Abstract
TrzF, the allophanate hydrolase from Enterobacter cloacae strain 99, was cloned, overexpressed in the presence of a chaperone protein, and purified to homogeneity. Native TrzF had a subunit molecular weight of 65,401 and a subunit stoichiometry of α2 and did not contain significant levels of metals. TrzF showed time-dependent inhibition by phenyl phosphorodiamidate and is a member of the amidase signature protein family. TrzF was highly active in the hydrolysis of allophanate but was not active with urea, despite having been previously considered a urea amidolyase. TrzF showed lower activity with malonamate, malonamide, and biuret. The allophanate hydrolase from Pseudomonas sp. strain ADP, AtzF, was also shown to hydrolyze biuret slowly. Since biuret and allophanate are consecutive metabolites in cyanuric acid metabolism, the low level of biuret hydrolase activity can have physiological significance. A recombinant Escherichia coli strain containing atzD, encoding cyanuric acid hydrolase that produces biuret, and atzF grew slowly on cyanuric acid as a source of nitrogen. The amount of growth produced was consistent with the liberation of 3 mol of ammonia from cyanuric acid. In vitro, TrzF was shown to hydrolyze biuret to liberate 3 mol of ammonia. The biuret hydrolyzing activity of TrzF might also be physiologically relevant in native strains. E. cloacae strain 99 grows on cyanuric acid with a significant accumulation of biuret.
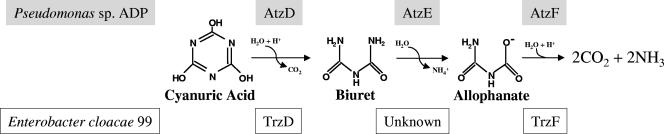
Genome sequencing projects and recent biochemical investigations have demonstrated that allophanate hydrolase is widely distributed in taxonomically diverse bacteria (3, 14, 18, 27). Previously, allophanate hydrolase was known in yeast and algae (15, 29) but was unknown in prokaryotes. It has now been determined that allophanate hydrolase has at least two distinct metabolic roles in bacteria. It functions in concert with a urea carboxylase to metabolize urea after its activation by carboxylation (17). This two-enzyme sequence provides bacteria with an alternative to urease that hydrolyzes urea directly to 2 mol of ammonia (14). Allophanate hydrolase also functions in the bacterial metabolism of cyanuric acid (3, 27). Recently, a number of cyanuric acid metabolizing bacteria have been shown to hydrolytically open the s-triazine ring of cyanuric acid to produce biuret, catalyzed by AtzD or TrzD (Fig. 1). It had been proposed that biuret was hydrolyzed to urea in different bacteria (4, 5, 8), but a more recent study showed that biuret hydrolysis in these bacteria produced allophanate (3). Allophanate was subsequently cleaved by an allophanate hydrolase. Thus, all three nitrogen atoms of the s-triazine ring are liberated to support bacterial growth on cyanuric acid as the sole nitrogen source.
FIG. 1.
Cyanuric acid catabolic pathway in Pseudomonas sp. strain ADP and E. cloacae 99.
One bacterium that has been demonstrated to grow on cyanuric acid, Enterobacter cloacae strain 99, was shown to have an allophanate hydrolase activity, but this strain was unusual in that it accumulated significant levels of biuret in the growth medium (4). The gene regions encoding cyanuric acid hydrolase (TrzD) and urea amidolyase have been reported previously (GenBank accession number AF342826), but no biuret hydrolase gene or enzyme has been found to date in that bacterium.
In the present work, the allophanate hydrolase from E. cloacae strain 99 was cloned with a chaperone protein and then purified and characterized. It was comparable in terms of a number of properties to AtzF from Pseudomonas sp. strain ADP (27) and malonamidase from Bradyrhizobium japonicum, a protein catalyzing a similar reaction with a structurally analogous substrate, malonamate (20, 28). Surprisingly, we found that purified, recombinant TrzF and AtzF proteins each showed a low, but significant, biuret hydrolase activity. In recombinant Escherichia coli, the biuret hydrolase activity was shown to function physiologically with cyanuric acid hydrolase to liberate ammonia from cyanuric acid and support growth.
