Abstract
Two highly enriched cultures containing Dehalococcoides spp. were used to study the effect of aceticlastic methanogens on reductive vinyl chloride (VC) dechlorination. In terms of aceticlastic methanogens, one culture was dominated by Methanosaeta, while the other culture was dominated by Methanosarcina, as determined by fluorescence in situ hybridization. Cultures amended with 2-bromoethanesulfonate (BES), an efficient inhibitor of methanogens, exhibited slow VC dechlorination when grown on acetate and VC. Methanogenic cultures dominated by Methanosaeta had no impact on dechlorination rates, compared to BES-amended controls. In contrast, methanogenic cultures dominated by Methanosarcina displayed up to sevenfold-higher rates of VC dechlorination than their BES-amended counterparts. Methanosarcina-dominated cultures converted a higher percentage of [2-14C]acetate to 14CO2 when concomitant VC dechlorination took place, compared to nondechlorinating controls. Respiratory indices increased from 0.12 in nondechlorinating cultures to 0.51 in actively dechlorinating cultures. During VC dechlorination, aqueous hydrogen (H2) concentrations dropped to 0.3 to 0.5 nM. However, upon complete VC consumption, H2 levels increased by a factor of 10 to 100, indicating active hydrogen production from acetate oxidation. This process was thermodynamically favorable by means of the extremely low H2 levels during dechlorination. VC degradation in nonmethanogenic cultures was not inhibited by BES but was limited by the availability of H2 as electron donor, in cultures both with and without BES. These findings all indicate that Methanosarcina (but not Methanosaeta), while cleaving acetate to methane, simultaneously oxidizes acetate to CO2 plus H2, driving hydrogenotrophic dehalorespiration of VC to ethene by Dehalococcoides.
Contamination with chlorinated ethenes, an almost ubiquitous class of pollutants, presently poses a serious threat to groundwater quality in industrialized countries (1). Microbial reductive dechlorination is the major pathway of degradation and detoxification of chloroethenes under anaerobic conditions (26). Dehalorespiring bacteria (DRB; synonym, chlororespiring bacteria) are able to use the energy available from reductive dechlorination in a respiratory process (27, 28); however, sequential dechlorination may result in transient buildup of the highly toxic metabolite vinyl chloride (VC) (15, 44). DRB growing with vinyl chloride as an electron acceptor are to date restricted to the Dehalococcoides group of bacteria (11, 12, 16, 23). All known Dehalococcoides isolates require hydrogen (H2) as a direct electron donor (3, 23, 34). However, in anaerobic substrate degradation, a considerable fraction of organic carbon is converted to methane via acetate. The latter may also be oxidized by sulfate-reducing, Fe(III)-reducing, or denitrifying bacteria (49), while known VC-dechlorinating DRB are unable to use acetate as a direct electron donor. H2 production from acetate is an obligately syntrophic process that may help to overcome this lack of reducing power for DRB. It can be mediated by two different microbial groups: (i) archaea capable of aceticlastic methanogenesis, as well as acetate oxidation, and (ii) syntrophic acetate-oxidizing bacteria. Acetate oxidation to CO2 plus H2 becomes thermodynamically favorable when parallel processes remove the produced hydrogen. These may include hydrogenotrophic methanogenesis, sulfate reduction, or reductive dechlorination.
Presently, there is only one study indicating syntrophic acetate oxidation as a process sustaining VC dechlorination (24), and even less is known about the influence of aceticlastic methanogens on dehalorespiration. Previous studies indicate a positive influence of methanogens on anaerobic dechlorination of chloroethenes (7, 20, 43), polychlorinated biphenyls (30), and chloroform (55). However, the underlying processes are often poorly understood or are related to nondehalorespiratory mechanisms such as oxidative acetogenesis of VC (6, 8) or beneficial influence of cofactors such as F430 and vitamin B12 (55), which are involved in cometabolic reductive dechlorination (26). The presence of aceticlastic methanogens has been shown to be beneficial for dehalogenation by bacteria dechlorinating 2,4,6-trichlorophenol (37); however, little is known about the actual processes or the organisms involved.
This lack of knowledge is remarkable, considering that acetate is the most abundant substrate of methanogenesis in nature (9, 51). Although Methanosarcina spp. are generally regarded as aceticlastic, it is well known that they are also capable of acetate oxidation (31, 33). The pathway is oxidation of the methyl carbon of acetate yielding CO2 and H2. Apparently, the production of H2 can be taken advantage of by hydrogenotrophic sulfate-reducers grown in coculture with Methanosarcina (5, 38). In response to H2 consumption by sulfate reducers, Methanosarcina spp. oxidize a higher proportion of the available methyl carbon to CO2. It was demonstrated that hydrogenotrophic sulfate reducers could scavenge up to 42% of available electron equivalents derived from acetate through this reaction (38). Similarly, Achtnich et al. (2) could demonstrate the occurrence of interspecies H2 transfer from aceticlastic methanogens to sulfate and Fe(III)-reducing bacteria in anoxic paddy soil. Consequently, coupling of H2 release from acetate oxidation, mediated by Methanosarcina, with H2 uptake by DRB seems feasible. By making reducing equivalents of acetate available in the form of H2, this would be highly beneficial to the detoxification of chlorinated ethenes. In terms of energetics, acetate oxidation to H2 plus CO2 should be thermodynamically favorable in dehalorespiratory systems, considering the extremely low hydrogen threshold concentrations of respiratory reductive dechlorination (approximately 0.1 nM) (22).
Accordingly, the objectives of this study were (i) to determine whether the presence of aceticlastic methanogens has any observable effect on hydrogenotrophic VC dechlorination, (ii) to evaluate the magnitude of this effect, and (iii) to elucidate which organisms are involved in this process. To this end, we conducted experiments using the mixed, dechlorinating culture KB-1. Fluorescence in situ hybridization (FISH) targeting the two genera capable of aceticlastic methanogenesis, Methanosarcina and Methanosaeta, as well as Dehalococcoides spp., was used to relate observed processes to the occurrence and dominance of specific microorganisms.
