Abstract
Treatment of either Mycobacterium tuberculosis or M. smegmatis with ethambutol results both in inhibition of arabinan synthesis and in copious loss of previously formed arabinan from the cell wall. The loss of arabinan has been shown to be due to the action of an endogenous arabinase. To better understand this phenomenon, a quantitative assay for endogenous arabinase was developed. Using the assay it was determined that various subcellular fractions of M. smegmatis showed significant amounts of endogenous arabinase activity. Surprisingly, treatment with ethambutol yielded only minor changes in the amounts of endogenous arabinase activities. Endogenous arabinase was present in the cell wall, and consistently, incubation of the M. smegmatis cell wall in only buffer resulted in the release of arabinan, mimicking the effect of ethambutol on whole cells. To determine if cell wall arabinan is rapidly turned over, the arabinan was labeled in the early log phase of culture by feeding [14C]glucose, followed by a “chase” with nonradioactive glucose. Most of the labeled arabinan remained in the cell wall after the culture was grown to late log phase. Thus, there is active arabinase in the cell wall, but arabinan is not rapidly removed unless ethambutol is present. Purification of the endogenous arabinase, using the assay described, is ongoing to help further discern its biological function.
The cell wall core of Mycobacterium tuberculosis consists of an inner peptidoglycan layer and an outer lipid mycolic acid layer (3). These two layers are connected by the polysaccharide arabinogalactan (AG) (3). The structure of AG is conveniently divided into three regions: the linker region (11), a d-galactofuranosyl region (4), and a d-arabinofuranosyl region (4). Thus, the arabinofuranosyl region is essential for the connection of the mycolate region to the peptidoglycan. The clinical anti-tuberculosis (TB) drug ethambutol has been demonstrated to inhibit the biosynthesis of arabinan, as first shown some time ago (14) and more recently confirmed (5, 9, 12). The target of ethambutol has been shown to be a protein known as EmbB, as revealed by identification of a plasmid containing M. avium embB which rendered M. smegmatis ethambutol resistant (1). This result has been extended to M. tuberculosis and studied further (8, 10, 15). In addition to its effect of inhibiting arabinan biosynthesis, ethambutol causes a dramatic release of arabinosyl residues present in the cell wall AG before addition of drug (2, 5) as well as in the arabinan present in lipoarabinomannan (7). This observation led to the recognition of the existence of an endogenous arabinase enzyme in mycobacterial extracts (16) capable of cleaving AG solubilized by base treatment from the mycobacterial cell wall. However, studies on the endogenous arabinase have been severely hampered due to the lack of a reliable and quantitative assay. Two pressing reasons for such an assay are to determine the arabinase activity in ethambutol-treated versus non-ethambutol-treated cells and to purify the arabinase so its gene can be located and the enzyme can be thoroughly studied as a potential TB drug target.
The endogenous arabinase is an endoenzyme that cuts deep inside the arabinan (16), yielding very large fragments with few reducing ends, and hence an assay based on the production of arabinosyl reducing ends, such as is often used for polysaccharide-degrading enzymes (17), will necessarily be insensitive. Thus, assay development has relied on separating the radioactive product from the reactant. This was initially attempted based on charge, considering that soluble AG is attached to peptidoglycan fragments and that after arabinase cleavage the arabinan should be neutral and flow through a Dowex anion-exchange column. Although this method worked to some extent, it was not reliable, sensitive, or quantitative. Another avenue that was explored was the separation of the smaller arabinan fragment from starting soluble AG based on solubility in 70% ethanol; the results were similar to those for the anion-exchange method. A third attempt was to separate the released arabinan from the much larger starting material by high-performance liquid chromatography (HPLC) sizing chromatography using an autoinjector. This method showed some promise, but the resolution of the HPLC sizing column degraded too quickly for the method to be practical. With the idea of separating products based on size, we then attempted to separate the product from the starting material by use of microcentrifugal filter units (Microcons) where the separation is effected by passage through membranes with appropriate molecular weight cutoffs. We report here the success of this approach and its use to analyze for the endogenous arabinase in various subcellular fractions of M. smegmatis that has been treated (or not treated) with ethambutol. We then go on to study the arabinan present in cell walls and suggest the possibility of a dynamic cleavage and reattachment of arabinan during mycobacterial growth.
