Abstract
Streptococcus pyogenes (group A streptococcus [GAS]) is a frequent cause of purulent infections in humans. As potentially important aspects of its pathogenicity, GAS was recently shown to aggregate, form intratissue microcolonies, and potentially participate in multispecies biofilms. In this study, we show that GAS in fact forms monospecies biofilms in vitro, and we analyze the basic parameters of S. pyogenes in vitro biofilm formation, using Streptococcus epidermidis as a biofilm-positive control. Of nine clinically important serotype strains, M2, M6, M14, and M18 were found to significantly adhere to coated and uncoated polystyrene surfaces. Fibronectin and collagen types I and IV best supported primary adherence of serotype M2 and M18 strains, respectively, whereas serotype M6 and M14 strains strongly bound to uncoated polystyrene surfaces. Absorption measurements of safranin staining, as well as electron scanning and confocal laser scanning microscopy, documented that primary adherence led to subsequent formation of three-dimensional biofilm structures consisting of up to 46 bacterial layers. Of note, GAS isolates belonging to the same serotype were found to be very heterogeneous in their biofilm-forming behavior. Biofilm formation was equally efficient under static and continuous flow conditions and consisted of the classical three steps, including partial disintegration after long-term incubation. Activity of the SilC signaling peptide as a component of a putative quorum-sensing system was found to influence the biofilm structure and density of serotype M14 and M18 strains. Based on the presented methods and results, standardized analyses of GAS biofilms and their impact on GAS pathogenicity are now feasible.
Single-species and multispecies biofilms are considered the most abundant ubiquitous life form among microorganisms from all branches of the phylogenetic tree (27). Biofilms appear to be ancient and integral components of the prokaryotic life cycle. Bacterial biofilms have a structurally complex and dynamic architecture and develop on many abiotic surfaces (plastic, glass, metal, and minerals) and biotic surfaces (plants, animals, and humans) in three developmental steps (27, 50, 58). The first step includes adhesion of planktonic bacteria to surfaces. This initial attachment is either mediated by electrostatic contacts or relies on interaction of bacterial surface structures (proteins and carbohydrates) with inert or protein-carbohydrate-coated surfaces. The second step is the proliferation of the primary colonizers and the maturation of the biofilm. During this step, either bacteria multiply without releasing progeny cells, or primary colonizers recruit and coaggregate planktonic members of the same species or other species (27). At the same time, most biofilm bacteria produce extracellular polymeric substances (EPS), which stabilize the biofilm architecture (27). In a final third step, previously sessile members of the mature biofilm detach and act as primary colonizers at different sites (27).
Many of the developmental, metabolic, genetic, and physical properties of biofilm establishment, maturation, and disintegration are regulated by intraspecies and interspecies communication systems that react to population densities (e.g., quorum-sensing [QS] regulation) (19, 22, 49-51).
Apart from their great impact on industrial and ecological aspects of human life, biofilms are of special significance in medicine. Many pathogenic and nosocomial bacteria with the ability to form biofilms are responsible for acute and chronic infections. Examples of typical biofilm-associated diseases are caries, gingivitis, periodontitis, endocarditis, and prostatitis (27). In particular, temporally or permanently implanted medical devices in the human host, like intravenous catheters, artificial joints, and cardiac pacemakers, become rapidly coated with human extracellular matrix and serum proteins and are prime targets for bacterial biofilm formation (20, 27, 44, 56). Because of the sharply decreased susceptibility of biofilm-forming bacteria to host defenses and antibiotic treatments, biofilms on implanted devices constitute a major medical problem (13, 34, 43, 57).
Monospecies biofilm formation and coaggregation with members of the local physiologic microflora have been demonstrated for members of the oral streptococci, such as Streptococcus mutans and Streptococcus gordonii (18, 21, 23). As found for other species, adhesin-like glucan-binding proteins and matrix-interacting factors such as SpaA (antigen I/II) and WapA (antigen A), as well as QS mechanisms, contribute to biofilm growth, structure creation, and regulation of the genes involved (1, 3, 5, 8, 39, 51, 60).
Among the beta-hemolytic streptococci, Streptococcus pyogenes (group A streptococcus [GAS]) is an important human pathogen associated with extensive human morbidity worldwide (17). GAS invades host cells (15) and causes primary infections of the skin, throat, and other mucosal surfaces, such as pharyngitis and impetigo. Particularly, pharyngitis is a primary infection that can occur in multiple episodes, due to antibiotic treatment failure in up to 30% of affected individuals. A recent study by Conley et al. (11) revealed that GAS strains recovered from patients with treatment failure in fact form biofilms in vitro with variable efficiency and that, compared to planktonic cultures, GAS organized in biofilms have higher MICs for all standard antibiotics used for treatment.
GAS biofilm-like structures (microcolonies) were also reported to exist in vivo by Hirota et al. (33) and Akiyama et al. (2). The latter study documented GAS microcolonies in skin sections from patients with impetigo and atopic dermatitis. Takemura and colleagues identified GAS as members of root canal multispecies biofilms (52). Additionally, another recent study published during the review process of this paper identified biofilm-like tissue communities in GAS-infected zebrafish, studied the transcriptome of a serotype M5 GAS strain from tissue communities and biofilms, and identified the M5 protein and Mga and CovR regulators as important for biofilm formation of this serotype (9). One step that could contribute to midstage biofilm formation by GAS has been studied in vitro with GAS serotypes M1, M4, M12, and M49, which form microcolonies when grown in liquid medium. The aggregation into microcolonies is mediated by a conserved 19-amino-acid residue peptide (designated AHP) present in M protein and protein H (24).
The present study investigated several aspects of in vitro GAS biofilm formation employing different clinically relevant GAS serotype strains. By using a variety of microscopic techniques under static and flow conditions, we demonstrate that some serotype strains have the capacity to form biofilms. Yet, these serotype strains have an adherence tendency toward different substrates, which constitutes the first step in their transition from planktonic to sessile lifestyle structures. Furthermore, isolates of the same serotype are heterogeneous in their biofilm-building capacity. Finally we show that silC, a component of the putative QS system (31, 32), plays an important role in biofilm formation by the M14 and M18 serotypes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The serotype M49 GAS strain 591 is a skin isolate originally obtained from R. Lütticken, Aachen, Germany. The different M serotype GAS strains (M1, M2, M3, M6, M12, M18, and M28) used in this study are all clinical isolates obtained from the collection of the Centre of Epidemiology and Microbiology, National Institute of Public Health, Prague, Czech Republic, and have been previously described by Podbielski et al. (46). GAS serotype strain M14 and its corresponding silC-deficient mutant were described by Hidalgo-Grass et al. (31). The biofilm formation of Staphylococcus epidermidis DSM 1798 was used as an internal control for all experiments. Escherichia coli strain DH5α was purchased from GibcoBRL (Eggenstein, Germany) and served as a host for plasmids pAT::egfp and pO-KK. E. coli was grown in Luria broth (LB) or on LB agar plates at 37°C for all experiments. All GAS strains were routinely grown in Todd-Hewitt (TH) broth or TH agar plates supplemented with 0.5% yeast extract. For biofilm experiments, GAS and S. epidermidis were grown in LB, TH agar plates supplemented with 0.5% yeast extract, brain heart infusion (BHI) medium, or chemically defined medium. The growth medium of recombinant E. coli strains and GAS mutants was supplemented with 100 and 60 mg of spectinomycin · liter−1, respectively. GAS strains were grown as standing cultures at 37°C under a 5% CO2-20% O2 atmosphere unless otherwise indicated.
