Abstract
Efficient killing of nematodes by Stropharia rugosoannulata Farlow ex Murrill cultures was observed. This fungus showed the ability to immobilize the free-living nematode Panagrellus redivivus Goodey within minutes and to immobilize the pine wilt nematode Bursaphelenchus xylophilus (Steiner & Buhrer) Nickle within hours on agar plates. Moreover, P. redivivus worms were completely degraded by the fungus within 24 to 48 h. The cultures of S. rugosoannulata studied shared the characteristic of abundantly producing cells with finger-like projections called acanthocytes. We showed that the nematode-attacking activity of this fungus is carried out by these spiny acanthocytes and that mechanical force is an important factor in the process. Furthermore, the growth and nematode-attacking activity of the fungus in soil were also determined, and our results suggest that acanthocytes are functional in soil.
In the past, mushrooms were considered saprobes only, but it has been known for several years now that many of them actually have the ability to take advantage of other organisms for nutritional purposes (including nematodes, plants, fungi, and bacteria). In this light, it is particularly interesting to investigate whether certain fungi can function as biocontrol agents against nematodes, because some worms can cause serious damage to crops and/or cause severe animal diseases. It has been discovered that so-called nematophagous fungi are among the fungi that have the potential to kill and decompose nematodes. There is a special group of fungi (nematophagous basidiomycetous fungi) which has clamp connections on the hyphae that are indispensable for their nematode-attacking ability. Many fungi have similar appendages on the hyphae. The appendages within the various species include hourglass-shaped adhesive knobs on members of the genus Nematoctonus (5, 6, 7, 8, 9), with its teleomorph in the genus Hohenbuehelia (2), secretory cells on Pleurotus spp. (3), secretory appendages on Conocybe lactea (11), and stephanocysts on Hyphoderma spp. (4, 21). There is some evidence that the spiny balls produced by Coprinus comatus have a similar role in attacking nematodes, but this should be studied in more detail (15).
Stropharia species have purple-brown to tobacco brown spores and appear in woods, grasslands, compost piles, and animal dung. Stropharia cultures can produce unique stellate cells, which are called acanthocytes. The ability of Stropharia spp. to form acanthocytes seems to be a characteristic of the whole genus, and the consistent occurrence of these structures suggests that they have taxonomic value (10, 16, 17, 18). Farr has made a detailed study of the morphology and development of acanthocytes (10). The acanthocytes produced by the fungus are large cells with a distinct and easily recognizable spiky shape, but the function has not yet been elucidated.
By pure chance we isolated a Stropharia sp. with good acanthocyte-producing ability and an outstanding capacity to destroy nematodes. We immediately wondered whether the nematode-trapping and -killing abilities of this strain were linked to the formation of acanthocytes. We therefore tested two strains of Stropharia rugosoannulata obtained from the Centraalbureau voor Schimmelcultures (CBS681.97 and CBS101784) for their ability to attack and kill the free-living nematode Panagrellus redivivus and the globally recognized harmful pest Bursaphelenchus xylophilus, both in vitro and in soil. We also investigated whether the ability to attack nematodes was due to the acanthocyte formation, and what kind of role the acanthocytes play during the interaction between the fungus and the nematode.
MATERIALS AND METHODS
Strain and cultivation conditions.
Two strains of S. rugosoannulata, designated CBS681.97 and CBS101784 (identical to their accession numbers), were obtained from the Centraalbureau voor Schimmelcultures (CBS). CBS681.97 and CBS101784 were cultivated on cornmeal agar (CMA, consisting of 20 g of cornmeal, 18 g of agar, and 1,000 ml of distilled water) at 24°C for 6 to 8 days in petri dishes (diameter, 9 cm). The free-living nematode P. redivivus was grown axenically in semiliquid oat medium (10 g of oats, 6 ml of distilled water) at 28°C for 4 to 6 days, stored at 4°C, and used within 15 days. The pine wilt nematode B. xylophilus was cultured by a previously described method (14).
Bioassays on cultures.
S. rugosoannulata strains subjected to the bioassay were grown under the conditions described above. The two nematodes were thoroughly rinsed four times before use in the bioassay. The washed nematodes were picked with an inoculating loop, and 50 to 100 worms were transferred to the culture plates at sites with abundant well-developed acanthocytes. Nematodes added to blank CMA plates served as negative controls. The plates were incubated at 24°C. Mobile and immobile P. redivivus and B. xylophilus worms were counted after 15 min and 2 h, respectively, at three random sites using a light microscope. This experiment was carried out with three replicates and conducted twice.
