Abstract
Sterol demethylation inhibitor (DMI) fungicides are widely used to control fungi pathogenic to humans and plants. Resistance to DMIs is mediated either through alterations in the structure of the target enzyme CYP51 (encoding 14α-demethylase), through increased expression of the CYP51 gene, or through increased expression of efflux pumps. We found that CYP51 expression in DMI-resistant (DMIR) isolates of the cherry leaf spot pathogen Blumeriella jaapii was increased 5- to 12-fold compared to that in DMI-sensitive (DMIS) isolates. Analysis of sequences upstream of CYP51 in 59 DMIR isolates revealed that various forms of a truncated non-long terminal direct repeat long interspersed nuclear element retrotransposon were present in all instances. Similar inserts upstream of CYP51 were not present in any of 22 DMIS isolates examined.
Sterol demethylation inhibitor (DMI) fungicides are the largest and most important group of antifungal agents and are used widely in both agriculture and medicine. These fungicides inhibit the sterol C-14 α-demethylation of 24-methylenedihydrolanosterol, a precursor of the cell membrane component ergosterol in fungi (4). DMIs were introduced for plant disease management in the 1970s. Since the 1980s, clearly defined field resistance to DMIs has been reported for powdery mildews of barley, cucumber, and grape (4, 12, 27) and reduced sensitivity to DMIs has been reported for several other fungal pathogens, including the apple scab pathogen Venturia inaequalis (21, 24).
Molecular mechanisms leading to DMI resistance in several important human and plant fungal pathogens have been studied intensively (reviewed in references 11 and 23). Common mechanisms of DMI resistance include (i) mutations in the DMI target enzyme, 14α-demethylase (CYP51), leading to a decreased affinity of DMIs to the target protein (3, 9, 10); (ii) overexpression or increased copy number of the CYP51 gene, leading to increased production of the target enzyme (16, 25, 30); and (iii) overexpression of ATP-binding cassette (ABC) transporters encoding efflux pumps (15, 17, 34). Additionally, some unknown mechanisms (4, 28, 32) may confer DMI resistance.
The cherry leaf spot pathogen, Blumeriella jaapii, is a major pathogen of tart cherry that is only effectively controlled through regular fungicide applications. DMI fungicides have been used intensively for leaf spot management in Michigan since 1989, and we have noted a decline in control with DMIs in research plots and commercial orchards (G. W. Sundin, unpublished). The objective of this study was to characterize the molecular mechanism of DMI resistance in B. jaapii. Our hypothesis was that the resistance mechanism in this fungal pathogen would be similar to mechanisms previously reported in other fungi, and thus our work focused on isolating and analyzing the B. jaapii CYP51 gene and flanking sequences. Additional knowledge of DMI resistance mechanisms will increase our understanding of the evolution of fungicide resistance, could result in the development of a detection method for DMIR B. jaapii, and should provide new clues for the management of fungicide-resistant pathogens.
MATERIALS AND METHODS
Sensitivity of B. jaapii to fenbuconazole fungicide.
Eighty-one isolates of B. jaapii were recovered from cherry leaf spot lesions from tart, sweet, and black cherry (Prunus cerasus L., P. avium L., and P. serotina Ehrh.) and purified through subculture from a single conidium. The isolates were collected from 20 locations in Ohio and Michigan in 2003 and 2004, including 12 commercial orchards with locations chosen to represent the geographic range of cherry orchards in Michigan. In cases where multiple isolates from a single orchard were included, each isolate was recovered from a separate tree.
Sensitivity of these isolates to the DMI fenbuconazole was tested on MMEA medium (20 g malt extract, 1 g yeast extract, 20 g agar, 1 liter water) amended with fenbuconazole (Indar 70% WSP; Rohm and Haas Company, Philadelphia, PA) at 0, 0.005, 0.01, 0.02, 0.04, 0.08, 0.16, 0.2, 3.0, or 5.0 μg active ingredient (a.i.)/ml. Mycelial plugs (1-mm in diameter) were taken from the edge of a 1-month-old colony of each isolate and placed onto MMEA plates (three replicates per isolate) amended with each of the above concentrations of fenbuconazole. The fungal cultures were incubated at 23°C for 3 weeks in the dark and then examined to identify the minimum concentration of fenbuconazole required to inhibit colony formation.
Cloning and sequencing of the CYP51 gene from B. jaapii.
