Abstract
In a previous study, we identified Congo red-binding and -nonbinding phase variants of Escherichia coli serotype O157:H7 strain ATCC 43895. The Congo red-binding variant, strain 43895OR, produced a dry, aggregative colony that was similar to the red, dry, and rough (rdar) phenotype characteristic of certain strains of Salmonella. In contrast, variant 43895OW produced a smooth and white colony morphology. In this study, we show that, similar to rdar strains of Salmonella enterica serovar Typhimurium, strain 43895OR forms large aggregates in broth cultures, firm pellicles at the air-medium interface on glass, and dense biofilms on glass and polystyrene. However, unlike S. enterica serovar Typhimurium, strain 43895OR does not stain positive for cellulose production. When strain 43895OR was fixed on agar, scanning electron microscopy showed cells expressing extracellular matrix (ECM) containing curli fibers. Strain 43895OW was devoid of any ECM or curli fibers on agar but showed expression of curli fibers during attachment to glass. Strain 43895OR produced >4-fold-larger amounts of biofilm than strain 43895OW on polystyrene, glass, stainless steel, and Teflon; formation was >3-fold higher in rich medium than in nutrient-limited medium. Biofilm-associated cells of both strains showed statistically greater resistance (P < 0.05) to hydrogen peroxide and quaternary ammonium sanitizer than their respective planktonic cells. This study shows that the rdar phenotype of E. coli O157:H7 strain 43895OR is important in multicellular growth, biofilm formation, and resistance to sanitizers. However, the lack of cellulose production by strain 43895OR indicates important differences in the ECM composition compared to that of Salmonella.
Enterohemorrhagic Escherichia coli (EHEC) is an important cause of food-borne illness. Food-producing animals, cattle in particular, are considered the primary source of EHEC, and most outbreaks have been associated with the consumption of contaminated meat or milk (11). In recent years, there has been an increase in the numbers of outbreaks associated with the consumption of produce such as fruit, juices, vegetables, and sprouts (11, 25). In 2000, the Centers for Disease Control and Prevention reported 69 confirmed outbreaks caused by Shiga toxin-producing E. coli from 26 states. All of the outbreaks were caused by serotype O157:H7, and they resulted in 1,564 cases of illness, 50 cases of hemolytic uremic syndrome, and four deaths (http://www.cdc.gov/foodborneoutbreaks/ecoli/2000_summaryLetter.pdf).
Bacterial attachment, colonization, and biofilm formation on food and food contact surfaces can serve as a source for biotransfer and cross-contamination of product, leading to health issues and increased product spoilage (3, 9). A biofilm is a community of microbes embedded in an organic polymer matrix, adhering to a surface (3). Early studies have shown that E. coli O157:H7 can form biofilms on a variety of surfaces (6, 29). In Salmonella spp. and E. coli, biofilm formation is associated with the expression of curli and exopolysaccharide (EPS), such as cellulose and colanic acid (1, 5, 27, 33). Both E. coli and certain Salmonella spp. are capable of producing curli (E. coli) or thin aggregative fimbriae (Salmonella) that bind the dye Congo red and mediate attachment to both inert surfaces and biological proteins (2, 4, 8, 13, 14, 26). In Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Enteritidis, the coexpression of thin aggregative fimbriae and cellulose leads to an aggregative colony phenotype (red, dry, and rough [rdar]) when grown on medium containing the dye Congo red (27, 34).
The rdar phenotype was described for strains of S. enterica serovar Typhimurium (21) as cells that form a rigid multicellular network expressing thin aggregative fimbriae, which mediate tight intracellular bonds. The rdar phenotype was associated with biofilm formation on both glass and polystyrene and formation of a tight pellicle at the air-liquid interface (19, 21). Zogaj et al. (34) showed that cellulose is a second component of the S. enterica serovar Typhimurium extracellular matrix (ECM) responsible for the rdar phenotype and biofilm formation. Cellulose-producing strains were identified by including the dye calcofluor in agar plates, which detected the β-1,4 glucose linkages of cellulose and caused stained colonies to fluoresce under a 366-nm UV light source (34). Salmonella strains producing curli, but not cellulose, produce a brown colony phenotype (brown, dry, and rough) on agar plates containing Congo red. Strains deficient in both cellulose and curli produce a smooth and white colony (saw). Some natural strains of S. enterica serovar Enteritidis and E. coli that produce the rdar phenotype were also shown to produce cellulose and thin aggregative fimbriae (curli) (27, 34).
In a previous study, we identified two phase-variable, Congo red-binding strains of E. coli O157:H7 with an aggregative behavior that produced a dry and rough colony phenotype (32). The phase-variable, Congo red-binding phenotype is a result of variations in the −10 region of the csgD promoter which alter the level of expression of the csgDEFG operon. These strains attach in greater numbers to cultured epithelial cells and are more virulent in a mouse model (31). Using these strains, Ryu et al. (22, 23) showed that curli expression is important in biofilm formation on stainless steel and that biofilm cells were more resistant to chlorine than planktonic cells. In the present study, we evaluated the rdar phenotype of strain 43895OR and showed that, although it has many of the characteristics of the rdar strains described in Salmonella, it does not produce cellulose as an additional component of the ECM. We also tested its ability to form biofilms on several surfaces in multiple media and compared the resistance of biofilm cells and planktonic cells to different antimicrobial compounds.
MATERIALS AND METHODS
Bacterial strains, media, and coupons.
The E. coli strains used in this work were described in a previous study (32). Strain 43895OR is a phase-variable, curli-producing strain isolated from a freezer stock of the noncurliated strain ATCC 43895 (strain MFS 2630; Centers for Disease Control and Prevention EDL 933) obtained from the American Type Culture Collection (Rockville, MD). The promoter of strain 43895OR contains a single base pair mutation at base −9 from the csgD transcriptional start site that increases transcription of csgD promoter fusions (32). Strain 43895OW is the noncurliated parent strain from the same culture. S. enterica serovar Enteritidis strains 3934 (curli positive, cellulose positive), 942 (curli positive, cellulose negative), and 1170/97 (curli negative, cellulose negative), used as controls in the calcofluor staining experiments, were gifts from Carlos Gamazo (Department of Microbiology, University of Navarra, Navarra, Spain). Bacterial strains were maintained on Congo red indicator (CRI) plates or YESCA plates (7). The propagation of strains on agar plates and during experiments requiring incubations of >24 h were performed at ≤30°C to minimize the phase switching of strain 43895OR to its curli-negative variant.