MATERIALS AND METHODS
Chemicals.
Potassium allophanate was prepared from diethyl allophanate (Acros Organics, Pittsburgh, PA) as described previously (31). Acetylurea was purchased from Lancaster Synthesis, Inc. (Pelham, NH), and all other commercially available compounds were purchased from Aldrich Chemical Co. (Milwaukee, WI).
Enzymatic assays.
The amount of ammonia released from allophanate and related compounds was determined using the Berthelot reaction as described previously (30). One unit of enzyme activity was defined as the amount of enzyme converting 1 μmol of allophanate to 2 μmol of ammonia in 1 min.
Construction of recombinant plasmid for the expression of TrzF with a His tag.
The putative urea amidolyase gene from E. cloacae strain 99 (GenBank accession number AF342826) was amplified, without its native promoter, by PCR using the primers trzFf-His (5′-CGGCTAGCATGAATTTGACAATTACA-3′) and trzFr-His (5′-CGAAGCTTCTATTGATCGGCAAGA-3′). Primers were synthesized by Integrated DNA Technologies (Coralville, IA). The primers contained NheI and HindIII restriction enzyme cloning sites at their 5′ and 3′ ends, respectively. The gene was cloned into vector pET28b+ (Novagen, Madison, WI), downstream of the lac promoter and His tag coding sequence, to create plasmid pGC1. Plasmid pGC1 was transformed into E. coli BLR(DE3) (Novagen, Madison, WI), and the sequence of the cloned gene was verified.
Expression and purification of TrzF.
Plasmid pAG containing the chaperone genes groEL and groES (24) was transformed into E. coli BLR(DE3)(pGC1) in order to increase the solubility of TrzF. E. coli BLR(DE3)(pAG, pGC1) was grown in LB medium (25) containing 50 μg of kanamycin and 30 μg of chloramphenicol per ml at 15°C with shaking at 150 rpm. l-Arabinose (0.0015%) was added when cultures reached an optical density of 0.5 at 600 nm, and 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added after an additional 90 min of incubation. Induced cells were grown for an additional 16 h under the same conditions. Cultures were centrifuged at 10,000 × g for 10 min at 4°C and washed three times with 0.85% NaCl, and cell pellets were resuspended in 20 ml of 50 mM 3-(N-morpholino)propanesulfonate (MOPS) buffer, pH 7.4. All buffers contained 10% glycerol. Cells were broken by passage three times through a chilled French pressure cell operated at 140 MPa. Cell extracts were obtained by centrifugation at 18,000 × g for 90 min at 4°C. Lysates were applied to a 5-ml HisTrap chelating column (Amersham Pharmacia Biotech, Piscataway, NJ) complexed with Ni2+ according to the manufacturer's instructions. The column was washed with 15 ml of 50 mM MOPS buffer, pH 7.4, followed by a second wash with the same buffer supplemented with 0.125 M imidazole. The enzyme was eluted from the column with 15 ml of 0.25 M imidazole in 50 mM MOPS buffer, pH 7.4. The purified enzyme was concentrated using a Centricon-30 filtration unit (Amicon, Beverly, MA), and enzyme purity and subunit molecular weight were estimated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (25).
Gel filtration chromatography.
The holoenzyme molecular weight was estimated by gel filtration chromatography using a Superose 12 HR column (Pharmacia, Uppsala, Sweden) and an FPLC System (Pharmacia). The column was equilibrated with 0.15 M KCl in 50 mM MOPS buffer, pH 7.6, and eluted at a flow rate of 0.3 ml min−1. The molecular mass of TrzF was estimated by comparing its elution to that of standards with well-defined molecular weights: thyroglobulin (670,000), gamma globulin (158,000), chicken ovalbumin (44,000), horse myoglobin (17,000), and vitamin B-12 (1,350).
Analysis of transition metal cofactors.
Purified TrzF (2.5 mg) was digested in 5 ml of 10% HCl, and samples were incubated overnight at 90°C. Metal content was determined by inductively coupled plasma emission spectroscopy at the University of Minnesota Soils Analytical Laboratory (St. Paul, MN).