Genomic DNA of B. jaapii was extracted from mycelium using the Tissue DNeasy kit (QIAGEN Inc, Valencia, CA) according to the manufacturer's instructions. Based on the conserved amino acid sequences encoded by CYP51 genes from Monilinia fructicola (31), Penicillium digitatum (16), and Tapesia yallundae (32), a pair of degenerate PCR primers, CYP51-Deg-F plus CYP51-Deg-R (Table 1), was developed to amplify an internal CYP51 gene fragment from B. jaapii. PCR amplification was performed in a 50-μl reaction volume containing 1× PCR buffer (Invitrogen, Carlsbad, CA), 2 mM MgCl2, 0.75 mM each deoxynucleoside triphosphate (dNTP), 1 pmol of each primer, 1.0 U Taq polymerase, and 50 ng genomic DNA of the DMIS isolate AH1-4. PCR amplification was conducted with the following parameters: one cycle at 94°C for 5 min; 35 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 1.5 min; and a final extension at 72°C for 10 min. A single ∼1.3-kb DNA fragment was amplified when using this pair of primers. The PCR product was purified with a gel extraction kit (QIAGEN Inc., Valencia, CA), directly ligated into the pGEM-T Easy vector (Promega, Madison, WI), and sequenced with the vector primers at the Genomics Technology Support Facility (GTSF), Michigan State University.
TABLE 1.
Oligonucleotide primers used in this study and their relevant characteristics
| Primer | Sequence (5′→3′) | Relevant characteristics |
|---|---|---|
| CYP51-Deg-F | GCC(C/T)CC(C/T)GT(C/T)GT(T/G)TTCCAC | Degenerate PCR primers for amplification of a |
| CYP51-Deg-R | AGC(A/G)CC(A/G)AA(A/G)GG(A/G)AG(A/G)TA | 1.3-kb CYP51 fragment from B. jaapii |
| RA2 | (A/C/T/G)CAGCT(A/T)(C/G)CT(A/C/T/GT)(C/G)CTT | Arbitrary primers for a TAIL-PCR for amplification |
| RA3 | GT(A/C/T/G)CGA(C/G)(A/T)CA(A/T/C/G)A(A/T)GTT | of upstream and downstream sequences of the |
| RA4 | CA(A/C/T/G)GCT(A/T)(C/G)GT(A/T/C/GT)(C/G)CAA | 1.3-kb CYP51 fragment from B. jaapii |
| TAIL-SP-Upstr-CYP51-1 | AGGTGAAGCAATCGCCGTACT | Specific primers for a TAIL-PCR for amplification |
| TAIL-SP-Upstr-CYP51-2 | CAGAAAGCAACTGACCTTCGC | of upstream sequence of the 1.3-kb CYP51 |
| TAIL-SP-Upstr-CYP51-3 | CTCGAAAAAGAACCTGTACGGG | fragment from B. jaapii |
| TAIL-SP-Downstr-CYP51-1 | ACTCGTCATTCCAACATCGCA | Specific primers for a TAIL-PCR for amplification |
| TAIL-SP-Downstr-CYP51-2 | AGGTTTTGAACAATCAAGTCGAGG | of downstream sequence of the 1.3-kb CYP51 |
| TAIL-SP-Downstr-CYP51-3 | CGACTATGGGTACGGTCTGGTC | fragment from B. jaapii |
| Bj-CYP51-F | GATCTGGCACACGTGGAAAGA | PCR primers for amplification of the complete |
| Bj-CYP51-R | AACACCGTCGGGGTGAGAAT | CYP51 gene from B. jaapii |
| Bj-CYP51-5-Upstr-F | AGGACCGGAAGGAGGAGTTGA | PCR primers for amplification of the 472-bp 5′ end |
| Bj-CYP51-5-Upstr-R | TGGCGTTTGGGCAATCGTATA | of CYP51 and 610-bp upstream DNA |
| GeneRacer 5′ primer | CGACTGGAGCACGAGGACACTGA | PCR primers for amplifying 5′-end sequences of |
| RACE-SP | TGTGATGGTAGACCCAACGA | cDNA of CYP51 |
| GeneRacer 5′ nested primer | GGACACTGACATGGACTGAAGGAGTA | Nested PCR primers for amplifying 5′-end sequences |
| RACE-nested-SP | TGGCTTAGCACATTGAGCAC | of cDNA of CYP51 |
| Det-Poly-F | AGCCCATCCTTGTGCTGACTT | PCR primers for detecting polymorphic upstream |
| Det-poly-R | TTGCAGACGCGACACGACT | sequences of the CYP51 genes from fenbuconazole-resistant B. jaapii isolates |
| Bj-CYP51-RT-F | CAAAGGTCAATCAGGAACTGCAGA | Real-time PCR primers for determining expression |
| Bj-CYP51-RT-R | AAGAGGAGCCCAGTGAAGCATAAA | level of the CYP51 gene in B. jaapii |
| Bj-Tub-RT-F2 | TCGTCCCATCTCCCAAGGTTT | Real-time PCR primers for determining expression |
| Bj-Tub-RT-R2 | AGACCAAGTGGTTGAGATCTCCG | level of β-tubulin gene in B. jaapii |
This sequence was further used to design six specific primers for the amplification of genomic regions in B. jaapii strains upstream and downstream of this fragment with the thermal asymmetric interlaced PCR (TAIL-PCR) method (22). In TAIL-PCR, three nested sequence-specific primers, TAIL-SP-Upstr-CYP51-1, -2, and -3, and three arbitrary primers, RA2, RA3, and RA4 (Table 1) (29), were used to amplify the region upstream of the 1.3-kb CYP51 fragment. Another three sequence-specific primers, TAIL-SP-Downstr-CYP51-1, -2, and -3, together with the three arbitrary primers were used to amplify the region downstream of the 1.3-kb fragment. TAIL-PCRs with the three arbitrary primers were performed independently. Thermal cycling conditions for TAIL-PCR were those of Liu and Whittier (22).