Purification of acanthocytes.
Strains CBS681.97 and CBS101784 were cultured on CMA plates as described above. Parafilm (Pechiney Plastic Packaging) was cut into pieces (1.5 cm by 3 cm). A drop of sterile water was placed on a cut piece of Parafilm, which was then turned over and placed on the agar surface with forceps. After 2 s, the film was gently picked up and immediately put back on the agar at the same site. In order to detach acanthocytes from hyphae, the movements were repeated eight times at one site and then the film was discarded. Each plate was treated with 10 film pieces at 10 sites (a total of 10 plates were treated). Subsequently, each plate received 2 ml of sterile water. The plates were slanted a little, and a pipette was used to wash the plates for 1 min. The resulting suspension was transferred to 1.5-ml Eppendorf tubes and spun for 2 min at 3,000 × g. The supernatant was transferred to new tubes. The sediment was washed twice with 1 ml of sterile water and centrifuged for 2 min at 3,000 × g to remove the water. Before use in the bioassay described below, 10 μl of the sediment was inspected under a light microscope. The acanthocyte suspension was adjusted to 105 cells per ml.
Bioassay with purified acanthocytes.
The acanthocyte suspension was gently mixed using a pipette, and 20 μl of the suspension was moved to a blank water agar (WA) plate. The plate was kept at room temperature for 15 min to allow absorption of excessive water. Panagrellus redivivus was prepared for the bioassay as described above. About 20 nematodes were picked and placed on the WA plate in the area containing acanthocytes. The immediate interaction between acanthocytes and nematodes was studied by microscope at room temperature. After 10 min, mobile and immobile nematodes were counted. This experiment was carried out with three replicates and repeated twice. Acanthocyte-free mycelia were picked with a needle and placed on the same WA plate to construct a mycelium disk. About 20 nematodes added to the mycelium disk served as the control system.
Staining for immobilized nematodes.
A stain solution was prepared by dissolving 0.05 g of crystal violet in 100 ml of distilled water. A drop of this solution was placed on a slide. Some immobilized nematodes in the bioassay with purified acanthocytes were picked with a hair and put into the stain to inspect for injury to the nematodes. The slide was kept at room temperature for 1 min and then observed under a microscope.
ESEM on nematodes immobilized by purified acanthocytes.
Fifty microliters of the purified acanthocyte suspension of CBS681.97 was placed on a cover slide. About 20 P. redivivus worms were then combined with the acanthocytes. They were kept in a moist chamber at room temperature for 30 min to allow for immobilization of nematodes. The cover slides were then quickly fixed on an aluminum stub and immediately put into the sample chamber of an environmental scanning electron microscope (ESEM; Philips XL30). The samples were then viewed in the environmental mode with the microscope operating at 10 to 20 kV.
Culturing of S. rugosoannulata in the field.
To study the behavior of the fungi in natural soil, a wood near Kunming Institute of Botany (Kunming, Yunnan Province, China) was selected to grow strains CBS681.97 and CBS101784. Inocula were obtained according to the method we described previously (15). Two sites (sites 1 and 2) with adequate organic material and satisfactory humidity were selected. Site 1 received three inocula of CBS681.97, whereas site 2 received three inocula of CBS101784. The inocula were buried about 3 cm deep from the ground surface. After the inocula were buried, the sites were humidified with water. The sites were inspected every day for the following 10 days, and the soil was prevented from drying out. Eventually, soil samples at a distance of 1 to 5 cm from the inocula were carefully collected for microscopic observation and bioassays.
Bioassay with soil colonized by S. rugosoannulata.
Soil samples of 50 g were divided into three different levels on the basis of the number of acanthocytes they contained. Soil samples assigned to level 1 contained 50 to 100 acanthocytes per visual field (magnification, ×40). Samples containing 100 to 200 acanthocytes per visual field were considered level 2, and samples considered level 3 contained more than 200 acanthocytes per visual field. Panagrellus redivivus was prepared as described above. Soil samples of 5 g and approximately 500 P. redivivus nematodes in 200 μl of water were combined in a petri dish. The soil samples and worms were then gently mixed and incubated at 25°C for 2 days. After 2 days, the worms were recovered from the soil using Barron's method (1). The number of nematodes retrieved was obtained under a microscope by determining the concentration and volume of nematode suspensions obtained after recovery. Negative controls were performed with soil samples taken 0.5 m away from sites 1 and 2. Very small nematodes (newly born) were ignored. This experiment was carried out with three replicates and conducted twice.