MATERIALS AND METHODS
Chemicals and analytical procedures.
Vinyl chloride gas (99.97%) was purchased from Gerling, Holz, & Co. (Hamburg, Germany), and ethene and methane were obtained as pure gases from Mikrolab (Aarhus, Denmark). Sodium acetate (Merck, pro analysi, min. 99%; Darmstadt, Germany) was used as electron donor and carbon source.
Concentrations of VC, ethene, and methane (CH4) were determined by headspace sampling directly from the culture bottles. A total of 100 μl of headspace gas sample was introduced into a Shimadzu 14A gas chromatograph equipped with a packed column (3% SP/500 Carbopack B) and a flame ionization detector. In each run, the oven temperature was maintained at 80°C for 1.2 min and then increased to 130°C at a rate of 20°C min−1 before being cooled. Calibration was performed by injection of different volumes (60 to 150 μl) of standard gas mixtures containing CH4, ethene, and VC in pure nitrogen (produced in 120-ml serum bottles with the chemicals mentioned above). These mixed standards covered the following concentration ranges (in micromoles per liter of gas): 0.6 to 84.2 for VC, 0.7 to 84.9 for ethene, and 1.7 to 12,480 for methane. Headspace hydrogen (H2) was analyzed with a reduction gas detector (RGD2; Trace Analytical, Menlo Park, CA). A total of 400 μl of each gaseous standard and sample were injected onto a 250-μl stainless steel sampling loop (Mikrolab, Aarhus, Denmark) fitted on a 10-port VICI valve (enabling back-flushing of the Carbosphere column to protect the detector from chlorinated compounds). Separation was performed on a stainless steel Carbosphere 80/100 column, measuring 6 ft by 1/8 in., followed by a stainless steel Molsieve 5A column, measuring 3 ft by 1/8 in. (both from Alltech, Deerfield, IL). Gaseous hydrogen standards were prepared by diluting pure H2 gas in 120-ml serum bottles containing pure nitrogen. Standards covered a range of 0.3 to 36.8 ppm by volume. As all gaseous samples were equalized to atmospheric pressure prior to injection, and measured concentrations of H2, CH4, ethene, and VC were corrected for overpressure as determined with a portable manometer (Manofix X30D; Frode Pedersen, Allerød, Denmark) to account for this loss in mass (e.g., a sample degassing from 1.1 to 1.0 atm experiences a loss of around 10%). Headspace concentrations were converted to aqueous concentrations and amount of substance per bottle by using Henry's law constants for vinyl chloride (46) and ethene, methane, and H2 (52). Error bars on all graphs represent ±1 standard deviation from the average value.
Aqueous samples for the analysis of acetate (0.3 to 1 ml) were filtered through 0.45-μm nylon filters (Whatman, Clifton, NJ), acidified with 50 μl of 17% H3PO4 per ml of sample and kept frozen until analysis by suppressed ion chromatography on a Dionex ICE-AS1 9- by 250-mm ion exclusion column (eluent, 4 mM heptafluorobutyric acid; chemical suppression, 10 mM tetrabutyl ammonium).
Headspace samples (each, 200 μl) for the analysis of CO2 were injected into a Mikrolab GC equipped with a packed column (1.1 m by 3/16 in. Molsieve 13X; 0.7 m by 1/4 in. Chromosorb 108) and a thermal conductivity detector. The temperature was maintained at 55°C.
Culture conditions.
The anaerobic microbial consortium KB-1 Dechlorinator (kindly provided by SiREM, Guelph, Canada) was used as a stock culture in all experiments. This enrichment culture was originally derived from trichloroethylene-contaminated soil and groundwater and contains Dehalococcoides bacteria that are able to grow on vinyl chloride as an electron acceptor (16, 17). All incubations were performed in sulfate-free medium as described by Heimann et al. (25) (major components were phosphate, ammonium, calcium, and magnesium salts; trace metals; vitamins; bicarbonate; iron sulfide; no yeast extract). Experiments were conducted with both a fresh KB-1 culture (used directly after shipping) and a culture that had been used in different experimental setups for a total of 315 days prior to the experiments reported here. The 315-day-old culture and the fresh culture are hereafter referred to as “the evolved culture” and “the original culture,” respectively (acknowledging that our experimental treatment likely also altered the microbial community composition of the initial KB-1 culture). In terms of aceticlastic methanogens, the original culture was dominated by Methanosaeta spp., whereas the evolved culture was dominated by Methanosarcina spp. (details follow below).
Since the preceding experiments (315 days prior to the experiments reported here) were likely responsible for this critical difference in population composition, we will here briefly describe the treatment of the evolved culture in this period. (i) In the first 139 days, KB-1 was fed on lactate plus trichloroethylene. (ii) It was then transferred into fresh medium, and fed on acetate plus VC for another 155 days. (iii) It was again transferred into fresh medium and fed on acetate plus VC for another 21 days. The medium in these experiments was identical to the one described above, except for period ii, in which we used a high-ionic-strength medium featuring high chloride and bromide levels (Cl−, 44 mM; Br−, 61 mM) but otherwise identical to the one described above. Some experiments reported here were repeated independently with a stock of the evolved culture that had not been transferred into fresh medium after 294 days. However, this culture displayed no observable difference in terms of degradation patterns or microbial composition (i.e., the dominance of Methanosarcina spp.).
Lactate, the electron donor used initially in the preceding experiments, is a rapidly fermenting substrate that can cause high H2 levels (18). Accordingly, we measured H2 levels of a few thousand nanomoles per liter in this period. Methanosaeta spp. are strict aceticlastic methanogens, while Methanosarcina spp. are capable of utilizing multiple substrates, such as CO2 plus H2 or methanol (49), which may explain why we observed an enrichment of Methanosarcina spp. in preference to Methanosaeta spp. in the evolved culture compared to the original KB-1 culture. Also, high (millimolar) concentrations of acetate favor the faster-growing Methanosarcina, whereas the slow-growing Methanosaeta is favored by low acetate levels (56). Since acetate levels in the preceding experiments were generally high (around 4 to 5 mM), this probably also contributed to the enrichment of Methanosarcina.