Colony size and surface spreading were determined on Luria-Bertani (LB; Difco Laboratories, Detroit, MI) agar (1.5%) made without salt (LB-NS). Plates were spotted with 2 μl of overnight cultures grown in tryptic soy broth (TSB; Difco) at 37°C and incubated at 28°C in a humid atmosphere (21). For colony size studies, three independent cultures of each strain were used to generate six replicate spots/plate. For swarming assays, three independent cultures of each strain were used to generate a single spot applied to the center of the plate, which was monitored daily for 96 h. Biofilm assays were carried out with TSB, 1/20 strength TSB (1/20-TSB), and LB-NS. The LB plates containing 200 mg/liter of calcoflour (fluorescent brightener 28; Sigma-Aldrich Corporation, St. Louis, MO) were used for detection of cellulose production. Biofilm assays on glass used microscope slides (3 by 1 in.; Corning Glass Works, Corning, NY). Stainless steel coupons of the same size were cut from no. 400 polish stainless steel provided by Seiichiro Isobe (Food Research Institute of Japan, Tsukuba, Ibaraki, Japan). Teflon coupons were cut to size from sheets of virgin polytetrafluoroethylene (McMaster-Carr, New Brunswick, NJ). Microscope slides and coupons were degreased with acetone, washed in Liqui-Nox (Alconox, White Plains, NY), rinsed with distilled water, and autoclaved.
Western blotting.
Strains were grown on YESCA agar for 48 h at 28°C and analyzed for curli protein as previously described (20). Polymerized curli required treatment with formic acid prior to gel separation as previously described (4). Cell pellets were collected from 4 ml of cells, suspended in sterile water to an optical density at 600 nm (OD600) of 1.0, resuspended in 200 μl of 98% formic acid for 10 m on ice, vacuum dried, and resuspended in 200 μl Laemmli buffer (Bio-Rad Laboratories, Hercules, CA). The samples were separated on an 8 to 16% Tris-HCl Ready gel (Bio-Rad) as previously described (10). The proteins were transferred to a 0.2-μm Immun-blot polyvinylidene difluoride membrane (Bio-Rad) and blotted with a polyclonal anti-E. coli csgA antibody of rabbit origin, kindly provided by Jorge Giron (University of Arizona). Polyclonal antibodies were detected using the Protein Detector Western blot kit (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD).
Electron microscopy and staining.
Bacterial colony morphology was studied using strains cultured on YESCA plates for 48 h at 28°C. Solid cultures were processed for microscopy by exposing the colonies on agar to a vapor of warm 25% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) solution and adsorbed to Whatman filter paper (Whatman, Ltd., Maidstone, England) in a closed petri dish for 1 h. Next, the agar-containing colonies were excised from the dish, and the agar slabs were immersed in 50% ethanol solution for dehydration through a graded series of ethanol solutions. Following dehydration in absolute ethanol, the agar slabs with colonies were critical point dried from liquid carbon dioxide, and the dry slabs were glued to specimen stubs with carbon adhesive tabs (Electron Microscopy Sciences), sputter coated with a thin layer of gold, and examined in a model Quanta 200 scanning electron microscope (EM; FEI Co., Inc., Hillsboro, OR) operated at high vacuum in the secondary electron imaging mode at various instrumental magnifications for digital imaging. The morphology of strains grown on glass was studied using biofilms generated in LB-NS broth as described above. After a 48-h incubation at 25°C, the glass slides were washed three times with sterile phosphate-buffered saline (PBS) and immersed in 2.5% glutaraldehyde in 0.1 M imidazole (Electron Microscopy Sciences) buffer (pH 7.0) for 2 h at room temperature before dehydration in a graded series of ethanol solutions (50%, 80%, and absolute), followed by critical point drying and sputter coating. The gold-coated microscope slides were mounted directly on a multiple-sample holder accessory and imaged in the microscope as described above. For transmission EM (TEM), patches of colonies on the surface of agar culture dishes were transferred with an open loop and dispersed through agitation into a 200-μl volume of Tris-buffered saline. Ten-microliter aliquots of the dispersions were adsorbed to a thin film of amorphous carbon on copper grids for about 30 s and then washed with a controlled stream of 5 to 8 drops of Tris-buffered saline, followed by a few drops of 2% uranyl acetate stain solution. Excess stain was adsorbed with Whatman filter paper, and grids were air dried before examination in a CM12 electron microscope (FEI Co., Inc., Hillsboro, OR), operated in the bright-field mode. Photographic images were made at various instrumental magnifications to resolve the details of filaments in the intercellular matrices.
Biofilm assays.
Detection and quantitation of biofilms on polystyrene were performed with 96-well polystyrene microtiter plates (Becton Dickinson, Franklin Lakes, N.J.) using the procedure of Mireles (12) with modifications (28). Tested strains were grown overnight at 37°C in each of three different media (TSB, 1/20-strength TSB, and LB-NS), diluted 1:10 in fresh medium, and used to inoculate eight replicate wells per microtiter plate. Controls consisted of wells containing sterile medium of each type. Plates were incubated for 48 h at 28°C, heat fixed (80°C for 30 min), stained with 200 μl 0.1% crystal violet for 20 min at 25°C, washed twice with 200 μl PBS, and solubilized in 200 μl of 95% ethanol. OD590 values were determined. Each experiment was performed on a single plate and consisted of a single trial of each strain (eight replicates) grown in each medium compared to their respective controls of noninoculated media. Three independent experiments were performed. The formation of biofilms on glass, Teflon, and stainless steel coupons was determined by a modification of the procedure of Prouty et al. (16). In brief, the coupons were cleaned, sterilized, and placed in disposable, 50-ml polypropylene, screw-topped centrifuge tubes. The tubes were filled with 20 ml TSB and inoculated with 100 μl of the appropriate test strain that had been grown overnight in TSB at 37°C. The tubes were incubated for 48 h at 28°C, the media were removed, and the biofilms were heat fixed as described above. The coupons were stained in 20 ml of 0.1% crystal violet for 20 min at 28°C, removed from the tubes, and washed under a stream of 25 ml of PBS. The coupons were returned to clean tubes, the stain was solubilized in 20 ml of 95% ethanol for 15 min, and the optical density was determined at 590 nm. Each experiment consisted of three independent samples of each strain. Each sample was tested in triplicate with each of the material types (glass, Teflon, and stainless steel). Duplicate control samples for each material consisted of the corresponding coupon in sterile medium. Experiments were run twice.
Assays of bacterial resistance to mild acid, hydrogen peroxide, and quaternary ammonium compounds.
