Abstract
An evolutionary algorithm was applied to study the complex interactions between medium parameters and their effects on the isolation of denitrifying bacteria, both in number and in diversity. Growth media with a pH of 7 and a nitrogen concentration of 3 mM, supplemented with 1 ml of vitamin solution but not with sodium chloride or riboflavin, were the most successful for the isolation of denitrifiers from activated sludge. The use of ethanol or succinate as a carbon source and a molar C/N ratio of 2.5, 20, or 25 were also favorable. After testing of 60 different medium parameter combinations and comparison with each other as well as with the standard medium Trypticase soy agar supplemented with nitrate, three growth media were highly suitable for the cultivation of denitrifying bacteria. All evaluated isolation conditions were used to study the cultivable denitrifier diversity of activated sludge from a municipal wastewater treatment plant. One hundred ninety-nine denitrifiers were isolated, the majority of which belonged to the Betaproteobacteria (50.4%) and the Alphaproteobacteria (36.8%). Representatives of Gammaproteobacteria (5.6%), Epsilonproteobacteria (2%), and Firmicutes (4%) and one isolate of the Bacteroidetes were also found. This study revealed a much more diverse denitrifying community than that previously described in cultivation-dependent research on activated sludge.
For nearly 2 decades, molecular biology has provided the tools to successfully overcome the “great plate count anomaly” and allow the study of uncultured microbial diversity (3). The growing awareness that molecular methods cannot or, in very few cases, can only indirectly investigate the function of specific microorganisms in the environment has raised interest in new cultivation efforts and approaches once again (14, 15, 34). Simple adjustments to the classical cultivation approach, such as prolonging the incubation time and avoiding complex or nutrient-rich growth media, have successfully resulted in cultivation of previously uncultured bacteria (12, 30).
A physiological trait such as denitrification, the respiratory reduction of nitrate and nitrite to N2O and nitrogen gas, is not limited to specific microbial taxa and is therefore studied independent of culture through the relevant functional genes (6, 25, 32, 38). To date, however, it is not clear to what extent, if at all, these functional genes contain phylogenetic information. Phillipot (22) showed that the phylogeny of nir and nor genes, coding for the key enzymes nitrite reductase and NO reductase in the denitrification pathway, does not always agree with the phylogeny of the 16S rRNA gene. New isolation and cultivation approaches are therefore imperative to provide the basis for further research on phylogenetic and functional gene diversity.
The isolation of specific physiological groups of bacteria, such as denitrifiers, requires knowledge of the interactions of a large number of medium components and growth conditions. Genetic or evolutionary algorithms (EAs) are heuristic optimization programs based on the Darwinistic principles of evolution by natural selection (10). An EA can aid in rationally deciding which fraction of all possible combinations of medium parameters needs to be tested in practice, with the advantage that it does not assume a model (10). Highly complex optimization problems in various domains as diverse as improvement of silage additives (8) and electricity estimations (21) have been resolved with EAs. In microbiology, their use so far has been limited to optimization of fermentation medium (36, 37) and conditions for transconjugant formation (5).
This paper discusses the optimization of the isolation conditions for denitrifying bacteria. The interactions between different medium parameters were investigated with an evolutionary algorithm. Using a minimal mineral medium as a basis, different combinations of medium parameters were applied as isolation medium for denitrifiers, with activated sludge of a municipal wastewater treatment plant (WWTP) as the inoculum, and the diversity of cultured denitrifiers was assessed.
MATERIALS AND METHODS
Inoculum.
Activated sludge samples were taken at a municipal wastewater treatment plant with subsequent anoxic and aerated tanks (Bourgoyen-Ossemeersen, Ghent, Belgium). Samples (20 ml) were collected from an anoxic tank at the start of each new batch of growth media and immediately processed. Homogenization of the flocs was performed using a needle (diameter, 0.8 mm) and a 50-ml syringe. After homogenization, dilution series of the samples (100 to 10−8) were made and spread plated on the growth media.
EA experimental design.
Each medium parameter can have different values, which can be different levels in concentration or temperature but also different sources of carbon or nitrogen. The combination of these values determines the composition of a growth medium. (The use of the term “growth medium” in this report refers to the composition of the medium and the culture conditions.) Different growth media were grouped into batches. Based on the success or fitness of the growth media from previous batches, a new batch was calculated by the EA. Therefore, the values of the medium parameters of the best scoring growth media were recombined in a new batch of growth media. As a result, the average fitness of each new batch should increase.
Eleven medium parameters with different values were selected as variables for the EA. The number of possible combinations of all parameters with their different values was 1,197,504. Each growth medium made up of a combination of medium parameter values was tested for suitability for isolating denitrifiers and was assigned a fitness value. The fitness value contained the following selection parameters: (i) the number of denitrifying isolates and (ii) the diversity of the denitrifying isolates. The first selection parameter was represented by the ratio between the number of isolated denitrifiers and the total number of isolates (Ratioden) per growth medium. The second selection parameter required knowledge of the identity of the isolated denitrifiers. For this purpose, fatty acid methyl ester (FAME) analysis was chosen as a fast identification method. The observed diversity at the genus level was represented for each growth medium by Simpson's reciprocal diversity index 1/D, calculated as follows:
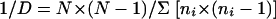 |
(1) |
with N representing the number of denitrifying isolates per medium and ni representing the number of denitrifying isolates per medium belonging to genus i. When only one denitrifier was isolated, the diversity index was 0; when all denitrifiers were assigned to the same genus by FAME analysis, the numerator in equation 1 was set to 1. A fitness value was calculated for each medium based on the results of both selection parameters, with both equally weighted, as follows:
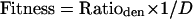 |
(2) |
The fitness of a given growth medium would increase if both the number of denitrifying isolates grown on this medium and the diversity of these denitrifying isolates increased. The combination of medium parameters with the highest fitness will therefore be most suited for use as a growth medium for denitrifiers.