Prior to the experiments reported here, the evolved culture always dechlorinated the added chloroethenes completely to ethene and also produced methane. Interestingly, VC dechlorination rates appeared to correlate positively with the rate of methanogenesis, which inspired us to further study this culture. All stock cultures were kept at 20°C.
Experimental setup.
For the experiments evaluating the effect of aceticlastic methanogens on dechlorination, the medium was modified by replacing mercaptoethanesulfonic acid (coenzyme M [CoM]) with 2-bromoethanesulfonic acid (BES) (a structural analogue of CoM and an inhibitor of methanogenesis) at a concentration of 5.28 g (of the sodium salt) per liter of vitamin stock solution (25 mM), resulting in a final BES concentration of 0.25 mM in the medium. This relatively low BES level has previously been shown not to affect vinyl chloride dechlorination rates in KB-1 (16). Experimental culture batches were set up in 120-ml serum bottles filled with 100 ml CoM- or BES-containing medium and sealed with 1-cm-thick butyl rubber stoppers (Apodan, Copenhagen, Denmark) and aluminum crimp caps. The headspace gas was N2/CO2 (80/20%). Each bottle received 400 μmol acetate as the only electron donor (0.4 ml of a 1 M sodium acetate solution, producing an initial acetate concentration of approximately 4 mM or 0.4 mmol per bottle) and 400 μl of a VC/N2 gas mixture (>95% VC) transferred with a gas-tight syringe (VICI Precision Sampling, Baton Rouge, LA), resulting in an initial aqueous VC concentration of approximately 140 μM (16 μmol per bottle). The experiments were initiated by inoculation with 1 ml stock culture (1% [vol/vol]). For each culture (original versus evolved), triplicate bottles were produced for each medium. To verify the results obtained from the evolved culture, the same experiment was repeated independently (again, in triplicate for all setups). Uninoculated bottles served as abiotic controls and were implemented as single bottles for both media. All bottles were incubated upside down in the dark at 20°C without agitation. Initial pH values were between 6.3 and 6.4. In the course of these experiments, VC and acetate were resupplied to the bottles as needed when near depletion.
To assess whether VC dechlorination was limited by available H2, two of the triplicates of a given setup were amended with 1 ml of pure H2 gas (corresponding to approximately 41 μmol per bottle), added via an anaerobic (flushed repeatedly with oxygen-free nitrogen) sterile syringe (BD Plastipak; Becton Dickinson, Brøndby, Denmark) after 117 days of incubation. The three bottles (H2-amended duplicates plus one control) were then incubated for another 20 days and analyzed for concentrations of VC and ethene. This experiment was conducted three times (twice with the original culture for both media, CoM and BES amended, and once with the evolved culture for the latter medium).
Rate calculations.
Dechlorination rates were determined for each sampling event (generally around 20 times in a 140-day experimental period) from the vinyl chloride consumption since the previous sampling event. VC consumption was corrected for sampling loss (withdrawal of aqueous and gaseous phase) and divided by the volume of culture (i.e., aqueous medium) at that time to obtain rates in the unit of micromoles per day per liter. Cumulative sampling loss of vinyl chloride over the whole experimental period was typically on the order of 3 μmol per bottle, or ∼20% of the initially added amount of VC. The significance of differences between maximum dechlorination rates mentioned hereafter was verified through Student's t test (α = 0.05; two tail).
Fluorescence in situ hybridization and microscopy.
FISH was performed according to the protocol described by Hugenholtz et al. (29). The oligonucleotide probes used in this study are shown in Table 1. The hybridization stringency was adjusted by formamide in the hybridization buffer and was 20% for all probes except for MS1414 (35% formamide, as for Sekiguchi et al.) (42). A mixture of probes EUB338 and EUB338+ was used to identify all Bacteria. EUB338 and EUB338+ were labeled with fluorescein isothiocyanate, ARC915 and DHE1259 were labeled with Cy3 or Cy5, and MS1414 and MX825 were labeled with Cy3. All probes were supplied by Thermo Electron, Ulm, Germany (http://www.thermohybaid.de).
TABLE 1.
Oligonucleotide probes used in this study
| Probe name | Target organism(s) | Probe sequence (5′-3′) | Reference |
|---|---|---|---|
| EUB338 | Most Bacteria | GCTGCCTCCCGTAGGAGT | 4 |
| EUB338+ | EUB338 supplement | GCWGCCACCCGTAGGTGT | 14 |
| ARC915 | Archaea | GTGCTCCCCCGCCAATTCCT | 45 |
| MS1414 | Methanosarcinaceae | CTCACCCATACCTCACTCGGG | 39 |
| MX825 | Methanosaetaceae | TCGCACCGTGGCCGACACCTAGC | 39 |
| DHE1259 | Dehalococcoides spp. | AGCTCCAGTTCWCACTGTTG | 53 |
Hybridized cells were examined with a Zeiss LSM 510 confocal laser scanning microscope employing an upright Axioplan 2 microscope and an ApoChromat microscope with a 63× objective and a 1.4 aperture. Excitation channels were 488 nm (green emissions), 545 nm (red emissions), and 670 nm (infrared emissions; presented in blue). For each sample, the scan rate and laser gain were adjusted; however, no digital postprocessing was applied.
Radiotracer experiments.