Construction of the silC mutants and GAS strains carrying enhanced green fluorescent protein gene (egfp).
The construction of the serotype M14 GAS strain silC mutant was described in a previous publication (31). To construct a plasmid that would allow an allelic replacement of silC by aad9 (spectinomycin resistance cassette) in serotype M18, we amplified a 1,572-bp fragment containing silC, 695 bp upstream and 511 bp downstream, using primers SilB-f2 (5′-TTATTGGATCGGAACTTACGC-3′) and SilD-r2 (5′-TGCTTCCCAACAACTTACCAC-3′), designed according to the serotype M14 chromosomal DNA. The PCR product was then cloned into pORI280, and a StyI-BspEI fragment encompassing silC was replaced by aad9. The resulting plasmid (pO-KK) was electroporated into serotype M18, and mutants with resistance to spectinomycin but susceptibility to erythromycin were selected. The fidelity of the replacement and the integrity of silB and silD were confirmed by PCR using primers SilB-f2 and SilD-r2. The PCR product was sequenced to confirm the sites of silC replacement.
For construction of the egfp expression plasmid, the egfp gene was released from vector pKen (12) by restriction digestion with XbaI and PstI. The fragment was cloned into pAT28 (55) downstream of a constitutively active recA promoter from GAS, which was cloned as an EcoRI/BamHI fragment into pAT28. The resulting plasmid pAT::egfp was sequenced to confirm its integrity.
Biofilm assays.
All biofilm experiments that used safranin staining as a detection method were performed in 96-well polystyrene microtiter plates (Greiner Bio-One, Frickenhausen, Germany). For all microscopic detection methods, biofilms were allowed to grow on round coverslips (Nunc, Wiesbaden, Germany) placed in 24-well polystyrene cell culture plates (Greiner Bio-One). Plastic surfaces of the 96-well plates and coverslips were either used uncoated or were coated with human fibronectin (Roche), human fibrinogen (Sigma), human collagen types I and IV (Biomol), or human laminin (Sigma) at a concentration of 50 μg · ml−1 overnight at 4°C. After being washed three times with phosphate-buffered saline (PBS), wells were used for inoculation with bacteria. GAS overnight cultures were harvested, and 100 μl of a suspension containing 104 CFU · ml−1 was inoculated into 100 μl fresh medium per well in 96-well plates and 900 μl fresh medium for 24-well plates, respectively. After desired incubation time points (from 12 h up to 120 h), the remaining planktonic bacteria were removed by aspiration of the liquid.
For quantitative measurements, we used a modified method of Christensen et al. (10). Essentially, 96-well plates with biofilms were washed twice with PBS and stained in a 0.1% safranin solution for 30 min. Stained biofilms were washed three times in PBS and allowed to dry, and staining was quantified in a spectrophotometer at 492 nm. In parallel experiments, unstained biofilm bacteria were removed from the wells by vigorous washing and vortexing, and bacterial numbers were quantified in a Thomae counting chamber by light microscopy. Like in many other studies that used safranin staining, we found a good correlation between increasing total cell numbers and increasing optical density of safranin-stained biofilms over the investigated time course (data not shown). Individual assays always consisted of three replicates. All experiments were performed at least four times. The data shown represent the means and standard deviations of four or more experiments.
Microscopic techniques.
For visualization of biofilms by light microscopy, the incubation of the bacteria on the uncoated or coated plastic coverslips was stopped at the indicated time points. Safranin staining was performed as described above. Stained coverslips were placed on slides with the biofilm pointing up and were inspected by light or fluorescence microscopy at magnifications of ×100 and ×200 (BX60 microscope; Olympus, Hamburg, Germany). Visible biofilms were documented with an attached digital camera (Leica, Solms, Germany).
Sample preparation for scanning electron microscopy (SEM) studies was performed as follows. The biofilms on the coverslips were fixed for 1 h in a solution containing 2.5% glutardialdehyde. Coverslips were washed in 0.1 M Na acetate buffer (pH 7.3) and dehydrated in a graded series of ethanol. Subsequently, coverslips were subjected to critical point drying with CO2, covered with gold to a 10-nm layer, and examined with a Zeiss DSM 960A electron microscope.
For confocal laser scanning microscopy (CLSM) studies, biofilms of GAS serotype strains M2, M6, and M18, all transformed with an egfp-expressing plasmid (the strains resulting were TM2, TM6, and TM18, respectively), were grown in glass-bottom chamber slides (Nunc). GAS serotype strain TM6 was grown on uncoated glass, TM2 was grown on fibronectin-coated glass, and TM18 was grown on collagen IV-coated glass. The coating was done as described above. A total of 200 μl of a suspension containing 104 CFU · ml−1 (each) TM2, TM6, and TM18 GAS bacteria was inoculated in 200 μl fresh BHI medium contained in the chamber slide wells. Preparations were inspected after desired time points of growth with a Zeiss inverted microscope attached to a Leica TCS SP2 AOBS laser scanning confocal imaging system with an Argon laser at a 488-nm excitation wavelength.
Flow chamber system.
For investigation of GAS biofilm development under constant flow conditions, a flow chamber system (FCS1c; Oligene, Berlin, Germany) was used. This chamber consisted of a main corpus with tubing adaptors, clip-on spring, silicon gaskets, plastic coverslips (diameter, 25 mm) and silicone tubing with a luer fitting on each side. The channel length was 15 mm, the channel width was 2 mm, and the channel height was 300 μm. Shear stresses of 0 to 3 Pa were applied. For a complete description of the system please refer to http://www.oligene.de/Flow_Chamber_System_FCS_1c.95.0.html. The plastic coverslips were coated with fibronectin and collagen IV for GAS serotype strains M2 and M18, respectively. The sterilized and assembled flow chamber system was attached to a pump, which was subsequently connected via tubing with an inlet of a flask containing fresh sterile BHI. The system was closed by connecting the flask outlet via tubing to the chamber inlet. The medium was inoculated with the respective GAS strains under sterile conditions, and the complete system was incubated at 37°C for 72 h. A constant medium flow of 1 ml · min−1 was found appropriate in preliminary experiments. To monitor biofilm growth after 72 h, the coverslips were removed from the flow chamber system and subsequently prepared for SEM as described above.
Statistics.
The two-tailed Mann-Whitney U test was used to calculate statistical significance between data sets.