The survival of biofilm and planktonic cells following exposure to low pH, hydrogen peroxide, or quaternary ammonium compounds was determined using biofilms generated on glass coupons. Biofilms were generated in either LB-NS or TSB for 48 h at 28°C as described above. Unattached cells growing in the broth surrounding the mature biofilm were expected to be in the stationary phase of growth. A 1.5-ml sample of the unattached cells was collected by centrifugation (5 min at 13,000 × g) and used as the planktonic cell sample. The glass slides with attached biofilms were exposed to 20 ml of the test compound in the appropriate diluent. Planktonic cells were resuspended in 1.5 ml of the test compound in diluent. Control samples were exposed to the diluent only. After incubation for the desired time, the test solutions were removed from the biofilm samples by being pipetted, and the samples were washed once with an equal volume of neutralization solution and once with sterile PBS. For the planktonic samples, the test solutions were immediately neutralized with 1.5 ml of 2× neutralization solution before being collected by centrifugation and then washed once with 1.5 ml of neutralization solution and once with 1.5 ml of PBS. After being washed, the planktonic samples were resuspended to their original volume in 0.1% peptone water (BBL/Becton Dickinson, Sparks, MD), serially diluted, and plated for enumeration of CFU per milliliter. The coupons, with attached biofilms, were moved to clean centrifuge tubes containing 20 ml of 0.1% peptone water and 0.25 g of glass beads. The cells were dislodged by being vortexed for 1 min, and cells were enumerated as for the planktonic samples. Determination of the resistance of strains to acidic conditions followed the procedure of Scher et al. (24). Cells were exposed to citrate phosphate buffer (40 mM citric acid [Amresco, Solon, OH], 20 mM dibasic sodium phosphate [Mallinkrodt Specialty Chemicals Co., Paris, KY], pH 3.0) for 2 h and neutralized with 0.1 M Na2S2O3 (Sigma-Aldrich). Exposure to hydrogen peroxide followed the procedure of Robbins et al. (17). Strains were exposed to 5% H2O2 (Sigma-Aldrich) in PBS (Boston Bioproducts, Inc., Worcester, MA) for 10 min and neutralized with 1% sodium pyruvate (Sigma-Aldrich). The concentrated BDD Backdown detergent disinfectant (Decon Laboratories, Inc., Bryn Mawr, PA), which contains alkyl dimethyl ethylbenzyl ammonium chloride and alkyl dimethyl benzyl ammonium chloride, each at 2.25%, was used to determine the resistance to quaternary ammonium compounds. Biofilm and planktonic cells were exposed to a 1:64 dilution in distilled H2O for either 60 s or 120 s and neutralized with Dey-Engley neutralizing broth as previously described (30). Three independent samples of each strain were tested under each condition, and samples were plated in duplicate for enumeration of resistant CFU. Experiments were run twice.
Statistical analyses.
The means of colony measurements were compared using Student's t tests. The data from biofilm formation and population reductions were compared by an analysis of variance; the means were separated by a Bonferroni least significant difference technique using SAS (SAS/STAT 9.1, Cary, NC). The level of significance for all comparisons was set at a P value of <0.05.
RESULTS
Phenotypic characterization of strains grown on solid agar.
In studies comparing colony characteristics, the mean colony diameter (MCD) of strain 43895OR (rdar) (MCD = 12.60 mm, standard deviation [SD] = 0.86) was statistically greater (P < 0.05) than that of strain 43895OW (saw) (MCD = 10.70 mm; SD = 0.50) after 48 h on 1.5% agar plates. This indicates that, analogous to the rdar strains of S. enterica serovar Typhimurium, E. coli strain 43895OR forms a larger colony than the saw variant strain 43895OW (21). When the surface spreading of single colonies spotted on individual 1.5% agar plates was monitored for 96 h, strain 43895OR increased from an MCD of 8.5 mm (SD = 1.80) after 24 h to an MCD of 16.3 mm (SD = 2.57) in 96 h, while strain 43895OW increased from an MCD of 7.5 mm (SD = 0.87) in 24 h to an MCD of 15.2 mm (SD = 2.02) in 96 h. The mean colony diameters of both strains essentially doubled during the period from 24 h to 96 h, indicating that there was little surface spreading in either strain 43895OR or strain 43895OW. This is in contrast to S. enterica serovar Typhimurium, where cells of the rdar strains were reported to reach colony diameters of 6 cm (six times the size of saw strains) after 48 h, with colonization of the entire plate by 96 h (21).
Electron microscopy of strains grown on agar.
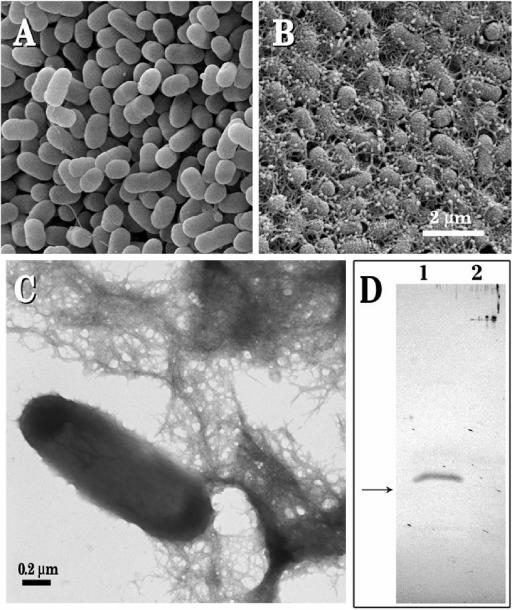
E. coli strain 43895OW grown on YESCA agar for 48 h at 30°C displayed a smooth-surfaced, rod-shaped morphology with the expression of an occasional single filamentous structure (Fig. 1A). However, cells of strain 43895OR, when grown under the same conditions, appeared as tightly packed, slightly longer rods with regular, roughened surfaces. The cells expressed large amounts of ECM projections, many terminating as smooth round droplets, that formed a dense, continuous network with the adjacent bacteria (Fig. 1B). Negative-staining transmission EM of 43895OR showed a network with fine filamentous curli fibers (Fig. 1C).
FIG. 1.
Topographical images of colonies of E. coli O157:H7 strains 43895OW (saw) (A) and 43895OR (rdar) (B) grown and fixed on YESCA agar for 48 h at 28°C. (C) Negatively stained TEM of dispersed colonies of strain 3895OR (rdar) harvested from a YESCA plate grown for 48 h at 28°C and stained with uranyl acetate. (D) Immunoblot of formic acid-treated cells collected from YESCA plates after 48 h at 28°C. Separated proteins of strains 43895OR (lane 1) and 43895OW (lane 2) reacted with anti-CsgA antibody. The arrow indicates the position of the 15-kDa marker. The predicted monomeric form of CsgA is 15.1 kDa.