After three successive nested PCRs with TAIL-PCR amplifications, the reaction products of the secondary and tertiary PCR steps were separated and compared on agarose gels. The bands from the tertiary PCRs with a decrease in length consistent with the differences between the positions of the primers TAIL-SP-Upstr-CYP51-2 and -3 or primers TAIL-SP-Downstr-CYP51-2 and -3 were assumed to be the target products and were purified, cloned, and sequenced. Thus, the complete sequence of the B. jaapii CYP51 gene was obtained from DMIS isolate AH1-4. Subsequently, primers Bj-CYP51-F and Bj-CYP51-R (Table 1) were used to amplify the complete CYP51 gene with Platinum Taq DNA Polymerase High Fidelity (Invitrogen) according to the manufacturer's instructions. All amplified PCR products were cloned in pGEM-T Easy and then sequenced at the GTSF at Michigan State University.
Analysis of regions upstream of CYP51 in DMIR B. jaapii.
The oligonucleotide primer pair Bj-CYP51-5-Upstr-F plus Bj-CYP51-5-Upstr-R (Table 1) was used to amplify a DNA fragment flanking the 472-bp 5′ end of the CPY51 gene and 610 bp of upstream DNA. PCRs were done for each of the 81 B. jaapii isolates with this primer pair. Amplified fragments were analyzed by agarose gel electrophoresis, and fragments of different sizes were cloned in pGEM-T Easy and sequenced. Sequences were compared to database sequences using BLAST searches.
Quantification of CYP51 expression in DMIS and DMIR isolates.
Two DMIS isolates, DSC1-4 and AH1-4, and four DMIR isolates, SIT4-8, SIT5-14, MF01-3, and RSS-2, were grown in liquid MMEB medium (20 g malt extract, 1 g yeast extract per liter) at 25°C for 20 days with continuous shaking. Mycelium of each isolate was harvested and frozen in liquid N2. RNA was extracted with a RNeasy Mini kit (QIAGEN) according to the manufacturer's instructions. For each RNA sample, 500 ng RNA was used for reverse transcription with the CYP51 gene-specific primers Bj-CYP51-RT-F and Bj-CYP51-RT-R and an Omniscript reverse transcription kit (QIAGEN) according to the manufacturer's recommendations.
Real-time PCR amplifications were performed in an ABI sequence detection system (Applied Biosystems, Foster, CA) using SYBR Green I fluorescent dye detection. Amplifications were conducted in 25-μl volumes containing 12.5 μl SYBR Green PCR master mix (Applied Biosystems), 1 μl reverse transcription product, and 1 μl each of the forward and reverse primers Bj-CYP51-RT-F and Bj-CYP51-RT-R (10 pmol each). To create a standard curve, 10-fold serial dilutions of genomic DNA of the isolate AH1-4 (ranging from 0.5 to 5,000 pg) were used for each experiment. There were two replicates for each sample, and the experiment was performed twice. The PCR amplifications were performed with the following parameters: an initial preheat for 2 min at 50°C and then 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. After the amplifications were completed, data were analyzed by using the ABI 7000 Prism 7000 SDS software (Applied Biosystems, Foster City, CA). PCR amplifications with primer pair Bj-Tub-RT-F plus Bj-Tub-RT-R for the β-tubulin gene were performed as the control for each sample in each experiment.
Determination of the transcriptional start site of CYP51.