To investigate the effect of VC dechlorination on the pathway of acetate utilization, we conducted an experiment employing 14C-labeled acetate (radiolabeled methyl group, 14CH3COONa; Amersham Life Science, Little Chalfont, United Kingdom). In this experiment, 21-ml headspace vials were sealed with 1-cm-thick butyl rubber stoppers and aluminum crimp caps, flushed with N2, and filled with 10 ml of the mineral salts medium (CoM amended). All biotic batches were inoculated with 3 ml of the evolved culture (∼23% [vol/vol] inoculum), while two abiotic controls were not inoculated. Of the biotic batches, nine replicates received 2.5 μmol VC (resulting in an initial aqueous VC concentration of 126 μM), and nine replicates received no VC. The initial acetate concentration was 4.6 mM. All vials were initially analyzed for VC, ethene, and CH4. Subsequently, three out of nine replicates for each setup were sacrificed for initial measurements of CO2, acetate, and pH. Approximately 25.4 kBq of 14CH3COONa was injected into each of three replicate vials for both setups (in total, six vials), which changed the overall acetate concentration by <1%. All vials were incubated in the dark for 65 days. Nonradioactive replicates were then analyzed for the parameters described above, while radioactive replicates were analyzed for radiolabeled compounds. 14CH4 and 14CO2 were separated by a stripping apparatus, using a slight modification of the procedure described by Hansen et al. (21) to minimize interference by radiolabeled acetic acid upon acidification of the sample. In this modified procedure, the headspace of each reaction vial was extracted in a prestep by addition of 6.5 ml mineral salts medium and simultaneous uptake of the displaced gas phase with a 10-ml disposable syringe (BD Plastipak; Becton Dickinson, Brøndby, Denmark). The content of this syringe was then injected into an N2-containing 120-ml serum bottle sealed with a 1-cm-thick butyl rubber stopper and aluminum crimp cap. This procedure recovered around 82% (±3%, 9 replicates; as tested by extraction of nonlabeled methane in preexperiments) of the total vial headspace. The 120-ml serum bottles were then connected to the stripping apparatus. The stripped gas passed through five traps in the first step, trapping CO2 (two traps of 10 ml 2-methoxyethanol and three traps of 10 ml Carbosorb E; Perkin-Elmer, Hvidovre, Denmark), and in the second step, following oxidation of CH4 to CO2, by passage through an 800°C CuO column, through four traps (one trap of 10 ml 2-methoxyethanol and three traps of 10 ml Carbosorb E). All samples were measured on a Wallac 1414 scintillation counter, following the addition of an appropriate scintillation fluid (10 ml Permafluor E+ for Carbosorb E traps and 10 ml Ultima Gold XR for 2-methoxyethanol traps). Measured radioactivities were corrected for extraction recovery (see above) and were back-calculated to total radioactivity of inorganic carbon (C) (4) and methane by use of Henry's law constants and speciation calculations performed with PHREEQC (36). Thus, all radioactive carbon species (aqueous and gaseous) were included in our calculations.
RESULTS
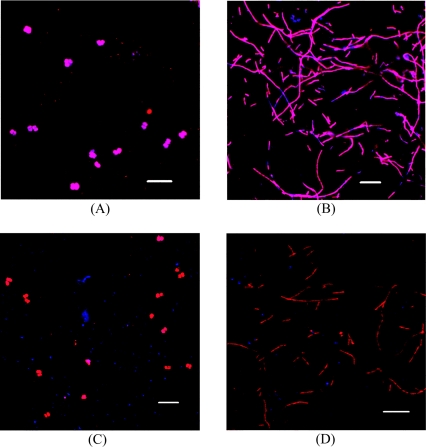
The archaeal composition of the two compared cultures was quite distinct. Close to 100% of all Archaea detected by FISH were either Methanosarcinaceae or Methanosaetaceae in the evolved and original cultures, respectively (Fig. 1A and B). Although the probe used to target Methanosarcinaceae, MS1414, also targeted individuals from Methanococcoides and Methanolobus, Methanosarcinaceae were easily distinguishable by their size and morphology. In addition, FISH suggested the presence of Dehalococcoides both in the evolved culture and to a lesser degree in the original culture (Fig. 1C and D). FISH samples that were simultaneously stained with the combined fluorescein isothiocyanate-labeled EUB probe exhibited increased autofluorescence in the channel for green emission (probably from iron sulfides present in the medium). Therefore, these results are not shown here. As expected, no or very few methanogens were observed in samples from either of the two cultures set up in the BES-amended medium.
FIG. 1.
FISH microscopic images from the methanogenic setups of the evolved culture (A and C) and the original culture (B and D) after 106 days of incubation. The following probe combinations were applied: ARC915 (Cy5, blue emissions) and MS1414 (Cy3, red emissions) (A), ARC915 (Cy5, blue emissions) and MX825 (Cy3, red emissions) (B), and ARC915 (Cy3, red emissions) and DHE1259 (Cy5, blue emissions) (C and D). The images are representative of general observations made in the different cultures, i.e., dominance of Methanosarcinaceae and Methanosaetaceae in the evolved culture and the original culture, respectively. Scale bars, 10 μm.
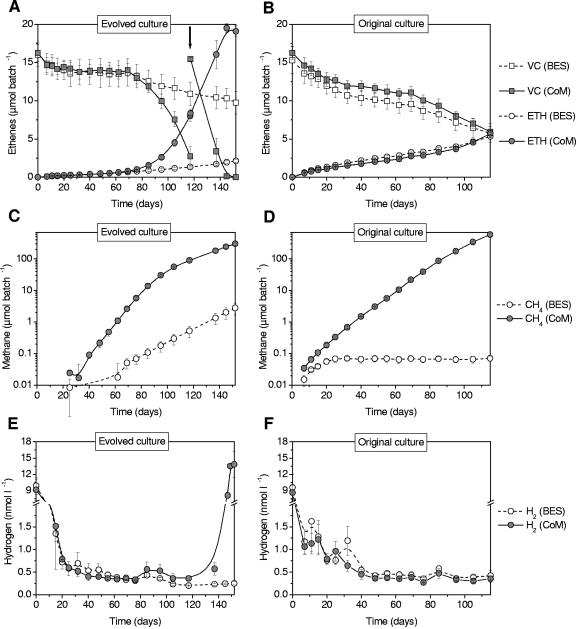
Dechlorination of vinyl chloride to ethene occurred in all cultures regardless of medium composition (Fig. 2A and B). However, amending the medium with 0.25 mM BES resulted in efficient inhibition of methanogenesis in both cultures and a conspicuous effect on dechlorination rates in the evolved culture (Fig. 2A to D). After approximately 70 days of incubation, average dechlorination rates in the CoM-amended bottles of the evolved culture started to increase and became significantly higher than in the BES-amended setup. This high rate was also maintained when vinyl chloride was resupplied after 117 days. This observation was confirmed in an independent repetition of the experiment. In contrast to this, the original culture showed no significant difference in dechlorination rate between CoM- and BES-amended media, despite methanogenesis proceeding at higher rates in this culture than in the evolved culture (after 115 days, approximately seven times more methane was produced in the original culture). Abiotic controls showed no dechlorination of VC and no production of methane.