Western blots.
The results of the immunoblot analysis comparing strains 43895OR (Congo red positive) and 43895OW (Congo red negative) following growth on YESCA agar for 48 h at 28°C are shown in Fig. 1D. A polyclonal antibody to E. coli CsgA reacted with a protein band from a formic acid-treated sample of strain 43895OR but not from strain 43895OW. The identified band migrated to a position corresponding to a molecular mass of slightly greater than 15 kDa, which is consistent with the predicted monomeric form of CsgA (15.1 kDa). Samples of both strains that were separated without using a formic acid treatment failed to react with the anti-curli antibody (data not shown). This indicates that strain 43895OR, but not 43895OW, generates detectable amounts of curli polymers when grown on YESCA agar. Minor signals were seen in the stacking portion of the gel for both strains 43895OR and 43895OW and could represent small amounts of a formic acid-insoluble CsgA protein. This suggests that although curli fibers may not be polymerized to a great extent on the surface of strain 43895OW during growth on YESCA agar, production of CsgA may still be occurring.
Calcofluor staining.
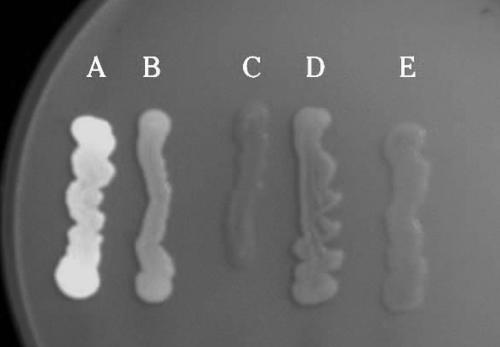
The results of the calcofluor staining experiments following incubation for 72 h are shown in Fig. 2. S. enterica serovar Enteritidis strain 3934 (curli positive, cellulose positive), displayed bright fluorescence when exposed to UV light at both 48 and 72 h. All other strains were negative for fluorescence following 48 h (results not shown). After 72 h, both strains 1170/97 (curli negative, cellulose negative) and 43895OW were not fluorescent; strains 942 (curli positive, cellulose negative) and 43895OR were only weakly fluorescent. These results indicate that unlike certain strains of Salmonella that displayed a red and dry colony phenotype, strain 43895OR did not produce significant quantities of extracellular cellulose concurrent with curli expression.
FIG. 2.
Fluorescence (366 nm) of bacterial strains grown on LB agar containing 200 mg/liter calcofluor at 28°C for 72 h. (A) S. enterica serovar Enteritidis strain 3934 (curli positive; cellulose positive); (B) S. enterica serovar Enteritidis strain 942 (curli positive; cellulose negative); (C) S. enterica serovar Enteritidis strain 1170/97 (curli negative; cellulose negative); (D) E. coli 0157:H7 strain 43895OR; (E) E. coli 0157:H7 strain 43895OW.
Characterization of strains adhered to glass slides.
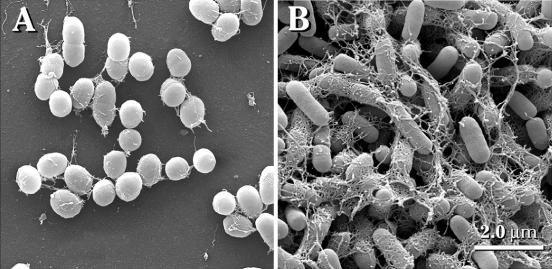
When strain 43895OR was allowed to generate a biofilm on a vertically oriented glass slide, a grossly visible pellicle which stained intensely with crystal violet (Fig. 3B) formed immediately below the surface of the medium. Microscopically, the bacteria in the pellicle (Fig. 4B) formed dense layers of tightly packed cells within a filamentous network and appeared similar in morphology to the same strain when cultured on YESCA agar. However, the surface of the cells appeared smooth. In the sections of the slide that were submerged deep within the medium during biofilm development, there were only scattered groups of bacteria adhered in shallow layers, which had less extracellular matrix and a less-extensive filamentous network composed of fine coiled fibers (results not shown). When slides cultured with strain 43895OW were examined grossly (Fig. 3A), there was no visible pellicle formation or crystal violet staining. Microscopically, on areas of the slide just below the medium-air interface, there were a few scattered clusters of bacteria attached to the slide in a monolayer (Fig. 4A). However, unlike the cells of 43895OW fixed and examined on YESCA plates, the cells attached to glass expressed moderate numbers of delicate coiled filaments that attached to the glass, bridged with adjacent cells, and occasionally formed a small network. The deeper areas of slides cultured with strain 43895OW showed essentially no attached cells. Strain 43895OR produced large visible aggregates, which settled to the bottom of the tubes during biofilm production (results not shown). However, strain 43895OW failed to produce any visible aggregates under the same conditions.
FIG. 3.
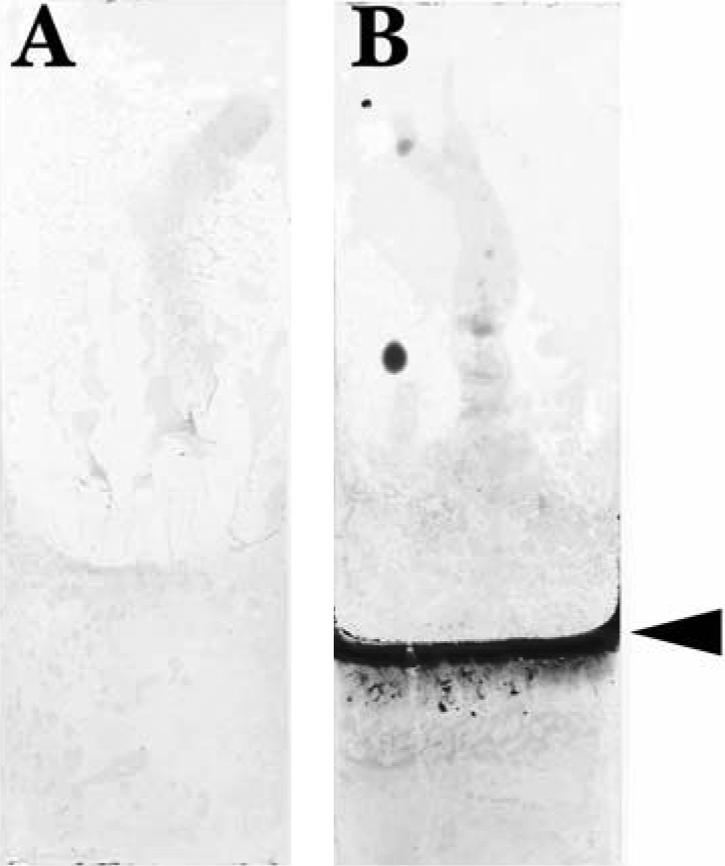
Crystal violet-stained glass coupons from biofilm assays with strains 43895OW (A) and 43895OR (B). Coupons were submerged in 50-ml tubes containing 20 ml LB-NS broth for 48 h at 28°C. The arrowhead indicates the medium fill line.