MATERIALS AND METHODS
Preparation of [14C]AG.
Soluble [14C]AG was prepared as the arabinase substrate. Thus, 200 μCi of d-[U-14C]glucose (320 μCi/μmol; American Radiolabeled Chemical Inc.) was added to a 200-ml culture of M. smegmatis mc2155 growing in 7H9 broth during logarithmic growth. After 3 days of labeling, the cells were harvested, and soluble [14C]AG was prepared by base treatment of the cell wall (4). Then the soluble [14C]AG was loaded onto 30-kDa Microcon filter units (Millipore, Billerica, MA) and centrifuged at 14,000 × g for 10 min. Milli-Q water was added to the retained [14C]AG at the top of the membrane, and the sample was centrifuged again; this addition of water and recentrifugation was then repeated twice. The concentration of the resulting purified [14C]AG was adjusted with Milli-Q water to 8,000 to 10,000 cpm per μl. The [14C]AG was divided into aliquots and was kept at −20°C.
Determination of the specific activity of [14C]AG.
[14C]AG (∼20,000 cpm) was hydrolyzed with 100 μl of 2 M trifluoroacetic acid at 110°C for 1 h. The product was dried and then twice dissolved in methanol and dried again. The hydrolyzed product was then dissolved in 100 μl Milli-Q water, 30 μl of which was mixed with 30 nmol ribose (monosaccharide standard) and analyzed by HPLC (Dionex Corporation, Sunnyvale, CA) using a Dionex PA-1 column with a pulsed amperometric detector (PAD; Dionex) and a radiometric detector (Beta Ram; IN/US Systems, Tampa, FL) as described previously (13). The specific activity of the arabinose (Ara) was calculated using the cpm for the Ara peak from the radiometric detector and the areas of the Ara and ribose from the PAD detector.
Crude enzyme preparation.
M. smegmatis mc2155 was cultivated in 8 liters of 7H9 medium at 37°C on a rotary shaker for 3 days. Cells were harvested and broken by a French press in 20 mM phosphate buffer (pH 7.0) containing 100 mM NaCl, 2 mM MgCl2, 2 mM dithiothreitol (DTT), 20% glycerol, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1 mM pepstatin A, and 0.1 mM leupeptin (buffer A). After centrifugation at 27,000 × g, the supernatant was made to contain 60% ammonium sulfate by addition of granular ammonium sulfate (with the pH kept at 7), and the pellet was recovered by centrifugation and resuspended in 10 mM (pH 7.0) phosphate buffer containing 2 mM MgCl2, 2 mM DTT, 0.1 mM PMSF, and 1 M ammonium sulfate. The enzyme solution obtained was applied to a hydrophobic interaction (HI) column (phenyl Sepharose; Amersham division of GE Healthcare, Piscataway, NJ) that was equilibrated with 10 mM phosphate buffer with 1.7 M ammonium sulfate at pH 7.0. The column was washed with the same buffer and eluted with a linear gradient of 1.7 M to 0.0 M ammonium sulfate in 10 mM phosphate buffer; 7.5-ml fractions were collected. The active fractions were pooled and frozen at −80°C. The protein concentration was estimated by using the Micro-Bradford method (Coomassie Plus protein assay reagent; Pierce Chemical, Rockford, IL) with bovine serum albumin as a standard.
Arabinase activity assay.