RESULTS
Evaluation of basic parameters of GAS biofilm formation.
To initially assess the effect of growth medium, oxygen partial pressure, and incubation time on potential biofilm assembly, S. epidermidis (positive control) and serotype M6 and M49 GAS strains were used with uncoated polystyrene microtiter plate surfaces as solid supports. Safranin staining was employed, as it is an established method for staphylococcal biofilm research and in parallel experiments the total number of biofilm bacteria was enumerated by light microscopy, giving concordant data (data not shown). Since the staining and quantification techniques did not allow the visualization of three-dimensional structures, potential biofilms will be referred to as “adherent bacteria” or “primary colonizers” unless experimentally proven otherwise.
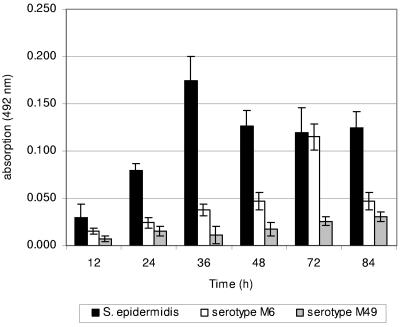
Preliminary results documented that BHI medium best supported adherence to uncoated plastic of all GAS strains tested. The oxygen partial pressure had no significant influence (data not shown). As illustrated in Fig. 1, the S. epidermidis-positive control measurably adhered as early as 24 h postinoculation (p.i.) to the plastic surface. Judged by safranin staining and absorption measurements, the serotype M49 GAS strain failed to adhere in detectable numbers during the whole investigation period (Fig. 1). Increasing numbers of bacteria, assembled in a potential biofilm, were detected for serotype M6 GAS up to 72 h p.i., when an absorption maximum was reached (Fig. 1). In addition, change of the growth medium at daily intervals did not increase the number of adhered bacteria (data not shown).
FIG. 1.
Quantification of primary adherence of different strains of S. pyogenes (serotypes M6 and M49) and S. epidermidis (positive control) to uncoated polystyrene surfaces. Bacteria were grown in BHI under static conditions at 37°C in ambient air. Adhesion of the bacteria was quantified by safranin staining of potential biofilms and subsequently by measuring absorbance at 492 nm at the indicated time points. The mean values of six independent experiments and standard deviations are shown.
As a consequence of these data, BHI medium and static cultures (unless stated otherwise) in ambient air at 37°C were used for all further experiments. Samples at 24, 48, and 72 h p.i. were routinely analyzed in the subsequent experiments. Whether the adherence led to formation of a multilayered biofilm needed further investigation.
The effect of matrix substratum on biofilm development by various GAS serotypes.
Primary adhesion of planktonic bacterial cells is a crucial step in biofilm formation. So far, GAS have not been described as colonizing implanted medical devices, but they bind to surfaces coated with extracellular matrix proteins during the infectious process. Thus, one isolate each of nine different clinically important GAS serotype strains (M1, M2, M3, M6, M12, M14, M18, M28, and M49), including an S. epidermidis strain as a positive control, was tested for its primary adhesion to uncoated plastic surfaces and plastic surfaces coated with fibronectin, fibrinogen, collagen types I and IV, and laminin, using the previously established assay conditions and quantitative measurements of safranin staining.
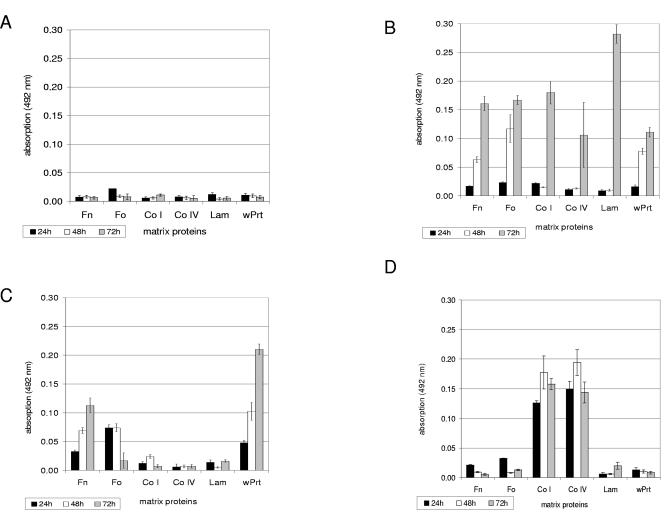
The experiments showed that the GAS isolates of serotypes M1 (Fig. 2A), M12, M28, and M49 (data not shown) did not display significant primary adhesion on any of the uncoated or matrix protein-coated plastic surfaces tested throughout the investigated sampling time points, suggesting that these strains are unable to form potential biofilms. In sharp contrast, the isolates of serotypes M2 (Fig. 2B), M6 (Fig. 2C), M14, and M18 (Fig. 2D) GAS strains were able to initially adhere to the different matrix protein surfaces in measurable amounts.
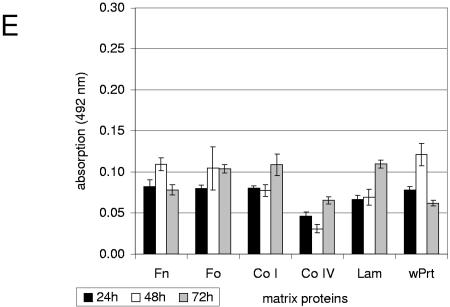
FIG.2.
Quantification of characteristic primary adhesion profiles of different S. pyogenes serotype strains and S. epidermidis (positive control) to immobilized matrix proteins and polystyrene surfaces. (A) Serotype M1 GAS strain; (B) serotype M2 GAS strain; (C) serotype M6 GAS strain; (D) serotype M18 GAS strain; (E) S. epidermidis (positive control strain). Bacteria were grown in BHI under static conditions at 37°C in ambient air. Adhesion of the bacteria was quantified by safranin staining of potential biofilms and subsequently by measuring absorbance at 492 nm at the indicated time points. The mean values of six independent experiments and standard deviations are shown. Immobilized matrix proteins were as follows: Fn, fibronectin; Fo, fibrinogen; Co I, collagen type I; Co IV; collagen type IV; Lam, laminin; wPrt, without proteins (i.e., polystyrene surface).
Fibronectin-coated surfaces supported the transition from planktonic to sessile cells of the serotype M2 (Fig. 2B) and M6 (Fig. 2C) GAS isolates and, to a lesser extent, those of serotype M14 and M3 GAS strains (data not shown). For all four strains, fibrinogen was equally as efficient as the primary adhesion substrate. Collagen I, collagen IV, and laminin supported the primary adhesion of the serotype M2 GAS isolate (Fig. 2B), but only after 72 h of incubation. The serotype M18 strain exclusively adhered to collagen type I- or IV-coated surfaces (Fig. 2D). Matrix proteins were dispensable for serotype M6 (Fig. 2C) and M14 GAS strains, since both isolates did adhere to uncoated plastic surfaces and thus qualified as potential biofilm builders. The S. epidermidis-positive control strain adhered equally well to the uncoated plastic surface and all substrates tested (Fig. 2E).
