Abstract
In Agaricus bisporus, traditional cultivars and most of the wild populations belong to A. bisporus var. bisporus, which has a predominantly pseudohomothallic life cycle in which most meiospores are heterokaryons (n + n). A lower proportion of homokaryotic (n) meiospores, which typify the heterothallic life cycle, also are produced. In wild populations, pseudohomothallism was thought previously to play a major role, but recent analyses have found that significant outcrossing also may occur. We inoculated a standard substrate for A. bisporus cultivation simultaneously with homokaryotic mycelium from one parent and spores from a second parent. Culture trays produced numerous sporocarps that could theoretically have resulted from five different reproductive modes (pseudohomothallism, selfing or outcrossing via heterothallism, and selfing or outcrossing via the Buller phenomenon [i.e., between a homokaryon and a heterokaryon]). Most or all of the sporocarps resulted from outcrossing between the inoculated homokaryon and the inoculated heterokaryotic spores (or mycelia that grew from them). These data broaden our understanding of population dynamics under field conditions and provide an outcrossing method that could be used in commercial breeding programs.
Agaricus bisporus (Lange) Imbach, the button mushroom, has a unifactorial mating system (18) with multiple alleles (11) at the MAT locus located on chromosome 1 (26). Most wild populations and all of the traditional cultivars of A. bisporus belong to A. bisporus var. bisporus. In this variety, the life cycle is amphithallic; i.e., both heterothallic genetic reproduction and (in this case, predominantly) pseudohomothallic (=secondary homothallic) genetic reproduction occur (22). Heterothallic basidiospores are homokaryotic (n) and generally give rise to self-sterile homokaryotic mycelia. Plasmogamy between two sexually compatible homokaryons restores a fertile heterokaryon. Pseudohomothallic basidiospores are heterokaryons (n + n) and contain two nonsister postmeiotic nuclei with different mating type alleles. Mycelia growing from these spores are sexually fertile and usually maintain the parental genotype in the offspring (15, 21, 23). Amphithallism is not rare, and 9% of ∼500 species of holobasidiomycetes with lamellae are considered amphithallic (16). In wild strains of A. bisporus var. bisporus only 1.3% of the basidia, on average, are tetrasporic (5) and produce homokaryotic basidiospores. In wild populations, successive generations of pseudohomothallism should generate pseudoclonal lineages (12). Little evidence of pseudohomothallic reproduction was found in a recent spatial-temporal analysis of two local French populations (25), suggesting that outcrossing and recombination played significant roles in the history of both populations. Determining the relative roles of heterothallism and pseudohomothallism in wild populations by genetic analysis of collected sporocarps is difficult because of migration between sites and because such samples do not include individuals that do not form sporocarps.
In the present study, we analyzed one event that could occur in a field setting: a homokaryon from one parent is growing, and spores from a second (heterokaryotic) parent arrive. When a homokaryon is cultivated on compost like a commercial heterokaryotic cultivar (Fig. 1), it cannot form sporocarps by itself and requires genetic information from the spores of a second parent to do so; on the other hand, mycelia derived from the heterokaryotic spores of the second parent can fructify by themselves. Our objectives were to evaluate the relative role played by outcrossing among the possible events leading to fructification and to develop a new breeding strategy for A. bisporus. Five events can potentially generate fertile heterokaryons (Fig. 2): outcrossing between homokaryons (heterothallism), outcrossing between a homokaryon and a heterokaryon via the Buller phenomenon, selfing between homokaryons (heterothallism), selfing via the Buller phenomenon, and intramixis (pseudohomothallism). During the Buller phenomenon (1), anastomosis between a homokaryon and a heterokaryon produces a hybrid heterokaryon that receives the nucleus of the homokaryon and one of the two nuclei of the heterokaryon (which must be sexually compatible with the nucleus of the homokaryon). This process occurs in many basidiomycetes and is generally accompanied by nuclear migration from the heterokaryon through the homokaryon, which becomes heterokaryotic. The Buller phenomenon occurs in A. bisporus, but so far there is no evidence for nuclear migration in this species (22).
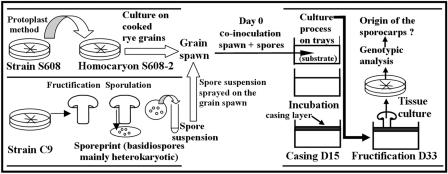
FIG. 1.
Schematic representation of the method used for the outcrossing experiments. The homokaryon S609-2 and spores of C9 are simultaneously inoculated into a standard substrate.
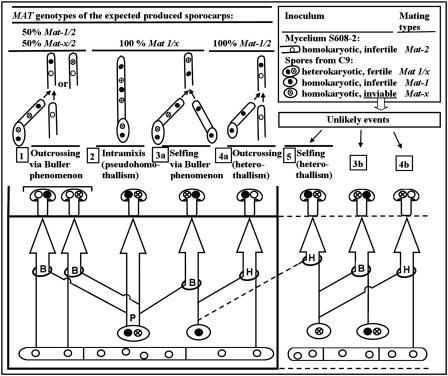
FIG. 2.