Evolutionary algorithm.
The Simple Evolutionary Algorithm for Optimization (seao) software (31) is available in an easy-to-use graphical user interface and can be freely downloaded (http://www.cran.r-project.org). The configuration and parameterization of the seao software for experimental optimization of the medium composition used the following settings: number of medium parameters, 11; number of growth media, 15; all previous batches were used for calculation of the next batch of growth media; the selection type was fitness based (rescaling = 0); recombination rate, 90%; and mutation followed a uniform distribution (i.e., all possible values have the same chance of being chosen), with a spread of 1.0 and a rate of 15. For the initial batch of growth media, the EA randomly combined medium parameter values into 15 different growth media.
Growth media.
All growth media were based on the mineral medium described by Stanier et al. (29). The following 11 medium parameters with different values were selected for optimization with the EA: pH at 6.5, 7, 7.5, or 8; temperature at 20°C or 37°C; sodium acetate-trihydrate, glycerol, sodium pyruvate, methanol, ethanol, glucose, or sodium succinate as the carbon source; molar C/N ratio of 1, 2.5, 5, 7.5, 10, 12.5, 15, 17.5, 20, 22.5, or 25; potassium nitrate or potassium nitrite as the nitrogen source; nitrogen concentration of 3 mM, 6 mM, 9 mM, 12 mM, 15 mM, or 18 mM; no addition of sodium chloride or a sodium chloride concentration of 0.34 M; 0-, 1-, or 2-ml addition of vitamin solution (17) containing 4 mg 4-aminobenzoic acid, 2 mg d-(+)-biotin, 10 mg nicotinic acid, 5 mg calcium d-(+)-panthothenate, 15 mg pyridoxine hydrochloride, 4 mg folic acid, and 1 mg lipoic acid in 100 ml 10 mM NaH2PO4 at pH 7.1; 0-, 1-, or 2-ml addition of riboflavin solution (17) containing 2.5 mg riboflavin in 100 ml 25 mM NaH2PO4 at pH 3.2; 0-, 1-, or 2-ml addition of thiamine solution (17) containing 10 mg thiamine hydrochloride in 100 ml 25 mM NaH2PO4 at pH 3.4; and cobalamin solution (17) containing 50 mg cyanocobalamin per liter distilled water. The following pH indicator was added (10 μM): bromothymol blue for growth media with a pH of 6.5 or phenol red for growth media with a pH of 7 or higher. Trypticase soy agar (TSA; Oxoid) was supplemented with 10 mM KNO3 and 10 μM phenol red.
Isolation.
A dilution series (100 to 10−8) of activated sludge was spread plated (100 μl) on 15 different growth media per batch, as determined by the EA. The inoculated growth media were incubated for 2 weeks in an anaerobic chamber (gas composition, 8% CO2, 8% H2, 84% N2). From each growth medium and supplemented TSA, 20 isolates were picked from the highest dilution still showing growth, further purified, and subcultured on the same medium (G4M3 was tested in triplicate).
Denitrification tests.
All purified isolates were incubated in liquid isolation medium for 1 week under isolation conditions. Tests for nitrate and nitrite reduction were performed using Griess reagents (27). Selection for denitrifiers was based on the results of the reduction tests and the pH indicator (19). This selection approach was validated by confirmation of the denitrifying activity of all isolates of the first batch with N2O measurements. All isolates of the first batch presumed to denitrify were grown in 50-ml culture flasks with 10 ml liquid isolation medium. The headspace of the vials was replaced with filter-sterilized argon by evacuating five times and refilling. Acetylene (10%) was added to stop the reduction of N2O to N2. After a 1-week incubation, a gas sample (1 ml) was taken with a gas-tight syringe, and N2O was measured with a gas chromatograph (Shimadzu GC-14B) equipped with an electron capture detector, a precolumn (1 m), and a Porapak column (2 m, 80- to 100-mesh).
FAME analysis.
A qualitative and quantitative analysis of cellular fatty acid compositions was performed by the gas-liquid chromatographic procedure described by Sasser (26). The resulting profiles were identified with microbial identification software (MIDI) using the TSBA database, version 5.0 (MIDI, Newark, Del.). In batch 4, some denitrifiers could not be grown under the standard conditions (medium and incubation time) for FAME analysis. Genus identification was then obtained by 16S rRNA gene sequence analysis and used in the same way for the determination of diversity.
DNA extraction.
DNA was extracted from each denitrifying isolate by the guanidium-thiocyanate-EDTA-sarkosyl method described by Pitcher et al. (23) for fast-growing strains and by alkaline lysis for slow-growing isolates. For alkaline lysis, one colony was suspended in an Eppendorf tube with 20 μl of lysis buffer (2.5 ml 10% sodium dodecyl sulfate, 5 ml 1 M NaOH, 92.5 ml MilliQ water). After 15 min at 95°C, 180 ml MilliQ water was added, the tube was centrifuged for 5 min at 13,000 × g, and the supernatant was transferred to a new tube. DNA extracts were stored at −20°C until use.