Additionally, investigation of four independent isolates each of the serotypes M1, M3, and M6 revealed heterogeneous biofilm formation behavior (see Fig. S1 in the supplemental material). This strongly suggested that biofilm formation is a trait of individual GAS isolates rather than a general attribute of defined GAS serotypes.
In conclusion, the majority of serotype M2, M6, M14, and M18 GAS strains revealed strong primary adhesion to their preferred substrates, suggesting that these isolates fulfill the first requirement in the process of biofilm formation. Whether this led to formation of typical biofilm architectures needed further investigation.
Evaluation and detection of GAS biofilm formation by microscopy.
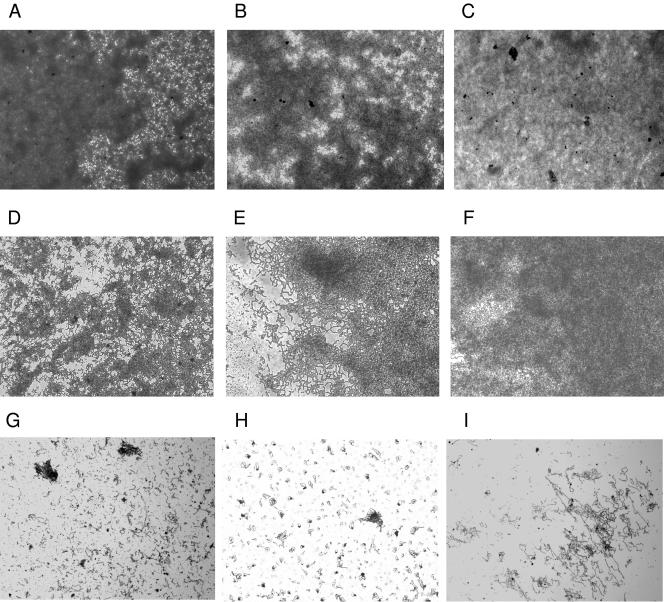
In the next series of experiments, we intended to determine whether the adherent bacteria were organized in structures resembling biofilms. As a first approach, we used light microscopy to study the architecture of potential GAS biofilms. Strains were allowed to adhere to and form putative biofilms on plastic coverslips coated with their preferred substrates for up to 72 h and were inspected by visual light microscopy at a magnification of ×200 after safranin staining of the bacterial coverslips.
As illustrated in Fig. 3A to C, the S. epidermidis-positive control strain revealed a strong and dense adherence after 24 h of incubation. The serotype M6 GAS strain formed a dense layer on uncoated polystyrene, which was increased in a time-dependent manner, reaching maximal levels at 48 h (Fig. 3D to F). Similar results were obtained for the serotype M2, M14, and M18 GAS strains exposed to fibronectin, uncoated plastic, and collagen types I and IV, respectively (data not shown). Serotype M1, M12, and M49 GAS strains did not form dense layers on any substrate (Fig. 3G to I). These data are consistent with the results presented in Fig. 1 and 2.
FIG. 3.
Inspection of adherence and biofilm development by safranin staining and light microscopy at a magnification of ×140. (A to C) S. epidermidis (positive control strain) biofilms grown in static culture on uncoated polystyrene coverslips at 24 h p.i. (A), 48 h p.i. (B), and 72 h p.i. (C). (D to E) Serotype M6 GAS biofilms grown in static culture on uncoated polystyrene coverslips at 24 h p.i. (D), 48 h p.i. (E), and 72 h p.i. (F). (G to I) Light microscopic images of potential biofilms of different S. pyogenes serotype strains grown in static culture. Serotype M1 GAS strain (72 h) (G), serotype M12 GAS strain (72 h) (H), and and serotype M 49 GAS strain (48 h) (I) are shown.
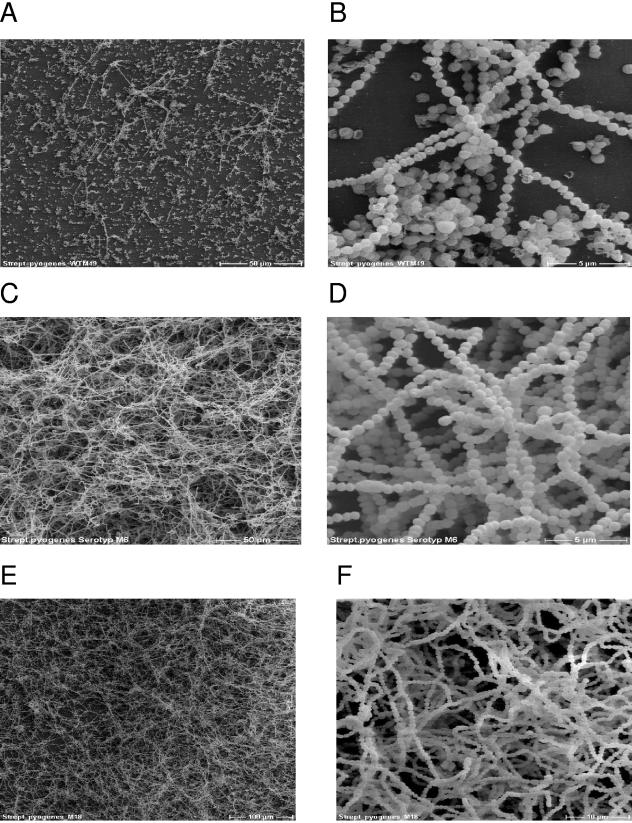
To ascertain that the structures visualized by light microscopy indeed represent biofilm-like structures, we employed SEM to elucidate the potential GAS biofilm architecture. The serotype M49 GAS strain did not bind to uncoated or coated surfaces, as revealed by microtiter plate assay (Fig. 1) and direct visualization by light microscopy (Fig. 3I). Therefore, we did not expect to see any biofilm-like structures. In fact, the SEM identified only a few bacterial chains on the surface and no multilayered meshwork for serotype M49 bacteria grown 72 h on a fibronectin-coated plastic coverslip (Fig. 4A and B).
FIG. 4.
SEM of S. pyogenes biofilms. (A and B) Serotype M49 GAS cells from a 72-h static culture on an uncoated plastic surface. Images reveal primary bacterial adherence without subsequent formation of typical biofilm structures, with magnifications of ×500 (A) and ×5,000 (B). (C and D) Serotype M6 GAS grown on plastic coverslips for 72 h in static culture, with magnifications of ×500 (C) and ×5,000 (D). (E and F) Biofilms of the serotype M18 GAS strain grown for 72 h in static culture on collagen type IV-coated coverslips, with magnifications of ×200 (E) and ×2,000 (F).