Theoretical expected origins and genotypes of sporocarps produced on a culture tray simultaneously inoculated with the homokaryon S608-2 and spores from a sporocarp of the heterokaryon C9. Five different processes that can produce fertile heterokaryons in competition in the substrate are represented schematically. B, Buller phenomenon; P, pseudohomothallism; H, heterothallism. The MAT alleles in spore nuclei are indicated as follows: •, Mat-1; ○, Mat-2; ⊗, Mat-x. A low proportion of the inoculated spores are homokaryotic. C9 is presumed not to produce viable homokaryotic spores that carry the Mat-x allele; therefore, unless recombination occurs, events 3b, 4b, and 5 do not occur, and hybrids resulting from outcrosses between homokaryons cannot carry a Mat-x allele.
In a previous experiment of this type, we used a homokaryon derived from a commercial cultivar and spores from a field strain from Greece that did not produce any viable homokaryotic spores. Numerous sporocarps were analyzed with codominant molecular markers (3). Some of these sporocarps resulted from outcrossing through the Buller phenomenon, and none of them had the same genotype as the heterokaryotic spores. However, no homokaryons were isolated from the Greek strain, so the genotype data could not be interpreted unambiguously. Additionally, many of the sporocarps resulted from crosses with contaminant inocula. In the present study, drastic precautions were taken to avoid such contamination. We used the commercial C9 strain of the traditional “brown” cultivar. Homokaryons recovered from this strain always have the same mating type allele, whether they are obtained from spores or from protoplasts. This result suggests that the “brown” cultivar carries a recessive deleterious or lethal allele at a locus linked to MAT. This characteristic of the C9 strain, specifically the behavior of a “restricted” class of nuclei, can be used to determine whether outcrossing proceeds through the Buller phenomenon or through heterothallism (Fig. 2).
Our objectives in this study were (i) to determine if outcrossing plays a role when a substrate is simultaneously inoculated with spores and mycelium and (ii) to develop a novel outcrossing method. Among the five different processes that could lead to fructification in this situation, we expected that pseudohomothallism and outcrossing, possibly via the Buller phenomenon, would occur. In a natural population outcrossing can alter the genetic structure and can favor genetic erosion via crop-to-wild gene flow. An outcrossing method that would allow controlled crosses between many strains of A. bisporus would broaden the available breeding strategies for this important fungal species, whose members cannot be easily crossed by using classical methods.
MATERIALS AND METHODS
Parental strains and their mating type genotypes.
Homokaryotic mycelium (S608-2) from S608 was confronted with spores from C9 (Fig. 1). Both of the parental strains, S608 and C9, are bisporic cultivars, but they produce sporocarps with white and brown caps, respectively. S608 (Somycel, Langeais, France) is a classic “white hybrid” that is genetically very similar to cultivar U1 (Mushroom Research Unit, Horst, The Netherlands). Homokaryons S608-2 and S608-6 were derived from S608 by protoplasting (14) and are genetically very similar to the U1-7 and U1-2 homokaryons, respectively. The U1-7 and U1-2 homokaryons have been used for genetic linkage analysis (3) and carry the Mat-2 and Mat-1 alleles, respectively. S608-6 was used only for mating tests. C9 (Société Blanc de Semi “Le Lion,” Saumur, France), the heterokaryotic parent of the spores, is a well-known traditional “brown” cultivar. All the homokaryons derived from C9 carry the Mat-1 allele. The mating type genotype of C9 is partially undetermined (Mat-1/x).
Tests for C9 offspring analysis.
Methods for spore germination and for evaluation of the proportion of homokaryons (mycelial growth rate test, multilocus genotype test, and mating tests) were conducted as previously described (14). For spore germination, we used stimulation by isovaleric acid or by a mycelial culture placed on the inner lid of a petri dish (17). The latent period before germination varies from 5 to 9 days, and the spore germination rate varies from ∼1% to ∼25%. Multilocus genotyping was performed with the PR4 and PR50 markers (see below), for which the C9 parent is heteroallelic. Mating tests were performed with the S608-2 (Mat-2) and S608-6 (Mat-1) homokaryotic testers.
Preparation of rye grain spawn.
A rye grain spawn culture of the S608-2 homokaryon was prepared under sterile conditions like a rye grain spawn culture is prepared for a commercial heterokaryon (9).
Simultaneous inoculation of rye grain spawn and spores into compost.