16S rRNA gene sequence analysis.
PCR amplification was performed as described by Heyrman and Swings (9). The PCR-amplified 16S rRNA gene products were purified using the Nucleofast 96 PCR system (Millipore). For each sequence reaction, a mixture was made using 3 μl purified and concentrated PCR product, 1 μl of BigDye Terminator RR mix, version 3.1 (Perkin-Elmer), 1.5 μl of BigDye buffer (5×), 1.5 μl sterile MilliQ water, and 3 μl (20 ng/μl) of one of the six sequencing primers used. The primers for partial sequencing (reverse 358-339 and reverse 536-519) and the PCR program were previously described by Heyrman and Swings (9). The sequencing products were cleaned up as described by Naser et al. (20). Sequence analysis of the partial 16S rRNA gene (first 300 to 500 bp) was performed using an Applied Biosystems 3100 DNA sequencer according to protocols provided by the manufacturer. Sequences were assembled using BioNumerics 4.0 software (Applied Maths). A reliable identification was obtained by the following two steps: (i) a BLAST search (2) with the 16S rRNA gene sequence of an isolate retrieved 50 sequences with the highest sequence similarities to the query sequence and (ii) all type strains of all species of all genera mentioned in the BLAST report were compared in an exhaustive pairwise manner with the query sequence of each strain in BioNumerics 4.0. The strains were assigned to a genus based on the obtained 16S rRNA gene sequence similarities.
Nucleotide sequence accession numbers.
The nucleotide sequence data generated in this study have been deposited in the GenBank/EMBL/DDBJ databases under accession numbers AM083989 to AM084186.
RESULTS
EA experiment.
An evolutionary algorithm was used to optimize the isolation conditions for denitrifiers. The influence of 11 medium parameters with different values and their combinations on the number and diversity of isolated denitrifying bacteria was examined. Sixty different growth media, i.e., combinations of medium parameter values, were investigated in four subsequent batches, with 15 growth media per batch. Activated sludge from a municipal wastewater treatment plant was used as the inoculum. An overview of the composition and the fitness results of each growth medium per batch is given in Table S1 in the supplemental material.
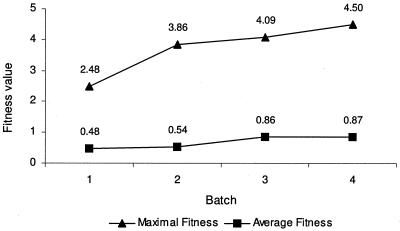
The success of a growth medium was determined as a fitness value (Fig. 1). This fitness selected for (i) a large number of denitrifying bacteria and (ii) a high diversity of denitrifying bacteria (see Materials and Methods). For the first batch, the EA randomly combined medium parameter values into 15 growth media. Batch 1 gave an average fitness of 0.48. In total, 269 isolates were examined and 34 were detected as denitrifiers. The maximal fitness of batch 1 (i.e., 2.48) was assigned to growth medium G1M1, with a nitrite concentration of 3 mM, a molar C/N ratio of 20, succinate as the carbon source, no sodium chloride or riboflavin added, the addition of 1 ml vitamin solution, 2 ml thiamine solution, and 2 ml cobalamin solution, a pH of 6.5, and incubation at 37°C. The EA calculated a second batch, selecting for those medium parameter values that contributed to high fitness in the previous batch. With batch 2, 217 isolates were examined, 33 isolates were detected as denitrifiers, and an average fitness of 0.54 was measured. The results of batches 1 and 2 appeared very similar, except for the maximal fitness, which increased to 3.86 in batch 2 (Fig. 1). Growth medium G2M11, giving the maximal fitness, differed from the best scoring medium of batch 1 only in the pH, which was 7 instead of 6.5. Some growth media in batches 1 and 2 showed no growth, not even from the undiluted activated sludge sample, while others showed growth, but with <20 colonies. This greatly limited the total number of isolates and, subsequently, the number of denitrifiers in these batches. Batch 3 was calculated based on the fitness results for batches 1 and 2. For the third batch, the average fitness increased to 0.86 (Fig. 1), 315 isolates were examined, and 56 denitrifiers were detected, which were clear increases for all three features compared to batches 1 and 2. The maximal fitness (i.e., 4.09) was found for growth medium G3M12, differing from the two former best scoring media in the values of most medium parameters, as follows: a pH of 7.5, ethanol as the carbon source, a low molar C/N ratio of 2.5, a nitrate concentration of 18 mM, 1 ml of thiamine solution, no cobalamin solution added, and an incubation temperature of 20°C. The EA calculated batch 4 based on the three preceding batches. Again, an increased number of denitrifying bacteria was isolated, with 69 denitrifiers from a total of 300 examined isolates. The maximal fitness of 4.50 was assigned to medium G4M3, which differed from G2M11 only in the use of nitrate instead of nitrite as a nitrogen source. This growth medium was arbitrarily chosen for testing in triplicate to investigate the reproducibility of the evolutionary algorithm. The fitness value differed between the three repeats due to a difference in diversity of the isolated denitrifiers (see Table S1 in the supplemental material). The average fitness value (i.e., 0.87) reached a plateau in batch 4, which led to the decision to stop the EA. Supplemented TSA was tested in parallel with each batch. The average fitness value for supplemented TSA was 0.625.
FIG. 1.