A different picture emerged from SEM of serotype M6 and M18 GAS strains, grown on plastic- and collagen type IV-coated surfaces, respectively. They revealed the formation of three-dimensional, multilayered, and dense biofilms (Fig. 4C to F). At high resolutions, it was apparent that at the lower surface only a single bacterial layer was in contact with the supporting matrix. The additional layers on top of this layer (“secondary colonizers”) were probably integrated into the biofilm architecture by interbacterial binding. Taken together, the results in Fig. 1 to 4 not only show that GAS attaches to coated or uncoated support matrices but document that this primary adhesion leads to the formation of typical biofilm structures. In contrast to the S. epidermidis biofilms (data not shown), no EPS matrix material could be detected for the biofilm-positive GAS serotype strains under these test conditions.
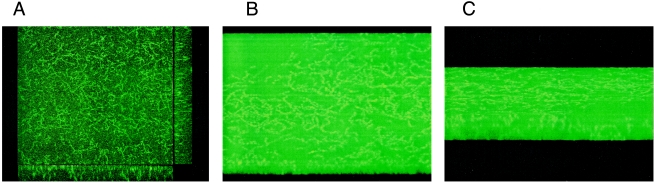
In the following experiments, we used confocal laser scanning microscopy to gain further insight into biofilm architecture and biofilm depth. The biofilm-forming serotype M2, M6, and M18 GAS strains were transformed with a plasmid constitutively expressing EGFP (12), yielding the strains TM2, TM6, and TM18, respectively. The fluorescent bacterial strains were allowed to form biofilms under the same conditions used for prior experiments. Figure 5A depicts a 72-h biofilm of TM2 taken from the x-y axis using the CLSM “snapshot” mode. Figures 5B and C illustrate a 72-h biofilm of GAS serotype strain TM6 taken in the “AnimTrans” mode. These experiments confirm the three-dimensional structure of GAS biofilms. Using the support software, we determined that the thickness of the biofilm layers was 13.3, 13.6, and 28 μm for TM2, TM6, and TM18, respectively. By analyzing four slides for each transformant and assuming that the GAS cell diameter ranges from 0.6 to 1 μm, we calculated that GAS biofilms contained 13 to 46 cell layers.
FIG. 5.
Confocal laser scanning microscopy images of different egfp-expressing S. pyogenes serotype strains assembled into biofilms. (A) This snapshot image shows a biofilm of a serotype M2 GAS strain grown statically for 72 h in chamber slides with integrated fibronectin-coated glass coverslips. The central portion of the picture gives an overview of the biofilm architecture. The side panels show the microcolonies associated with the support matrix. (B and C) Three-dimensional reconstruction of the biofilm formed by the egfp-expressing serotype M6 GAS strain, with two different views of the biofilm shown. Dimensions of the regions in all panels are 238.1 by 238.1 μm (x-y perspective).
Biofilm dynamics during extended incubations.
To study the GAS biofilm structure and stability after longer incubation periods, we extended our experimental setup to 120 h. Safranin staining and adsorption assays or SEM of biofilms of serotype M2, M6, and M18 GAS strains was used. Quantitative measurements and SEM were performed after 72 h, 96 h, and 120 h of incubation. As shown in Fig. S2 in the supplemental material, the attachment of serotype M2 and M6 GAS strains reached a peak at 96 h, after which it appeared to decline. However, the attachment of the serotype M18 GAS strain reached its maximum at 72 h of incubation. In accordance with results shown in Fig. S2 in the supplemental material, the biofilm mass formed by the serotype M2 GAS strain increased during incubation from 72 h to 96 h (see Fig. S3 in the supplemental material). After 120 h of incubation, a partial disintegration of the biofilm was observed, and it was accompanied by formation of significantly shorter chains (see Fig. S3 in the supplemental material). Together, these results show morphological changes in GAS biofilms during lengthy incubation times. The partial disintegration of the biofilm observed after 120 h could be required for a return to planktonic life style initiated by nutrient limitation (42).
GAS biofilm formation in a flow chamber.
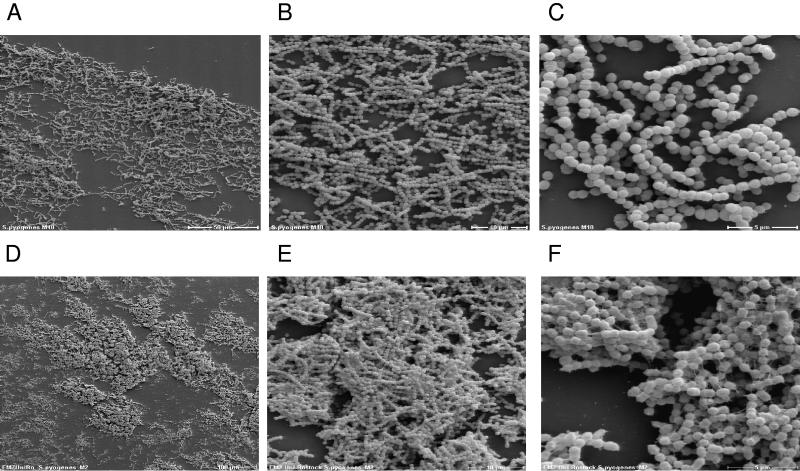
GAS are exposed to a constant flow of secretions at their colonization or infection sites, i.e., the upper respiratory tract of their host. Thus, formation of biofilms in a flow chamber, rather than formation under static conditions, is more related to the situation which exists in vivo during the GAS infection process. Consequently, we monitored the biofilm architecture of serotype M2 and M18 GAS strains under in vitro flow conditions by SEM. For this purpose, serotype M2 and M18 GAS strains were allowed to grow and form biofilms on plastic coverslips mounted in a flow chamber system. The slips were precoated with fibronectin or collagen type IV, respectively, and were incubated for 72 h under constant flow conditions of 1.2 ml · min−1 BHI medium. Again, the S. epidermidis strain was used as a positive control (data not shown). SEM analysis shown in Fig. 6 clearly demonstrates that GAS form biofilms under flow conditions. The structure of the serotype M18 GAS biofilm (Fig. 6A to C) was not as dense as observed under static conditions (Fig. 4E and F). The individual serotype M18 GAS chains within the biofilm were oriented in the direction of the medium flow. No obvious EPS material could be detected (Fig. 6A to C). Compared with serotype M18 GAS, serotype M2 GAS displayed a more compact biofilm structure under flow conditions (Fig. 6D and E). In addition, unlike the M18 strain, the chains of the serotype M2 strain were connected by threadlike structures of an as-yet-unknown chemical composition (magnification, ×3,500) (Fig. 6F).
FIG. 6.
Scanning electron microscopy of S. pyogenes biofilm development under continuous flow conditions in a flow chamber system. (A to C) Serotype M18 GAS strain; (D to F) serotype M2 GAS strain. Biofilms were formed for 72 h on coverslips coated with collagen type IV (A to C) or fibronectin (D to F) in a flow chamber system under continuous flow conditions for the medium. The development of the biofilm architecture is shown at magnifications of ×140 (D), ×350 (A), ×1,400 (B and E), and ×3,500 (C and F).