Spores from a single sporeprint of a C9 sporocarp (∼109 spores) were used to equally inoculate four culture trays at the same time as spawn inoculation. Spawn was spread on a table in a room sterilized by UV light, and the spore suspension (16 ml) was sprayed directly onto the spawn. The mixture of S608-2 grain spawn and C9 spores was divided into four bags. To avoid contamination, one person sprayed the spores in the sterile room, a second person took the bags from this room to the culture room, and a third person inoculated compost with the mixture of spawn and spores in the culture room. Compost was brought from the production site to the culture room in closed containers. When the compost was inoculated, the trays were filled with successive layers of grain spawn and compost to reduce or eliminate any airborne inoculum. The level of inoculation was 2% (0.13 kg of spawn for 6.5 kg of standard pasteurized compost in each tray); this was twice the level usually used for a commercial heterokaryon in order to compensate for the lower growth rate of the homokaryon.
First experiment: culture process and fructification.
In the first experiment, four trays were inoculated simultaneously with grain spawn and spores, and 12 control trays were inoculated only with grain spawn. The control trays were colonized by the S608-2 homokaryon, which could not produce sporocarps by itself and produced sporocarps only if the trays were contaminated by extraneous inoculum. A standard culture process (Fig. 1) was used. To avoid outside contamination, the culture process was performed entirely in a single culture room with a controlled environment, which included inoculation, incubation (15 days), casing and postincubation (8 days) at 25°C, and fructification at 17°C. The air entering the culture room was filtered (99.999% at 0.3 μm) with an airflow rate of 2,000 m3/h (or 35 room volumes per h). People working in the room wore sterile overclothes. The plastic trays were arbitrarily distributed at the same level and covered by plastic film for 22 days (i.e., until 7 days after casing). Casing soil was divided into 16 plastic bags that were treated at 65°C and 100% relative humidity for 72 h and then opened in a greenhouse for 2 weeks before casing to enable microbial growth. No heterokaryons were cultivated in the room as controls for the fruiting conditions because heterokaryotic mycelium could have contaminated the other trays with airborne inoculum. Sporocarps were picked before caps opened to avoid spore dissemination. Transfers were made in petri dishes on compost agar medium for further analyses.
Second experiment.
In the second experiment 16 trays were inoculated simultaneously with grain spawn and spores. Five control trays were inoculated only with grain spawn, and four trays were inoculated only with spores. The latter four trays were treated exactly as if they had been inoculated simultaneously with grain spawn and spores (using one complete sporeprint) but with rye grain that was not colonized by S608-2. These control trays should have produced only C9 sporocarps. The culture process was similar in both experiments.
Molecular markers.
Eight sequence-characterized amplified region (20) markers were used to determine the origin of the sporocarps (Table 1). DNA was extracted with an RPN8510 Nucleon Phytopure plant DNA extraction kit (Amersham Pharmacia Biotech), and a PCR was performed as previously described (10). After digestion with an appropriate restriction endonuclease, DNA fragments were separated by electrophoresis in 1.2% (wt/vol) agarose.
TABLE 1.
Primer sequences, restriction enzymes, fragment sizes, and parental genotypes for eight sequence-characterized amplified region markers
| Locus | Primer sequence (5′-3′)a | Restriction enzyme | Allele | Characteristic DNA fragment (bp)b | Genotypes
|
|
|---|---|---|---|---|---|---|
| S608-2 | C9 | |||||
| PR3 | CGCCACATGTTTCCCTTCAAT | HinfI | 1 | 630, 280 | 1 | 2/2 |
| TGGAGCATCATCAGGACTTGG | 2 | 400, 210 | ||||
| PR21 | GTCAAGAGCGTTAATCAA | HinfI | 1 | 270, 110 | 1 | 2/2 |
| ACGTCGGTGAAATCCATT | 2 | 380 | ||||
| PR28 | GAACCAAATTTACTGTGA | HinfI | 1 | 750, 540 | 1 | 4/4 |
| GAGAAGCAACAAAATATG | 4 | 1,100, 300 | ||||
| PR46 | CAGGCAGTTCAAAAGCCAGT | HinfI | 1 | 330, 240 | 1 | 3/3 |
| ACAATTTCCGAGATCACCGA | 3 | 480, 240 | ||||
| PR4 | TTGCCGCTTCACTCGCCGTT | HinfI | 1 | 230, 210 | 1 | 1/2 |
| CCGTTGGTGAACCAGCGGAGGGATC | 2 | 340, 230, 210 | ||||
| PR6 | CAATCTCAAGCTTGCCTGG | None | 1 | 830 | 1 | 1/2 |
| AGGTGACATGTCAGAAGCGC | 2 | 1,250 | ||||
| PR30 | GACTAACCGTCGGAAT | RsaI | 1 | 400, 160 | 1 | 1/2 |
| TCACACAGGAAACAGC | 2 | 260, 160 | ||||
| PR50 | CCATCTCTCGTGATGAGCTTG | HpaII | 1 | 730, 250 | 1 | 1/3 |
| TAAGCAAAAGCGACAGCGAT | 3 | 900, 250 | ||||
PR3, PR21, PR28, and PR46 were used to detect outcrossing because the cultivated homokaryon S608-2 and the parent of the C9 spores have different alleles at these loci (Table 1). C9 is heteroallelic at PR4, PR6, PR30, and PR50, which were used to analyze segregation resulting from crosses. PR6 is derived from restriction fragment length polymorphism marker P1N150 and is tightly linked to MAT on chromosome 1 (2, 15). PR30 also is on chromosome 1. PR4 and PR50 are located on different chromosomes.