Substrate specificity and kinetic studies.
All substrate assays and enzyme kinetic studies were done in 50 mM MOPS buffer, pH 7.4, at 25°C. Solutions containing allophanate and structural analogs were prepared in MOPS buffer, pH 7.4, and added to reaction mixtures to final concentrations of 250 μM to 10 mM. Ammonia release was measured as described above. Control analyses without enzyme were done in parallel. Kinetic parameters were calculated from the initial hydrolysis rates at different concentrations using the Hanes-Woolf equation: [S]/V0 = [S]/Vmax + Km/Vmax (26), where S is the substrate concentration, V0 is the rate a fixed substrate concentration, and Vmax is the rate at saturating substrate. Linear regression of the plot of [S]/V0 versus [S] was used to determine the Vmax and Km parameters. The kcat value was calculated by dividing Vmax by the moles of TrzF, based on the subunit molecular mass. When only low activity was detected, such as with biuret as a substrate, levels of activity 10-fold higher were used, and incubations were conducted over a period of hours. In these experiments, less than 10% of initial substrate was consumed in determining initial rates. Biuret and malonamide were also tested as substrates for AtzF, purified as previously described (27).
Inhibition of TrzF by phenyl phosphorodiamidate.
TrzF (30 μM subunit concentration) was incubated at 25°C with 0.15 or 3 mM phenyl phosphorodiamidate in 0.1 M sodium phosphate buffer, pH 8.0. Subsamples were withdrawn at several time points, and degradation of 20 mM allophanate was measured using the ammonia release assay as described above.
Growth of E. coli containing the atzD and atzF genes on minimal medium with cyanuric acid as a nitrogen source.
An E. coli strain containing the atzD and atzF genes from Pseudomonas sp. strain ADP was constructed by transforming plasmid pKT230::atzD (11, 21) into E. coli BLR(DE3) containing pET28b+::atzF (27). The ability of this recombinant E. coli to use AtzF to grow on biuret as a nitrogen source (produced by the hydrolysis of cyanuric acid by AtzD) was measured. Starter cultures grown in LB medium were centrifuged at 10,000 × g at 4°C, washed three times in 0.85% NaCl, and inoculated into 100 ml of minimal R medium (10) containing 1 mM proline and supplemented with 5 mM cyanuric acid as the nitrogen source. The culture was incubated at 30°C, with shaking at 150 rpm, and growth was measured by following optical density at 600 nm (OD600). Cultures of E. coli containing each of the plasmids separately were used as negative controls. Minimal medium without cyanuric acid served as a control to measure the contribution of proline as a nitrogen source for growth. Medium containing 15 mM ammonium chloride, providing an equivalent amount of nitrogen as 5 mM cyanuric acid, was used to measure the optimum growth potential of the recombinant strain.
RESULTS
TrzF optimized expression and purification.
TrzF activity was 0.06 umol/min per mg of protein in initial clones containing plasmid pGC1. Subsequent transformation of plasmid pGC1 into E. coli BLR(DE3) expressing the chaperone proteins GroEL and GroES led to a 20-fold increase in allophanate hydrolase activity in crude lysates. In crude extracts, a major protein band was observed by SDS-PAGE that migrated slightly more slowly than the intense high-molecular-weight chaperone protein. In initial purification efforts, significant amounts of the chaperone protein migrated with TrzF. Ultimately, conditions were found to elute the His-tagged TrzF uniquely from a nickel column to yield a homogeneous protein with a 10-fold purification and a 20% yield. Yield was sacrificed to obtain purity.
Physical characterization of TrzF.
The purified TrzF protein appeared homogeneous by SDS-PAGE, with an estimated subunit molecular weight of 68,000. Based on translation of the gene sequence, the native TrzF protein without the His tag was calculated to have a subunit molecular weight of 65,401 (Table 1). This is very similar to the subunit molecular weight of AtzF and allophanate hydrolase from Oleomonas sagaranensis. The protein had a calculated pI of 5.1, comparable to other allophanate hydrolases. Gel filtration indicated a holoenzyme molecular weight of 144,000, indicating a subunit stoichiometry of α2, similar to malonamidase. No transition metals were observed; a stoichiometry of <0.1 metal per subunit was observed for Al, B, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, Pb, and Zn.