Arabinase activity was determined using [14C]AG as a substrate. The reaction mixture, containing 2 μl (16,000 to 20,000 cpm) of purified [14C]AG and typically 30 μg (except for the enzyme concentration titration of Fig. 3) of HI-purified enzyme solution in a total of 90 μl of 100 mM phosphate buffer (pH 7.0), was incubated at 37°C for 1 h (except for the various times in the time course reaction [see Fig. 2]). The reaction was terminated by boiling for 10 min, and the reaction mixture was transferred to a 10-kDa Microcon filter unit. Then 200 μl Milli-Q water was added, and the reaction mixture was centrifuged at 14,000 × g for 45 min at room temperature, followed by the addition of 300 μl Milli-Q water and recentrifugation under the same conditions. The radioactivity in the fraction that went through the Microcon was determined by liquid scintillation counting. The counts per minute were converted to nanomoles of Ara18 by using the known specific activity of the Ara in each [14C]AG preparation and to specific activity by using the known amount of protein.
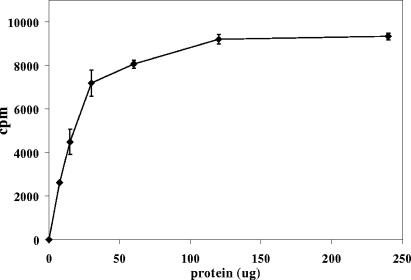
FIG. 3.
Protein concentration dependence of product formation by the arabinase assay. Each data point is the average of three determinations. Error bars, standard deviations.
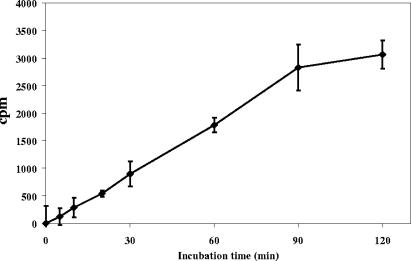
FIG. 2.
Time dependence of product formation as monitored by the arabinase assay. Counts per minute is plotted instead of nanomoles of product, because the former more directly shows the sensitivity of the assay. Each data point is the average of three determinations, except for the data points at 0 and 5 min, which are averages of two determinations. Error bars, standard deviations. The amount of product (in nanomoles) is readily obtained by division of the counts per minute by the specific activity (for this particular preparation of [14C]AG, the specific activity of Ara18 is 4,741).
Effect of pH on arabinase activity.
The optimum pH of the arabinase activity was found by determining the activity with 30 μg crude enzyme at 37°C for 1 h in 100 mM phosphate buffer with different pHs.
Release of arabinan from the cell wall.
A 2-liter culture of M. smegmatis was prepared and broken by a French press as described above for the endogenous arabinase. After centrifugation at 27,000 × g, the pellet (cell wall) was suspended in 1 ml of buffer A, and a 100-μl aliquot was incubated with 200 μl of 50 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 8 with 10 mM MgCl2 and 5 mM β-mercaptoethanol) for various times ranging from none to 48 h at 37°C. Chloroform-methanol (1:1) was added to reach a final extraction ratio of 10:10:3 (CHCl3-methanol-H2O), followed by centrifugation at 5,000 rpm for 15 min. (We had previously determined the surprising fact that the released arabinan fragments were soluble in this solvent.) Subsequently, a part of the supernatant thus obtained (containing the released arabinan) was taken for sugar analysis by the alditol acetate method (17).
Arabinase activity assay on subcellular fractions of ethambutol-treated and control M. smegmatis.
M. smegmatis mc2 155 in four 2-liter flasks was grown in 7H9 medium (0.05% Tween 80) at 37°C on a rotary shaker to an A600 of ∼0.175. Each 2-liter flask was split in half, and ethambutol (100 μg/ml) was added to one half. Pairs of control cells and ethambutol-treated cells were then harvested 0, 1, 2, and 4 h later by centrifugation. Cells were washed once with buffer A and were broken by a French press. Cell walls were obtained by centrifugation at 27,000 × g for 30 min. Supernatants and cell membranes were obtained by ultracentrifugation at 100,000 × g for 2 h. AG was analyzed from part of the cell wall of the 4-h point for glycosyl composition as described previously (4). The arabinase was extracted from the cell wall by suspending it in buffer A containing detergent {0.5% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS]} on ice for 2 h. The solubilized proteins were obtained by centrifugation. The activity assay for supernatants, membranes, and soluble proteins of cell walls of control cells and ethambutol-treated cells was performed as described above.