Role of the GAS SilC signaling peptide in biofilm development.
The development of biofilms is usually regulated by QS systems (20, 50, 59). The GAS sil gene locus (streptococcal invasion locus) is a putative QS system demonstrated to play (for serotype M14 strains) an important role in the pathogenesis of highly invasive soft tissue infection (31, 32). A similar locus exists in serotype M18 GAS strains, but its role in virulence has not been elucidated (48). silC is a component of the sil locus, which is required for virulence; recently, it was discovered to encode a protein that regulates gene expression (Y. Eran and E. Hanski, unpublished data).
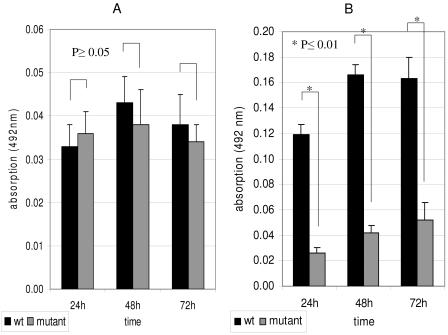
To test a possible participation of silC in biofilm development, we compared the biofilms of M14 and M18 wild-type (wt) strains to those of the corresponding silC-deficient mutants. Using the quantitative safranin-staining measurements, we could demonstrate a reduced adherence to fibronectin, fibrinogen, and plastic of the M14 silC mutant compared to the corresponding wt strain (Fig. 7A). However, the differences were not statistically significant. In contrast, the M18 silC-deficient mutant displayed significantly reduced primary adhesion on collagen type I and IV substrates, compared to the corresponding wt (Fig. 7B).
FIG. 7.
Comparative biofilm formation of serotype M14 and M18 GAS strains with their corresponding silC-deficient peptide mutants. Biofilms were grown in static culture on fibronectin-coated plastic wells (serotype M14 GAS strain) (A) or collagen type IV-coated plastic wells (serotype M18 GAS strain) (B) for 24, 48, and 72 h. Biofilms were detected by safranin staining. The Mann-Whitney U test was used to calculate statistical significance from six independent assays with three replicates each time. An asterisk indicates a significant difference between the two isogenic strains at a given incubation period.
The SEM analysis (see Fig. S4A to H in the supplemental material) confirmed these results. The biofilm structure of the M14 silC mutant was more cleft than that of the M14 wt strain (see Fig. S4E to H in the supplemental material). Much more pronounced differences were discovered for the M18 silC mutant, which displayed a patchy, thin biofilm compared to the solid and thick biofilm of the corresponding parental strain (magnification, ×500) (see Fig. S4C and D in the supplemental material).
Together, these experiments documented an involvement of the potential signaling peptide SilC in GAS biofilm assembly and architecture. The molecular background of this involvement needs to be determined in future experiments.
DISCUSSION
Streptococcus pyogenes is an important human pathogen, but it is not considered a classical biofilm-building species. The facts that GAS can be found in microcolony-like structures in skin sections from impetigo patients (2), as tissue communities in skin sections of GAS-infected zebrafish (9), and as members of multispecies biofilms in root canals (52) prompted us to investigate the basic parameters of GAS biofilm development.
This study revealed that the majority of clinically important serotype M2, M6, M14, and M18 GAS strains produced detectable biofilms on different coated and uncoated plastic surfaces in vitro. As documented for typical biofilm-forming bacteria (24), the developmental process of GAS biofilm formation can be characterized as a three-step process of adherence of planktonic cells to the surface, biofilm maturation, and return to the planktonic life style after complete or partial disintegration of the biofilm. These three steps were all documented in our study by quantification of safranin-stained cells and various microscopic techniques.
Uncoated plastic or glass surfaces supported primary adherence of serotype M6 and M14 GAS isolates, whereas serotype M2 and M18 GAS isolates preferentially adhered to human matrix protein-coated surfaces. After comparison with results obtained for initial adherence of the S. epidermidis-positive control strain, the latter four isolates were designated biofilm positive. For S. epidermidis, the major adhesins important for initial adherence during biofilm formation are AtlE (major autolysin), Bap (biofilm-associated protein), and AAP (accumulation-associated protein) (16, 25, 29, 30). So far, homologous genes have not been detected in the eight GAS genomes that have been completely sequenced (B. Kreikemeyer, M. Nakata and A. Podbielski, unpublished data).
The heterogeneous primary adherence behavior of the GAS isolates in this study therefore raised the question of whether (i) a specific but variable adhesin or (ii) a serotype- or even strain-specific array of several adhesins could be responsible for this initial step in the biofilm process.
The obvious candidate molecule for the first option would be the GAS M protein, which is expressed by almost every clinical isolate and displays an N-terminal sequence variability that allows the discrimination of >120 variants (7). The M protein of a serotype M5 GAS strain was recently implicated in primary adherence during biofilm development (9). Consistent with the idea of M protein as a general primary adhesion in biofilm formation, Conley and coworkers reported biofilm-positive phenotypes of 99 GAS isolates representing nine different M serotypes (11). However, these authors defined biofilms by the number of recovered CFU after 24 h of incubation and only randomly performed SEM to correlate CFU and biofilm thickness or architecture.
In contrast to the statements on the association of M protein expression and biofilm building, our consistent results from safranin staining and microscopic techniques revealed the presence of biofilm-negative serotype strains (M1, M12, M28, and M49) and heterogeneous behavior of isolates with identical serotypes. These findings argue against the M protein as a general primary biofilm adhesin.
The current most probable group of candidate molecules for the second option are commonly termed MSCRAMMs (for microbial surface components recognizing adhesive matrix molecule) (45). At present, more than a dozen surface proteins have been characterized that enable GAS to adhere to surfaces coated with matrix molecules and to eukaryotic cells (14, 15, 36). Considering the binding strength of such GAS MSCRAMMs for matrix molecules, the genes of the most important surface molecules are located in the FCT region (6) and are controlled by specific transcriptional regulators (4, 26, 35, 40, 41, 47). The FCT region has a serotype-specific gene content, with genes encoding MSCRAMMs responsible for fibronectin and collagen binding (28, 37, 38, 53, 54). At present, both molecule groups could explain the serotype-specific primary binding of GAS to matrix-protein-coated surfaces. The isolate-specific patterns within a certain serotype could be due to diverse regulatory mechanisms leading to differential expression of the primary adhesins. The adhesins and corresponding regulators that enable the minority of the tested strains to directly interact with uncoated plastic are currently uncharacterized.
During the three-dimensional development of biofilms formed by serotype M2, M6, M14, and M18 GAS isolates, we observed an aggregation of cells into microcolonies by SEM and CLSM. Microcolony formation appears to be a common feature of many GAS strains, among them serotype M1 and M49 strains, when grown in vitro in liquid medium (24). This aggregation is mediated by homophilic protein-protein interactions between AHP-containing surface proteins of neighboring bacteria (24). However, serotype M49 GAS strains form aggregates under in vitro conditions in liquid medium but do not assemble into stable and detectable biofilms under the experimental setup used in this study. This suggested that additional mechanisms responsible for interbacterial binding and subsequent biofilm maturation probably exist.