Expected sporocarps and their theoretical genotypes.
In A. bisporus, most homokaryotic strains cannot form sporocarps (4, 6), and only the heterokaryons in this study could produce mature sporocarps. These heterokaryons had five theoretical origins (Fig. 2). PR3, PR21, PR28, and PR46 could be used to determine whether these heterokaryons resulted from outcrossing. Outcrossing heterokaryons resulting from the Buller phenomenon or heterothallism could be distinguished by the distribution of the alleles inherited from the C9 parent at the PR6 locus (tightly linked to MAT). Heterokaryons that did not result from outcrossing (i.e., from pseudohomothallism or selfing) could be distinguished by the alleles present at PR4 and PR50. Selfing via homokaryotic spores, which could not be distinguished from selfing via the Buller phenomenon, was not expected since Mat-x spores of C9 are not viable.
RESULTS
Analysis of C9 offspring.
Of 96 single-spore isolates, 90 were heteroallelic Pr6-1/2 (and therefore heterokaryotic), while six carried only Pr6-2 alleles. The six presumed homokaryons were analyzed with the PR50 marker; five had a haploid genotype, which confirmed their homokaryotic status, and the remaining presumed homokaryon was heterokaryotic and its genotype was therefore Pr6-2/2 Pr50-1/3; its homoallelism at PR6 resulted from recombination between PR6 and the centromere, possibly between PR6 and MAT, which is hypothetically close to the centromere (15). In a second set of 100 single-spore isolates, 15 of the isolates were selected by using the mycelium growth rate test and then analyzed with the PR6 and PR50 markers; seven of these isolates were haploid at both loci and therefore putatively homokaryotic, and eight were Pr6-1/2 and therefore heterokaryotic. The genotype at PR50 was also determined for seven of the eight heterokaryons, and all were Pr50-1/3.
Finally, we obtained 12 homokaryotic single-spore isolates from C9; 7 of these isolates were Pr6-2 Pr50-1, and 5 were Pr6-2 Pr50-3. None of the 12 homokaryons had the parental Pr6-1 allele. All of them were negative when they were mated with the Mat-1 tester, S608-6. When mated with the Mat-2 tester, S608-2, 10 of these isolates gave positive reactions (fluffy mycelium), one gave an ambiguous reaction, and one gave no reaction, which we interpreted as a false negative and which is not uncommon in A. bisporus.
Thus, at most, C9 rarely produces viable spores that carry only its unidentified Mat-x allele, and MAT and PR6 are tightly linked. The deduced genotypes of the two nuclei of C9 were Mat-x Pr6-1 and Mat-1 Pr6-2. These two genotypes were the genotypes of the nuclei of C9's heterokaryotic descendants, while the genotype of the homokaryotic descendants was Mat-1 Pr6-2. Recombination could have occurred between the centromere, MAT, PR6, and the unknown linked recessive lethal gene to give other nuclear types, but only one such recombinant was detected.
Production of sporocarps.
On the four trays that were simultaneously inoculated with spores and spawn, 50 sporocarps were produced between 33 and 47 days after inoculation (Fig. 1). Control trays inoculated only with grain spawn were completely colonized by the inoculated homokaryon at the end of the incubation period. On all 12 of these control trays a total of five sporocarps were produced, one on day 43 and four on day 47. Sporocarps were not harvested from either the crossed or the control trays after day 47 to avoid analyzing sporocarps that might have resulted from contamination. Mycelia were isolated from 20 experimental sporocarps. We chose one sporocarp from each tray each time that we entered and harvested, usually every 3 days. For two trays, there were four well-separated sporocarps and a fifth sporocarp that was close to one of the other four. We selected only four sporocarps from the third tray, which produced fewer sporocarps than the other trays due to localized contamination by Trichoderma, and six sporocarps from the fourth tray, which produced more sporocarps than the other trays.
In the second experiment, 1 of 16 coinoculated trays became contaminated with Trichoderma. From the 15 remaining trays 138 sporocarps were collected between 36 and 62 days after inoculation. Mycelia were isolated from 67 of these sporocarps. Unexpected sporocarps, presumably due to contamination, appeared on only one of the five control trays inoculated with only S608-2 grain spawn. The four control trays inoculated with only C9 spores were not colonized by any mycelia and did not produce any sporocarps during the 67-day experiment. Thus, in this substrate the spores apparently could not germinate in the absence of stimulation by another mycelium, and the pseudohomothallic mode of reproduction failed.
Phenotypic and genotypic evidence for outcrossing.