RESULTS
Use of a Microcon for separation of arabinase-generated product from starting material.
It was found that radioactive arabinan, released from soluble [14C]AG by arabinase, passed through a Microcon with a 10-kDa cutoff. However, variable amounts of background 14C material went through the Microcon when [14C]AG that had not been treated with enzyme was used. Therefore, the [14C]AG substrate was preapplied to a 30-kDa Microcon and the retained material used for arabinase substrate. This pretreatment reduced the background of [14C]AG with no enzyme treatment from about 9% of the radioactivity loaded onto the Microcon to about 1.3% of the radioactivity loaded onto the Microcon and made the detection of small amounts of arabinase activity possible.
Conversion of released radioactivity to nanomoles of product.
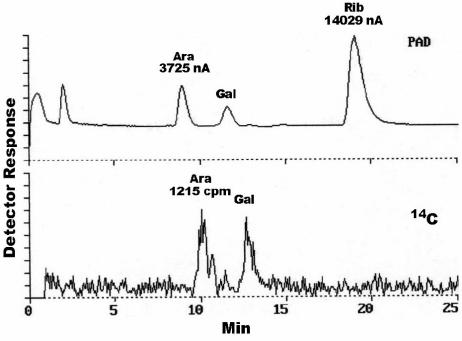
It is desirable to have some absolute scale of activity rather than merely radioactive counts, and for this reason the cpm was correlated to nmol of product released. Two pieces of information were needed: the specific activity of the arabinosyl residues in [14C]AG and the number of arabinosyl residues in the product. The specific activity of the arabinosyl residues in the [14C]AG was calculated using HPLC (after hydrolysis) with both PAD and radiometric detection. The amount of Ara (nmol) was determined by comparison of the areas of the Ara and ribose (internal standard) peaks detected by PAD (Fig. 1). The radioactivity (cpm) in the Ara was determined by measuring the radioactivity of the Ara peak on the Beta-Ram HPLC radioactive counter (Fig. 1). The ratio of radioactivity per nmol of Ara (specific activity) was then readily calculated (see the legend to Fig. 1). The specific activity of the Ara varies from one preparation of [14C]AG to another but typically was about 150 cpm/nmol. With every new preparation of [14C]AG, the specific activity needs to be determined. Recent mass spectral analysis of the released product (data not shown) showed that the major initial product was an “18-mer” of arabinan (Ara18). Thus, one nmol of product would have 18 nmol of arabinosyl units, or typically 2,700 to 4,500 cpm/nmol depending on the [14C]AG preparation. With this information in hand, we defined 1 unit as the amount of arabinase that will release of 1 nmol of Ara18/h at 37°C, and the specific arabinase activity as the units/mg of protein.
FIG. 1.
Determination of the specific activity of the arabinosyl residues in [14C]AG. The amount of Ara is determined by comparison of its area (in nanoamperes) to the area of the known amount of ribose (in nanomoles). The counts per minute of that much Ara is determined directly from the counts per minute calculated by the radiometric detector. Thus, in this specific case the specific activity of Ara is 1,215/[(3,725/14,029) · 30], which equals 152 cpm/nmol Ara or 2,746 cpm/nmol Ara18.
Characterization of the arabinase assay.
For the assay to be quantitative and reliable, it needs to be linear with respect to time. Since the reaction is nonreversible, it was expected that most of the arabinosyl residues could be released before nonlinearity. Considering the amount of radioactivity in the [14C]AG substrate that is present as arabinosyl residues, this leads to a rule of thumb that about 20% of the counts in the starting AG can be converted to product before linearity is lost. The release of product versus time (Fig. 2) and versus protein concentration (Fig. 3) was indeed shown to have a substantial linear component.
pH profile of arabinase.
With the quantitative assay in hand, we determined that the pH optimum of arabinase was pH 7 (data not presented).
Quantitation of the amounts of arabinase in ethambutol- and non-ethambutol-treated M. smegmatis.
