at ambient temperature. Inside a glove box (Coy Laboratory Products Inc., Grass Lake, MI), approximately 2 g of sediment was transferred to 20-ml (nominal capacity) glass vials, and 9 ml of sterile, anoxic, reduced, bicarbonate-buffered (30 mM) medium amended with 10 mM acetate was added (46). All the vials received 0.5 μl neat PCE as the electron acceptor and were sealed with Teflon-lined rubber stoppers. Sediment-free, nonmethanogenic, PCE-dechlorinating cultures were obtained after sequential transfers (1% [vol/vol]) to fresh medium. Dilution-to-extinction series were established in liquid and semisolid medium (0.5% [wt/vol] SeaPlaque agarose; Cambrex Bio Science Rockland, Inc., Rockland, ME). PCE (2 μl dissolved in 100 μl hexadecane) was added by syringe after the agarose had solidified. The vials were incubated upside down, and PCE was available to the bacteria only via the headspace.
16S rRNA gene sequencing and phylogenetic analysis.
Genomic DNA was obtained from PCE-acetate-, Fe(III)-acetate-, and nitrate-acetate-grown cultures. Individual genomic DNA was extracted with a QIAamp DNA mini kit (QIAGEN, Santa Clarita, CA). 16S rRNA genes were PCR amplified using a universal bacterial primer pair (8F and 1525R) and 50 ng of genomic DNA as the template and sequenced as described previously (31, 43). Related 16S rRNA gene sequences from cultured organisms and environmental clones were identified by BLAST analysis and obtained from GenBank. Distance matrices and phylogenetic trees were generated following sequence alignment using the MegAlign program of the Lasergene software package (DNA Star Inc., Madison, MI). Bootstrap values were calculated for 1,000 replicates with the MEGA software program (27). Geobacter sulfurreducens and Geobacter metallireducens were kindly provided by Kelly Nevin, University of Massachusetts, Amherst, and Benjamin Griffin, Michigan State University, East Lansing, provided a culture of Geobacter (formerly Trichlorobacter) thiogenes strain K1 (10, 22). Desulfuromonas michiganensis strain BB1 was isolated in our laboratory (46).
RFLP analysis of PCR-amplified 16S rRNA genes.
Genomic DNA was extracted from acetate-fed cultures amended with Fe(III) citrate, nitrate, PCE, or fumarate as the electron acceptor. PCR with primer pair 8F and 1525R and restriction fragment length polymorphism (RFLP) analysis of the amplicons using HhaI, MspI, and RsaI were performed as described previously (31, 43). Controls included genomic DNA from G. thiogenes, G. sulfurreducens, and D. michiganensis strain BB1.
DNA-DNA hybridization.
Genomic DNA was isolated from fumarate-acetate-grown strain SZ and G. thiogenes cultures using a French pressure cell and chromatographic purification on hydroxyapatite (5). DNA-DNA hybridization was carried out as described previously (8) under consideration of the modifications introduced by Huss et al. (23), using a model Cary 100 Bio UV/Vis spectrophotometer equipped with a Peltier-thermostatted 6 × 6 multicell changer and a temperature controller with an in situ temperature probe (Varian). DNA-DNA hybridization was performed at the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany).
Rep-PCR.
To discriminate between strain SZ and its closest relative, G. thiogenes, repetitive extragenic palindromic PCR (Rep-PCR) was conducted using the REP-1R/REP-2I primer pair and 100 to 150 ng of genomic DNA as the template as described previously (42, 48). PCR-amplified fragments were resolved on 1.5% agarose gels run in 1× Tris-acetate-EDTA buffer at 100 V (3.3 V/cm of gel length) for 6 h. The fragments were visualized with UV light following staining for 30 min with aqueous ethidium bromide solution (3 μg/ml) and destaining for 20 min in distilled water.
G+C content.
The mole percent G+C content was determined by a modification of the method described by Mesbah et al. (41). DNA (6 to 11 μg) obtained from acetate-fumarate-grown (10 mM each) cultures of strain SZ and G. thiogenes was diluted to a final volume of 70 μl with sterile distilled water. The solution was heated in a boiling water bath for 2 min and then immediately placed on ice. Then, 5 μl of 0.3 M sodium acetate buffer (pH 5.0), 5 μl of 20 mM ZnSO4, and 5 μl S1 nuclease (0.2 U μl−1 in 30 mM sodium acetate buffer) were added. The samples were incubated at 37°C for 2 h, and 10 μl of bovine intestinal mucosal alkaline phosphatase (0.1 U μl−1) in 0.1 M glycine hydrochloride buffer (pH 10.4) was added. The samples were incubated at 37°C overnight, centrifuged at 14,000 rpm for 4 min, and stored at −20°C until chromatographic analysis.
Characterization of substrate utilization and growth conditions.
Electron acceptor utilization was tested in bicarbonate-buffered medium amended with 5 mM acetate. The following compounds were added to duplicate 160-ml serum bottles containing 100 ml of medium (aqueous concentrations are given in parentheses): PCE (0.17 to 0.33 mM), TCE (0.32 to 0.63 mM), cis-1,2-dichloroethene (DCE) (0.28 mM), trans-1,2,-dichloroethene (0.21 mM), 1,1,-dichloroethene (0.19 mM), vinyl chloride (0.1 mM), carbon tetrachloride (0.1 mM), 1-chloroethane (0.1 mM), 1,1-dichloroethane (0.1 mM), 1,2-dichloroethane (0.1 mM), 1,1,1-trichloroethane (0.1 mM), 1,1,2-trichloroethane (0.1 mM), 1,1,2,2-tetrachloroethane (0.1 mM), trichloroacetate (0.5 to 2 mM), trifluoroacetate (1 mM), U(VI) (0.1 to 2 mM), Fe(III) citrate (5 to 10 mM), NO3− (5 to 10 mM), NO2− (1 to 10 mM), fumarate (5 to 10 mM), malate (5 mM), SO42− (5 to 10 mM), SO3− (0.5 to 5 mM), poorly crystalline Fe(III) oxide (4 to 12 mM, nominal concentration), MnO2 (1 to 2 mM), and oxygen (5 to 10% [vol/vol]). Liquid chloroethenes were added undiluted or from autoclaved hexadecane stock solutions 1 day prior to inoculation (46). All other halogenated solvents were added undiluted using a gas-tight Hamilton syringe (1800 series; Hamilton, Reno, NV). Gaseous compounds were added by plastic syringe. Nonvolatile compounds were added from aqueous, anoxic, neutralized, sterilized stock solutions by syringe. Oxygen was added as sterilized air (breathing quality) (Airgas, Inc., Randor, PA) to medium that did not receive reductants and resazurin. Elemental sulfur was added as an aqueous, repeatedly pasteurized (at 90°C), and powdered S0 suspension. Soluble ferric iron was added from an aqueous, neutralized, and autoclaved ferric citrate stock solution (0.5 M). Poorly crystalline Fe(III) oxide and MnO2 were synthesized and added as described previously (36, 37). A U(VI) stock solution was prepared by dissolving 0.635 g uranyl acetate in 50 ml of a 30 mM bicarbonate solution (17). All cultures received a 1% (vol/vol) inoculum from an acetate-PCE-grown culture that had consumed all PCE.