To determine the transcriptional start site of the CYP51 gene in DMIR and DMIS isolates, the 5′-end sequences of cDNA of CYP51 were obtained from one DMIS and two DMIR isolates by using a GeneRacer kit (Invitrogen) according to the manufacturer's instructions. Briefly, 2 μg of total RNA was treated with calf intestinal alkaline phosphatase to remove 5′ phosphates and subsequently with tobacco acid pyrophosphatase to remove the 5′ cap structure from intact, full-length mRNA. The decapped mRNA was joined to the GeneRacer RNA oligonucleotide (5′-CGACUGGAGCACGAGGACACUGACAUGGACUGAAGGAGUAGAAA-3′) with RNA ligase. Reverse transcription of the ligated mRNA was done with Superscript III reverse transcriptase and the gene-specific primers Bj-CYP51-RT-F and Bj-CYP51-RT-R. The initial PCR was performed with GeneRacer 5′ primer and as a gene-specific primer, RACE-SP. Nested PCR amplifications were then conducted using GeneRacer 5′ nested primer and a gene-specific nested primer, RACE-nested-SP (Table 1). Amplified products of the nested PCR were purified and sequenced.
Nucleotide sequence accession number.
The complete sequence of CYP51 from the B. jaapii isolate AH1-4 was deposited in GenBank under accession no. DQ389077. The sequences upstream of CYP51 from the isolates SIT4-8, SIT5-14, MF01-3, and RSS-2 were deposited under accession no. DQ389078 to DQ389081.
RESULTS
Sensitivity of B. jaapii isolates to fenbuconazole.
After incubation at 23°C for 21 days, 22 of the 81 isolates tested did not grow on MMEA amended with fenbuconazole at 0.02 μg a.i./ml. Each of these 22 isolates had been collected from a cherry tree with no known history of fungicide exposure. Thus, these 22 isolates were considered sensitive to fenbuconazole and designated DMIS. The remaining 59 isolates could grow on MMEA plus fenbuconazole at ≥0.2 μg/ml and were considered to be resistant to fenbuconazole and designated DMIR.
Isolation and characterization of CYP51 from B. jaapii.
Based on Southern hybridization analysis, CYP51 was present in a single copy in the genome of B. jaapii (data not shown). Using GenScan (http://genes.mit.edu/GENSCAN.html), this gene was predicted to encode 522 amino acids and to have introns of 50 bp and 60 bp located after nucleotide positions 246 and 494, respectively. The deduced amino acid sequence of CYP51 from B. jaapii was 83% identical to that of Tapesia acuformis (GenBank accession no. AAF18468), 75% identical to that of Monilinia fructicola (AAL79180), 74% identical to that of Botryotinia fuckeliana (AAF85983), and 68% identical to that of Blumeria graminis (CAE18103).
Comparison of the deduced amino acid sequences of the translated CYP51 protein from four DMIS and four DMIR isolates identified only a single difference. DMIS isolates AH1-4, AH1-7, DSC1-4, and RL6 had a serine at codon position 508, while DMIR isolates SIT4-8, SIT5-14, SIT6-15, and SIT9-10 had a tyrosine at this position. The deduced amino acid sequences of CYP51 from other DMIS fungi Botrytis cinerea (GenBank accession no. AAF85983) and M. fructicola (GenBank accession no. AAL79180) also have a tyrosine at position 508. Thus, we concluded that differences at position 508 were not associated with DMI resistance/sensitivity and decided to examine other possible mechanisms conferring DMI resistance in the B. jaapii strains.
Transcriptional start site of CYP51 in DMIR and DMIS isolates of B. jaapii.
The 5′-end sequences of cDNA of CYP51 genes from two DMIR isolates, SIT5-14 and MF01-3, and one DMIS isolate, AH1-4, were amplified with the GeneRACER kit. The CYP51 transcripts from all three examined isolates had the same transcriptional start site, which was located 68 bp upstream of the ATG start codon (data not shown).
Analysis of the region upstream of CYP51 in DMIR B. jaapii.