TABLE 1.
Comparison of physicochemical properties of TrzF, AtzF, malonamidase, and allophanate hydrolase from O. sagaranensis
| Property | TrzFa | AtzFb | MAE2c | OSAHd |
|---|---|---|---|---|
| Mol wt | 65,401 | 64,058 | 43,682 | 61,999 |
| Subunit stoichiometry | α2 | α4 | α2 | α2 |
| pI | 5.1 | 5.3 | 7.8 | 4.8 |
| Metal | None | None | NDe | ND |
| Km (mM) | 0.68 | 1.5 | 10 | 0.04 |
| kcat/Km (s−1 M−1) | 8.8 × 103 | 1.1 × 104 | NDf | 2.7 × 106 |
| PPDAg inhibition | Time dependent | Time dependent | Covalenth | ND |
Allophanate hydrolase from E. cloacae strain 99.
Allophanate hydrolase from Pseudomonas sp. strain ADP.
Malonamidase from Bradyrhizobium japonicum USDA 110.
Allophanate hydrolase from O. sagaranensis.
ND, not determined.
Kinetic parameters are reported for the nonphysiological hydroxylaminolysis reaction catalyzed by malonamidase MAE2, but not the physiological amide hydrolysis reaction.
PPDA, phenyl phosphorodiamidate.
Tested with crystals and shown to react with active-site serine.
Characterization of TrzF enzyme activity.
TrzF was incubated with potassium allophanate at pH 7.4, and the specific activity was found to be 9.3 μmol per min per mg protein. The kcat was 6 s−1, the Km was 0.68 mM, and the kcat/Km was 8.8 × 103 s−1 M−1. This was comparable with values previously determined for AtzF from Pseudomonas sp. strain ADP (27), but kcat was higher and Km was lower with the allophanate hydrolase from Oleomonas (Table 1). When the reaction was allowed to proceed to completion for 20 min with limiting concentrations of allophanate, 2.0 mol of ammonia was detected per mol of substrate consumed.
TrzF showed strong time-dependent inhibition with phenyl phosphorodiamidate, a compound shown to covalently modify the homologous enzymes AtzF (27) and malonamidase (28) (Table 1). These data are consistent with the structural relatedness of TrzF with other members of the amidase signature family. With both AtzF and malonamidase, phenyl phosphorodiamidate was shown to react specifically with a single serine residue. Other data have demonstrated that this serine residue acts as the nucleophile that displaces the leaving amino group, a mechanism characteristic of members of the amidase signature family (23, 28).
Nonsubstrates and slow substrates for TrzF.
Urea was not observed to be a substrate for TrzF. In addition, the following urea derivatives were not found to be substrates: N-methylurea, acetylurea, 1-acetyl-2-thiourea, methyl carbamate, and semicarbazide. The following allophanate analogs were not detectably reactive with TrzF: methyl allophanate, hydantoic acid, and oxamic acid. Thus, TrzF was observed here to have stringent substrate specificity.
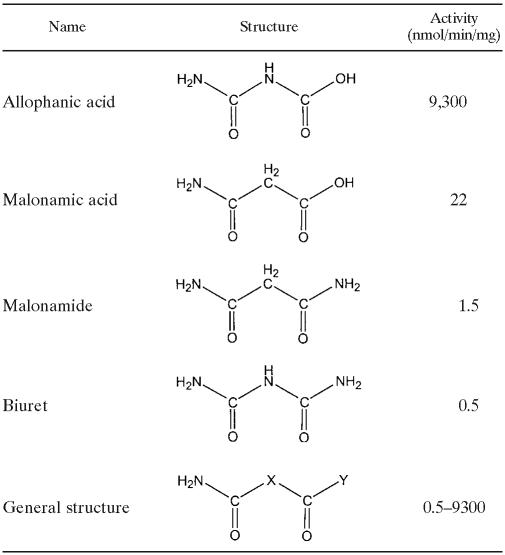
Subsequently, other substrates for TrzF were uncovered, but they were all highly isosteric with allophanate, and activity was significantly lower than that observed with allophanate (Table 2). All compounds showing reactivity contained the following string of five functional groups in the precise order: (i) amino group, (ii) carbonyl carbon, (iii) amino group or methylene carbon, (iv) carbonyl carbon, and (v) hydroxyl or amino group.