The ability of the new isolate to use alternate electron donors was evaluated with duplicate bottles amended with 0.33 mM PCE or 5 mM Fe(III) citrate. The following substrates were tested at the concentrations given in parentheses: H2 (50,000 ppmv, 123 μmol), pyruvate (5 mM), yeast extract (0.01 to 0.1%, wt/vol), formate (10 mM), propionate (10 mM), lactate (10 mM), citrate (5 mM), succinate (5 mM), butyrate (10 mM), benzoate (10 mM), glucose (10 mM), methanol (0.08 to 0.4 mM), toluene (0.5 mM), benzene (0.5 mM), and ethanol (0.1 to 0.3 mM). Sodium salts were added from anoxic, sterile stock solutions before inoculation. Sterile H2 gas was added by syringe, and undiluted alcohols, toluene, and benzene were added with a Hamilton syringe. Electron donor oxidation was judged by its consumption and/or the reduction of PCE or Fe(III) compared to that of control cultures that received no electron donor. All cultures received a 1% (vol/vol) inoculum from an acetate-PCE-grown culture that had consumed all acetate.
Possible carbon sources were examined in duplicate cultures amended with PCE or Fe(III) citrate and H2 (50,000 ppmv, 123 μmol). Formate, propionate, lactate, citrate, succinate, butyrate, glucose (5 mM each), and yeast extract (0.1%, wt/vol) were tested. The reduction of PCE and Fe(III) was monitored and compared with that of duplicate control cultures that did not contain H2. The bottles received a 1% (vol/vol) inoculum from an acetate-PCE-grown culture that had consumed all acetate.
Temperature and pH optima were determined in 27-ml anaerobic culture tubes containing 15 ml medium amended with acetate and fumarate (5 mM each). Triplicate vials were incubated at temperatures of 4, 10, 15, 22, 30, 35, and 40°C. To test the effect of pH on the growth of strain SZ, bicarbonate-free medium was amended with 20 mM MES [2-(N-morpholino)ethanesulfonic acid] or 20 mM HEPES. The medium pH values were adjusted to 5.5, 6.0, and 6.5 (MES) and 7.0, 7.5, and 8.0 (HEPES). All vials received a 1% (vol/vol) inoculum from an acetate-fumarate-grown culture and were incubated at 35°C. Growth was judged by measuring the consumption of fumarate and associated succinate formation in triplicate cultures.
PCE dechlorination by phylogenetically related species.
G. thiogenes, G. sulfurreducens, and G. metallireducens were tested for their abilities to dechlorinate PCE. Duplicate cultures were amended with 0.1 mM PCE as the electron acceptor and 5 mM acetate as the electron donor. Experiments were initiated by adding 5% (vol/vol) inoculum from Fe(III) citrate-acetate-grown G. sulfurreducens and G. metallireducens cultures or fumarate-acetate-grown G. thiogenes cultures. Reductive dechlorination of PCE was monitored over a 3-month incubation period. All cultures were incubated statically at room temperature in the dark.
Determination of electron donor consumption threshold concentrations.
Triplicate bottles were amended with an excess electron acceptor [0.33 mM PCE, 5 mM Fe(III) citrate, or 5 mM nitrate], H2 as the sole electron donor (8,333 ppmv, 20.4 μmol), and 5 mM lactate as a carbon source. One set of triplicate control cultures was amended with each electron acceptor and lactate as the carbon source and had N2 and CO2, but no hydrogen, in the headspace. The inoculum (1%, vol/vol) was transferred from dechlorinating cultures that had consumed all acetate. Concentrations of chlorinated compounds, Fe(III), Fe(II), nitrate, ammonium, and H2, were monitored over time. After a constant H2 threshold concentration was reached, another 20.4 μmol of H2 was added to all cultures, and H2 consumption was monitored again.
The acetate consumption threshold concentration for strain SZ was determined using the same three terminal electron acceptors, PCE, Fe(III) citrate, and nitrate. Strain SZ cultures were amended with 0.1 mM acetate and 0.33 mM PCE, 7.5 mM Fe(III) citrate, or 2 mM nitrate. The cultures were incubated at room temperature until reduction of the respective electron acceptor ceased due to electron donor limitation. For the dechlorinating cultures, [14C]acetate (59 μCi/mmol; Sigma-Aldrich, MO) was added to triplicate cultures at concentrations of 447 nM and 894 nM. Triplicate cultures amended with Fe(III) or nitrate received 894 nM [14C]acetate as the electron donor. After no further acetate consumption occurred in dechlorinating cultures, an additional 894 nM [14C]acetate was added and its consumption monitored. All cultures were incubated at room temperature in the dark without agitation.
Rate and yield measurements.
Reduction rates were determined in triplicate 100-ml cultures amended with 5 mM acetate and 0.33 mM PCE, 3 mM Fe(III) citrate, or 3.5 mM NO3− that had received a 3% (vol/vol) inoculum from acetate-PCE-, Fe(III) citrate-, or nitrate-pregrown cultures, respectively. After reduction of the electron acceptor provided was complete, reduced products (cis-DCE, ferrous iron, and ammonium) and acetate concentrations were determined, and the protein increase (difference between initial and final protein concentrations) was estimated.
Influence of different electron acceptors on PCE dechlorination.