FIG. 2.
Concentrations of VC and ethene (A and B), methane (C and D), and hydrogen (E and F) over time in both cultures (left versus right columns) for CoM-amended (gray symbols) and BES-amended (white symbols) media. Note the logarithmic scale (C and D) and the broken y axis (E and F). Error bars are ±1 standard deviation of the average from three replicate cultures. The arrow in panel A indicates readdition of VC to the CoM-amended batches after 117 days of incubation.
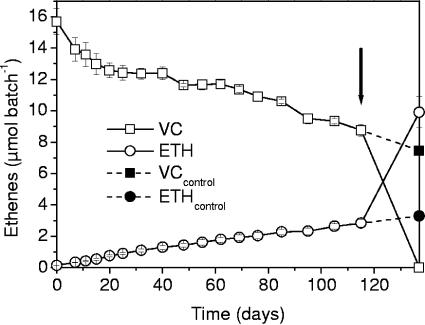
In all cultures, vinyl chloride dechlorination resulted in a decrease of aqueous H2 concentrations by >1 order of magnitude in the first 20 days, thereafter approaching levels of around 0.3 to 0.5 nM (Fig. 2E and F). When all vinyl chloride was consumed in the evolved culture (after about 140 days), the H2 level increased >20 fold to levels of around 14 nM. The same observation was repeatedly made during preexperiments (data not shown). When H2 (41 μmol per bottle) was supplied to the BES-amended setup of the evolved culture after 117 days, it was able to completely dechlorinate the remaining VC to ethene within only 20 days (Fig. 3). The same was true for both the BES-amended and the methanogenic setups of the original culture (data not shown), demonstrating that dechlorination was not inhibited by BES in our experiments (BES can potentially inhibit dechlorination of chloroethenes, as was shown by Löffler et al.) (32).
FIG. 3.
VC dechlorination in a BES-amended setup of the evolved culture. The arrow indicates addition of H2 to two out of three replicates after 115 days, with the unamended bottle (no H2 addition) serving as a control.
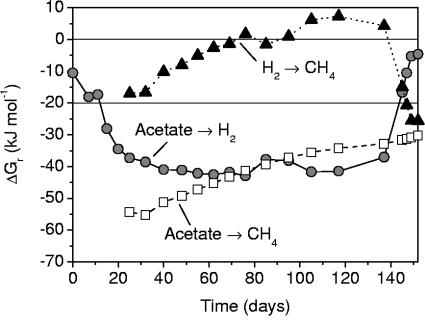
Methane formation was accounted for by acetate consumption at a nearly 1:1 molar ratio, as expected for aceticlastic methanogenesis (Table 2, reaction 2). The apparent lack of hydrogenotrophic methanogenesis was confirmed by calculations of the available Gibbs free energy (ΔGr) from this process. The results of free energy calculations for in situ conditions observed in the evolved (CoM-amended) culture (for the data depicted in Fig. 2A, C, and E) are shown in Fig. 4, along with the in situ Gibbs free energy values for aceticlastic methanogenesis and acetate oxidation to CO2 and H2 (Table 2, reactions 1 to 3). The free energy yield for hydrogenotrophic methanogenesis was lower (more-positive ΔGr) at nearly all times than the assumed threshold energy of about −20 kJ (mol reaction−1) necessary for ATP formation (40) and even became positive from around day 100 to 140. The energy yield for acetate oxidation became increasingly higher (more-negative ΔGr) in response to the decreasing H2 levels. From day 70 on, acetate oxidation to H2 and CO2 was thermodynamically equally or even more favorable than aceticlastic methanogenesis. The changes in energy yield towards the end of the observation period (around 140 days) were due to the increase of H2 concentrations in response to the ceasing VC dechlorination (Fig. 2).
TABLE 2.
Relevant reactions discussed in this study
| Reaction no. | Reaction | ΔG0′ (kJ mol−1)a |
|---|---|---|
| 1 | Acetate− + 4H2O → 2HCO3− + 4H2 + H+ | +104.6 |
| 2 | Acetate− + H2O → CH4 + HCO3− | −31.1 |
| 3 | 4H2 + H+ + HCO3− → CH4 + 3H2O | −135.7 |
| 4 | VC + H2 → Ethene + H+ + Cl− | −146.0 |
FIG. 4.
In situ Gibbs free energy yields for reactions 1 to 3 in Table 2 in the methanogenic setup of the evolved culture. In all calculations, the pH is 6.5, and the activity of HCO3− is 1.1 × 10−2 (as computed with PHREEQC (36); CH4, H2, and acetate vary according to the observed variation over time.
In the evolved culture (CoM amended), VC dechlorination rates were about 12% (±2.4%) of methane production rates on a molar basis (as determined by linear regression over time; R2 = 0.89 and P < 0.001; data not shown). Considering the stoichiometry of acetate oxidation (Table 2, reaction 1), this translates into approximately 3 mol% of utilized acetate being oxidized to CO2 and H2 (instead of being utilized for methane production), the produced H2 being consumed by VC dechlorination.