FIG. 4.
Scanning electron micrographs of glass coupons with biofilms generated in LB-NS medium for 48 h at 28°C. (A) E. coli O157:H7 strain 43895OW (saw) from the region of the slide immediately below the medium-air interface. (B) E. coli O157:H7 strain 43895OR (rdar) from the biofilm pellicle located below the medium-air interface.
Biofilm formation on polystyrene in multiple media.
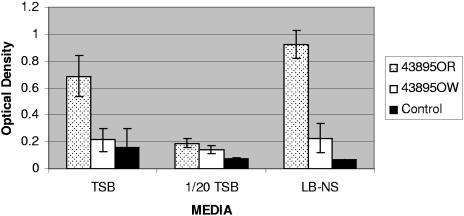
The results of the assays comparing biofilm formation on polystyrene, in each of three different media, by strains 43895OR and 43895OW are shown in Fig. 5. When grown in TSB, strain 43895OR (mean OD at 590 nm [MOD] = 0.69; SD = 0.15) retained >3-fold more crystal violet dye than both strain 43895OW (MOD = 0.21, SD = 0.09, P < 0.05) and the medium control (MOD = 0.15, SD = 0.14, P < 0.05). There was no significant difference between strain 43895OW and the TSB control. When grown in LB-NS, strain 43895OR (MOD = 0.92, SD = 0.10) retained >4-fold more dye than either strain 43895OW (MOD = 0.23, SD = 0.11, P < 0.05) or the medium control (MOD = 0.06, SD = 0.01, P < 0.05). There was a slight but significant (P < 0.05) difference between 43895OW and the LB-NS control. In 1/20-TSB, strain 43895OR (MOD = 0.19, SD = 0.03) retained slightly larger amounts of dye than the medium control (MOD = 0.07, SD = 0.01, P < 0.05). However, there was no significant difference (P > 0.05) between strain 43895OR and strain 43895OW (MOD = 0.14, SD = 0.03) or between strain 43895OW and the 1/20-TSB control.
FIG. 5.
Biofilm formation of E. coli O157:H7 strains 43895OR and 43895OW on polystyrene in TSB, 1/20 tryptic soy broth, and LB-NS. Each bar represents the mean optical density measured at 590 nm ± standard deviation of eight replicates from a single sample taken from each of three independent trials for strains 43895OR and 43895OW or two independent trials from the medium controls.
Biofilm formation on glass, Teflon, and stainless steel.
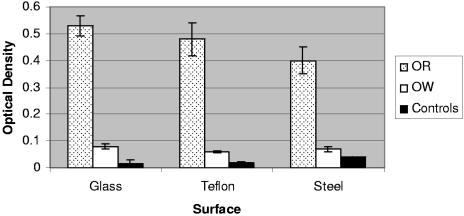
The results of the assays for biofilm formation on glass, Teflon, and stainless steel by strains 43895OR and 43895OW grown in TSB are shown in Fig. 6. When grown on glass, strain 43895OR (MOD = 0.53, SD = 0.04) retained >6-fold-larger amounts of crystal violet dye than either strain 43895OW (MOD = 0.08, SD = 0.01, P < 0.05) or the medium control (MOD = 0.07, SD = 0.02, P < 0.05). On Teflon, strain 43895OR (MOD = 0.48, SD = 0.06) retained approximately eight-fold-higher amounts of dye than either strain 43895OW (MOD = 0.06, SD = 0.003, P < 0.05) or the TSB control (MOD = 0.02, SD = 0.001, P < 0.05). Finally, on stainless steel, strain 43895OR (MOD = 0.40, SD = 0.05) retained >5-fold more dye than either strain 43895OW (MOD = 0.07, SD = 0.01, P < 0.05) or the TSB control (MOD = 0.04, SD = 0.0, P < 0.05). There was no significant difference in the dye retention of strain 43895OW compared to the controls following growth on each of the individual surfaces. When the different surfaces were compared for any within-strain differences in biofilm formation, strain 43895OR retained more dye when grown on glass than on stainless steel (P < 0.05), but there were no differences between the dye retention of 43895OR on glass compared to Teflon or on stainless steel compared to Teflon. There were no within-group differences for strain 43895OW or the medium controls.
FIG. 6.
Biofilm formation of E. coli O157:H7 strains 43895OR and 43895OW on glass, Teflon, and stainless steel in LB broth (without salt). Each bar represents the mean optical density measured at 590 nm ± standard deviation of a single sample taken from each of three independent trials for strains 43895OR and 43895OW or two independent trials from the medium controls.
Resistance of strain 43895OR and 43895OW planktonic and biofilm cells to hydrogen peroxide, quaternary ammonium sanitizer, and acid.
The results of assays measuring survival of strains 43895OR and 43895OW following exposure to antimicrobial agents are shown in Table 1. When planktonic cells of strains 43895OR and 43895OW were exposed to 5% H2O2 for 10 min, no viable cells could be recovered compared to ca. 8 log10 CFU/ml recovered from the untreated controls. When strains 43895OR and 43895OW were allowed to form biofilms on glass coupons and then exposed to 5% H2O2 for 10 min, >3 log10 CFU cells/ml were recovered from the 43895OR biofilm. This was statistically less (P < 0.05) than the >7 log10 CFU/ml recovered from the untreated controls and indicates that the biofilm cells of strain 43895OR have some but not complete resistance to the effect of treatment with 5% H2O2. However, >4 log10 CFU/ml were recovered from the attached cells of strain 43895OW, a result statistically indistinguishable from that for the untreated controls, indicating that strain 43895OW was completely resistant to the effects of H2O2 at that concentration.
TABLE 1.