To test the influence of alternate electron acceptors on PCE reductive dechlorination, duplicate cultures amended with PCE (0.33 mM) plus Fe(III) citrate (5 mM), PCE plus U(VI) (0.3 mM), and PCE plus nitrate (5 mM), with acetate (10 mM) as the electron donor, were established. The experiments were initiated by adding a 3% (vol/vol) inoculum of a strain SZ culture grown with PCE and acetate that had consumed all PCE. Control cultures were amended with the same electron acceptors but received the same amount of filter-sterilized inoculum. Consumption of electron acceptors and production of cis-DCE, Fe(II), and ammonium were monitored over time. In addition, the effects of sulfur oxyanions (sulfate, up to 10 mM; and sulfite, up to 5 mM) on reductive dechlorination were examined.
Microscopy.
Light micrographs were obtained with an Olympus BX40 microscope after the cells and flagella were stained with silver nitrate (51). Cells were collected by centrifugation (1,288 × g for 20 min at room temperature) from cultures grown with soluble (nitrate, PCE, and fumarate) and insoluble [poorly crystalline Fe(III) oxide, elemental sulfur, and MnO2] electron acceptors. Scanning electron micrographs were obtained from fumarate-grown cultures as described previously (18).
Analytical procedures.
Chloroethenes, chloroethanes, and volatile fatty acids were quantified by gas chromatography as described previously (19). Dehalogenation of trichloroacetic acid (TCA) and trifluoroacetic acid was monitored by chloride and fluoride ion releases. Oxygen concentrations in the headspace were measured with a Hewlett-Packard (HP) model 5890 GC equipped with a thermal conductivity detector and a Chrompack Molsieve 5-Å fused silica column (10 m by 0.53 μm). For analysis of [14C]acetate, 1 ml of culture suspension was made basic by adding 10 μl of 1 M NaOH and pushed through a 0.2-μm Millipore cellulose membrane filter. Radiolabeled acetate was quantified with an HP 1050 high-performance liquid chromatography system equipped with a C-61OH carbohydrate Supelcogel column (Supelco, Bellefonte, PA) and a 500 TR series flow scintillation analyzer (Packard Instrument, Meriden, CT). The solvent system was 0.1% H3PO4 at a flow rate of 0.5 ml/min. Inorganic anions and cations were analyzed with an ion chromatograph equipped with a CD 20 conductivity detector and two AS11-HC 2-mm columns for anions and a CS12A column for cations (Dionex, Sunnyvale, CA). Formation of N2O, a potential denitrification product, was monitored on an HP 6890 gas chromatograph equipped with an HP-1 column (30-m length, 0.32-mm diameter, and 0.25-μm film thickness) and a 63Ni electron capture detector. Sulfide was determined colorimetrically as described previously (7). Fe(II) production was measured using the ferrozine method (45). The decrease of Mn(IV) and U(VI) was monitored spectrophotometrically (4, 25). Growth was monitored by measuring electron acceptor consumption or protein increase. The Coomassie Plus protein assay reagent kit (Pierce Biotechnology, Rockford, Il) was used in accordance with the manufacturer's recommendations to estimate the protein content (18).
Nucleotide sequence accession number.
The nearly complete 16S rRNA gene sequence (1,478 bp) of strain SZ has been deposited in GenBank under accession number AY914177.
RESULTS
Enrichment and isolation.
Microcosms established with Su-Zi Creek sediment amended with acetate and H2 completely dechlorinated PCE to ethene after 6 weeks of incubation. A sediment-free, nonmethanogenic, and cis-DCE-producing culture was obtained after 20 sequential transfers (1% [vol/vol]) to completely synthetic, defined bicarbonate-buffered medium, with acetate as the electron donor and PCE as the electron acceptor. Isolation of a dechlorinating organism, designated strain SZ, was achieved through three consecutive dilution-to-extinction series in semisolid medium. Small (diameter, <0.2 mm) ellipsoid-shaped, white colonies formed in 10−5 dilution tubes after 2 months with PCE provided as the electron acceptor. Larger (diameter, 2 to 3 mm) ellipsoid-shaped, reddish colonies developed down to the 10−6 dilution tubes within 2 weeks with fumarate as the electron acceptor. Isolated colonies grown with PCE and fumarate were transferred to liquid medium, and PCE-to-cis-DCE dechlorination, Fe(III)-to-Fe(II) reduction, and nitrate-to-ammonium reduction activities were recovered within 1 week of incubation at room temperature.
Culture purity.
Microscopic uniformity, recovery of PCE, Fe(III), and nitrate reduction activities from single colonies, identical 16S rRNA gene sequences obtained with genomic DNA extracted from PCE-, Fe(III)-, and nitrate-grown cultures, and indistinguishable RFLP patterns that matched those predicted from in silico digests suggested culture purity. RFLP patterns of MspI- and RsaI-digested amplicons generated from G. thiogenes, G. metallireducens, and G. sulfurreducens genomic DNA showed patterns different from those of strain SZ. HhaI-digested amplicons corroborated culture purity but failed to distinguish strain SZ from G. thiogenes, G. metallireducens, and G. sulfurreducens.
Morphology of strain SZ.
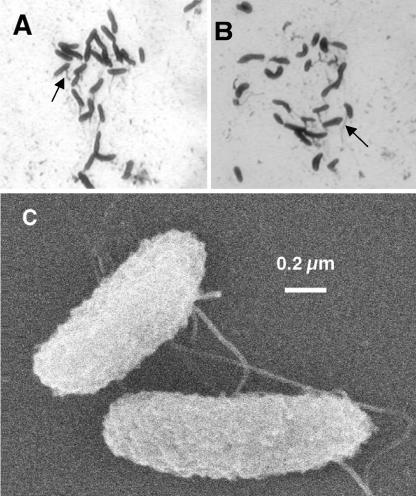
Cells were rod shaped and 1 to 1.4 μm long and 0.4 μm wide. Cell morphology was constant under different growth conditions and during different growth phases. Motility was observed under all growth conditions, including in cultures grown with insoluble electron acceptors [i.e., poorly crystalline Fe(III) oxide and MnO2]. Figures 1A and B illustrate silver nitrate-stained flagella of cells grown with PCE and nitrate, respectively. Scanning electron micrographs revealed flagellated, rough-surface, and rod-shaped cells in the exponential growth phase (Fig. 1C). Spores were never observed.
FIG. 1.