We repeated this experiment, and samples were analyzed at certain time points to illustrate the activity of the fungi in soil. Approximately 200 P. redivivus nematodes were added to 2 g of soil with level 2 acanthocytes. The samples were examined by microscope every hour for the first 8 h, and every 8 h for the following 5 days. The petri dishes were kept in a container for the course of the experiments to maintain a stable humid environment.
RESULTS AND DISCUSSION
Immobilization and degradation of nematodes on agar.
To determine the immobilization and killing effects of S. rugosoannulata strains CBS681.97 and CBS101784 on the nematodes P. redivivus and B. xylophilus, we performed a bioassay. The worms were placed on an agar plate containing one of the S. rugosoannulata strains. As shown in Table 1, 95.5 and 92.9% of the P. redivivus worms added were immobilized after 15 min by S. rugosoannulata strains CBS681.97 and CBS101784, respectively. Bursaphelenchus xylophilus is more resistant to the immobilization effect of the strains than P. redivivus; only 84.3 and 80.6% of the B. xylophilus worms were immobilized by CBS681.97 and CBS101784, respectively, after 2 h of contact with the strains (Table 1). There are highly significant differences between the samples treated and the controls (P < 0.005). As acanthocytes distribute unevenly on the plates, we observed that sites with more acanthocytes killed nematodes more rapidly than those with fewer acanthocytes or without acanthocytes.
TABLE 1.
Immobilization of P. redivivus and B. xylophilus by S. rugosoannulata
| Strain | Nematode | Incubation time | No. of treated nematodes
|
% Immobilized nematodes | No. of control nematodes
|
Statisticsa
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Immobile | Mobile | Immobile | Mobile | χ2 | P | ||||
| CBS681.97 | P. redivivus | 15 min | 85 | 4 | 95.5 | 0 | 61 | 130.6 | <0.005 |
| CBS101784 | P. redivivus | 15 min | 79 | 6 | 92.9 | 2 | 105 | 157.4 | <0.005 |
| CBS681.97 | B. xylophilus | 2 h | 43 | 8 | 84.3 | 0 | 22 | 41.7 | <0.005 |
| CBS101784 | B. xylophilus | 2 h | 79 | 19 | 80.6 | 2 | 37 | 62.7 | <0.005 |
χ2 tests (df = 1) comparing frequencies of mobilized and immobilized nematodes in treated versus control samples.
After the P. redivivus nematodes were immobilized and killed, we observed a strong occurrence of decomposition within 12 to 24 h. After approximately 48 h, the nematodes were completely digested. Then a great number of acanthocytes formed, and that formation was accompanied by the growth of hyphae. The hyphae of the fungus grew out of the digested nematodes within 36 h, and we observed enrichment of the acanthocytes on and around the decomposed worms.
In this bioassay S. rugosoannulata exhibited a high activity to destroy the two nematodes and digested P. redivivus very quickly. It has been suggested that some mushrooms have developed this function of feeding on microfauna to obtain nitrogen supplementation for their survival (15, 19, 20, 21). Stropharia rugosoannulata could be another example. Interestingly, the fungus could kill but not digest B. xylophilus. The dead nematodes could still be distinguished without obvious changes, even after 4 days.
Immobilization of nematodes by purified acanthocytes.
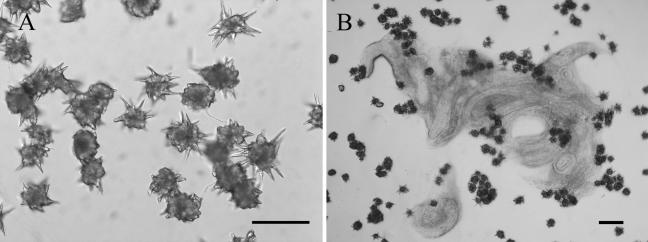
Intact acanthocytes were obtained in reasonable quantity, and the acanthae of the acanthocytes were also kept complete (Fig. 1A). Purified acanthocytes exhibited very high nematicidal activity. As shown in Table 2, more than 95% of P. redivivus worms added were immobilized in 10 min by the two S. rugosoannulata strains. Acanthocytes from the two strains exhibited very close efficacies. Highly significant differences were observed between the treated samples and the controls (P < 0.005). In the bioassay, purified acanthocytes caged the nematodes, but on the control plates, the nematodes moved freely and finally escaped. Figure 1B shows that P. redivivus nematodes were immobilized and killed 2 h after their addition. We noticed that killed nematodes looked like a pasty mass, resembling partially degraded nematodes, on the fungal culture.