In biofilm-forming bacteria expressing QS regulatory systems, these systems have consistently been shown to be involved in one or several steps of biofilm development (18, 59). Sil, a putative QS system in GAS, has so far exclusively been found in serotype M14 and M18 GAS strains (31, 48). The sil locus encodes an ABC transporter composed of the proteins SilD and SilE, a two-component regulatory system (SilAB), and two small converging, overlapping open reading frames termed SilC and SilCR. The latter open reading frame encodes a competence-like stimulating peptide, but in serotype M14 it is not formed due to a missense mutation in the start codon (31). silC is required for virulence of the serotype M14 GAS strains (31), and it was recently shown to encode a small peptide that regulates gene expression (Eran and Hanski, unpublished). A synthetic SilCR peptide counteracts SilC virulence activity. In this study, we demonstrate that deletion of silC in the serotype M14 GAS strain has small effects on primary adhesion, biofilm assembly, and architecture. However, in the serotype M18 GAS strain, a deletion of silC causes a drastically decreased ability to form biofilms. It would be interesting to compare the profiles of genes that are regulated by SilC in M14 and M18 strains, in an attempt to delineate the cause for such pronounced strain-specific differences in biofilm formation. So far, such prominent strain-specific differences in the influence of a QS peptide have not been observed in other gram-positive cocci.
In summary, this study has collected significant evidence that individual isolates of several GAS serotypes grow in biofilm structures in vitro, under either static or flow conditions. Preferential primary adhesion substrates have been identified, the three-dimensional structure of GAS biofilms was documented, and the thicknesses of the biofilms were determined. The clinical significance of these observations for GAS pathogenesis is underscored by recent preliminary reports showing GAS microcolonies in skin sections from patients with impetigo or atopic dermatitis, implications of GAS biofilm formation in antibiotic treatment failure cases (11), and identification of GAS as members of multispecies biofilms in root canals (2, 52). It has to be considered that this newly emerging GAS virulence trait potentially renders these important pathogens more resistant to antibiotic therapy and to innate, as well as adaptive, immune responses. In particular, GAS biofilms could play a significant role in recurrent and chronic infections and should be investigated. With the basic parameters evaluated in this study, it will be possible to analyze the biofilm phenotype of GAS isolates from more M serotypes at the transcriptome and proteome levels to develop novel prevention strategies and therapy concepts.
Supplementary Material
Acknowledgments
The work was supported by BMBF grant BE031-03U213B, as a part of the German PathoGenomik Competence Network, awarded to A.P. and B.K., and by a grant (I-741-106.2/2002) from the German-Israel Science Foundation (GIF) awarded to A.P. and E.H.
We especially thank Jana Normann for expert technical assistance. We also thank Peter Lorenz, Institute of Immunology, University Hospital Rostock, for his instructions when using the confocal laser scanning microscope, and we thank Brendan Cormack, Johns Hopkins University, for providing the EGFP expression plasmids.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama, H., S. Morizane, O. Yamasaki, T. Oono, and K. Iwatsuki. 2003. Assessment of Streptococcus pyogenes microcolony formation in infected skin by confocal laser scanning microscopy. J. Dermatol. Sci. 32:193-199. [DOI] [PubMed] [Google Scholar]
- 3.Banas, J. A., and M. M. Vickerman. 2003. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 14:89-99. [DOI] [PubMed] [Google Scholar]
- 4.Beckert, S., B. Kreikemeyer, and A. Podbielski. 2001. Group A streptococcal rofA gene is involved in the control of several virulence genes and eukaryotic cell attachment and internalization. Infect. Immun. 69:534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bensing, B. A., J. A. López, and P. M. Sullam. 2004. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect. Immun. 72:6528-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessen, D. E., and A. Kalia. 2002. Genomic localization of a T serotype locus to a recombinatorial zone encoding extracellular matrix-binding proteins in Streptococcus pyogenes. Infect. Immun. 70:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisno, A. L., M. O. Brito, and C. M. Collins. 2003. Molecular basis of group A streptococcal virulence. Lancet Infect. Dis. 3:191-200. [DOI] [PubMed] [Google Scholar]
- 8.Blehert, D. S., R. J. Palmer, Jr., J. B. Xavier, J. S. Almeida, and P. E. Kolenbrander. 2003. Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions. J. Bacteriol. 185:4851-4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho, K. H., and M. G. Caparon. 2005. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol. Microbiol. 57:1545-1556. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conley, J., M. E. Olson, L. S. Cook, H. Ceri, V. Phan, and H. D. Davies. 2003. Biofilm formation by group A streptococci: is there a relationship with treatment failure? J. Clin. Microbiol. 41:4043-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 13.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 14.Courtney, H. S., D. L. Hasty, and J. B. Dale. 2002. Molecular mechanisms of adhesion, colonization, and invasion of group A streptococci. Ann. Med. 34:77-87. [DOI] [PubMed] [Google Scholar]
- 15.Courtney, H. S., and A. Podbielski. 2004. Group A streptococcal invasion of host cells, p. 239-273. In R. J. Lamont (ed.), Bacterial invasion of host cells. Cambridge University Press, Cambridge, United Kingdom.