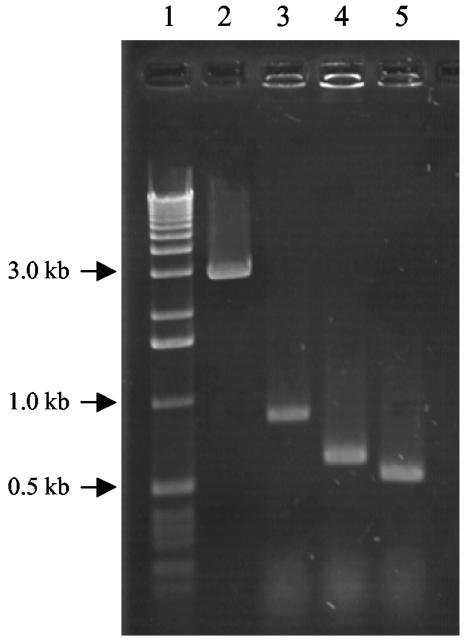
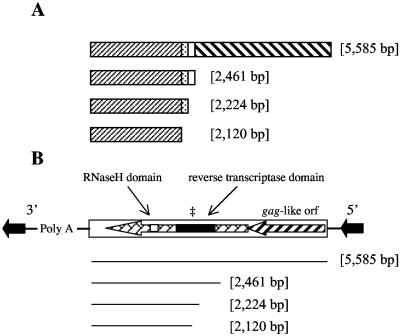
PCR analyses of the region upstream of CYP51 from each of the 81 B. jaapii isolates indicated that one of four DNA inserts ranging from ∼2.1 to 5.6 kb was present upstream of CYP51 in all of the DMIR isolates but in none of the DMIS isolates (Fig. 1). These four inserts were sequenced. The three shorter sequences (2,120 bp to 2,461 bp) were inserted 102 bp upstream of CYP51, while the 5,585-bp insert was inserted 181 bp upstream of CYP51. Comparison of each insert sequence suggested that deletions contributed to their observed size, as all inserts contained a common 2,120-bp sequence (Fig. 2A).
FIG. 1.
Detection of different insert sequences upstream of CYP51 in representative DMIR strains of B. jaapii obtained using the oligonucleotide primers Det-Poly-F and Det-Poly-R (Table 1). Lane 1, molecular size markers; lane 2, B. jaapii RSS-2; lane 3, B. jaapii MF01-3; lane 4, B. jaapii SIT4-8; lane 5, B. jaapii SIT5-14.
FIG. 2.
(A) Schematic presentation of the four inserts of different sizes located upstream of the 14α-demethylase (CYP51) gene from fenbuconazole-resistant (DMIR) isolates of Blumeriella jaapii. Sequences conserved among the different inserts are shaded similarly. (B) Genetic depiction of a LINE-like retrotransposon and depiction of the features of LINE-like retrotransposons present in the various B. jaapii CYP51 upstream inserts. Two open reading frames (orf) are denoted by the large arrows, and the locations of the reverse transcriptase and RNase H domains within the larger open reading frame are shown by the open and shaded boxes, respectively. The ‡ symbol denotes the location of the conserved YADD amino acid motif within the reverse transcriptase domain.
The 5,585-bp fragment contained two open reading frames (ORFs). The ORFs were homologous to genes present in retrotransposon elements from other fungi, e.g., Magnaporthe grisea (GenBank accession no. AAB71688) and Colletotrichum gloeosporioides (GenBank accession no. L76205), and from other organisms, e.g., Anopheles gambiae (GenBank accession no. BAC57907) and Caenorhabditis elegans (GenBank accession no. NP_503033). The ORFs were organized similarly to retrotransposon elements that do not include long terminal direct repeats and that are referred to as long interspersed nuclear element (LINE)-like retrotransposons (Fig. 2B) (7, 8). The deduced amino acid sequence of the first ORF was similar to gag-like proteins and contained a cysteine-rich motif that is thought to function in nucleotide binding (18). The deduced amino acid sequence of the second larger ORF had homology to a multidomain protein from retrotransposons including a reverse transcriptase domain and an RNase H domain (Fig. 2B). The reverse transcriptase domain motif is similar to that of proteins from M. grisea and in the CgT1 element from C. gloeosporioides. It contains the YXDD motif (YADD in B. jaapii) which is a signature sequence for reverse transcriptase of viral origin (33). In addition, a 25-amino-acid RNase H domain with 52% identity to the RNase H signature motif from S. cerevisiae (19) was present in the C-terminal region of orf2 (Fig. 2B).
The 5,585-bp insert from B. jaapii was truncated relative to a full-length LINE retrotransposon and did not contain a poly(A) region downstream of orf2 or 5′ and 3′ repeat regions (Fig. 2B). The three shorter inserts all were similar truncations of this LINE-like retrotransposon. The truncated inserts lacked sequences from the 5′ end of the retrotransposon including orf1 and part of orf2 (Fig. 2B).
The frequency of isolation of the CYP51 upstream inserts within the B. jaapii population in Michigan and Ohio was evaluated with PCR. No upstream insert was detected in any of the 22 DMIS isolates. Inserts were detected in all 59 DMIR isolates, with the 2,120-bp and 2,222-bp inserts the most common (Table 2). There was no correlation between host of isolation and insert size. All five isolates from eastern Michigan had the same 2,471-bp insert, and the 5,585-bp insert was detected in only two B. jaapii isolates (Table 2).
TABLE 2.