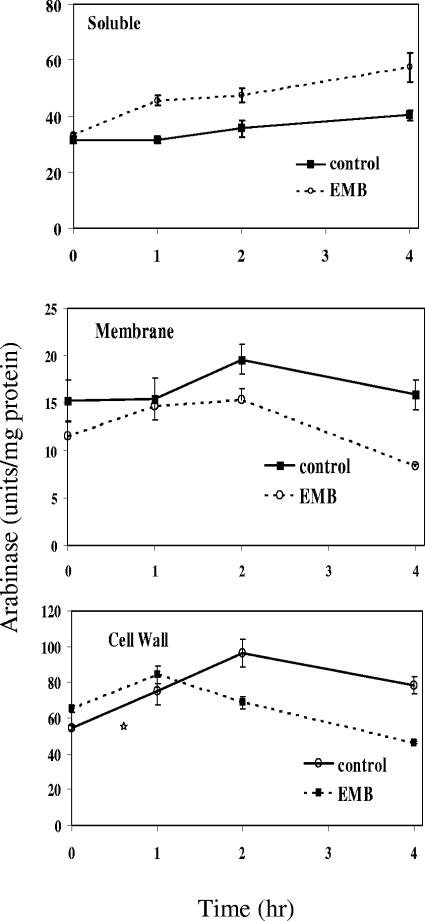
We have previously shown that treatment with ethambutol removes approximately 1/3 of the Ara from the cell wall in 1 to 4 h (5). Therefore, it might be expected that the levels of arabinase go up markedly with treatment with ethambutol. To test this, growing M. smegmatis was treated with 100 μg/ml of ethambutol, and cells were harvested at 0, 1, 2, and 4 h. The cells were then fractionated into cell wall, cell membrane, and soluble fractions. Glycosyl composition analysis of the cell wall fraction at 4 h showed, as expected, that the ratio of Ara to galactose in the cell wall decreased from 1.9 to 1.4 (data not presented). All fractions were then assayed for arabinase. The cell walls were assayed in the presence of 0.5% CHAPS detergent to solubilize the arabinase (preliminary experiments on soluble arabinase showed that this detergent did not interfere with activity). The results are shown in Fig. 4. The major finding is that arabinase was not induced by ethambutol but was present in all three fractions without ethambutol treatment. There was some decrease in the level of arabinase in the membrane and cell wall fractions and a concomitant increase in the soluble fraction with drug treatment. This may be due to leakage of the enzyme, as the cells are damaged, but the major point is the presence of the enzyme under all conditions and its lack of upregulation by ethambutol.
FIG. 4.
Arabinase activity in various subcellular fractions of M. smegmatis with and without ethambutol treatment. After incubation with and without ethambutol, cells were harvested at the times indicated, broken with a French press, and fractionated by centrifugation. The cell wall is the 27,000 × g pellet, the membrane is the 100,000 × g pellet, and soluble material is the 100,000 × g supernatant. Each data point is the average of three determinations, and the entire experiment was repeated (data not shown) with similar results. Error bars, standard deviations.
Ability of endogenous arabinase to release arabinan from isolated cell walls.
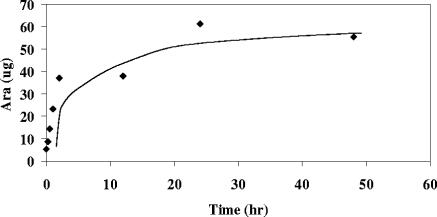
The presence of substantial amounts of arabinase in the cell wall even without ethambutol treatment led to testing whether this arabinase could release arabinan from the isolated cell walls. Incubation of cell walls of M. smegmatis (and M. tuberculosis [results not presented]) with just buffer released arabinan effectively, as shown in Fig. 5. Glycosyl compositional analysis of the remaining insoluble cell wall in similar experiments showed that the final release of arabinan was similar to what is seen in vivo after treatment with ethambutol (5). Thus, in cell walls isolated after use of a French press and centrifugation, the arabinase is both active and capable of releasing arabinan from the cell wall.
FIG. 5.