In A. bisporus, a recessive white allele (Ppc1-w) determines the white color of the cap (7). The brown allele (Ppc1-b) is generally not completely dominant. C9 is a relatively dark brown cultivar and is expected to be Ppc1-b/b, since none of the homokaryons recovered from this strain produce white hybrids when they are crossed with homokaryons from white strains (Ppc1 is not linked to Mat, and homokaryotic spores of C9 should receive both alleles).
All of the sporocarps had a cap color that ranged from light brown to cream. The absence of dark brown caps, like those of the brown parent C9, strongly suggests that these sporocarps were not derived solely from C9 via pseudohomothallism or selfing. The absence of sporocarps with white caps, like those of the white parent S608, indicates that there was no contamination by inocula carrying a Ppc1-w allele. The observed cap color of all the sporocarps is consistent with their origin, outcrossing between mycelia of the S608 homokaryon (Ppc1-w) and spores of C9 (presumably Ppc1-b/b).
All 20 sporocarps analyzed had the same heteroallelic genotype, Pr3-1/2 Pr21-1/2 Pr28-1/4 Pr46-1/3, at the four loci for which S608-2 and C9 carry different alleles. Thus, these sporocarps were produced by hybrid heterokaryons that resulted from outcrossing between the S608-2 homokaryon and the spores of C9. The absence of nonparental alleles at these four loci and at the four loci used to study allelic segregation (PR4, PR6, PR30, and PR50) suggests that none of these sporocarps resulted from fertilization with any other inocula. For the second experiment, only the PR3, PR21, PR6, and PR50 markers were used. Of the 67 sporocarps analyzed, all were Pr3-1/2 and Pr21-1/2, and only alleles from S608 and C9 were found at all four loci.
Vegetative clonality.
For three independent loci, PR3, PR6, and PR50, six of eight possible genotypic classes were recovered, in agreement with the low frequency of the Pr50-1 allele (present in only 2 of the 20 fructifying hybrids). Redundancy within individual trays was limited to five pairs of sporocarps. Identical genotypes within single trays were found at distances of 39, 39, 24, 19, and 7 cm, and the average distance was 26 cm. The average distance between individuals with nonidentical genotypes was 17 cm. If identical genotypes resulted from resampling sporocarps arising from the same contiguous individual mycelium, we would expect genotype duplications to increase with decreasing distance between sporocarps; however, our data contradict this expectation. For this reason we believe that resampling of individuals played little or no role in determining the genotypes observed and that all or almost all of the 20 sporocarps represented unique individuals.
Allelic segregation.
For the 20 hybrid sporocarps examined in the first experiment, the segregation ratios of the C9 alleles at PR4, PR6, PR30, and PR50 were 11:9, 11:9, 11:9, and 18:2, respectively, and only the segregation at PR50 was not consistent with the expected Mendelian 1:1 ratio. There was no recombination between PR6 and PR30, both of which are on chromosome 1. In the second experiment the alleles at PR6 again segregated in a 1:1 manner (40:27), but the alleles at PR50 did not (53:14).
Evidence for outcrossing via the Buller phenomenon.
C9 cannot produce viable homokaryotic spores carrying Pr6-1 and Mat-x, so the only way to generate a heterokaryon with the nucleus of S608-2 and a nucleus carrying Pr6-1 is through a cross between S608-2 and a mycelium derived from a heterokaryotic spore of C9. A 1:1 ratio at PR6 and, by inference at MAT, indicates that the Buller phenomenon played the greatest, if not the only, role in heterokaryon establishment. If heterokaryons had been formed between S608-2 and mycelia derived from homokaryotic spores, then most of the heterokaryons would have been Pr6-1/2 hybrids. In fact, there was a statistically insignificant excess of Pr6-1 alleles among the sporocarps sampled, underscoring the fact that most of the heterokaryons could not possibly have arisen through mating between homokaryons. The 51 hybrids that presumably had the Mat-2/x genotype represented genetic variability that cannot be explained by the classic method of hybridization. We noted that, incidentally, our data showed that the Mat-x allele was not the Mat-2 allele.
DISCUSSION
We found that experimental sporocarps could be derived from outcrossing, mainly via the Buller phenomenon. Although some sporocarps might have resulted from outcrossing via classical heterothallism, it is certain that none of the sporocarps resulted from selfing or pseudohomothallism. All (or almost all) of the hybrid sporocarps represented unique individuals. Possibly hybrid heterokaryons were too numerous and/or lacked sufficient time to extend their individual territory enough to produce multiple sporocarps. These data are consistent with recent data which we obtained with spores of a field strain from Greece (3). In both cases the pseudohomothallic life cycle was absent since sporocarps directly derived from heterokaryotic spores were not detected. In the present experiments we used stringent conditions to avoid contamination and got reliable genetic results that were supported by informative genetic markers.