Micrographs of Geobacter lovleyi strain SZ. Light micrographs of silver nitrate-stained cells grown with PCE and acetate (A) and nitrate and acetate (B). The arrows indicate silver-stained flagella. (C) Scanning electron micrograph of cells grown with fumarate and acetate.
Substrate utilization.
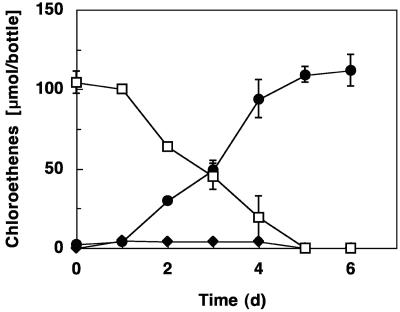
Strain SZ coupled acetate oxidation to the reduction of PCE (Fig. 2), TCE, Fe(III) citrate (Fig. 3), poorly crystalline Fe(III) oxide, nitrate, fumarate, malate, elemental sulfur, U(VI), and Mn(IV). cis-DCE was the only product detected from PCE and TCE dechlorination. Succinate accumulated in cultures that received fumarate and malate, and sulfide was produced in cultures amended with sulfur flower. Nitrate was reduced to ammonium, and the intermediate formation of nitrite was not observed. No N2O formation occurred during growth with nitrate. Strain SZ did not reduce TCA, trifluoroacetic acid, cis-DCE, trans-DCE, 1,1-DCE, vinyl chloride, 1-chloroethane, 1,1-dichloroethane, 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2-trichloroethane, 1,2-dichloropropane, nitrite, sulfate, thiosulfate, or sulfite, with acetate as the electron donor, under the conditions tested. In addition to acetate, pyruvate and H2 served as alternate electron donors. The following compounds were not utilized as electron donors for PCE and Fe(III) reduction: yeast extract, formate, propionate, lactate, citrate, succinate, butyrate, benzoate, glucose, toluene, benzene, methanol, and ethanol. Sustained PCE and Fe(III) reduction with H2 as the electron donor required the presence of acetate or lactate. H2 oxidation and electron acceptor reduction ceased in cultures lacking acetate (or lactate) and could not be stimulated through the addition of formate, propionate, citrate, succinate, butyrate, glucose, or yeast extract, suggesting that these organic compounds cannot fulfill the carbon source requirements of strain SZ.
FIG. 2.
Dechlorination of PCE to cis-DCE with the intermediate formation of small amounts of TCE by strain SZ, with acetate as the electron donor. Data were averaged from triplicates, and error bars depict the standard deviations. □, PCE; •, cis-DCE; and ♦, TCE.
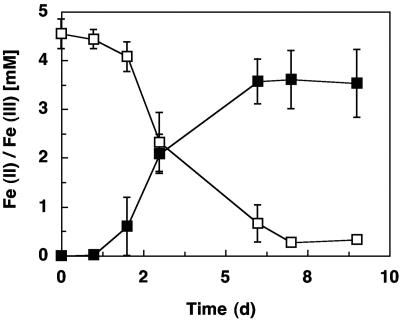
FIG. 3.
Reduction of soluble ferric iron by strain SZ, with acetate as the electron donor. Data from triplicate cultures were averaged, and the error bars depict the standard deviations. ▪, Fe(II); and □, Fe(III).
Under the conditions applied, strain SZ dechlorinated PCE at a maximum rate (average ± standard deviation) of 56.5 ± 0.84 nmol min−1 mg of protein−1 with acetate as the electron donor. Soluble Fe(III) was reduced at a maximum rate of 164 ± 43 nmol min−1 mg of protein−1. Closely related species, including G. thiogenes, G. metallireducens, and G. sulfurreducens, were unable to dechlorinate PCE. Optimum growth of strain SZ occurred between pH 6.5 and 7.2 and a temperature of 35°C. Growth was also observed at 10 and 40°C.
Electron donor consumption threshold concentrations.
In the presence of excess PCE, Fe(III) citrate, and nitrate, strain SZ consumed H2 to concentrations (averages ± standard deviations) of 0.08 ± 0.03, 0.16 ± 0.07, and 0.5 ± 0.06 nM, respectively. No cis-DCE, Fe(II), or ammonium formation was observed in control cultures that did not receive H2. Consistent residual acetate concentrations (averages ± standard deviations) of 3.0 ± 2.1, 1.2 ± 0.5, and 3.6 ± 0.25 nM were observed when PCE, Fe(III) citrate, and nitrate, respectively, were present in excess.
Dechlorination in presence of alternate electron acceptors.
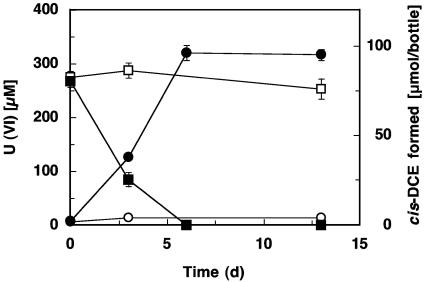
As depicted in Fig. 4, the presence of U(VI) did not inhibit PCE dechlorination, and both electron acceptors were reduced simultaneously. At day 6, both PCE and U(VI) were completely consumed. Neither PCE nor U(VI) reduction was observed in control cultures. Similarly, strain SZ reduced Fe(III) or nitrate concomitantly with PCE (data not shown). Sulfate (10 mM) and sulfite (5 mM) did not affect PCE dechlorination, whereas the presence of oxygen completely inhibited growth of strain SZ.
FIG. 4.
Concomitant reduction of PCE and U(VI) by strain SZ, with acetate as the electron donor. Control cultures received a filter-sterilized inoculum. •, cis-DCE formation; ○, cis-DCE formation in control cultures; ▪, U(VI) depletion; and □, U(VI) depletion in control cultures. Data are averaged from duplicate cultures, and the error bars depict the standard deviations.
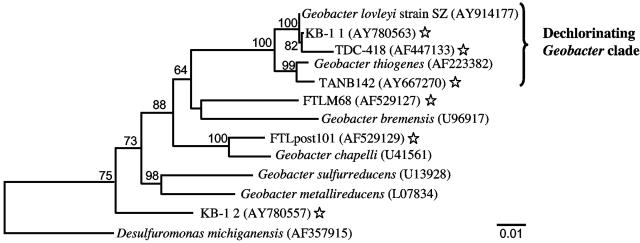
Phylogeny and taxonomy of strain SZ.