FIG. 1.
Purified acanthocytes and immobilization of P. redivivus by these cells on WA. (A) Many purified acanthocytes with complete acanthae. Bar, 20 μm. (B) Nematodes immobilized by purified acanthocytes 12 h after addition of the nematodes. Bar, 100 μm.
TABLE 2.
Immobilization of P. redivivus by purified acanthocytes of S. rugosoannulata
| Straina | No. of treated nematodes
|
% Immobilized nematodes | No. of control nematodes
|
Statisticsb
|
|||
|---|---|---|---|---|---|---|---|
| Immobile | Mobile | Immobile | Mobile | χ2 | P | ||
| CBS681.97 | 19 | 1 | 95.0 | 0 | 22 | 34.4 | <0.005 |
| CBS101784 | 22 | 1 | 95.7 | 0 | 17 | 32.4 | <0.005 |
For both strains, the nematode used was P. redivivus and the incubation time was 10 min.
χ2 tests (df = 1) comparing frequencies of mobilized and immobilized nematodes in treated versus control samples.
Acanthocytes are spiny cells that are produced on the vegetative hyphae of Stropharia species. Possession of this unique cell is a common characteristic of the species of this genus. However, there is limited information with regard to the function of the acanthocytes formed by Stropharia species. Barron provided the following suggestion: “Their function is possibly to protect the succulent nutrient rich hyphae from marauding microfauna” (http://www.uoguelph.ca/∼gbarron/MISC2003/acanthoc.htm). In this test, acanthocytes immobilized and killed nematodes very quickly, indicating that they were effective weapons against nematodes.
Mechanical injury of nematodes by acanthocytes.
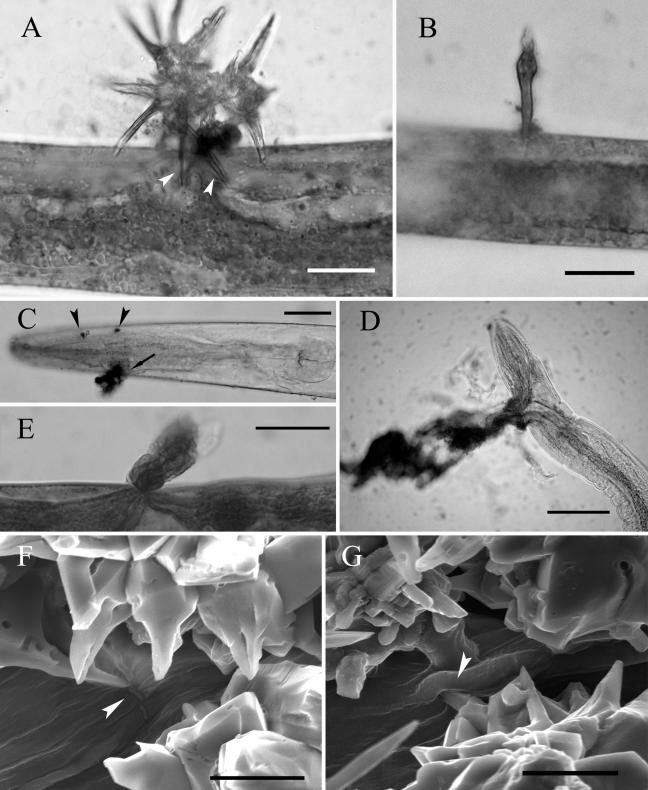
Most of the immobilized and violet-stained nematodes showed obvious wounds caused by the acanthocytes. Under a microscope, wounds were stained quickly, and the color was dark blue. On some selected nematodes, acanthocytes or broken acanthae were found to have pierced the nematode body, with leakage of its inner materials at injured sites (Fig. 2A and B). Slight hurt could leave small cuts without leakage of the inner materials (Fig. 2C). Severe damage caused the serious loss of nematode inner materials (Fig. 2D and E). It was the leakage of inner materials that made the immobilized nematodes look as though they were partially degraded. ESEM study of the interaction between purified acanthocytes and nematodes confirmed the mechanical injury in more detail. Figure 2F shows a long cut on nematode cuticle caused by sharp acanthae. Figure 2G visualizes an acantha that has pierced the nematode body.
FIG. 2.