Frequency of occurrence of sequences of various sizes upstream of CYP51 in DMIR B. jaapiia
| Source of isolateb | No. of isolates | Host plant | DMI phenotype | No. of isolates containing insert of:
|
|||
|---|---|---|---|---|---|---|---|
| 2,120 bp | 2,222 bp | 2,471 bp | 5,585 bp | ||||
| Central MI | 1 | BC | S | 0 | 0 | 0 | 0 |
| Central MI | 2 | TC | S | 0 | 0 | 0 | 0 |
| Ohio | 11 | SC | S | 0 | 0 | 0 | 0 |
| Ohio | 8 | TC | S | 0 | 0 | 0 | 0 |
| Central MI | 10 | TC | R | 4 | 6 | 0 | 0 |
| Eastern MI | 5 | SC | R | 0 | 0 | 5 | 0 |
| Southwest MI | 5 | TC | R | 1 | 4 | 0 | 0 |
| Western MI | 9 | TC | R | 7 | 0 | 2 | 0 |
| Northwest MI | 3 | SC | R | 1 | 1 | 0 | 1 |
| Northwest MI | 27 | TC | R | 14 | 12 | 0 | 1 |
Abbreviations: BC, black cherry; SC, sweet cherry; TC, tart cherry; R, resistant; S, sensitive.
Central MI, Ingham and Ionia Counties; Eastern MI, Tuscola County; Southwest MI, van Buren County; Western MI, Oceana County; Northwest MI, Benzie, Grand Traverse, and Leelanau Counties.
Overexpression of CYP51 in DMIR isolates of B. jaapii.
DMIR isolates representing each of the four insert sizes upstream of CYP51 were analyzed: SIT5-14 (2,120 bp), SIT4-8 (2,222 bp), MF01-3 (2,471 bp), and RSS-2 (5,585 bp). Expression of CYP51 in each DMIR isolate was 5- to 12-fold higher than that in DMIS isolates (data not shown). Expression of CYP51 in DMIR isolates was not dependent on the size of the upstream insert sequence, as similar expression levels were found for isolates SIT5-14, SIT4-8, MF01-3, and RSS-2 (data not shown).
DISCUSSION
In this study, we found that resistance to DMI fungicides in B. jaapii resulted from increased expression of the CYP51 target gene. Overexpression of the CYP51 gene in fungi conferring DMI resistance may result from different mechanisms. In the clinical fungus Candida glabrata, overexpression of CYP51 resulted from a chromosomal duplication and therefore an increase in copy number of the CYP51 gene (25). Increased expression of CYP51 in V. inaequalis was correlated with the presence of a 553-bp insertion located in the promoter region of this gene in some, but not all, DMIR isolates (30). In a study of DMIR field isolates of P. digitatum, a unique 126-bp sequence in the CYP51 promoter region hypothesized to act as a transcriptional enhancer was tandemly repeated five times in resistant isolates and was present only once in sensitive isolates (16).
In our study, the overexpression of CYP51 in B. jaapii was positively correlated with the upstream insertion of various truncated derivatives of a LINE-like retrotransposon in all 59 DMIR isolates examined. Since the CYP51 transcriptional start site was not located within the LINE element in DMIR isolates, the role of this element in CYP51 overexpression is not proven. In addition, our attempts to transform a DMIS isolate with a construct containing CYP51 and associated upstream insert sequences have yet to succeed.
The possible involvement of transposable elements in eukaryotic resistance to fungicides, herbicides, or insecticides is a rare event, and we are aware of only two other instances. In Drosophila melanogaster, resistance to the insecticide DDT is conferred via overtranscription of Cyp6g1, which carries an Accord transposable element insertion in the 5′ end of the gene (6). Resistance to organophosphate insecticides in D. melanogaster can be conferred via the insertion of the LINE-like element Doc in the CHKov1 gene, resulting in the generation of a hybrid transcript creating a new functional protein (1). Other examples in fungi of increased levels of transcription of adjacent genes as a consequence of transposon insertion have been described (14, 26). For example, Ty elements in Saccharomyces cerevisiae can function as enhancers, and insertion of a Ty1 element in the 5′ regulatory region of the CYC-7 locus resulted in a 20-fold increase in CYC-7 expression (13).