In the radiotracer experiments, about 98% of the initially added VC was dechlorinated within 65 days. While addition of VC reduced the production of methane by a factor of 10 (inhibition of methanogenesis by VC), utilization of [2-14C]acetate resulted in relatively more 14CO2 than in the setup containing no VC (Table 3). The respiratory index, equal to 14CO2/(14CO2 + 14CH4), increased from 0.12 in the nondechlorinating culture to approximately 0.51 during VC dechlorination. In the dechlorinating setup, about 10% of the initially added [2-14C]acetate was converted to 14CO2 in 65 days, which when back-calculated to the total unlabeled carbon pools would provide roughly 10 times the amount of H2 needed for complete dechlorination of VC (4 × 6.0 μmol; 1 mol of acetate oxidized yielded 4 mol of H2).
TABLE 3.
Results from the radiotracer experimenta
| Treatment | CH4 produced (μmol batch−1)
|
RI = 14CO2/ (14CO2 + 14CH4)
|
||
|---|---|---|---|---|
| Avg | SE | Avg | SE | |
| Acetate + VC | 5.4 | 1.3 | 0.51 | 0.05 |
| Acetate, no VC | 54.6 | 0.1 | 0.12 | 0.003 |
The evolved culture was employed in all treatments (n = 3 for all results). RI, respiratory index.
DISCUSSION
Acetate-driven dechlorination of vinyl chloride was found in all biotic setups, regardless of the abundance of methanogens. This indicates that a small population of methanogens might have been present in the BES-amended cultures providing sufficient hydrogen to drive the observed low-rate VC dechlorination. Alternatively, nonarchaeal organisms could have supplied H2 from acetate to dechlorinators (thermodynamically facilitated by low H2 levels). The occurrence of bacterial syntrophic acetate oxidizers under mesophilic conditions is well known (10, 35, 41). Interestingly, a recent study indicates that this could be a relevant process in the reductive dechlorination of chlorinated ethenes (24). However, while BES-amended cultures could sustain dechlorination only at low rates in our experiments, the presence of Methanosarcina considerably increased VC dechlorination rates by a factor of up to 7, indicating they may be producing H2 by acetate oxidation. Although the fraction of acetate utilized in our experiments for CO2 and H2 production was not very high (∼3%), this may still provide a sufficient amount of reducing equivalents to drive dechlorination of chlorinated species in field situations.
The fact that the presence of Methanosaeta did not have a positive influence on dechlorination rates suggests that this genus is not able to produce H2 from acetate. Valentine et al. (50) showed that Methanosarcina barkeri transiently produced H2 under low-hydrogen conditions, even without any organic substrate (presumably derived from previously produced metabolites). It was also found that Methanosaeta concilii produced no hydrogen in these experiments, a result which is supported by our findings. It is significant that Methanosaeta spp. are strict aceticlastic methanogens, while Methanosarcina spp. are capable of utilizing multiple substrates such as CO2 plus H2, or methanol (49). Since acetate-grown Methanosarcina species contain low levels of enzymes that are utilized in the CO2-H2 pathway of methanogenesis (19), the difference in H2 production capabilities between Methanosarcina and Methanosaeta is presumably related to differences in enzymology.
Methane production from acetate is not necessarily reflective of an aceticlastic pathway. Several studies have shown that methane can be also produced by syntrophic acetate oxidation with subsequent hydrogenotrophic methanogenesis (41). However, the unfavorable thermodynamics, caused by reductive dechlorination, favor aceticlastic methanogenesis as a major methane source in our experiments.
Slow, fortuitous dehalogenation of VC or other chlorinated ethenes has been related to metabolic by-products, e.g., via cofactors such as vitamin B12, which is abundant in methanogens (15). However, our results suggest that VC is dehalogenated in a respiratory process, as indicated by the sustained low hydrogen levels during dechlorination. These are in the range of hydrogen concentrations during chloroethene halorespiration observed by others (13, 22). Moreover, the observation that H2 concentrations do not change considerably during growth of Methanosarcina spp. suggests that H2 production from acetate limits the overall rate of dechlorination, which again suggests the presence of active DRB. This assumption is supported by recent findings by Duhamel et al. (16), who demonstrated that KB-1 features organisms capable of growth on VC. Hydrogen production via acetate oxidation is also documented by the respiratory indices (0.51 versus 0.12 for dechlorinating versus nondechlorinating methanogenic systems). The values we found are very similar to results from Achtnich et al. (2), who added sulfate to an aceticlastic methanogenic population.
In summary, we present here the first evidence of H2 production from acetate-grown methanogens driving chloroethene dechlorination. The interpretation of our findings as interspecies hydrogen transfer from Methanosarcina spp. to dehalorespiring Dehalococcoides spp. is supported by (i) the lack of fortuitous effects (cometabolic degradation) that might increase dechlorination rates (i.e., no effect of Methanosaeta), (ii) the favorable energetics of acetate oxidation to CO2 and H2 under our experimental conditions, (iii) the 10- to 100-fold increase in H2 levels upon complete consumption of VC, (iv) the fact that dechlorination in nonmethanogenic cultures was not inhibited by BES but was limited by H2, and (v) the conspicuous increase in the respiratory index when VC was added to methanogenic cultures. These results give exciting insights into the microbial ecology of methanogenic systems impacted by a very common class of groundwater contaminants and thus may be relevant for bioremediation of contaminated environments. Since their minimum thresholds for acetate utilization are on the order of 0.5 mM (56), Methanosarcina spp. may contribute to contaminant degradation in bioengineered systems where acetate concentrations are high (i.e., in the millimolar range) and H2 production from acetate limits the overall dechlorination process.
Acknowledgments
This work was supported by the Danish Research Training Council (FUR).
We thank SiREM (Guelph, Ontario, Canada) for kindly providing the KB-1 culture and Biocentrum DTU for permission to use their confocal LSM. The manuscript was improved through the helpful comments of three anonymous reviewers.