Mechanical injury of P. redivivus by purified acanthocytes. (A) Two acanthae of acanthocytes pierced the body of an immobilized nematode (arrowheads), and some inner material of the nematode was leaking out at the injured sites. Bar, 10 μm. (B) A broken acantha penetrated a nematode, with a small quantity of inner material leaking out. Bar, 10 μm. (C) A nematode hurt by acanthocytes showed two slight cuts (arrowheads) without leakage and a serious one (arrow) with leakage. Bar, 20 μm. (D and E) Severe damage by the acanthocytes caused serious leakage of inner material of nematodes. Bars, 30 μm. (F) A cut (arrowhead) on a nematode caused by an acantha viewed by the ESEM technique. Bar, 20 μm. (G) An acantha (arrowhead) was shown by the ESEM technique to have pierced the nematode body. Bar, 20 μm.
The results showed that some acanthae resemble a sharp sword causing damage to the nematode cuticle, resulting in leakage of nematode inner materials. This indicates that mechanical force is an important factor in the immobilization and killing of nematodes. On the basis of our observations, we are convinced that the acanthocytes are more than an antifeedant structure for protection. They function as a nematode-attacking device that helps the fungus to obtain nutritional supplementation. Furthermore, this device shows a difference—damaging nematode cuticle—from other known nematode-attacking devices.
Growth of S. rugosoannulata in soil.
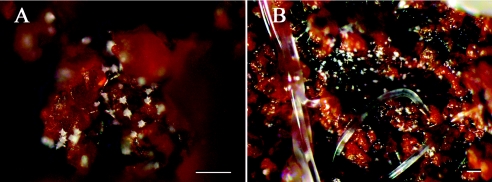
Methods to visualize growth and nematode-attacking activity in soil are limited and instrument dependent (12, 13). With S. rugosoannulata there is a simple way to visualize the growth of the fungus, by tracking the unique spiny acanthocytes. During the growth of S. rugosoannulata in soil, acanthocytes were formed abundantly, and it was not difficult to find these cells on soil particles (Fig. 3A). Acanthocytes were also found in clearance between soil particles and on wood chips and leaf debris in soil. We also found that S. rugosoannulata produced more acanthocytes in soil than it did on agar medium.
FIG. 3.
Growth of S. rugosoannulata and its nematode-attacking ability in natural soil. (A) Some acanthocytes produced on soil particles. (B) Immobilized nematodes at the site with many acanthocytes in field soil. Bars, 100 μm.
Immobilization and degradation of nematodes in soil.
As shown in Fig. 3B, S. rugosoannulata immobilized P. redivivus in soil. Immobilized nematodes would eventually die and decompose, and acanthocytes formed abundantly on the degraded worms. As shown in Table 3, percentages of immobilization ranged from 22.8 to 43.4% for the soil samples tested. Obviously, the soil samples with more acanthocytes (soil levels 2 and 3) had higher immobilization efficiencies than soil samples at level 1 (Table 3). Highly significant differences existed between the treated samples and the controls (P < 0.005).
TABLE 3.
Percentages of nematodes trapped in soil samples colonized by S. rugosoannulata
| Straina | Soil level | No. of nematodes
|
% Trapped nematodes | Statisticsb
|
||
|---|---|---|---|---|---|---|
| Recovered | Control | χ2 | P | |||
| CBS681.97 | 1 | 372 | 466 | 25.6 | 63.7 | <0.005 |
| CBS681.97 | 2 | 352 | 448 | 29.6 | 56.4 | <0.005 |
| CBS681.97 | 3 | 283 | 489 | 43.4 | 238.8 | <0.005 |
| CBS101784 | 1 | 386 | 425 | 22.8 | 9.4 | <0.005 |
| CBS101784 | 2 | 341 | 504 | 31.8 | 188.1 | <0.005 |
| CBS101784 | 3 | 303 | 478 | 39.4 | 177.0 | <0.005 |
In all cases, the nematode used was P. redivivus and the incubation time was 2 days.
χ2 tests (df = 1) comparing frequencies of extracted and trapped nematodes versus control samples.
In soil we did not observe any effect of the mycelia against the worms. The nematodes touching the mycelia were not killed within 5 days as long as they did not touch the acanthocytes. Panagrellus redivivus added to the soil samples was immobilized only at sites containing acanthocytes, suggesting that these structures were functional in their natural habitat. It could be of interest to further investigate the behavior of the fungus in soil and to see whether there is a potential use for S. rugosoannulata to control certain pests, such as Meloidogyne spp., in the future.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grants 30470067 and 30230020) and the Science and Technology Department of Yunnan Province (2005NG05).