Average and maximal fitness values for each batch of growth media. The fitness value of a growth medium represents the success of a combination of medium parameters in rendering a large (relative) number of denitrifying isolates that are highly diverse in genus assignments.
Experimental course of medium parameters.
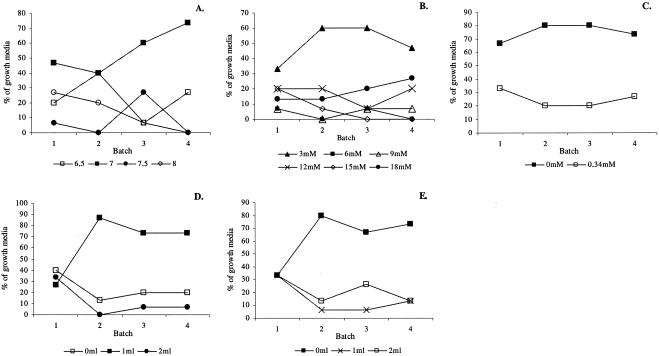
A detailed look at the experimental course of each medium parameter defined by the EA revealed convergence to one optimal value for five medium parameters (Fig. 2). The percentage of growth media with the same medium parameter value is directly correlated with the parameter's contribution to high fitness in the preceding batches. Thus, a pH value of 7, a nitrogen concentration of 3 mM, the addition of 1 ml of vitamin solution, and the exclusion of sodium chloride and riboflavin solution contributed to the success of an elective growth medium for denitrifiers (Fig. 2). The other medium parameters diverged to different values. Both temperature values were equally selected over four batches, with an increasing preference for 20°C in batches 3 and 4. Cobalamin converged to either exclusion or the addition of 2 ml. For the nitrogen source, both nitrite and nitrate were equally selected, with an increasing preference for the latter in batch 4. For thiamine, all three possible values were equally selected. Although no optimal value could be determined, the carbon source and molar C/N ratio diverged to two (i.e., ethanol and succinate) and three (i.e., 2.5, 20, and 25) values, respectively, which were more favorable for isolation of denitrifiers than the other possible values. The best scoring growth medium in batches 1, 2, and 4 incorporated most or all of the optimal values determined for the medium parameters; only the composition of the best scoring medium in batch 3 deviated from these values.
FIG. 2.
Percentages of growth media with certain values for medium parameters for each batch. The experimental course of the following five medium parameters converged to one value: pH (A), nitrogen concentration (B), sodium chloride concentration (C), vitamin solution (D), and riboflavin solution (E). The percentage of growth media with the same value for a medium parameter is directly correlated with its contribution to high fitness in the preceding batches.
Diversity of denitrifying populations in activated sludge.
One hundred ninety-two denitrifying isolates were distinguished in a total of 1,101 isolates obtained on the 60 evaluated growth media, while 7 of 80 isolates obtained on supplemented TSA were able to denitrify. After FAME analysis, 198 denitrifying isolates were reliably identified to the genus level (Table 1) via partial 16S rRNA gene sequence analysis (no 16S rRNA gene amplicon could be obtained for one isolate). The majority of the denitrifiers belonged to the Betaproteobacteria (50.5%, or 100 isolates). Sixty-eight strains were assigned to the Acidovorax, Alicycliphilus, Comamonas, and Diaphorobacter genera of the Comamonadaceae and were isolated predominantly from growth media with ethanol or succinate as the carbon source, coupled with nitrate or nitrite as the nitrogen source, respectively. Thirty-one isolates were assigned to the Azospira, Azovibrio, Dechloromonas, Thauera, and Zoogloea genera of the Rhodocyclaceae, the majority of which were isolated on growth medium with succinate as the carbon source and a pH value of 7. One isolate belonged to the genus Aquaspirillum of the Neisseriaceae. The second biggest group of denitrifiers belonged to the Alphaproteobacteria (37.3%, or 74 isolates): 22 isolates belonged to the Brucella and Ochrobactrum genera of the Brucellaceae, 8 isolates belonged to the Rhizobium and Sinorhizobium genera of the Rhizobiaceae, 43 isolates belonged to the Paracoccus and Pannonibacter genera of the Rhodobacteraceae, and 1 isolate belonging to the genus Methylobacterium represented the Methylobacteraceae. The Gammaproteobacteria were represented by 11 isolates belonging to the genus Pseudomonas (5.6%). Four isolates (2%) belonging to Arcobacter represented the Epsilonproteobacteria. Eight isolates (4%) belonging to the Bacillus, Trichococcus, Enterococcus, Paenibacillus, and Staphylococcus genera represented the Firmicutes. One isolate of the genus Chryseobacterium belonging to the Flavobacteriaceae represented the Bacteroidetes. No clear trends were observed in the compositions of the growth media used for isolation of members of the Alpha-, Gamma-, and Epsilonproteobacteria and Firmicutes.
TABLE 1.