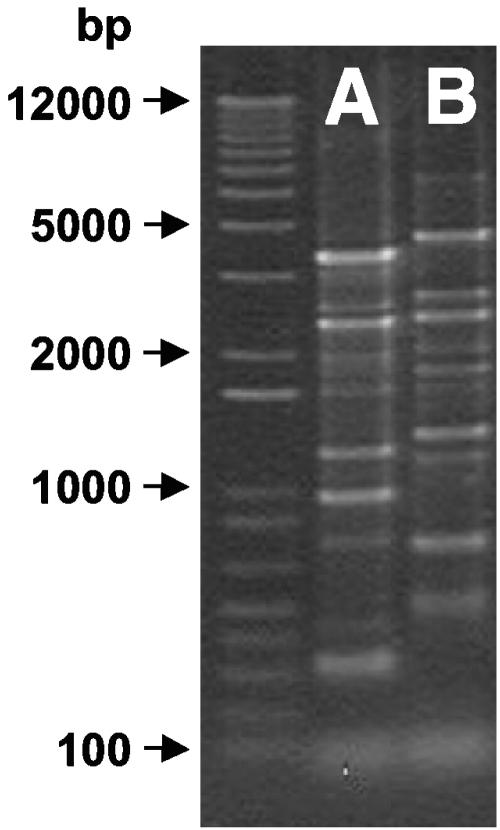
Analysis of strain SZ's 16S rRNA gene sequence indicated an affiliation with the genus Geobacter (Fig. 5). The 16S rRNA molecule of strain SZ shares the secondary structure characteristics of the 16S rRNA molecule typical for members of the Geobacter cluster (33). The closest relative, the TCA dechlorinator G. thiogenes, shares a 98.4% similar 16S rRNA gene sequence. Rep-PCR performed on G. thiogenes and strain SZ genomic DNA revealed unique fingerprints (Fig. 6), and a DNA-DNA similarity value of 25.4% ± 5.8% also suggested that G. thiogenes and the new isolate were different Geobacter species. Phenotypic characteristics further corroborate classifying strain SZ as a distinct species within the Geobacter cluster. Table 1 summarizes properties of G. thiogenes and the new isolate, strain SZ.
FIG. 5.
Inferred phylogenetic tree of strain SZ and related species and environmental clones based on 16S rRNA gene sequences. The bootstrap values at the nodes are based on 1,000 replicates, and only values of >50% are shown. The tree was generated for 1,290-bp aligned positions using Clustal W (MegAlign). Stars indicate environmental clone sequences retrieved from dechlorinating enrichment cultures or chloroethene-contaminated sites. The scale bar represents a 1-bp substitution per 100 nucleotides.
FIG. 6.
Rep-PCR fingerprint patterns of Geobacter lovleyi strain SZ (A) and Geobacter thiogenes strain K1 (B). The first lane shows the 1-kb Plus size marker (Invitrogen).
TABLE 1.
Properties of Geobacter thiogenes strain K1 and Geobacter lovleyi strain SZa
| Organism | Electron donor(s) for dechlorination | Chlorinated electron acceptor(s) | Sulfur-sulfide redox cycleb | Formation of sulfur granules | Acetate consumption threshold for chlororespiration | Cell morphology and size | Motility | Source | G+C content ± SD (mol%) (n = 4) |
|---|---|---|---|---|---|---|---|---|---|
| Geobacter lovleyi strain SZ | Acetate, H2 | PCE, TCE | ND | No | 3 nM | Curved, rod shaped, 1 to 1.4 μm by 0.4 μm | Flagellar motility | Noncontaminated freshwater sediment | 56.7 ± 0.3 |
| Geobacter thiogenes strain K1c | Acetate | TCA | Yes | Yes | 0.1 mM | Curved, rod shapedd | Nonmotile | Contaminated soil | 55.1 ± 0.3e |
The table lists only properties for which data for both species are available.
Apparently, a sulfur-sulfide redox cycle functions as an electron shuttle between acetate and the chlorinated electron acceptor (TCA). ND, not detected.
Data from De Wever et al. (10).
Size has not been reported; our studies suggested that G. thiogenes cells are slightly smaller that strain SZ cells.
Determined in this study.
DISCUSSION
The Geobacter group attracts considerable attention because its members are widespread in anoxic environments and play key roles in environmentally relevant processes (34). Geobacter species affect iron geochemistry in anoxic subsurface environments, reduce toxic metals and radionuclides, and oxidize a variety of organic compounds (e.g., toluene and phenol) (1, 34). Although Geobacter species display versatility with regard to electron donor and electron acceptor utilization, metabolic reductive dechlorination (chlororespiration) has been described for only one species to date: G. thiogenes coupled growth to the reductive dechlorination of TCA to dichloroacetic acid (10). The new isolate, strain SZ, is the first Geobacter species that uses PCE and TCE as metabolic electron acceptors. Growth with PCE as the electron acceptor has been demonstrated previously for members of the Desulfuromonas cluster (i.e., Desulfuromonas chloroethenica and Desulfuromonas michiganensis [26, 46]), and the discovery of strain SZ suggests that metabolic reductive dechlorination is more widely distributed among the family Geobacteraceae.
Microbial reduction of soluble U(VI) to insoluble U(IV) is a promising strategy for containment of uranium plumes. Laboratory and field studies demonstrated that adding acetate can promote activity of native Geobacter species and result in U(VI) reduction and immobilization (2, 15, 21). PCE and nitrate are frequently encountered cocontaminants at uranium-contaminated sites. The changes in free energy under standard conditions associated with PCE-to-cis-DCE dechlorination, nitrate reduction to ammonium, and U(VI) reduction to U(IV) are −72.5, −61.9, and −37.0 kJ per mol of electrons transferred, respectively (free energy of formation values are from references 11, 28, and 50). Electron acceptors are typically oxidized sequentially, with the energetically more favorable electron acceptor consumed first. Hence, one would predict that PCE dechlorination precedes ammonification and U(VI) reduction. Although standard conditions apply rarely to real- world conditions, thermodynamic calculations, assuming environmentally relevant concentrations of reactants and products and low pH values (e.g., groundwater at the Natural and Accelerated Bioremediation Research Field Research Center [www.lbl.gov/NABIR]), predict the same sequence of terminal electron-accepting processes (TEAPs). Such patterns of sequential reduction of electron acceptors have been observed at field sites and confirmed in pure culture studies (3, 16, 49). Strain SZ reduced nitrate, Fe(III) citrate, or U(VI) and PCE simultaneously, suggesting no tight control over consumption of reducing equivalents in less-favorable TEAPs. It is currently unclear whether energy is captured from all simultaneously operating reductive pathways, and the significance of this electron acceptor utilization pattern is unclear. From an ecological point of view, however, this type of behavior might give strain SZ an edge over competing populations in oligotrophic, heterogeneous subsurface environments with periodically changing influx/availability of substrates.