We thank Zhuliang Yang for helpful information on the genus Stropharia and the acanthocytes.
REFERENCES
- 1.Barron, G. L. 1977. The nematode-destroying fungi, p. 122-125. Canadian Biological Publications Ltd., Guelph, Ontario, Canada.
- 2.Barron, G. L., and Y. Dierkes. 1977. Nematophagous fungi: Hohenbuehelia the perfect state of Nematoctonus. Can. J. Bot. 55:3054-3062. [Google Scholar]
- 3.Barron, G. L., and R. G. Thorn. 1987. Destruction of nematodes by species of Pleurotus. Can. J. Bot. 65:774-778. [Google Scholar]
- 4.Burdsall, H. H., Jr. 1969. Stephanocysts: unique structures in the Basidiomycetes. Mycologia 61:915-923. [PubMed] [Google Scholar]
- 5.Drechsler, C. 1941. Some hyphomycetes parasitic on free-living terricolous nematodes. Phytopathology 31:773-801. [Google Scholar]
- 6.Drechsler, C. 1943. Two new basidiomycetous fungi parasitic on nematodes. J. Wash. Acad. Sci. 33:183-189. [Google Scholar]
- 7.Drechsler, C. 1946. A clamp-bearing fungus parasitic and predacious on nematodes. Mycologia 38:1-23. [Google Scholar]
- 8.Drechsler, C. 1949. A nematode-capturing fungus with anastomosing clamp-bearing hyphae. Mycologia 41:369-387. [Google Scholar]
- 9.Drechsler, C. 1954. A nematode-capturing fungus with clamp-connections and curved conidia. J. Wash. Acad. Sci. 44:82-85. [Google Scholar]
- 10.Farr, D. F. 1980. The acanthocyte, a unique cell type in Stropharia (Agaricales). Mycotaxon 11:241-249. [Google Scholar]
- 11.Hutchison, L. J., S. E. Madzia, and G. L. Barron. 1996. The presence and antifeedent function of toxin-producing secretory cells on hyphae of the lawn-inhabiting agaric Conocybe lactea. Can. J. Bot. 74:431-434. [Google Scholar]
- 12.Jansson, H.-B., C. Persson, and R. Odeslius. 2000. Growth and capture activities of nematophagous fungi in soil visualized by low temperature scanning electron microscopy. Mycologia 92:10-15. [Google Scholar]
- 13.Jansson, H.-B. 2001. Methods to monitor growth and activity of nematode-trapping fungi in soil. IOBC/WPRS Bull. 24:65-68. [Google Scholar]
- 14.Kano, S., T. Aimi, S. Masumoto, Y. Kitamoto, and T. Morinaga. 2004. Physiology and molecular characteristics of a pine wilt nematode-trapping fungus, Monacrosporium megalosporum. Curr. Microbiol. 49:158-164. [DOI] [PubMed] [Google Scholar]
- 15.Luo, H., M. H. Mo, X. W. Huang, X. Li, and K. Q. Zhang. 2004. Coprinus comatus: a basidiomycete fungus forms novel spiny structures and infects nematodes. Mycologia 96:1218-1225. [PubMed] [Google Scholar]
- 16.Norvell, L. L., and S. A. Redhead. 2000. Stropharia albivelata and its basionym Pholiota albivelata. Mycotaxon 76:315-320. [Google Scholar]
- 17.Redhead, S. A. 1984. Additional Agaricales on wetland Monocotyledoneae in Canada. Can. J. Bot. 62:1844-1851. [Google Scholar]
- 18.Redhead, S. A. 1984. Mycological observations, 4-12: on Kuehneromyces, Stropharia, Marasmius, Mycena, Geopetalum, Omphalopsis, Phaeomarasmius, Naucoria and Prunulus. Sydowia 37:246-270. [Google Scholar]
- 19.Thorn, R. G., and G. L. Barron. 1984. Carnivorous mushrooms. Science 224:76-78. [DOI] [PubMed] [Google Scholar]
- 20.Thorn, R. G., and G. L. Barron. 1986. Nematoctonus and the tribe Resupinateae in Ontario, Canada. Mycotaxon 25:321-453. [Google Scholar]
- 21.Tzean, S. S., and J. Y. Liou. 1993. Nematophagous resupinate basidiomycetous fungi. Phytopathology 83:1015-1020. [Google Scholar]