TABLE 2.
Structurally analogous compounds shown to be hydrolyzed by TrzF
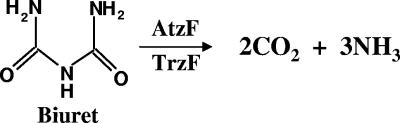
Allophanate was by far the most reactive compound. However, malonamate was hydrolyzed by TrzF to yield 1 mol of ammonia per mol of substrate consumed, with a specific activity of 22 nmol per min per mg of protein. In contrast, malonamide was hydrolyzed by TrzF to yield 2 mol of ammonia per mol of substrate consumed with a specific activity of 1.5 nmol per min per mg of protein. Biuret was hydrolyzed by TrzF to yield 3 mol of ammonia per mol of substrate consumed (Fig. 2), and it was hydrolyzed with a specific activity of 0.5 nmol per min per mg of protein.
FIG. 2.
Biuret hydrolysis liberating 3 mol of ammonia by TrzF from E. cloacae 99 and AtzF from Pseudomonas sp. strain ADP.
AtzF also hydrolyzes biuret.
We had previously reported that biuret was not a substrate for AtzF, the allophanate hydrolase homolog of TrzF (27), but the low activity of TrzF with biuret observed here caused us to reexamine this. With longer incubation times, we determined that AtzF was similarly active with biuret, showing a specific activity of 1 nmol per min per mg of protein.
Potential physiological relevance of low reactivity of allophanate hydrolase with biuret.
E. cloacae strain 99 grew with 5 mM cyanuric acid as the sole nitrogen source and accumulated 4.7 mM biuret, slightly less than stoichiometric accumulation. The difference, 0.3 mM cyanuric acid, can give rise to 0.9 mM ammonia, and that is sufficient nitrogen to support substantial growth. However, the accumulation of biuret indicates that it is inefficiently hydrolyzed by E. cloacae strain 99. This bacterium did not show a gene that amplified via PCR using atzE gene primers (data not shown), and a trzE gene has not been identified in this or any other bacterium (Fig. 1).
In this context, we considered the idea that the low level of biuret hydrolase activity conferred by TrzF or AtzF could support slow bacterial growth on biuret since consecutive hydrolysis reactions will liberate 3 mol of ammonia (Fig. 2). Biuret is poorly taken up by bacteria (4, 8), but it can be generated in situ from cyanuric acid by cyanuric acid hydrolase (AtzD or TrzD). To test this, along with controls to rule out indigenous activities, the atzD and atzF genes were transformed into E. coli, either individually or together, and the recombinant strains were tested for growth on cyanuric acid (Table 3). The growth of the clone, a proline auxotroph that required proline in the medium, was compared to the wild-type strain that did not contain atzD and atzF. The E. coli clone grew to an OD three- to fourfold higher than controls containing only atzD or atzF. Growth was slow but increased steadily, with the final absorbance reported after 21 days. The level of growth, indicated by an OD of 0.8, was consistent with the idea that the liberation of all three nitrogen atoms in cyanuric acid supported growth, suggesting that the recombinant AtzF catalyzed both biuret and allophanate hydrolysis in vivo. The failure of the atzD clone to grow ruled out the possibility that E. coli contained an endogenous biuret hydrolase activity as this reaction liberates ammonia (Fig. 1). These data suggest that an atzD-atzF or trzD-trzF gene couple may be sufficient to allow slow growth on cyanuric acid and thus provide selective pressure to maintain those enzymes in an environmental bacterium exposed to cyanuric acid as the sole nitrogen source.
TABLE 3.