Strain SZ exhibited flagellar motility when grown with insoluble (i.e., amorphous ferric iron and MnO2) and soluble electron acceptors. This contrasts with observations made for G. thiogenes, which never displayed motility, and G. metallireducens. Childers et al. (6) suggested that flagellar synthesis in G. metallireducens is regulated by the available electron acceptor and occurs only when insoluble substrates are used. Apparently, the synthesis of the flagellar apparatus underlies different regulatory mechanisms in members of the Geobacter group, and further research is warranted to elucidate motility controls and how motility affects contaminant transformation.
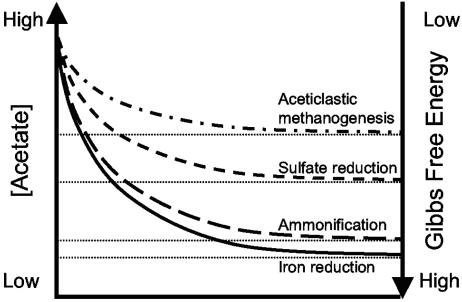
Acetate and H2 are key intermediates in the anaerobic degradation of organic matter, and fluxes of both H2 and acetate control microbial redox processes in subsurface environments. A variety of materials have been suggested and used for delivery of reducing equivalents to support a desired TEAP, such as reductive dechlorination (14). Biostimulation ultimately increases the flux of H2 and acetate (19), but competition for reducing equivalents, in particular for H2, often limits its success. Obviously, organisms with greater electron donor versatility, in particular those that utilize acetate and H2, are more desirable for bioremediation applications aimed at stimulating reductive processes. To our knowledge, strain SZ is the first chlorinated ethene-dechlorinating organism described as utilizing both H2 and acetate as electron donors. This physiological feature offers a distinct advantage in subsurface environments where competition for reducing equivalents is fierce. Further, strain SZ consumes H2 and acetate to low concentrations. It has been shown previously that hydrogenotrophic chloroethene-respiring populations exhibit low H2 consumption threshold concentrations and maintain H2 concentrations below those needed to sustain other TEAPs, such as methanogenesis, acetogenesis, and sulfate reduction (30, 32, 40, 44, 53). Indeed, the H2 consumption threshold of 0.08 nM measured in PCE-fed cultures of strain SZ is in agreement with reported threshold values for chlororespiration (30, 32). Analogously, acetate consumption threshold concentrations depend on the thermodynamics of the TEAP (Fig. 7). Although obviously relevant, information on acetate threshold concentrations is scarce. Lovley and Phillips (35) showed that acetate threshold concentrations were lower in Fe(III)-reducing sediments than in sulfate-reducing and methanogenic sediments. Under mesophilic conditions, the acetate threshold concentration for aceticlastic methane formation ranged from 69 to 1,180 μM (52). Desulfobacter postgatei, an acetate-oxidizing sulfate reducer, consumed acetate to concentrations below 1 μM (24). Recently, He and Sanford (20) determined acetate threshold concentrations in Anaeromyxobacter dehalogenans strain 2CP-C cultures for two different TEAPs. Strain 2CP-C consumed acetate to concentrations of 69 nM with 2-chlorophenol as the electron acceptor (chlororespiration) and an even lower value (<1 nM) when grown with soluble ferric iron. The observed acetate threshold value of 3.0 nM in strain SZ cultures grown with PCE is at least 3 orders of magnitude lower than those reported for methanogens and sulfidogens. According to the threshold model, acetate-oxidizing dechlorinators should outcompete acetotrophic methanogens and sulfidogens for acetate. Hence, analogous to the H2 consumption threshold model, acetate consumption threshold concentrations could serve as a diagnostic tool for the presence and activity of acetotrophic dechlorinators and for the delineation of TEAPs in subsurface environments.
FIG. 7.
Conceptual acetate consumption threshold model. Analogous to the hydrogen consumption threshold model, the energetics of the TEAP control the minimum acetate concentration (i.e., the threshold) that can be consumed. Acetate is consumed to a lower threshold concentration as the change in Gibbs free energy associated with the TEAPs increases.
Interestingly, 16S rRNA gene sequences with high similarity to strain SZ were detected in a TCE-contaminated, deep fractured basalt (38) and in two PCE-to-ethene-dechlorinating mixed cultures, including consortium KB-1 (Fig. 5) (9, 12). The KB-1 consortium was maintained for many years with PCE as the electron acceptor, suggesting that the Geobacter organisms present are capable of capturing energy from chloroethene reductive dechlorination. Although we failed to detect PCE dechlorination in other available Geobacter isolates, further studies are needed to explore whether the ability to dechlorinate is more widely distributed among the Geobacter cluster or restricted to a single clade, currently comprising two isolates, G. thiogenes strain K1 and Geobacter lovleyi strain SZ.
Description of Geobacter lovleyi sp. nov.
Geobacter lovleyi was named to recognize the contributions of Derek R. Lovley to our understanding of microbial metal and radionuclide reduction. Geobacter lovleyi is a rod-shaped, motile, gram-negative, and anaerobic bacterium with cell dimensions of 1 to 1.4 μm by 0.4 μm. The G+C content of strain SZ is 56.7 ± 0.3 mol%. Electron donors include acetate, pyruvate, and H2. PCE, TCE, nitrate, soluble and insoluble forms of ferric ion, manganic ion, sulfur, fumarate, malate, and U(VI) are used as electron acceptors. PCE is reduced to cis-DCE as the final product. Optimum growth occurs at 35°C and pH 6.8. Strain SZ was isolated from noncontaminated freshwater sediment collected from Su-Zi Creek, South Korea. Phylogenetic, genotypic, and phenotypic characteristics place strain SZ in the Geobacter cluster within the family Geobacteraceae in the δ-subclass of the Proteobacteria and warrant classifying strain SZ as the type strain of a new species, Geobacter lovleyi sp. nov. Strain SZ has been deposited at the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSM 17278) and the ATCC (BAA-1151).