Release of arabinan from the cell wall of M. smegmatis by merely incubating isolated cell walls in buffer. (Each data point is the result of a single determination, but the release of arabinan by incubating cell walls in buffer was demonstrated repeatedly.)
Stability of arabinosyl residues in the cell wall of M. smegmatis.
The data above (Fig. 4 and 5) suggest the possibility that arabinosyl residues in the cell wall have a very short half-life in vivo. For this reason, the stability of cell wall arabinosyl residues in growing M. smegmatis was investigated. Thus, M. smegmatis was inoculated into 25 ml of 7H9 medium containing 100 μCi of [14C]glucose. After 3 h, the cells were washed with nonradioactive medium containing 1 mg/ml of nonradioactive glucose. Half of the culture was harvested after 6 h and the remainder after 36 h. The isolated cell walls had 186,400 cpm and 152,800 cpm for the 6-h and 36-h cultures, respectively. Hydrolysis followed by HPLC showed that of the cell wall radioactivity, 18,450 and 12,360 cpm were in Ara in the 6- and 36-h samples, respectively, and as a control, 14,700 and 7,860 cpm of galactose were in the two samples, respectively. Thus, most dramatically, the arabinosyl residues were not removed, as would be expected from the robust arabinase activity—indeed, only one-third were lost, no more than the galactosyl residues. Hence, essentially complete turnover of arabinosyl residues during 30 h of growth, as might be expected from the activity of arabinase found in the cell wall (Fig. 5), was not found.
DISCUSSION
A simple and quantitative arabinase assay has been developed.
The assay presented here can be performed quickly and on multiple samples; it is quantitative and allowed for the amounts of arabinase in various subcellular fractions with and without ethambutol to be determined. It is also useful for purification of the arabinase, as discussed further below.
Endogenous arabinase in cell walls is an enigma.
The arabinase assay clearly showed that the amounts of active arabinase in ethambutol-treated and non-ethambutol-treated cells are similar (Fig. 4). Furthermore, the arabinase in these walls is capable of releasing the arabinan from them in a fairly short time (Fig. 5). Earlier experiments (5) showed that some arabinan is released into the culture supernatant (as indeed are other cell wall components [6]). This release increases with ethambutol treatment (5). However, without ethambutol, the arabinan in the cell wall was shown here to be turned over very slowly by a radioactive pulse-chase experiment. Thus, the active arabinase in cell walls is an enigma. It is clear that somehow the inhibition of EmbB with ethambutol results in the arabinase having the immediate and dramatic effect of releasing arabinan from the wall (5). One possible explanation is that under ethambutol-free conditions, the arabinan released by arabinase is quickly reattached via the EmbB protein. However, for that reattachment to occur, some type of activation of the reducing end of the released arabinan would need to occur. Various scenarios are possible; for example, displacement of one of the arabinosyl residues at the reducing end could provide the energy to reattach the arabinan. A fundamentally different possibility is that the arabinase, though in the cell wall, is somehow physically sequestered from its substrate (until in vitro cell breakage or ethambutol treatment). However, this begs the questions of why the enzyme is there during logarithmic growth in the first place. A major tool for answering these intriguing biological questions is the purification of the enzyme. This work is ongoing, and thus far anion-exchange chromatography, HI chromatography, and what appears to be affinity chromatography on a Sephacryl S-100 column have resulted in the purification of the enzyme from about 5 to 35 units/mg in the crude form to approximately 25,000 units/mg, showing that the enzyme is a quantitatively minor protein. Efforts are ongoing to obtain sufficient purity and amounts of protein for sequencing and gene identification.
Acknowledgments
This work was supported by grant AI33706 from the National Institute of Allergy and Infectious Diseases, NIH.
We greatly appreciate the early help with the Microcon assay by Delphi Chatterjee.