The effect of fungicide resistance on the ecological fitness of strains is an important factor in the long-term maintenance of this phenotype in fungal populations (reviewed in reference 2). Slight fitness costs of DMI resistance phenotypes have been observed (20); in experimental populations, these fitness costs can be reduced following continued evolution of DMIR strains (5). We have not yet evaluated the fitness of the DMIR B. jaapii strains, but since only 2/59 DMIR isolates carried the large 5.6-kb insert, this larger insert might reduce fitness more than the smaller 2.1-kb inserts. Since the 5.6-kb and 2.1-kb inserts are found at slightly different upstream insertion sites, these insertions occurred independently and the 2.1-kb insertion may be present in a B. jaapii strain background of increased fitness. We plan to conduct further analyses of the fitness of the distinct DMIR B. jaapii isolates to assess the potential for long-term retention of these genotypes in the pathogen population in the absence of selection pressure.
Acknowledgments
This research was supported by grants from the United States Department of Agriculture’s Risk Avoidance and Mitigation Program, the Michigan Cherry Committee, and the Michigan Agricultural Experiment Station.
We thank Jim Nugent for assistance in locating orchards where fungal isolates were recovered.
REFERENCES
- 1.Aminetzach, Y. T., J. M. Macpherson, and D. A. Petrov. 2005. Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila. Science 309:764-767. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J. B. 2005. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nat. Rev. Microbiol. 3:547-556. [DOI] [PubMed] [Google Scholar]
- 3.Asai, K., N. Tsuchimori, K. Okonogi, J. R. Perfect, O. Gotoh, and Y. Yoshida. 1999. Formation of azole-resistant Candida albicans by mutation of sterol 14-demethylase P450. Antimicrob. Agents Chemother. 14:1163-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brent, K. J. 1995. Fungicide resistance in crop pathogens, how can it be managed. Global Crop Protection Federation, Brussels, Belgium.
- 5.Cowen, L. E., L. M. Kohn, and J. B. Anderson. 2001. Divergence in fitness and evolution of drug resistance in experimental populations of Candida albicans. J. Bacteriol. 183:2971-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daborn, P. J., J. L. Yen, M. R. Bogwitz, G. Le Goff, E. Feil, S. Jeffers, N. Tijet, D. Heckel, P. Batterham, R. Feyereisen, T. G. Wilson, and R. H. French-Constant. 2002. A single P450 allele associated with insecticide resistance in Drosophila. Science 297:2253-2256. [DOI] [PubMed] [Google Scholar]
- 7.Daboussi, M. J. 1997. Fungal transposable elements and genome evolution. Genetica 100:253-260. [PubMed] [Google Scholar]
- 8.Daboussi, M. J., and P. Capy. 2003. Transposable elements in filamentous fungi. Annu. Rev. Microbiol. 57:275-299. [DOI] [PubMed] [Google Scholar]
- 9.Délye, C., F. Laigret, and M.-F. Corio-Costet. 1997. A mutation in the 14α-demethylase gene of Uncinula necator that correlates with resistance to a sterol biosynthesis inhibitor. Appl. Environ. Microbiol. 63:2966-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delye, C., L. Bousset, and M. F. Corio-Costet. 1998. PCR cloning and detection of point mutations in the eburicol 14α-demethylase (CYP51) gene from Erysiphe graminis f. sp. hordei, a “recalcitrant” fungus. Curr. Genet. 34:399-403. [DOI] [PubMed] [Google Scholar]
- 11.de Waard, M. A. 1996. Molecular genetics of resistance in fungi to azole fungicides. ACS Symp. Ser. 645:62-71. [Google Scholar]
- 12.Erickson, E. O., and W. F. Wilcox. 1997. Distributions of sensitivities to three sterol demethylation inhibitor fungicides among populations of Uncinula necator sensitive and resistant to triadimefon. Phytopathology 87:784-791. [DOI] [PubMed] [Google Scholar]
- 13.Errede, B., M. Company, and C. Hutchinson. 1987. Ty1 sequence with enhancer and mating-type-dependent regulatory activities. Mol. Cell. Biol. 7:258-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard, L., and M. Freeling. 1999. Regulatory changes as a consequence of transposon insertion. Dev. Genet. 25:291-296. [DOI] [PubMed] [Google Scholar]
- 15.Gisi, U., K. M. Chin, G. Knapova, R. K. Färber, U. Mohr, S. Parisi, H. Sierotzki, and U. Steinfeld. 2000. Recent developments in elucidating modes of resistance to phenylamide, DMI and strobilurin fungicides. Crop Prot. 19:863-872. [Google Scholar]