Survival of biofilm and planktonic cells of strains 43895OR and 43895OW following exposure to hydrogen peroxide (5.0% for 10 min at 28°C), quaternary ammonium sanitizer (1:64 dilution for 1 min and 2 min at 28°C), or citric acid (pH = 3.0 for 2 h at 28°C)a
| Treatment (time) | Planktonic 43895OR | Planktonic 43895OW | Biofilm 43895OR | Biofilm 43895OW |
|---|---|---|---|---|
| H2O2 | NDb,c | NDc | 3.56 ± 0.57c | 4.18 ± 0.78 |
| H2O2 control | 7.79 ± 0.09 | 7.98 ± 0.27 | 7.70 ± 0.22 | 5.04 ± 0.33 |
| Sanitizer (1 min) | 7.43 ± 0.14 | 4.83 ± 0.83c | 7.09 ± 0.20 | 4.40 ± 0.54 |
| Sanitizer control (1 min) | 8.03 ± 0.06 | 7.81 ± 0.05 | 7.63 ± 0.22 | 5.17 ± 0.32 |
| Sanitizer (2 min) | 3.70 ± 0.21c | 0.73 ± 0.34c | 6.68 ± 0.52 | 5.42 ± 0.77 |
| Sanitizer control (2 min) | 7.50 ± 0.26 | 8.15 ± 0.19 | 7.52 ± 0.42 | 4.76 ± 0.30 |
| Citric acid | 7.66 ± 0.37 | 8.13 ± 0.09 | 7.29 ± 0.08 | 5.20 ± 0.32 |
| Citric acid control | 7.27 ± .012 | 7.31 ± 0.01 | 7.35 ± 0.10 | 4.92 ± 0.47 |
Each value represents the mean log10 CFU/ml ± standard deviation of two replicates of a single sample from each of three independent trials.
No colonies detected in undiluted samples.
Indicates treatment and control pairs with means that are statistically different from each other by Bonferroni least significant difference.
The assays using the quaternary ammonium compound were recorded for two different exposure periods. Following exposure for 1 min, the planktonic cells of 43895OW were recovered in significantly smaller numbers than the untreated control, indicating a susceptibility to quaternary ammonium compounds under those conditions. However, the planktonic cells of strain 43895OR and the biofilm cells of both strains were recovered in numbers indistinguishable from their controls indicating that they were resistant. When the time of exposure to the sanitizer was doubled to 2 min, the planktonic cells of both strains 43895OR and 43895OW showed susceptibility to the sanitizer. The numbers of strain 43895OW were reduced >7 log10 CFU/ml compared to the control, while strain 43895OR was reduced >3 log10 CFU/ml. As with the 1-min exposure, the biofilm cell numbers of both strains were not significantly different from those of the untreated controls. These findings indicate that cells attached in a biofilm, whether strain 43895OW or 43895OR, are more protected from the lethal effects of quaternary ammonium compounds than the corresponding planktonic cells under the same conditions. In addition, the planktonic cells of 43895OR (>3 log10 CFU/ml reduction compared to nontreated controls), were more resistant to the sanitizer than planktonic cells of strain 43895OW (>8 log10 CFU/ml reduction compared to nontreated controls) following a 2-min exposure.
The results of the exposure of biofilm and planktonic cells of 43895OR and 43895OW to citric acid (pH = 3.0) are also shown in Table 1. There was no statistical difference in the survival of either strain compared to their untreated controls for either planktonic or biofilm cells following exposure to acid for 2 h (P > 0.05).
DISCUSSION
The rdar phenotype, associated with the expression of curli fibers and cellulose, is well described for certain strains of Salmonella spp. However, the rdar phenotype has not been well defined for E. coli O157:H7. The extremely aggregative behavior of a naturally occurring mutant strain (43895OR) on agar containing Congo red dye is similar to the rdar phenotype described for Salmonella (32). In this study, we showed that strain 43895OR is also similar to the rdar strains of S. enterica serovar Typhimurium in characteristics such as colony size, pellicle formation on solid surfaces, aggregate formation in broth, and cellular ultrastructure following growth on agar (18, 21). Collectively, these findings suggest that the rdar phenotype in E. coli strain 43895OW is similar to that of strains of certain Salmonella spp. However, while immunoblot assays confirmed the expression of polymerized curli fibers on strain 43895OR grown on YESCA media, the results of calcofluor staining indicated that cellulose is not a component of the rdar phenotype in E. coli strain 43895OR. Zogaj et al. (34) have shown that certain natural isolates of E. coli, which displayed the characteristics of the rdar phenotype, also produced cellulose as an additional component of the ECM. Our findings indicate that components other than cellulose could be important in generating the rdar phenotype in E. coli O157:H7. This difference in the components of the extracellular matrix might also explain the differences in swarming motility between strain 43895OR and rdar-producing strains of S. enterica serovar Typhimurium.
We also looked at the ability of the variants of strain 43895 to form biofilms on different surfaces. Our finding that strain 43895OR (rdar) produced significantly more biofilm than strain 43895OW (saw) on stainless steel is in agreement with that of Ryu et al. (23), who tested these same strains on stainless steel coupons and found greater attachment of the curli-producing strain, with the level of attachment reaching near maximum by 48 h. The current study shows that strain 43895OR also produces significantly more biofilm than strain 43895OW on three additional surfaces: polystyrene, glass, and Teflon. In a recent study (15), several pairs of curli-expressing and non-curli-expressing variants of EHEC isolated from the same stock culture were tested on polystyrene, glass, stainless steel, and rubber. The results indicated that curliated strains of some pairs, but not others, attached in greater numbers than their noncurliated isogenic variants. There were also differences between the curliated strains and their noncurliated variants in attachment on certain surfaces, but no differences on others. However, the EM characterization of the cells in that study clearly indicates differences in the ECM between the curli-producing strains used by Pawar et al. (15) and the rdar strain used in this study. These differences in the ECM may explain the differences in biofilm formation between the strains in these two studies. It is clear that the expression of curli and the ability to form biofilms vary greatly among strains and even among isolates within a strain.
Strain 43895OR produced a dense biofilm on polystyrene in two different rich media but produced little biofilm in 1/20-TSB. These findings are in contrast to those of Dewanti and Wong (6), who found that low-nutrient media enhanced the development of E. coli O157:H7 biofilms on stainless steel. Our study indicates that biofilm formation by strain 43895OR is greatly reduced when generated in a low-nutrient environment. Whether this difference is due to a greater nutrient requirement resulting from a constitutive ECM expression in strain 43895OR is unclear.