Denitrifying organisms determined in this study
| Taxonomic position (class, family, or genus) | Type strain with highest 16S rRNA gene sequence similarity to query sequence
|
Growth medium (no. of isolates) | |||
|---|---|---|---|---|---|
| Species name | Strain number | % Sequence similarity | Accession number | ||
| Alphaproteobacteria | |||||
| Brucellaceae | |||||
| Brucella | Brucella ovis | ATCC 25840T | 99.5 | L26168 | G3M5 (1) |
| Ochrobactrum | Ochrobactrum anthropi | DSM 6882T | 97.8-100 | D12794 | G1M3 (1), G1M14 (3), G2M6 (1), G2M7 (1), G3M4 (5), G3M5 (3), G3M7 (2), G4M3 (1) |
| Ochrobactrum intermedium | LMG 3301T | 99.2 | U70978 | G2M11 (1), G2M12 (1) | |
| Ochrobactrum tritici | DSM 13340T | 100 | AJ242584 | G3M5 (2) | |
| Methylobacteraceae | |||||
| Methylobacterium | Methylobacterium suomiense | DSM 14458T | 95.1 | AY009404 | G2M4 (1) |
| Rhizobiaceae | |||||
| Rhizobium | Rhizobium giardinii | CIP 105503T | 97.2 | U86344 | G3M12 (1) |
| Rhizobium gallicum | MSDJ1109T | 97.6 | U86343 | G1M15 (1) | |
| Rhizobium radiobacter | ATCC 19358T | 97.2-100 | AJ389904 | G3M2 (1), G1M15 (1) | |
| Rhizobium sullae | DSM 14623T | 97.6 | Y10170 | G1M15 (1) | |
| Sinorhizobium | Sinorhizobium morelense | LC04T | 97.0-97.2 | AY024335 | G1M1 (1), G2M11 (1), G2M12 (1) |
| Rhodobacteraceae | |||||
| Paracoccus | Paracoccus aminophilus | ATCC 49673T | 97.6 | AY014176 | G1M3 (2) |
| Paracoccus alcaliphilus | ATCC 51199T | 97.6-97.8 | AY014177 | G1M3 (1), G2M7 (1), G3M4 (1), G3M7 (1), G3M12 (1), G4M15 (2) | |
| Paracoccus aminovorans | ATCC 49632T | 97.4-99.7 | D32240 | G1M5 (2), G1M14 (3), G2M3 (3), G3M4 (1), G3M5 (1), G4M3 (1), G4M12 (5), G4M15 (3) | |
| Paracoccus carotinifaciens | E-396T | 98.4-98.7 | AB006899 | G3M4 (1), G3M7 (2) | |
| Paracoccus pantotrophus | ATCC 35512T | 100 | Y16933 | G3M5 (1), G3M14 (2) | |
| Paracoccus yeei | CCUG 46822T | 97.8 | AY014173 | G3M4 (1), G3M12 (1), G3M13 (1) | |
| Paracoccus versutus | ATCC 25364T | 99.9-100 | AY014174 | G1M7 (1), G2M4 (1), G2M7 (1), G3M5 (1) | |
| Pannonibacter | Pannonibacter phragmitetus | DSM 14782T | 100 | AJ400704 | G3M2 (1) |
| Betaproteobacteria | |||||
| Comamonadaceae | |||||
| Acidovorax | Acidovorax avenae subsp. citrulli | ATCC 29625T | 98.1-98.3 | AF078761 | G2M6 (1), G2M7 (1) |
| Acidovorax defluvii | DSM 12644T | 99.4-100 | Y18616 | G2M15 (1), G3M2 (1), G3M10 (2), G3M11 (1), G3M12 (1), G3M13 (2), G4M13 (1), G4M14 (1) | |
| Acidovorax temperans | ATCC 49665T | 97.8-99.5 | AF078766 | G1M1 (4), G1M8 (1), G2M15 (2) | |
| Alicycliphilus | Alicycliphilus denitrificans | DSM 14773T | 97.7-99.8 | AJ418042 | G1M1 (3), G3M1 (1) |
| Comamonas | Comamonas aquatica | ATCC 11330T | 99.0-99.8 | AJ430344 | G3M1 (2) |
| Comamonas denitrificans | ATCC 700936T | 98.3-99.9 | AF233877 | G1M15 (1), G2M4 (1), G2M7 (2), G2M9 (1), G2M11 (1), G3M1 (2), G3M3 (2) | |
| G3M9 (1), G3M15 (1), G4M4 (1), G4M5 (3), G4M8 (3), G4M10 (17) | |||||
| Diaphorobacter | Diaphorobacter nitroreducens | DSM 15985T | 99.2-99.8 | AB064317 | G1M1 (3), G2M11 (2), G3M1 (1), G3M12 (1), G4M14 (1) |
| Neisseraceae | |||||
| Aquaspirillum | Aquaspirillum metamorphum | DSM 1837T | 98.9 | Y18618 | G4M3 (1) |
| Rhodocyclaceae | |||||
| Azospira | Azospira oryzae | LMG 9096T | 99.9-100 | AF011347 | G2M11 (1), G4M3 (1) |
| Azovibrio | Azovibrio restrictus | LMG 9099T | 100 | AF011346 | G2M9 (1) |
| Dechloromonas | Dechloromonas agitata | ATCC 700666T | 100 | AF047462 | G4M3 (4), G4M7 (2) |
| Dechloromonas denitrificans | DSM 15892T | 97.2-99.8 | AJ318917 | G4M3 (1), G4M6 (1) | |
| Thauera | Thauera aromatica | DSM 6984T | 99.2-99.3 | X77118 | TSA (2) |
| Thauera aminoaromatica | DSM 14742T | 99.5-100 | AJ315677 | G4M3 (5), G4M7 (2), G4M8 (2) | |
| Thauera chlorobenzoica | ATCC 700723T | 99.4 | AF123264 | TSA (1) | |
| Thauera mechernichensis | DSM 12266T | 98.0 | Y17590 | G2M13 (1) | |
| Thauera phenylacetica | DSM 14743T | 95.0-99.5 | AJ315678 | G2M13 (1), G3M15 (1), G4M6 (2), TSA (1) | |
| Thauera selenatis | ATCC 55363T | 99.1 | Y17591 | TSA (1) | |
| Zoogloea | Zoogloea ramigera | ATCC 19544T | 96.4 | X74913 | G4M6 (1) |
| Species name | Strain number | % Sequence similarity | Accession number | ||
| Gammaproteobacteria | |||||
| Pseudomonadaceae | |||||
| Pseudomonas | Pseudomonas aeruginosa | ATCC 10145T | 99.9-100 | AF094713 | G1M1 (1), G2M11 (1) |
| Pseudomonas alcaligenes | ATCC 14909T | 100 | Z76653 | G2M6 (2), G2M7 (1) | |