- 16.Hamamoto, H., K. Hasegawa, R. Nakaune, Y. J. Lee, Y. Makizumi, K. Akutsu, and T. Hibi. 2000. Tandem repeat of a transcriptional enhancer upstream of the sterol 14α-demethylase gene (CYP51) in Penicillium digitatum. Appl. Environ. Microbiol. 66:3421-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi, K., H. Schoonbeek, and M. A. De Waard. 2002. Expression of the ABC transporter BcatrD from Botrytis cinerea reduces sensitivity to sterol demethylation inhibitor fungicides. Pestic. Biochem. Physiol. 73:110-121. [Google Scholar]
- 18.He, C., J. P. Nourse, S. Kelemu, J. A. G. Irwin, and J. M. Manners. 1996. CgT1: a non-LTR retrotransposon with restricted distribution in the fungal phytopathogen Colletotrichum gloeosporioides. Mol. Gen. Genet. 252:320-331. [DOI] [PubMed] [Google Scholar]
- 19.Itaya, M., D. McKelvin, S. K. Chatterjee, and R. J. Crouch. 1991. Selective cloning of genes encoding RNase H from Salmonella typhimurium, Saccharomyces cerevisiae, and Escherichia coli rnh mutant. Mol. Gen. Genet. 227:438-445. [DOI] [PubMed] [Google Scholar]
- 20.Karaoglanidis, G. S., C. C. Thanassoulopoulos, and P. M. Ioannidis. 2001. Fitness of Cercospora beticola field isolates—resistant and—sensitive to demethylation inhibitor fungicides. Eur. J. Plant Pathol. 107:337-347. [Google Scholar]
- 21.Köller, W., W. F. Wilcox, J. Barnard, A. L. Jones, and P. G. Braun. 1997. Detection and quantification of resistance of Venturia inaequalis populations to sterol demethylation inhibitors. Phytopathology 87:184-190. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y., and R. F. Whittier. 1995. Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674-681. [DOI] [PubMed] [Google Scholar]
- 23.Lupetti, A., R. Danesi, M. Campa, M. Del Tacca, and S. Kelly. 2002. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 8:76-81. [DOI] [PubMed] [Google Scholar]
- 24.Ma, Z., and T. J. Michailides. 2005. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop. Prot. 24:853-863. [Google Scholar]
- 25.Marichal, P., V. Vanden Bossche, F. C. Odds, G. Nobels, D. W. Warnock, V. Timmerman, C. Van Broeckhoven, S. Fay, and P. Mose-Larsen. 1997. Molecular biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob. Agents Chemother. 41:2229-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marino-Ramirez, L., K. C. Lewis, D. Landsman, and I. K. Jordan. 2005. Transposable elements donate lineage-specific regulatory sequences to host genomes. Cytogenet. Genome Res. 110:333-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGrath, M. T. 2001. Fungicide resistance in cucurbit powdery mildew, experiences and challenges. Plant Dis. 85:236-245. [DOI] [PubMed] [Google Scholar]
- 28.Mellado, E., T. M. Diaz-Guerra, M. Cuenca-Esterella, and J. L. Rodriguez-Tudela. 2001. Identification of two different 14-α sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 39:2431-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motomura, M., N. Chihaya, T. Shinozawa, T. Hamasaki, and K. Yabe. 1999. Cloning and characterization of the O-methyltransferase I gene (dmtA) from Aspergillus parasiticus associated with the conversions of demethylsterigmatocystin to sterigmatocystin and dihydrodemethylsterigmatocystin to dihydrosterigmatocystin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 65:4987-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnabel, G., and A. L. Jones. 2001. The 14α-demethylase (CYP51A) gene is overexpressed in Venturia inaequalis strains resistant to myclobutanil. Phytopathology 91:102-110. [DOI] [PubMed] [Google Scholar]
- 31.Schnabel, G., and Q. Dai. 2004. Heterologous expression of the P450 sterol 14α-demethylase gene from Monilinia fructicola reduces sensitivity to some but not all DMI fungicides. Pestic. Biochem. Physiol. 78:31-38. [Google Scholar]
- 32.Wood, H. M., M. J. Dickinson, J. A. Lucas, and P. S. Dyer. 2001. Cloning of the CYP51 gene from the eyespot pathogen Tapesia yallundae indicates that resistance to the DMI fungicide prochloraz is not related to sequence changes in the gene encoding the target site enzyme. FEMS Microbiol. Lett. 196:183-187. [DOI] [PubMed] [Google Scholar]
- 33.Yuki, S., Y. Inouye, S. Ishimura, and K. Saigo. 1986. Nucleotide sequence characterisation of a Drosophila retrotransposon 412. Eur. J. Biochem. 158:403-410. [DOI] [PubMed] [Google Scholar]
- 34.Zwiers, L.-H., I. Stergiopoulos, J. G. M. Van Nistelrooy, and M. A. De Waard. 2002. ABC transporters and azole susceptibility in laboratory strains of the wheat pathogen Mycosphaerella graminicola. Antimicrob. Agents Chemother. 46:3900-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]