REFERENCES
- 1.Belanger, A. E., G. S. Besra, M. E. Ford, K. Mikusova, J. Belisle, P. J. Brennan, and J. M. Inamine. 1996. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc. Natl. Acad. Sci. USA 93:11919-11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boshoff, H. I. M., T. G. Myers, B. R. Copp, M. R. McNeil, M. A. Wilson, and C. E. Barry. 2004. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J. Biol. Chem. 279:40174-40184. [DOI] [PubMed] [Google Scholar]
- 3.Crick, D. C., P. J. Brennan, and M. R. McNeil. 2004. The cell wall of Mycobacterium tuberculosis, p. 115-134. In W. N. Rom and S. M. Garay (ed.), Tuberculosis. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 4.Daffe, M., P. J. Brennan, and M. McNeil. 1990. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C-NMR analyses. J. Biol. Chem. 265:6734-6743. [PubMed] [Google Scholar]
- 5.Deng, L., K. Mikusova, K. G. Robuck, M. Scherman, P. J. Brennan, and M. R. McNeil. 1995. Recognition of multiple effects of ethambutol on metabolism of mycobacterial cell envelope. Antimicrob. Agents Chemother. 39:694-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock, I. C., S. Carman, G. S. Besra, P. J. Brennan, and E. Waite. 2002. Ligation of arabinogalactan to peptidoglycan in the cell wall of Mycobacterium smegmatis requires concomitant synthesis of the two wall polymers. Microbiology 148:3059-3067. [DOI] [PubMed] [Google Scholar]
- 7.Khoo, K. H., E. Douglas, P. Azadi, J. M. Inamine, G. S. Besra, K. Mikusova, P. J. Brennan, and D. Chatterjee. 1996. Truncated structural variants of lipoarabinomannan in ethambutol drug-resistant strains of Mycobacterium smegmatis. Inhibition of arabinan biosynthesis by ethambutol. J. Biol. Chem. 271:28682-28690. [DOI] [PubMed] [Google Scholar]
- 8.Lee, A. S. G., S. N. K. Othman, Y. M. Ho, and S. Y. Wong. 2004. Novel mutations within the embB gene in ethambutol-susceptible clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 48:4447-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, R. E., W. Li, D. Chatterjee, and R. E. Lee. 2005. Rapid structural characterization of the arabinogalactan and lipoarabinomannan in live mycobacterial cells using 2D and 3D HR-MAS NMR: structural changes in the arabinan due to ethambutol treatment and gene mutation are observed. Glycobiology 15:139-151. [DOI] [PubMed] [Google Scholar]
- 10.Lety, M. A., S. Nair, P. Berche, and V. Escuyer. 1997. A single point mutation in the embB gene is responsible for resistance to ethambutol in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 41:2629-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeil, M., M. Daffe, and P. J. Brennan. 1990. Evidence for the nature of the link between the arabinogalactan and peptidoglycan components of mycobacterial cell walls. J. Biol. Chem. 265:18200-18206. [PubMed] [Google Scholar]
- 12.Mikusova, K., R. A. Slayden, G. S. Besra, and P. J. Brennan. 1995. Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob. Agents Chemother. 39:2484-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherman, M., A. Weston, K. Duncan, A. Whittington, R. Upton, L. Deng, R. Comber, J. D. Friedrich, and M. McNeil. 1995. The biosynthetic origin of the mycobacterial cell wall arabinosyl residues. J. Bacteriol. 177:7125-7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takayama, K., and J. O. Kilburn. 1989. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 33:1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telenti, A., W. J. Philipp, S. Sreevatsan, C. Bernasconi, K. E. Stockbauer, B. Wieles, J. M. Musser, and W. R. Jacobs. 1997. The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat. Med. 3:567-570. [DOI] [PubMed] [Google Scholar]
- 16.Xin, Y., Y. Huang, and M. R. McNeil. 1999. The presence of an endogenous endo-d-arabinase in Mycobacterium smegmatis and characterization of its oligoarabinoside product. Biochim. Biophys. Acta 1473:267-271. [DOI] [PubMed] [Google Scholar]
- 17.York, W. S., A. G. Darvill, M. McNeil, T. T. Stevenson, and P. Albersheim. 1986. Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol. 118:3-40. [Google Scholar]