- 16.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cvitkovitch, D. G., Y. H. Li, and R. P. Ellen. 2003. Quorum sensing and biofilm formation in streptococcal infections. J. Clin. Investig. 112:1626-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 20.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egland, P. G., R. J. Palmer, Jr., and P. E. Kolenbrander. 2004. Interspecies communication in Streptococcus gordonii-Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc. Natl. Acad. Sci. USA 101:16917-16922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federle, M. J., and B. L. Bassler. 2003. Interspecies communication in bacteria. J. Clin. Investig. 112:1291-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster, J. S., and P. E. Kolenbrander. 2004. Development of a multispecies oral bacterial community in a saliva-conditioned flow cell. Appl. Environ. Microbiol. 70:4340-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frick, I. M., M. Morgelin, and L. Bjorck. 2000. Virulent aggregates of Streptococcus pyogenes are generated by homophilic protein-protein interactions. Mol. Microbiol. 37:1232-1247. [DOI] [PubMed] [Google Scholar]
- 25.Goetz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 26.Granok, A. B., D. Parsonage, R. P. Ross, and M. G. Caparon. 2000. The RofA binding site in Streptococcus pyogenes is utilized in multiple transcriptional pathways. J. Bacteriol. 182:1529-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95-108. [DOI] [PubMed] [Google Scholar]
- 28.Hanski, E., and M. Caparon. 1992. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 89:6172-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Goetz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 30.Heilmann, C., M. Hussain, G. Peters, and F. Goetz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 31.Hidalgo-Grass, C., M. Ravins, M. Dan-Goor, J. Jaffe, A. E. Moses, and E. Hanski. 2002. A locus of group A streptococcus involved in invasive disease and DNA transfer. Mol. Microbiol. 46:87-99. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo-Grass, C., M. Dan-Goor, A. Maly, Y. Eran, L. A. Kwinn, V. Nizet, M. Ravins, J. Jaffe, A. Peyser, A. E. Moses, and E. Hanski. 2004. Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet 363:696-703. [DOI] [PubMed] [Google Scholar]
- 33.Hirota, K., K. Murakami, K. Nemoto, T. Ono, T. Matsuo, H. Kumon, and Y. Miyake. 1998. Fosfomycin reduces CD15s-related antigen expression of Streptococcus pyogenes. Antimicrob. Agents Chemother. 42:1083-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyle, B. D., and J. W. Costerton. 1991. Bacterial resistance to antibiotics: the role of biofilms. Prog. Drug Res. 37:91-105. [DOI] [PubMed] [Google Scholar]
- 35.Kreikemeyer, B., S. Beckert, A. Braun-Kiewnick, and A. Podbielski. 2002. Group A streptococcal RofA-type global regulators exhibit a strain-specific genomic presence and regulation pattern. Microbiology 148:1501-1511. [DOI] [PubMed] [Google Scholar]
- 36.Kreikemeyer, B., M. Klenk, and A. Podbielski. 2004. The intracellular status of Streptococcus pyogenes: role of extracellular matrix-binding proteins and their regulation. Int. J. Med. Microbiol. 294:177-188. [DOI] [PubMed] [Google Scholar]
- 37.Kreikemeyer, B., S. Oehmcke, M. Nakata, R. Hoffrogge, and A. Podbielski. 2004. Streptococcus pyogenes fibronectin-binding protein F2: expression profile, binding characteristics, and impact on eukaryotic cell interactions. J. Biol. Chem. 279:15850-15859. [DOI] [PubMed] [Google Scholar]
- 38.Kreikemeyer, B., M. Nakata, S. Oehmcke, C. Gschwendtner, J. Normann, and A. Podbielski. 2005. Streptococcus pyogenes collagen type I-binding Cpa surface protein: expression profile, binding characteristics, biological functions, and potential clinical impact. J. Biol. Chem. 280:33228-33239. [DOI] [PubMed] [Google Scholar]
- 39.Li, Y., and R. A. Burne. 2002. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiology 147:2841-2848. [DOI] [PubMed] [Google Scholar]
- 40.Molinari, G., M. Rohde, S. R. Talay, G. S. Chhatwal, S. Beckert, and A. Podbielski. 2001. The role played by the group A streptococcal negative regulator Nra on bacterial interactions with epithelial cells. Mol. Microbiol. 40:99-114. [DOI] [PubMed] [Google Scholar]
- 41.Nakata, M., A. Podbielski, and B. Kreikemeyer. 2005. MsmR, a specific positive regulator of the Streptococcus pyogenes FCT pathogenicity region and cytolysin-mediated translocation system genes. Mol. Microbiol. 57:786-803. [DOI] [PubMed] [Google Scholar]
- 42.O'Toole, G. A., K. A. Gibbs, P. W. Hager, P. V. Phibbs, Jr., and R. Kolter. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pajkos, A., K. Vickery, and Y. Cossart. 2004. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J. Hosp. Infect. 58:224-229. [DOI] [PubMed] [Google Scholar]
- 44.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677-701. [DOI] [PubMed] [Google Scholar]
- 45.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 46.Podbielski, A., A. Kaufhold, and P. P. Cleary. 1993. PCR-mediated amplification of group A streptococcal genes encoding immunoglobulin-binding proteins. ImmunoMethods 2:55-64. [Google Scholar]
- 47.Podbielski, A., M. Woischnik, B. A. Leonard, and K. H. Schmidt. 1999. Characterization of nra, a global negative regulator gene in group A streptococci. Mol. Microbiol. 31:1051-1064. [DOI] [PubMed] [Google Scholar]
- 48.Smoot, J. C., K. D. Barbian, J. J. Van Gompel, L. M. Smoot, M. S. Chaussee, G. L. Sylva, D. E. Sturdevant, S. M. Ricklefs, S. F. Porcella, L. D. Parkins, S. B. Beres, D. S. Campbell, T. M. Smith, Q. Zhang, V. Kapur, J. A. Daly, L. G. Veasy, and J. M. Musser. 2002. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc. Natl. Acad. Sci. USA 99:4668-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanley, N. R., and B. A. Lazazzera. 2004. Environmental signals and regulatory pathways that influence biofilm formation. Mol. Microbiol. 52:917-924. [DOI] [PubMed] [Google Scholar]
- 50.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 51.Suntharalingam, P., and D. G. Cvitkovitch. 2005. Quorum sensing in streptococcal biofilm formation. Trends. Microbiol. 13:3-6. [DOI] [PubMed] [Google Scholar]
- 52.Takemura, N., Y. Noiri, A. Ehara, T. Kawahara, N. Noguchi, and S. Ebisu. 2004. Single species biofilm-forming ability of root canal isolates on gutta-percha points. Eur. J. Oral Sci. 112:523-529. [DOI] [PubMed] [Google Scholar]
- 53.Talay, S. R., P. Valentin-Weigand, P. G. Jerlstrom, K. N. Timmis, and G. S. Chhatwal. 1992. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect. Immun. 60:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terao, Y., S. Kawabata, M. Nakata, I. Nakagawa, and S. Hamada. 2002. Molecular characterization of a novel fibronectin-binding protein of Streptococcus pyogenes strains isolated from toxic shock-like syndrome patients. J. Biol. Chem. 277:47428-47435. [DOI] [PubMed] [Google Scholar]
- 55.Trieu-Cuot, P., C. Carlier, C. Poyart-Salmeron, and P. Courvalin. 1990. A pair of mobilizable shuttle vectors conferring resistance to spectinomycin for molecular cloning in Escherichia coli and in gram-positive bacteria. Nucleic Acids Res. 18:4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaudaux, P. E., D. P. Lew, and F. A. Waldvogel. 1994. Host factors predisposing to and influencing therapy of foreign body infections, p. 1-29. In A. L. Bisno and F. A. Waldvogel (ed.), Infections associated with indwelling medical devices, 2nd ed. ASM Press, Washington, D.C.
- 57.Vickery, K., A. Pajkos, and Y. Cossart. 2004. Removal of biofilm from endoscopes: evaluation of detergent efficiency. Am. J. Infect. Control 32:170-176. [DOI] [PubMed] [Google Scholar]
- 58.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yarwood, J. M., and P. M. Schlievert. 2003. Quorum sensing in Staphylococcus infections. J. Clin. Investig. 112:1620-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida, A., T. Ansai, T. Takehara, and H. K. Kuramitsu. 2005. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 71:2372-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.