One important question is whether crosses via the Buller phenomenon use the nuclei from the heterokaryon at random. Mendelian allelic segregation was observed among the hybrids for Pr4 and Pr6. Parental heterozygosity is highly conserved in the heterokaryotic spores of A. bisporus (15). We expected the parental genotype at PR4 and PR6 (Pr 6-1/2 Pr 4-1/2) to be conserved in most of the heterokaryotic spores. Heterokaryotic spores from C9 with the Pr 6-1/2 Pr 4-1/2 genotype theoretically can be two types: Pr 4-1 Pr6-2 + Pr4-2 Pr6-1 or Pr 4-1 Pr6-1 + Pr4-2 Pr6-2. We identified this variation following the Buller phenomenon, which associated nuclei from the heterokaryotic spores with nuclei from the S608-2 homokaryon. The numbers of hybrids that received the four nuclear types were 4, 4, 7, and 5. These data are consistent with the assumption that chromosome segregation is random in bisporic basidia and that nuclear selection following the Buller phenomenon occurred at random for the PR4 and PR6 loci since each of the four nuclei participated equally in the crosses.
In contrast, the inequality in the segregation at PR50 could have resulted from a nonrandom process. A similar lower frequency of the PR-50-1 allele was not detected for the homokaryotic spores of C9 (seven Pr50-1 and five Pr50-3) or among the heterokaryotic spores (eight Pr50-1/3). Therefore, this inequality originated after spore germination. It could have resulted from a nonrandom crossing system (e.g., there could have been a locus linked to PR50 that determined the rules of the association of nuclei during the Buller phenomenon), or it could have resulted from selection after the crosses (e.g., if the Pr50-1/1 hybrid heterokaryons grew more slowly or fruited less easily than the Pr50-1/3 heterokaryons).
There are five possible origins of the sporocarps in the compost trays (Fig. 2). The homokaryon inoculated with the grain spawn was not a limiting factor since it completely colonized the compost during the incubation step. We inoculated at least 200 million spores onto each tray (one sporeprint of ∼109 spores used for four trays), so even with a germination rate of 0.1% (much less than the 1 to 25% observed for C9 in vitro) and 6% homokaryons (12 homokaryons found among 196 single-spore isolates) among the C9 viable offspring, we still expected 1,200 viable C9 homokaryons in each tray, which is 16-fold lower than the number of heterokaryons (18,800 = 1,200 × 94/6). The lower number of homokaryons is consistent with the relatively low proportion of tetrasporic basidia observed in A. bisporus var. bisporus (5). The lower growth rate of the homokaryons could have been due to hemizygosity of unfavorable recessive alleles, which also could explain the lack of success in selfing and heterothallism. Inbreeding depression, which has been reported in A. bisporus (24), could affect heterokaryons resulting from selfing but not heterokaryons resulting from pseudohomothallic reproduction. Thus, the heterokaryotic spores participate in the pseudohomothallic process and in outcrossing via the Buller phenomenon. Pseudohomothallism seems to be faster and more efficient than the other processes since no cross is needed and since heterokaryons that are similar to C9 should be quite vigorous and able to fruit easily. Thus, the relative lack of pseudohomothallic spores was unexpected.
The relative rapidity of the fruiting process also was unexpected. In commercial cultivation it takes at least 30 days for cultivars such as C9 or S608 to colonize the substrate and fruit. With a latent period of 5 days, the minimum time is 35 days, and we observed hybrid sporocarps after only 33 days. We think that the hybrid heterokaryons would not have been able to colonize the substrate and then fruit without the participation of the “parental” homokaryon S608-2 that was omnipresent in the substrate.
On trays inoculated simultaneously with spores and mycelium, no sporocarps attributable to contamination were detected, even though such sporocarps were numerous in a previous experiment (3) and could have resulted from outcrossing via the Buller phenomenon. Determining the rules of incompatibility or selectional priority in the Buller phenomenon could explain the fruiting success of the unexpected inocula, which was a priori quantitatively much less important than the fruiting success of the inoculum intentionally introduced.
In the control trays inoculated only with spores, we expected pseudohomothallism to occur, but no sporocarps were produced. In these trays a low rate of germination could have been due to the absence of stimulation by volatile components produced by a mycelium (17). Thus, the spores appeared to be more likely to produce sporocarps following outcrossing with a preexisting mycelium than to produce sporocarps by themselves. Under field conditions, most sporocarps of A. bisporus collected at a single site are genetically different. Whether these sporocarps share nuclei from previously established mycelia is not known, but if they do, then the hypothesis that airborne mycelia have an important role in establishing field populations requires more in-depth testing and evaluation.