In the challenge studies with microbicidal agents, we noticed that large numbers of strain 43895OW cells were dislodged from the glass slides of untreated control tubes following the 48-h incubation. Although the numbers were >2 log10 CFU/ml lower than numbers recovered from the untreated controls of strain 43895OR, we found these numbers to be unexpectedly high, in light of the fact that the crystal violet biofilm assays showed that the adhesion of strain 43895OW was often not statistically different from that of the medium controls. This prompted us to verify the presence of cells on the glass slides by scanning electron microscopy. While strain 43895OR formed a dense, well-organized biofilm, strain 43895OW formed only scattered clusters of monolayer cells. The numbers of cells we found on the slides with strain 43895OW were lower than expected, but it may be that the 43895OW cells were more loosely attached and dislodged more easily during the EM processing. Nevertheless, the finding that strain 43895OW (saw) expressed ECM and attached to glass indicates that the expression of curli fibers and ECM can be induced in saw strains upon exposure to solid surfaces under the right conditions. We also dislodged the cells from slides growing with either strain 43895OR or strain 43895OW during biofilm studies and immediately plated the cells (and the corresponding planktonic cells) on CRI plates, which were incubated for 48 h at 28°C (data not shown). We found that ca. 5% of the planktonic cells of strain 43895OR had phase switched to the white variant. This is not surprising, as we have previously shown (32) that a phase switch of the Congo red-binding (rdar) variant to the nonbinding (saw) variant will occur after several passages in rich broth. However, when we plated cells that were dislodged from the biofilm of strain 43895OR, we found that all cells displayed the rdar phenotype on CRI plates. This could indicate that white variants are less likely to incorporate into an existing biofilm and/or that the cells already existing within the biofilm are under pressure to remain in the rdar phase. When the planktonic cells of strain 43895OW were plated on CRI agar, all colonies were of the saw phenotype. Again, this is not surprising, as previous studies have shown that the white variants do not revert back to a dry, red phenotype with the promoter mutation (32). When strain 43895OW cells were dislodged from the glass slide, a few colonies (<1%) showed a Congo red-binding phenotype that, unlike the rdar phenotype, was pale red and smooth (results not shown). These colonies could reflect some of the cells of strain 43895OW that express curli fibers when attached to glass (Fig. 4A). However, the different colony phenotype on CRI agar, compared to that for strain 43895OR, indicates differences in the surface structure. This suggests that while certain mutant strains of E. coli O157:H7 are capable of producing dense biofilms, as shown by the highly aggregative, rdar phenotype of strain 43895OR, the full expression of all factors required for maximum biofilm formation by the parent strain (ATCC 43895) may remain repressed under laboratory conditions. While curli expression may be induced, other factors required for full biofilm development may not be. In a study of E. coli EPS and biofilm formation, colanic acid was shown to be important for biofilm development but not required for attachment (5). Whether colanic acid, rather than cellulose, is important for the robust development of strain 43895OR biofilms is unknown. We are currently working on identifying the EPS components involved in the highly aggregative behavior and biofilm formation of strain 43895OR.
We also tested the affects of microbicidal agents on both planktonic and biofilm cells of strains 43895OR and 43895OW. Our finding that both 43895OW and 43895OR showed complete resistance to the killing effects of acid (pH = 3.0), whether in a biofilm or as planktonic cells, is in agreement with the findings of Scher et al. (24), who found that pellicle cells of S. enterica serovar Typhimurium (rdar) and the associated stationary-phase planktonic cells were completely resistant to the effects of acid. Although there is little information in the literature comparing the effects of hydrogen peroxide on planktonic cells versus biofilm cells from strains of E. coli O157:H7, studies have shown that biofilm cells of Listeria monocytogenes were more resistant than planktonic cells to hydrogen peroxide exposure (17). Our results confirmed a similar pattern of resistance for the biofilm cells of E. coli O157:H7. Likewise, our finding that biofilm cells were more resistant to a quaternary ammonium compound is in agreement with that of Stopforth et al. (30), who showed similar results for E. coli O157:H7 growing in biofilms on stainless steel. Collectively, these studies indicate that cells of both of the variants of strain 43895 (43895OR and 43895OW), when attached to solid surfaces, are more resistant to sanitizers than the corresponding planktonic cells. As 43895OR cells formed a larger biofilm, there was a protective advantage in numbers for strain 43895OR over strain 43895OW. Surprisingly, at the levels tested, the scattered clusters of strain 43895OW cells attached to glass did not seem to be more sensitive to hydrogen peroxide or quaternary ammonium sanitizer than the densely packed biofilm cells of strain 43895OR. In fact, strain 43895OW biofilm cells were more resistant to the effects of hydrogen peroxide, based on cell viability comparison to the untreated controls, than strain 43895OR biofilm cells. This suggests that factors in strain 43895OR that result in a more robust biofilm may contribute to a slightly increased susceptibility to environmental oxidative stress by an unknown mechanism. In planktonic cell form, strain 43895OR was more resistant to quaternary ammonium sanitizer, compared to controls, than strain 43895OW. This may indicate that components of the constitutively produced ECM of strain 43895OR may provide protection for unattached cells against certain sanitizers.
In this study, we have shown that the rdar phenotype of strain 43895OR is similar to that described for certain strains of Salmonella and is important in multicellular behavior, biofilm production, and resistance to certain bactericidal compounds. However, strain 43895OR does not produce cellulose as part of the ECM. The studies of strain 43895OW attached to glass indicate that curli fibers can be expressed during attachment to glass. However, the difference in the colony phenotype between strain 43895OW recovered from glass and strain 43895OR indicates differences other than the expression of curli. Strain 43895OR may serve as a valuable strain for investigation of these composition differences and characterization of other factors involved in biofilm formation. Moreover, strain 43895OW may serve as a valuable strain for the study of the selective expression of factors involved in biofilm formation under different conditions.
Acknowledgments
We thank John Phillips for assistance with statistical analyses and Mary Ubbens, Bryan Cottrell, and Paul Pierlott for technical assistance.
Mention of trade names or commercial products in this document is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
REFERENCES
- 1.Austin, J. W., G. Sanders, W. W. Kay, and S. K. Collinson. 1998. Thin aggregative fimbriae enhance Salmonella enteritidis biofilm formation. FEMS Microbiol. Lett. 162:295-301. [DOI] [PubMed] [Google Scholar]
- 2.Ben Nasr, A., A. Olsén, U. Sjöbring, W. Müller-Esterl, and L. Björck. 1996. Assembly of human contact phase proteins and release of bradykinin at the surface of curli-expressing Escherichia coli. Mol. Microbiol. 20:927-935. [DOI] [PubMed] [Google Scholar]
- 3.Carpentier, B., and O. Cerf. 1993. Biofilms and their consequences, with particular reference to hygiene in the food industry. J. Appl. Bacteriol. 75:499-511. [DOI] [PubMed] [Google Scholar]
- 4.Collinson, S. K., L. Emödy, K.-H. Müller, T. J. Trust, and W. W. Kay. 1991. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J. Bacteriol. 173:4773-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewanti, R., and A. C. L. Wong. 1995. Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. Int. J. Food Microbiol. 26:147-164. [DOI] [PubMed] [Google Scholar]
- 7.Hammar, M., Z. Bian, and S. Normark. 1996. Nucleator-dependent intercellular assembly of adhesive curli organelles in Escherichia coli. Proc. Natl. Acad. Sci. USA 93:6562-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammar, M., A. Arnqvist, Z. Bian, A. Olsén, and S. Normark. 1995. Expression of two csg operons is required for production of fibronectin- and Congo red-binding curli polymers in Escherichia coli K12. Mol. Microbiol. 18:661-670. [DOI] [PubMed] [Google Scholar]
- 9.Kumar, C. G., and S. K. Anand. 1998. Significance of microbial biofilms in food industry: a review. Int. J. Food Microbiol. 42:9-27. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Lund, B. M., T. C. Baird-Parker, and G. W. Gould. 2000. The microbiological safety and quality of food. Aspen Publishers, Inc., Gaithersburg, Md.