Acknowledgments
This research was funded by the Natural and Accelerated Bioremediation Research (NABIR) Program, Biological and Environmental Research (BER), U.S. Department of Energy (grant no. DE-FG02-04ER63718). Part of this material is based upon work supported by the National Science Foundation under grant no. 0090496 (Career Award to F.E.L.).
We thank Soojin Yi for guidance with the bootstrap analysis, Robert Apkarian from Emory University's Integrated Microscopy and Microanalytical Facility for help with electron microscopy, Jon Holt for technical assistance in determining acetate threshold concentrations, and Barny Whitman for helpful comments on the manuscript.
Footnotes
In memory of Rob Apkarian. His microscopy skills and enthusiasm will truly be missed.
REFERENCES
- 1.Anderson, R. T., and D. R. Lovley. 2002. Microbial redox interactions with uranium: an environmental perspective, p. 205-223. In M. J. Keith-Roach and F. R. Livens (ed.), Interactions of microorganisms with radionuclides. Elsevier Science Limited, Amsterdam, The Netherlands.
- 2.Anderson, R. T., H. A. Vrionis, I. Ortiz-Bernad, C. T. Resch, P. E. Long, R. Dayvault, K. Karp, S. Marutzky, D. R. Metzler, A. Peacock, D. C. White, M. Lowe, and D. R. Lovley. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beliaev, A. S., D. K. Thompson, T. Khare, H. Lim, C. C. Brandt, G. Li, A. E. Murray, J. F. Heidelberg, C. S. Giometti, J. Yates III, K. H. Nealson, J. M. Tiedje, and J. Zhou. 2002. Gene and protein expression profiles of Shewanella oneidensis during anaerobic growth with different electron acceptors. OMICS J. Integr. Biol. 6:39-60. [DOI] [PubMed] [Google Scholar]
- 4.Burnes, B. S., M. J. Mulberry, and T. J. DiChristina. 1998. Design and application of two rapid screening techniques for isolation of Mn(IV) reduction-deficient mutants of Shewanella putrefaciens. Appl. Environ. Microbiol. 64:2716-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cashion, P., M. A. Hodler-Franklin, J. McCully, and M. Franklin. 1977. A rapid method for base ratio determination of bacterial DNA. Anal. Biochem. 81:461-466. [DOI] [PubMed] [Google Scholar]
- 6.Childers, S. E., S. Ciufo, and D. R. Lovley. 2002. Geobacter metallireducens accesses insoluble Fe(III) oxide by chemotaxis. Nature 416:767-769. [DOI] [PubMed] [Google Scholar]
- 7.Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-458. [Google Scholar]
- 8.De Ley, J., H. Cattoir, and A. Reynaerts. 1970. The quantitative measurement of DNA hybridization from renaturation rates. Eur. J. Biochem. 12:133-142. [DOI] [PubMed] [Google Scholar]
- 9.Dennis, P. C., B. E. Sleep, R. R. Fulthorpe, and S. N. Liss. 2003. Phylogenetic analysis of bacterial populations in an anaerobic microbial consortium capable of degrading saturation concentrations of tetrachloroethylene. Can. J. Microbiol. 49:15-27. [DOI] [PubMed] [Google Scholar]
- 10.De Wever, H., J. R. Cole, M. R. Fettig, D. A. Hogan, and J. R. Tiedje. 2000. Reductive dehalogenation of trichloroacetic acid by Trichlorobacter thiogenes gen. nov., sp. nov. Appl. Environ. Microbiol. 66:2297-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolfing, J., and J. E. M. Beurskens. 1995. The microbial logic and environmental significance of reductive dehalogenation, p. 143-206. In J. G. Jones (ed.), Advances in microbial ecology, vol. 14. Plenum Press, New York, N.Y. [Google Scholar]
- 12.Duhamel, M., K. Mo, and E. A. Edwards. 2004. Chracterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl. Environ. Microbiol. 70:5538-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis, D. E., E. J. Lutz, J. M. Odom, J. Ronald, J. Buchanan, C. L. Bartlett, M. D. Lee, M. R. Harkness, and K. A. Deweerd. 2000. Bioaugmentation for accelerated in situ anaerobic bioremediation. Environ. Sci. Technol. 34:2254-2260. [Google Scholar]
- 14.Fennell, D. E., J. M. Gossett, and S. H. Zinder. 1997. Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ. Sci. Technol. 31:918-926. [Google Scholar]
- 15.Finneran, K. T., R. T. Anderson, K. P. Nevin, and D. R. Lovley. 2002. Potential for bioremediation of uranium-contaminated aquifers with microbial U(VI) reduction. Soil Sediment. Contam. 11:339-357. [Google Scholar]
- 16.Finneran, K. T., M. E. Housewright, and D. R. Lovley. 2002. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 4:510-519. [DOI] [PubMed] [Google Scholar]
- 17.Gorby, Y. A., and D. R. Lovley. 1992. Enzymatic uranium precipitation. Environ. Sci. Technol. 26:205-207. [Google Scholar]
- 18.He, J., K. M. Ritalahti, K.-L. Yang, S. S. Koenigsberg, and F. E. Löffler. 2003. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62-65. [DOI] [PubMed] [Google Scholar]
- 19.He, J., Y. Sung, M. E. Dollhopf, B. Z. Fathepure, J. M. Tiedje, and F. E. Löffler. 2002. Acetate versus hydrogen as direct electron donors to stimulate the microbial reductive dechlorination process at chloroethene-contaminated sites. Environ. Sci. Technol. 36:2945-3952. [DOI] [PubMed] [Google Scholar]
- 20.He, Q., and R. A. Sanford. 2004. Acetate threshold concentrations suggest varying energy requirements during anaerobic respiration by Anaeromyxobacter dehalogenans. Appl. Environ. Microbiol. 70:6940-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes, D. E., K. T. Finneran, R. A. O'Neil, and D. R. Lovley. 2002. Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl. Environ. Microbiol. 68:2300-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes, D. E., K. Nevin, and D. R. Lovley. 2004. Comparison of 16S rRNA, nifD, recA, gyrB, rpoB and fusA genes within the family Geobacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 54:1591-1599. [DOI] [PubMed] [Google Scholar]
- 23.Huss, V. A. R., H. Festl, and K. H. Schleifer. 1983. Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst. Appl. Microbiol. 4:184-192. [DOI] [PubMed] [Google Scholar]
- 24.Ingvorsen, K., A. J. B. Zehnder, and B. B. Jørgensen. 1984. Kinetics of sulfate and acetate uptake by Desulfobacter postgatei. Appl. Environ. Microbiol. 47:403-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, D. A., and T. M. Florence. 1971. Spectrophotometric determination of uranium(VI) with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol. Anal. Chim. Acta 53:73-79. [Google Scholar]
- 26.Krumholz, L. R., R. Sharp, and S. Fishbain. 1996. A freshwater anaerobe coupling acetate oxidation to tetrachloroethene dehalogenation. Appl. Environ. Microbiol. 62:4108-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: Molecular Evolutionary Genetics Analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 28.Langmuir, D. 1997. Aqueous environmental geochemistry. Prentice Hall, Upper Saddle River, N.J.