Our first objective was to determine if outcrossing played a role when heterokaryotic spores were placed on a homokaryon. Clearly, outcrossing played a major role. Our conclusions are similar to those obtained for French local populations but are for a special situation that probably does not occur frequently under field conditions. In A. bisporus var. bisporus populations, heterokaryotic mycelia probably commonly encounter heterokaryotic spores or other heterokaryotic mycelia, and hybridization could occur. Testing this hypothesis under semicontrolled conditions will not be easy since all the mycelia used would be heterokaryotic and therefore fertile. It often is not possible to recover both types (with respect to MAT) of expected homokaryons following protoplasting, and sometimes it is not possible to recover either of them, suggesting that numerous haploid nuclei may not be viable by themselves. For example, one of the nuclei in the present experiment, C9, and apparently both nuclei from the Greek field strain Bs 598 used previously (3) appear to have such problems. Nuclei that cannot support independent homokaryotic growth could easily survive in a heterokaryon as long as their partner carried an allele that complemented their lethal or deleterious allele(s). If outcrossing is important in A. bisporus populations, then gametic selection should eliminate some of these alleles. However, gametic selection would be inefficient if outcrossing occurred mainly via the Buller phenomenon or via hypothetical heterokaryon-heterokaryon hybridization since lethal or deleterious alleles could be transmitted through multiple outcrossed generations by these processes.
Our second objective was to develop a new outcrossing method for A. bisporus. From a theoretical point of view, the new method has three advantages. First, genetic resources can be more efficiently exploited. Classical methods for recovering homokaryons are laborious and difficult, so only a relatively low proportion of the several hundred currently available wild strains of A. bisporus var. bisporus have been used in breeding programs. With the new method, any wild strain that can yield a sporeprint can be used as a parent. However, the efficiency of this process for any wild strain has yet to be demonstrated. Second, the high level of heterozygosity generally present in the field strains is poorly exploited in classical crosses because few, if any, homokaryons generally are obtained. With the new method the homokaryons are not required, and the field strains can be used directly. Third, hybrid heterokaryons are selected on a standard substrate under standard conditions for commercial mushroom production, which should exert selection pressure for useful hybrids that cannot be exerted with the current in vitro screening method. The outcrossing method also could be modified by mixing sporeprints from different strains or by mixing several grain spawns inoculated with sexually incompatible homokaryotic mycelia. Thus, depending on the number and relationship of the parents, the heterokaryons produced are full-sib or half-sib progeny that could provide information on the breeding value of the parents; for example, their performance could be used for genotypic evaluation of the parents in the framework of recurrent selection. This method also might be used with the pseudohomothallic and economically important species Agaricus subrufescens Peck (=Agaricus blazei Murrill sensu Heineman) (13).
This method used for hybridization and selection also has a disadvantage. Since crosses occur via the Buller phenomenon without gametic selection, undesirable parental alleles can be transmitted to the hybrids. Thus, the best use of this method probably is in the early generations of selection. One solution to this problem is to inoculate the grain spawn with a homokaryon that carries the Bsn-t allele, which results in a high percentage of tetrasporic basidia (4, 10). All of the hybrid sporocarps would then carry this dominant allele and should produce primarily homokaryotic spores that can be used in the next generations of selection.
In conclusion, by simultaneously inoculating spores and the grain spawn of a homokaryon into a culture substrate, numerous sporocarps are produced via outcrossing. The Buller phenomenon plays a major role. The involvement of the homokaryon in the fruiting process could explain the relative speed of this process and the relatively poor success of pseudohomothallic reproduction. Our data can partially explain how outcrossing occurs in field populations. We do not know if crosses between heterokaryons also are possible. Whether this process occurs under field conditions or not, it can be exploited in breeding programs and can enable utilization of much more of the germplasm of A. bisporus var. bisporus than is accessible at this time.
Acknowledgments
We thank Simone Rextoueix, Magalie Monmarson, and Patrick Castant for technical assistance, Richard W. Kerrigan for useful comments, and Mark Loftus for providing primer sequences for PR30.
This research was supported in part by the Bureau des Resources Génétiques (BRG).
REFERENCES
- 1.Buller, A. H. R. 1931. Researches on fungi, vol. IV. Longmans, Green and Co., London, United Kingdom.
- 2.Callac, P., C. Desmerger, R. W. Kerrigan, and M. Imbernon. 1997. Conservation of genetic linkage with map expansion in distantly related crosses of Agaricus bisporus. FEMS Microbiol. Lett. 146:235-240. [DOI] [PubMed] [Google Scholar]
- 3.Callac, P., J. Gaubert, M. Imbernon, J. Guinberteau, C. Desmerger, and J. M. Olivier. 2003. Ressources génétiques chez les agarics: résultats récents et premiers essais expérimentaux d'interfécondation libre chez le champignon de Paris. Actes Bureau Resources Génétiques 4:331-346. [Google Scholar]
- 4.Callac, P., S. Hocquart, M. Imbernon, C. Desmerger, and J. M. Olivier. 1998. Bsn-t allele from French field strains of Agaricus bisporus. Appl. Environ. Microbiol. 64:2105-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callac, P., M. Imbernon, R. W. Kerrigan, and J. M. Olivier. 1996. The two life cycles of Agaricus bisporus, p. 57-66. In D. J. Royse (ed.), Mushroom biology and mushroom product. Proceeding of the Second International Conference 1996. The Pennsylvania State University, University Park, Pa.