REFERENCES
- 1.Abelson, P. H. 1990. Volatile contaminants of drinking water. Science 247:141. [DOI] [PubMed] [Google Scholar]
- 2.Achtnich, C., A. Schuhmann, T. Wind, and R. Conrad. 1995. Role of interspecies H2 transfer to sulfate and ferric iron-reducing bacteria in acetate consumption in anoxic paddy soil. FEMS Microbiol. Ecol. 16:61-70. [Google Scholar]
- 3.Adrian, L., U. Szewzyk, J. Wecke, and H. Görisch. 2000. Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580-583. [DOI] [PubMed] [Google Scholar]
- 4.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boone, D. R., J. A. G. F. Menaia, J. E. Boone, and R. A. Mah. 1987. Effects of hydrogen pressure during growth and effects of pregrowth with hydrogen on acetate degradation by Methanosarcina species. Appl. Environ. Microbiol. 53:83-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley, P. M., and F. H. Chapelle. 2000. Acetogenic microbial degradation of vinyl chloride. Environ. Sci. Technol. 34:2761-2763. [Google Scholar]
- 7.Bradley, P. M., and F. H. Chapelle. 1999. Methane as a product of chloroethene biodegradation under methanogenic conditions. Environ. Sci. Technol. 33:653-656. [Google Scholar]
- 8.Bradley, P. M., and F. H. Chapelle. 1999. Role for acetotrophic methanogens in methanogenic biodegradation of vinyl chloride. Environ. Sci. Technol. 33:3473-3476. [Google Scholar]
- 9.Conrad, R. 1999. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol. Ecol. 28:193-202. [Google Scholar]
- 10.Cord-Ruwisch, R., D. R. Lovley, and B. Schink. 1998. Growth of Geobacter sulfurreducens with acetate in syntrophic cooperation with hydrogen-oxidizing anaerobic partners. Appl. Environ. Microbiol. 64:2232-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cupples, A. M., A. M. Spormann, and P. L. McCarty. 2004. Comparative evaluation of chloroethene dechlorination to ethene by Dehalococcoides-like microorganisms. Environ. Sci. Technol. 38:4768-4774. [DOI] [PubMed] [Google Scholar]
- 12.Cupples, A. M., A. M. Spormann, and P. L. McCarty. 2003. Growth of a Dehalococcoides-like microorganism on vinyl chloride and cis-dichloroethene as electron acceptors as determined by competitive PCR. Appl. Environ. Microbiol. 69:953-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupples, A. M., A. M. Spormann, and P. L. McCarty. 2004. Vinyl chloride and cis-dichloroethene dechlorination kinetics and microorganism growth under substrate limiting conditions. Environ. Sci. Technol. 38:1102-1107. [DOI] [PubMed] [Google Scholar]
- 14.Daims, H., A. Bruhl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 15.Dolfing, J. 2003. Thermodynamic considerations for dehalogenation, p. 89-114. In M. M. Häggblom and I. D. Bossert (ed.), Dehalogenation: microbial processes and environmental applications. Kluwer Academic Publishers, Boston, Mass.
- 16.Duhamel, M., K. Mo, and E. A. Edwards. 2004. Characterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl. Environ. Microbiol. 70:5538-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duhamel, M., S. D. Wehr, L. Yu, H. Rizvi, D. Seepersad, S. Dworatzek, E. E. Cox, and E. A. Edwards. 2002. Comparison of anaerobic dechlorinating enrichment cultures maintained on tetrachloroethene, trichloroethene, cis-dichloroethene and vinyl chloride. Water Res. 36:4193-4202. [DOI] [PubMed] [Google Scholar]
- 18.Fennell, D. E., J. M. Gossett, and S. H. Zinder. 1997. Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ. Sci. Technol. 31:918-926. [Google Scholar]
- 19.Ferry, J. G. 1993. Fermentation of acetate, p. 536. In J. G. Ferry (ed.), Methanogenesis—ecology, physiology, biochemistry, and genetics. Chapman & Hall, New York, N.Y.
- 20.Freedman, D. L., and J. M. Gossett. 1989. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl. Environ. Microbiol. 55:2144-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen, L. K., R. Jakobsen, and D. Postma. 2001. Methanogenesis in a shallow sandy aquifer, Romo, Denmark. Geochim. Cosmochim. Acta 65:2925-2935. [Google Scholar]
- 22.He, J., K. M. Ritalahti, M. R. Aiello, and F. E. Löffler. 2003. Complete detoxification of vinyl chloride by an anaerobic enrichment culture and identification of the reductively dechlorinating population as Dehalococcoides species. Appl. Environ. Microbiol. 69:996-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, J., K. M. Ritalahti, K.-L. Yang, S. S. Koenigsberg, and F. E. Löffler. 2003. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62-65. [DOI] [PubMed] [Google Scholar]
- 24.He, J., Y. Sung, M. E. Dollhopf, B. Z. Fathepure, J. M. Tiedje, and F. E. Löffler. 2002. Acetate versus hydrogen as direct electron donors to stimulate the microbial reductive dechlorination process at chloroethene-contaminated sites. Environ. Sci. Technol. 36:3945-3952. [DOI] [PubMed] [Google Scholar]
- 25.Heimann, A. C., A. K. Friis, and R. Jakobsen. 2005. Effects of sulfate on anaerobic chloroethene degradation by an enriched culture under transient and steady-state hydrogen supply. Water Res. 39:3579-3586. [DOI] [PubMed] [Google Scholar]
- 26.Holliger, C., C. Regeard, and G. Diekert. 2003. Dehalogenation by anaerobic bacteria, p. 115-157. In M. M. Häggblom and I. D. Bossert (ed.), Dehalogenation: microbial processes and environmental applications. Kluwer Academic Publishers, Boston, Mass.