| Pseudomonas mendocina | ATCC 25411T | 95.3-95.6 | AJ308310 | G3M9 (1), TSA (1) | |
| Pseudomonas nitroreducens | ATCC 33634T | 99.1-99.3 | D84021 | G1M10 (1), G3M9 (1) | |
| Pseudomonas putida | ATCC 12633T | 98.9 | AJ308313 | G4M9 (1) | |
| Pseudomonas stutzeri | ATCC 17588T | 100 | AF094748 | G2M11 (1) | |
| Epsilonproteobacteria | |||||
| Campylobacteraceae | |||||
| Arcobacter | Arcobacter cryaerophilus | CCUG 17801T | 99.3-99.8 | L14624 | G4M6 (2) |
| Arcobacter skirrowii | ATCC 51132T | 94.6 | L14625 | G4M6 (1) | |
| Arcobacter nitrofigilis | ATCC 33309T | 95.4 | L14627 | G4M6 (1) | |
| Firmicutes | |||||
| Bacillaceae | |||||
| Bacillus | Bacillus clausii | ATCC 700160T | 99.7 | X76440 | G4M3 (1) |
| Bacillus mojavensis | ATCC 51516T | 98.6-98.7 | X68416 | G1M4 (1), G1M8 (1) | |
| Carnobacteraceae | |||||
| Trichococcus | Trichococcus flocculiformis | DSM 2094T | 100 | AJ306611 | G4M6 (1) |
| Enterococcaceae | |||||
| Enterococcus | Enterococcus casseliflavus | ATCC 25788T | 99.2-100 | AF039903 | G1M5 (1) |
| G2M11 (1) | |||||
| Paenibacillaceae | |||||
| Paenibacillus | Paenibacillus agaridevorans | DSM 1355T | 98.6 | AJ345023 | G3M12 (1) |
| Staphylococcaceae | |||||
| Staphylococcus | Staphylococcus hominis subsp. hominis | ATCC 27844T | 99.9 | L37601 | TSA (1) |
| Bacteroidetes | |||||
| Flavobacteriaceae | |||||
| Chryseobacterium | Chryseobacterium gleum | ATCC 35910T | 94.8 | AY468449 | G2M6 (1) |
DISCUSSION
Little is known about the denitrifying diversity present in activated sludge, as straightforward cultivation-independent approaches are not suitable and cultivation-dependent research is limited. Magnusson et al. (18) performed an isolation campaign on nutrient agar with activated sludge from five different municipal WWTPs and found only denitrifying proteobacteria belonging to the Rhodobacteraceae, Comamonadaceae, and Pseudomonadaceae. After applying 60 different defined isolation conditions, a much more important denitrifier diversity was found, although proteobacteria were still predominant. Denitrifying representatives ofAlpha-, Beta-, Gamma-, and Epsilonproteobacteria, Firmicutes, and Bacteroidetes were found, and apart from genera classically known to harbor denitrifiers, such as Pseudomonas, Ochrobactrum, Comamonas, and Acidovorax, genera less frequently observed in cultivation studies of denitrifiers were also encountered. The Rhodocyclaceae were well represented, encompassing, besides the genus Thauera, the recently described genera Azospira and Azovibrio (24) and Dechloromonas (1, 11). Furthermore, possibly new species belonging to Thauera and Zoogloea were retrieved. Recent efforts to identify denitrifiers in activated sludge in a cultivation-independent manner by combining fluorescence in situ hybridization with microautoradiography (35) recognized the Azoarcus-Thauera group of the Rhodocyclaceae as probably the most abundant denitrifiers in industrial WWTPs. The genus Arcobacter was previously found in significant numbers in activated sludge (28), but its function was undetermined. In this study, four denitrifying Arcobacter strains were isolated, demonstrating that the genus can contribute to the denitrification process in activated sludge systems. The denitrifying potential of Bacteroidetes and Firmicutes strains, including Bacillus, Paenibacillus, Staphylococcus, Trichococcus, and enterococci, known from cultivation-independent studies to be numerically less important in WWTPs than the proteobacteria (13), was also established.
This study shows the applicability of an EA for the optimization of growth media. The progressive improvement of the average and maximal fitness values in each successive batch confirms the iterative nature of an EA. The maximal fitness value of each batch of newly designed media was significantly higher than the average fitness of supplemented TSA, which is still the standard growth medium for denitrifiers (33). Highly suitable elective growth media were developed, rendering between 40 and 80% denitrifiers. Comparable data are unavailable for cultivation-dependent studies on activated sludge; for soil, 10% of all isolates on supplemented nitrate broth were denitrifiers (7). After evaluations of 60 different combinations of medium parameters, the three best scoring growth media, G2M11, G3M12, and G4M3, can be recommended for the isolation of denitrifiers in the future.