- 6.Callac, P., I. Jacobe de Haut, M. Imbernon, J. Guinberteau, and I. Theochari. 2003. A novel homothallic variety of Agaricus bisporus comprises rare tetrasporic isolates from Europe. Mycologia 95:222-231. [PubMed] [Google Scholar]
- 7.Callac, P., F. Moquet, M. Imbernon, M. Ramos Guedes-Lafargue, M. Mamoun, and J. M. Olivier. 1998. Evidence for PPC1, a determinant of the pilei-pellis color of Agaricus bisporus fruit bodies. Fungal Genet. Biol. 23:181-188. [DOI] [PubMed] [Google Scholar]
- 8.Couture, C., A. Michel, M. Imbernon, and P. Callac. 2004. Inheritance of the haploid fruiting ability in Agaricus bisporus. Mushroom Sci. 16:45-52. [Google Scholar]
- 9.Elliott, T. J. 1985. Spawn-making and spawns, p. 131-139. In P. B. Flegg, D. M. Spencer, and D. A. Wood (ed.), The biology and technology of the cultivated mushroom. John Wiley & Sons, Chichester, United Kingdom.
- 10.Imbernon, M., P. Callac, P. Gasqui, R. W. Kerrigan, and, A. J. Velcko, Jr. 1996. BSN, the primary determinant of basidial spore number and reproductive mode in Agaricus bisporus, maps to chromosome 1. Mycologia 88:749-761. [Google Scholar]
- 11.Imbernon, M., P. Callac, S. Granit, and L. Pirobe. 1995. Allelic polymorphism at the mating type locus in Agaricus bisporus var. burnettii, and confirmation of the dominance of its tetrasporic trait. Mushroom Sci. 14:11-19. [Google Scholar]
- 12.Kerrigan, R. W. 1990. Evidence of genetic divergence in two populations of Agaricus bisporus. Mycol. Res. 94:721-733. [Google Scholar]
- 13.Kerrigan, R. W. 2005. Agaricus subrufescens, a cultivated edible and medicinal mushroom, and its synonyms. Mycologia 97:12-24. [DOI] [PubMed] [Google Scholar]
- 14.Kerrigan, R. W., M. Imbernon, P. Callac, C. Billette, and J. M. Olivier. 1994. The heterothallic life cycle of Agaricus bisporus var. burnettii, and the inheritance of its tetrasporic trait. Exp. Mycol. 18:193-210. [Google Scholar]
- 15.Kerrigan, R. W., J. C. Royer, L. M. Baller, Y. Kohli, P. A. Horgen, and J. B. Anderson. 1993. Meiotic behavior and linkage relationships in the secondarily homothallic fungus Agaricus bisporus. Genetics 133:225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamoure, D. 1989. Indices of useful information for incompatibility tests in basidiomycetes. V. Agaricales sensu lato. Cryptogam. Mycol. 10:41-80. [Google Scholar]
- 17.Lôsel, D. 1964. The stimulating of spore germination in Agaricus bisporus by living mycelium. Ann. Bot. 28:541-554. [Google Scholar]
- 18.Miller, R. E., and D. L. Kananen. 1972. Bipolar sexuality in the mushroom. Mushroom Sci. 8:713-718. [Google Scholar]
- 19.Moquet, F., C. Desmerger, M. Mamoun, M. Ramos Guedes-Lafargue, and J. M. Olivier. 1999. Resistance to Pseudomonas tolaasi: the first QTL of Agaricus bisporus is closely related to natural cap color. Fungal Genet. Biol. 28:34-42. [DOI] [PubMed] [Google Scholar]
- 20.Paran, I., and R. W. Michelmore. 1993. Development of reliable PCR-based markers linked to downy mildew resistance genes in lettuce. Theor. Appl. Genet. 85:985-993. [DOI] [PubMed] [Google Scholar]
- 21.Pelham, J. 1967. Techniques for mushroom genetics. Mushroom Sci. 6:49-64. [Google Scholar]
- 22.Raper, C. A., J. R. Raper, and R. E. Miller. 1972. Genetic analysis of the life cycle of Agaricus bisporus. Mycologia 64:1088-1117. [Google Scholar]
- 23.Summerbell, R. C., Z. J. Castle, P. A. Horgen, and J. B. Anderson. 1989. Inheritance of restriction fragment length polymorphisms in Agaricus brunnescens. Genetics 123:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu, J. 1995. Analysis of inbreeding depression in Agaricus bisporus. Genetics 141:137-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, J., C. Desmerger, and P. Callac. 2002. Fine-scale genetic analyses reveal unexpected spatial-temporal heterogeneity in two natural populations of the commercial mushroom Agaricus bisporus. Microbiology 148:1253-1262. [DOI] [PubMed] [Google Scholar]
- 26.Xu, J., R. W. Kerrigan, P. A. Horgen, and J. B. Anderson. 1993. Localization of the mating-type gene in Agaricus bisporus. Appl. Environ. Microbiol. 59:3044-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]