- 27.Holliger, C., and W. Schumacher. 1994. Reductive dehalogenation as a respiratory process. Antonie Leeuwenhoek 66:239-246. [DOI] [PubMed] [Google Scholar]
- 28.Holliger, C., G. Wohlfarth, and G. Diekert. 1999. Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol. Rev. 22:383-398. [Google Scholar]
- 29.Hugenholtz, P., G. W. Tyson, and L. L. Blackall. 2001. Design and evaluation of 16S rRNA-targeted oligonucleotide probes for fluorescence in situ hybridization, p. 29-41. In B. A. Lieberman (ed.), Methods in molecular biology, vol. 176. Steroid receptor methods: protocols and assays. Humana Press, Inc., Totowa, N.J. [DOI] [PubMed] [Google Scholar]
- 30.Kim, J., and G.-Y. Rhee. 1999. Reductive dechlorination of polychlorinated biphenyls: interactions of dechlorinating microorganisms with methanogens and sulfate reducers. Environ. Toxicol. Chem. 18:2696-2702. [Google Scholar]
- 31.Krzycki, J. A., J. B. Morgan, R. Conrad, and J. G. Zeikus. 1987. Hydrogen metabolism during methanogenesis from acetate by Methanosarcina barkeri. FEMS Microbiol. Lett. 40:193-198. [Google Scholar]
- 32.Löffler, F. E., K. M. Ritalahti, and J. M. Tiedje. 1997. Dechlorination of chloroethenes is inhibited by 2-bromoethanesulfonate in the absence of methanogens. Appl. Environ. Microbiol. 63:4982-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovley, D. R., and J. G. Ferry. 1985. Production and consumption of H2 during growth of Methanosarcina spp. on acetate. Appl. Environ. Microbiol. 49:247-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maymó-Gatell, X., Y. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 35.Nüsslein, B., K.-J. Chin, W. Eckert, and R. Conrad. 2001. Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel). Environ. Microbiol. 3:460-470. [DOI] [PubMed] [Google Scholar]
- 36.Parkhurst, D. L., and C. A. J. Appelo. 1999. User's guide to PHREEQC (version 2)— a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water-resources investigations report 99-4259. U.S. Geological Survey, Denver, Colo.
- 37.Perkins, P. S., S. J. Komisar, J. A. Puhakka, and J. F. Ferguson. 1994. Effects of electron donors and inhibitors on reductive dechlorination of 2,4,6-trichlorophenol. Water Res. 28:2101-2107. [Google Scholar]
- 38.Phelps, T. J., R. Conrad, and J. G. Zeikus. 1985. Sulfate-dependent interspecies H2 transfer between Methanosarcina barkeri and Desulfovibrio vulgaris during coculture metabolism of acetate or methanol. Appl. Environ. Microbiol. 50:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raskin, L., J. M. Stromley, B. E. Rittmann, and D. A. Stahl. 1994. Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl. Environ. Microbiol. 60:1232-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schink, B. 1997. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Rev. 61:262-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnürer, A., F. P. Houwen, and B. H. Svensson. 1994. Mesophilic syntrophic acetate oxidation during methane formation by a triculture at high ammonium concentration. Arch. Microbiol. 162:70-74. [Google Scholar]
- 42.Sekiguchi, Y., Y. Kamagata, K. Nakamura, A. Ohashi, and H. Harada. 1999. Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Appl. Environ. Microbiol. 65:1280-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skeen, R. S., J. Gao, and B. S. Hooker. 1995. Kinetics of chlorinated ethylene dehalogenation under methanogenic conditions. Biotechnol. Bioeng. 48:659-666. [DOI] [PubMed] [Google Scholar]
- 44.Smidt, H., and W. M. De Vos. 2004. Anaerobic microbial dehalogenation. Annu. Rev. Microbiol. 58:43-73. [DOI] [PubMed] [Google Scholar]
- 45.Stahl, D. A., and R. Amann. 1991. Development and application of nucleic acid probes, p. 205-248. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Inc., New York, N.Y.
- 46.Staudinger, J., and P. V. Roberts. 2001. A critical compilation of Henry's law constant temperature dependence relations for organic compounds in dilute aqueous solutions. Chemosphere 44:561-576. [DOI] [PubMed] [Google Scholar]
- 47.Stumm, W., and J. J. Morgan. 1996. Aquatic chemistry, 3rd ed. Wiley, New York, N.Y.
- 48.Thauer, R. K., K. Jungermann, and K. Decker. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41:100-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thauer, R. K., D. Möller-Zinkhan, and A. M. Spormann. 1989. Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Annu. Rev. Microbiol. 43:43-67. [DOI] [PubMed] [Google Scholar]
- 50.Valentine, D. L., D. C. Blanton, and W. S. Reeburgh. 2000. Hydrogen production by methanogens under low-hydrogen conditions. Arch. Microbiol. 174:415-421. [DOI] [PubMed] [Google Scholar]
- 51.Vogels, G. D., J. T. Keltjens, and C. van der Drift. 1988. Biochemistry of methane production, p. 707-769. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley & Sons, New York, N.Y.
- 52.Wilhelm, E., R. Battino, and R. J. Wilcock. 1977. Low-pressure solubility of gases in liquid water. Chem. Rev. 77:219-262. [Google Scholar]
- 53.Yang, Y., and J. Zeyer. 2003. Specific detection of Dehalococcoides species by fluorescence in situ hybridization with 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 69:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yaws, C. L. 1992. Thermodynamic and physical property data. Gulf Publishing Company, Houston, Tex.
- 55.Yu, Z., and G. B. Smith. 1997. Chloroform dechlorination by a wastewater methanogenic consortium and cell extracts of Methanosarcina barkeri. Water Res. 31:1879-1886. [Google Scholar]
- 56.Zinder, S. H. 1993. Physiological ecology of methanogens, p. 536. In J. G. Ferry (ed.), Methanogenesis—ecology, physiology, biochemistry, and genetics. Chapman & Hall, New York, N.Y.