The isolation conditions for denitrifiers were optimized heuristically. Convergence of a medium parameter to one value indicates no interaction with other medium parameters. The EA determined that five medium parameters converged to one optimal value. Because of their independence of the overall medium composition, these parameters can be fixed at these values in further optimization studies while other medium parameters are varied. Although halotolerant and halophilic denitrifiers are known (16), the exclusion of sodium chloride appeared to increase the isolation of denitrifiers. This observation may be correlated with the use of activated sludge as the inoculum. Riboflavin did not result in an enhanced retrieval of denitrifiers, which contradicts an earlier report on the reduction of the doubling time for Paracoccus denitrificans when riboflavin was added under denitrifying conditions (4). The same study showed an increase in the nitrite reductase activity, thus decreasing the accumulation of nitrite, with ethanol as the carbon source. The suitability of ethanol as a carbon source for denitrifiers was also confirmed here. In contrast to previous optimization studies in microbiology with EAs (5, 8, 36), the reproducibility of fitness was assessed. The observed nonreproducibility of the genus diversity determination was probably attributed to (i) the limited number of investigated strains per growth medium due to logistics and time, (ii) the use of FAME analysis for genus identification, and/or (iii) other possible parameters not included in the EA.
Weuster-Botz (37) stated that “a combination of highly directed random searches to explore the n-dimensional variable space with a genetic algorithm, and subsequent application of classical statistical experimental design is recommended for media development.” The work reported here can be seen as the initial step for elective medium design and development for denitrifying bacteria and provides the basis for further cultivation-dependent research on denitrifiers. Furthermore, through this study, new growth media are available that favor the growth of denitrifiers exhibiting high natural diversity. Also, a large set of denitrifying isolates has been obtained that can be further subjected to research concerning denitrification, e.g., functional gene sequence analysis. Similar large-scale cultivation studies could have future value for physiologically interesting bacterial groups that are difficult to study, e.g., filamentous or nitrifying bacteria.
Supplementary Material
Acknowledgments
The constructive comments on earlier versions of the manuscript from the anonymous reviewers were highly appreciated.
This work was supported by project grant G.O.A. 1205073 (2003-2008) of the Ministerie van de Vlaamse Gemeenschap, Bestuur Wetenschappelijk Onderzoek (Belgium), and by FWO project G20156.02.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Achenbach, L. A., U. Michaelidou, R. A. Bruce, J. Fryman, and J. D. Coates. 2001. Dechloromonas agitata gen. nov., sp. nov. and Dechloromonas suillum gen. nov., two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. Evol. Microbiol. 51:527-533. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Amann, R. I., W. Ludwig, and K.-H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaszczyk, M. 1993. Effect of medium composition on the denitrification of nitrate by Paracoccus denitrificans. Appl. Environ. Microbiol. 59:3951-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon, N., S. Depuydt, and W. Verstraete. 2006. Evolutionary algorithms and flow cytometry to examine the parameters influencing transconjugant formation. FEMS Microbiol. Ecol. 55:17-27. [DOI] [PubMed] [Google Scholar]
- 6.Braker, G., J. Zhou, L. Wu, A. H. Devol, and J. M. Tiedje. 2000. Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in Pacific Northwest marine sediment communities. Appl. Environ. Microbiol. 66:2096-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chèneby, D., L. Philippot, A. Hartmann, C. Hénault, and J.-C. Germon. 2000. 16S rDNA analysis for characterisation of denitrifying bacteria isolated from three agricultural soils. FEMS Microbiol. Ecol. 34:121-128. [DOI] [PubMed] [Google Scholar]
- 8.Davies, Z. S., R. J. Gilbert, R. J. Merry, D. B. Kell, M. K. Theodorou, and G. W. Griffith. 2002. Efficient improvement of silage additives by using genetic algorithms. Appl. Environ. Microbiol. 66:1435-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heyrman, J., and J. Swings. 2001. 16S rDNA sequence analysis of bacterial isolates from biodeteriorated mural paintings in the Servilia tomb (Necropolis of Carmona, Seville, Spain). Syst. Appl. Microbiol. 24:417-422. [DOI] [PubMed] [Google Scholar]
- 10.Holland, J. 1992. Adaptation in natural and artificial systems: an introductory analysis with applications to biology, control and artificial intelligence. MIT Press, Cambridge, Mass.