- 12.Mireles, J. R., A. Toguchi, and R. M. Harshey. 2001. Salmonella enterica serovar Typhimurium swarming mutants with altered biofilm-forming abilities: surfactin inhibits biofilm formation. J. Bacteriol. 183:5848-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsén, A., M. J. Wick, M. Mörgelin, and L. Björck. 1998. Curli, fibrous surface proteins of Escherichia coli, interact with major histocompatability complex class I molecules. Infect. Immun. 66:944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsén, A., A. Jonsson, and S. Normark. 1989. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature 338:652-655. [DOI] [PubMed] [Google Scholar]
- 15.Pawar, D. M., M. L. Rossman, and J. Chen. 2005. Role of curli fimbriae in mediating the cells of enterohemorrhagic Escherichia coli to attach to abiotic surfaces. J. Appl. Microbiol. 99:418-425. [DOI] [PubMed] [Google Scholar]
- 16.Prouty, A. M., W. H. Schwesinger, and J. S. Gunn. 2002. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect. Immun. 70:2640-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins, J. B., C. W. Fisher, A. G. Moltz, and S. E. Martin. 2005. Elimination of Listeria monocytogenes biofilms by ozone, chlorine, and hydrogen peroxide. J. Food Prot. 68:494-498. [DOI] [PubMed] [Google Scholar]
- 18.Römling, U., M. Rhode, A. Olsén, S. Normark, and J. Reinköster. 2000. AgfD, the checkpoint of multicellular and aggregative behavior in Salmonella typhimurium regulates at least two independent pathways. Mol. Microbiol. 36:10-32. [DOI] [PubMed] [Google Scholar]
- 19.Römling, U., and M. Rhode. 1999. Flagella modulate the multicellular behavior of Salmonella typhimurium on the community level. FEMS Microbiol. Lett. 180:91-102. [DOI] [PubMed] [Google Scholar]
- 20.Römling, U., Z. Bian, M. Hammar, W. D. Sierralta, and S. Normark. 1998. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 180:722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Römling, U., W. D. Sierralta, K. Eriksson, and S. Normark. 1998. Multicellular and aggregative behavior of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249-264. [DOI] [PubMed] [Google Scholar]
- 22.Ryu, J.-H., and L. R. Beuchat. 2005. Biofilm formation by Escherichia coli O157:H7 on stainless steel: effect of exopolysaccharide and curli production on its resistance to chlorine. Appl. Environ. Microbiol. 71:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryu, J.-H., H. Kim, J. F. Frank, and L. R. Beuchat. 2004. Attachment and biofilm formation on stainless steel by Escherichia coli O157:H7 as affected by curli production. Lett. Appl. Microbiol. 39:359-362. [DOI] [PubMed] [Google Scholar]
- 24.Scher, K., U. Römling, and S. Yaron. 2005. Effect of heat, acidification, and chlorination on Salmonella enterica serovar Typhimuriun cells in a biofilm formed at the air-liquid interface. Appl. Environ. Microbiol. 71:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivapalasingam, S., C. R. Friedman, L. Cohen, and R. V. Tauxe. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67:2342-2353. [DOI] [PubMed] [Google Scholar]
- 26.Sjöbring, U., G. Pohl, and A. Olsén. 1994. Plasminogen, absorbed by Escherichia coli expressing curli or by Salmonella enteritidis expressing thin aggregative fimbriae, can be activated by simultaneously captured tissue-type plasminogen activator (t-PA). Mol. Microbiol. 14:443-452. [DOI] [PubMed] [Google Scholar]
- 27.Solano, C., B. Garcia, J. Valle, C. Berasain, J.-M. Ghigo, C. Gamazo, and I. Lasa. 2002. Genetic analysis of Salmonella enteritidis biofilm formation: critical roll of cellulose. Mol. Microbiol. 43:793-808. [DOI] [PubMed] [Google Scholar]
- 28.Solomon, E. B., B. A. Niemira, G. M. Sapers, and B. A. Annous. 2005. Biofilm formation, cellulose production, and curli biosynthesis by Salmonella originating from produce, animal, and clinical sources. J. Food Prot. 68:906-912. [DOI] [PubMed] [Google Scholar]
- 29.Somers, E. B., J. L. Schoeni, and A. C. L. Wong. 1994. Effect of trisodium phosphate on biofilm and planktonic cells of Campylobacter jejuni, Escherichia coli O157:H7, Listeria monocytogenes and Salmonella typhimurium. Int. J. Food Microbiol. 22:269-276. [DOI] [PubMed] [Google Scholar]
- 30.Stopforth, J. D., J. Samelis, J. N. Sofos, P. A. Kendall, and G. C. Smith. 2002. Biofilm formation by acid-adapted and nonadapted Listeria monocytogenes in fresh beef decontamination washings and its subsequent inactivation with sanitizers. J. Food Prot. 65:1717-1727. [DOI] [PubMed] [Google Scholar]
- 31.Uhlich, G. A., J. E. Keen, and R. O. Elder. 2002. Variations in the csgD promoter of Escherichia coli O157:H7 associated with increased virulence in mice and increased invasion of HEp-2 cells. Infect. Immun. 70:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlich, G. A., J. E. Keen, and R. O. Elder. 2001. Mutations in the csgD promoter associated with variations in curli expression in certain strains of Escherichia coli O157:H7. Appl. Environ. Microbiol. 67:2367-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidal, O., R. Longin, C. Prigent-Combaret, C. Dorel, M. Hooreman, and P. Lejeune. 1998. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 180:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zogaj, X., M. Nimtz, M. Rohde, W. Bokranz, and U. Römling. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39:1452-1463. [DOI] [PubMed] [Google Scholar]