- 29.Lendvay, J. M., F. E. Löffler, M. Dollhopf, M. R. Aiello, G. Daniels, B. Z. Fathepure, M. Gebhard, R. Heine, R. Helton, J. Shi, R. Krajmalnik-Brown, C. L. Major, Jr., M. J. Barcelona, E. Petrovskis, J. M. Tiedje, and P. Adriaens. 2002. Bioreactive barriers: bioaugmentation and biostimulation for chlorinated solvent remediation. Environ. Sci. Technol. 37:1422-1431. [Google Scholar]
- 30.Löffler, F. E., and R. A. Sanford. 2005. Analysis of trace hydrogen metabolism. Methods Enzymol. 397:222-237. [DOI] [PubMed] [Google Scholar]
- 31.Löffler, F. E., Q. Sun, J. Li, and J. M. Tiedje. 2000. 16S rRNA gene-based detection of tetrachloroethene-dechlorinating Desulfuromonas and Dehalococcoides species. Appl. Environ. Microbiol. 66:1369-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Löffler, F. E., J. M. Tiedje, and R. A. Sanford. 1999. Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl. Environ. Microbiol. 65:4049-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonergan, D. J., H. L. Jenter, J. D. Coates, E. J. P. Phillips, T. M. Schmidt, and D. R. Lovley. 1996. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J. Bacteriol. 178:2402-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovley, D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:119-286. [DOI] [PubMed] [Google Scholar]
- 35.Lovley, D. R., and E. J. P. Phillips. 1987. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl. Environ. Microbiol. 53:2636-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovley, D. R., and E. J. P. Phillips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macbeth, T. W., D. E. Cummings, S. Spring, L. M. Petzke, and K. S. Sorenson. 2004. Molecular characterization of a dechlorinating community resulting from in situ biostimulation in a trichloroethene-contaminated deep, fractured basalt aquifer and comparison to a derivative laboratory culture. Appl. Environ. Microbiol. 70:7329-7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Major, D. W., M. L. McMaster, E. E. Cox, E. A. Edwards, S. M. Dworatzek, E. R. Hendrickson, M. G. Starr, J. A. Payne, and L. W. Buonamici. 2002. Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environ. Sci. Technol. 36:5106-5116. [DOI] [PubMed] [Google Scholar]
- 40.Mazur, C. S., and W. J. Jones. 2001. Hydrogen concentrations in sulfate-reducing estuarine sediments during PCE dehalogenation. Environ. Sci. Technol. 35:4783-4788. [DOI] [PubMed] [Google Scholar]
- 41.Mesbah, M., U. Permachandran, and W. B. Whitman. 1989. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int. J. Syst. Bacteriol. 39:159-167. [Google Scholar]
- 42.Rademaker, J. L., B. Hoste, F. J. Louws, K. Kersters, J. Swings, L. Bauterin, P. Vauterin, and F. J. de Bruijn. 2000. Comparison of AFLP and rep-PCR genomic fingerprinting with DNA-DNA homology studies: Xanthomonas as a model system. Int. J. Syst. Evol. Microbiol. 50:665-677. [DOI] [PubMed] [Google Scholar]
- 43.Ritalahti, K. M., and F. E. Löffler. 2004. Populations implicated in anaerobic reductive dechlorination of 1,2-dichloropropane in highly enriched bacterial communities. Appl. Environ. Microbiol. 70:4088-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smatlak, C. R., and J. M. Gossett. 1996. Comparative kinetics of hydrogen utilization for reductive dechlorination of tetrachloroethene and methanogenesis in an anaerobic enrichment culture. Environ. Sci. Technol. 30:2850-2858. [Google Scholar]
- 45.Stookey, L. L. 1970. Ferrozine-a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 46.Sung, Y., K. M. Ritalahti, R. A. Sanford, J. W. Urbance, S. J. Flynn, J. M. Tiedje, and F. E. Löffler. 2003. Characterization of two tetrachloroethene-reducing, acetate-oxidizing anaerobic bacteria and their description as Desulfuromonas michiganensis sp. nov. Appl. Environ. Microbiol. 69:2964-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.U.S. Department of Energy. 1999. From cleanup to stewardship. Office of Environmental Management, U.S. Department of Energy, Washington, D.C.
- 48.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan, X.-F., N. C. VerBerkmoes, L. A. McCue, D. Stanek, H. Connelly, L. J. Hauser, L. Wu, X. Liu, T. Yan, A. Leaphart, R. L. Hettich, J. Zhou, and D. K. Thompson. 2004. Transcriptomic and proteomic characterization of the Fur modulon in the metal-reducing bacterium Shewanella oneidensis. J. Bacteriol. 186:8385-8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weast, R. C. 1986. CRC handbook of chemistry and physics, 67th ed. CRC Press, Boca Raton, Fla.
- 51.West, M., N. M. Burdash, and F. Freimuth. 1977. Simplified silver-plating stain for flagella. J. Clin. Microbiol. 6:414-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westermann, P., B. K. Ahring, and R. A. Mah. 1989. Threshold acetate concentrations for acetate catabolism by aceticlastic methanogenic bacteria. Appl. Environ. Microbiol. 55:514-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, Y., and P. L. McCarty. 1998. Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ. Sci. Technol. 32:3591-3597. [Google Scholar]