- 11.Horn, M. A., J. Ihssen, C. Matthies, A. Schramm, G. Acker, and H. L. Drake. 2005. Dechloromonas denitrificans sp. nov., Flavobacterium denitrificans sp. nov., Paenibacillus anaericanus sp. nov. and Paenibacillus terrae strain MH72, N2O-producing bacteria isolated from the gut of the earthworm Aporrectodea caliginosa. Int. J. Syst. Evol. Microbiol. 55:1255-1565. [DOI] [PubMed] [Google Scholar]
- 12.Joseph, S. J., P. Hugenholtz, P. Sangwan, C. A. Osborne, and P. H. Janssen. 2003. Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl. Environ. Microbiol. 69:7210-7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juretschko, S., A. Loy, A. Lehner, and M. Wagner. 2002. The microbial community of a nitrifying-denitrifying activated sludge from an industrial sewage treatment plant analysed by the full-cycle rRNA approach. Syst. Appl. Microbiol. 25:84-99. [DOI] [PubMed] [Google Scholar]
- 14.Kaeberlein, T., K. Lewis, and S. S. Epstein. 2002. Isolating ‘uncultivable’ microorganisms in pure culture in a simulated environment. Science 296:1127-1129. [DOI] [PubMed] [Google Scholar]
- 15.Keller, M., and K. Zengler. 2004. Tapping into microbial diversity. Nat. Rev. Microbiol. 2:141-150. [DOI] [PubMed] [Google Scholar]
- 16.Kim, S.-G., H.-S. Bae, H.-M. Oh, and S.-T. Lee. 2003. Isolation and characterisation of novel halotolerant and/or halophilic denitrifying bacteria with versatile metabolic pathways for the degradation of trimethylamine. FEMS Microbiol. Lett. 225:263-269. [DOI] [PubMed] [Google Scholar]
- 17.Kniemeyer, O., C. Probian, R. Roselló-Mora, and J. Harder. 1999. Anaerobic mineralization of quaternary carbon atoms: isolation of denitrifying bacteria on dimethylmalonate. Appl. Environ. Microbiol. 65:3319-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnusson, G., H. Edin, and G. Dalhammar. 1998. Characterisation of efficient denitrifying bacterial strains isolated from activated sludge by 16S rDNA analysis. Water Sci. Technol. 38:63-68. [Google Scholar]
- 19.Mazoch, J., and I. Kučera. 2002. Detection, with a pH indicator, of bacterial mutants unable to denitrify. J. Microbiol. Methods 51:105-109. [DOI] [PubMed] [Google Scholar]
- 20.Naser, S., F. L. Thompson, B. Hoste, D. Gevers, K. Vandemeulebroecke, I. Cleenwerk, C. C. Thompson, M. Vancanneyt, and J. Swings. 2005. Phylogeny and identification of enterococci using atpA gene sequence analysis. J. Clin. Microbiol. 43:2224-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozturk, H. K., H. Ceylan, O. E. Canyurt, and A. Hepbasli. 2005. Electricity estimation using genetic algorithm approach: a case study of Turkey. Energy 30:1003-1012. [Google Scholar]
- 22.Phillipot, L. 2002. Denitrifying genes in bacterial and archaeal genomes. Biochim. Biophys. Acta 1577:355-376. [DOI] [PubMed] [Google Scholar]
- 23.Pitcher, D. G., L. A. Saunders, and N. A. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thio-cyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 24.Reinhold-Hurel, B., and T. Hurek. 2000. Reassessment of the taxonomic structure of the diazotrophic genus Azoarcus sensu lato and description of three new genera and new species, Azovibrio restrictus gen. nov., sp. nov., Azospira oryzae gen. nov., sp. nov. and Azonexus fungiphilus gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 50:649-659. [DOI] [PubMed] [Google Scholar]
- 25.Rösch, C., A. Mergel, and H. Bothe. 2002. Biodiversity of denitrifying and dinitrogen-fixing bacteria in an acid forest soil. Appl. Environ. Microbiol. 68:3818-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasser, M. 1990. Identification of bacteria by gas chromatography of cellular fatty acids. MIDI technical note 101. MIDI Inc., Newark, Del.
- 27.Smibert, R. M., and N. R. Krieg. 1994. Phenotypic characterization, p. 649. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 28.Snaidr, J., R. Amann, I. Huber, W. Ludwig, and K. H. Schleifer. 1997. Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl. Environ. Microbiol. 63:2884-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanier, R. Y., N. J. Palleroni, and M. Doudoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson, B. S., S. A. Eichorst, J. T. Wertz, T. M. Schmidt, and J. A. Breznak. 2004. New strategies for cultivation and detection of previously uncultured microbes. Appl. Environ. Microbiol. 70:4748-4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sys, K., N. Boon, and W. Verstraete. 2004. Development and validation of evolutionary algorithm software as an optimisation tool for biological and environmental applications. J. Microbiol. Methods 57:309-322. [DOI] [PubMed] [Google Scholar]
- 32.Throbäck, I. N., K. Enwall, Å. Jarvis, and S. Hallin. 2004. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 49:401-417. [DOI] [PubMed] [Google Scholar]
- 33.Tiedje, J. M. 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium, p. 179-244. In A. J. B. Zehnder (ed.), Environmental microbiology of anaerobes. John Wiley & Sons, New York, N.Y.
- 34.Tyson, G. W., and J. F. Banfield. 2005. Cultivating the uncultivated: a community genomics perspective. Trends Microbiol. 13:411-415. [DOI] [PubMed] [Google Scholar]
- 35.Wagner, M., and A. Loy. 2002. Bacterial community composition and function in sewage treatment systems. Curr. Opin. Biotechnol. 13:218-227. [DOI] [PubMed] [Google Scholar]
- 36.Weuster-Botz, D., and C. Wandrey. 1995. Medium optimisation by genetic algorithm for continuous production of formate dehydrogenase. Process Biochem. 30:563-571. [Google Scholar]
- 37.Weuster-Botz, D. 2002. Experimental design for fermentation media development: statistical design or global random search? J. Biosci. Bioeng. 90:473-483. [DOI] [PubMed] [Google Scholar]
- 38.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.