Growth of E. coli containing the atzD and atzF genes on cyanuric acid as a nitrogen source
| Plasmid | Nitrogen source | Final OD600 |
|---|---|---|
| pET28b+::atzF | 5 mM cyanuric acid | 0.23 |
| pKT230::atzD | 5 mM cyanuric acid | 0.27 |
| pET28b+::atzF, pKT230::atzD | 0.22 | |
| pET28b+::atzF, pKT230::atzD | 15 mM NH4Cl2 | 1.07 |
| pET28b+::atzF, pKT230::atzD | 5 mM cyanuric acid | 0.79 |
DISCUSSION
Bacterial allophanate hydrolases participate in two distinct metabolic processes: (i) urea metabolism, in concert with urea carboxylase, and (ii) the catabolism of s-triazine ring compounds. The catabolism of s-triazine pesticides and the resin intermediate melamine occurs via cyanuric acid as an intermediate. For 20 years, cyanuric acid metabolism was thought to proceed through urea (4, 19). Recently, bacterial metabolism of cyanuric acid was shown to proceed through allophanate, and urea was found not to be an intermediate in this metabolism (3). A significant number of soil bacteria are capable of growing on cyanuric acid (32), suggesting that the involvement of allophanate hydrolases in cyanuric acid metabolism is widespread. Only one such enzyme, AtzF, had been purified previously, and the present study extends our knowledge of this broadly distributed enzyme class. TrzF is principally, or completely, involved in cyanuric acid metabolism in vivo, based on its measured activities and linkage of the trzF gene with the trzD and trzC genes involved in the metabolism of s-triazine compounds. TrzC encodes ammelide hydrolase, and TrzD encodes cyanuric acid hydrolase. These two enzymes catalyze consecutive metabolic reactions and produce biuret (9).
The allophanate hydrolases, the enzyme malonamidase (28) (Table 1), and AtzE (21) work on the structurally analogous substrates shown in Table 2, and they are evolutionarily related members of the amidase signature protein family. TrzF is clearly identified as a member of this family by sequence comparison and time-dependent inhibition by phenyl phosphorodiamidate, as shown in this study. Phenyl phosphorodiamidate has been shown to covalently modify an essential serine residue in the active site of certain members of this superfamily (13, 28). Both allophanate hydrolases, AtzF (27) and TrzF, which was tested here, are highly sensitive to this reagent.
Data derived from hundreds of microbial genome sequencing projects also contribute to the idea that allophanate hydrolases are widespread. It was previously reported that bacteria from diverse taxonomic groups contain homologs to AtzF (27) and to the allophanate hydrolase from Oleomonas sp. (18). Allophanate hydrolase genes that are closely linked with a urea carboxylase gene are typically found on a chromosomal element. The allophanate hydrolase from Pseudomonas sp. strain ADP, involved in cyanuric acid metabolism, is found on plasmid pADP-1 (21). It comprises part of a cyanuric acid catabolic operon where it is flanked by atzD, encoding cyanuric acid hydrolase, and atzE, encoding biuret hydrolase. The allophanate hydrolase TrzF characterized in this study is encoded by a gene also found on plasmid DNA, and the flanking sequences show evidence for a cynanuric acid hydrolase, but nothing resembling the known biuret hydrolase gene, atzE, has been identified.
The functioning of allophanate hydrolase in the catabolism of cyanuric acid, derived from s-triazine herbicides that have only lately been present in the environment, suggests that this may be a more recently acquired function for these enzymes. The plasmid localization of the allophanate hydrolase genes that act in s-triazine metabolism is also consistent with recent recruitment of these genes for this function. To our knowledge, there is no known natural product s-triazine compound. In contrast to these observations, the allophanate hydrolase gene, atzF, in Pseudomonas sp. strain ADP is clustered with the atzD and atzE genes in an operon. The cyanuric acid metabolism in Pseudomonas sp. strain ADP has been shown to be expressed optimally in the presence of cyanuric acid and under general nitrogen starvation conditions (12). In contrast, E. cloacae strain 99 showed similar levels of allophanate hydrolase expression in the presence or absence of cyanuric acid and regardless of the presence of ammonium ion (3). Furthermore, the allophanate hydrolase levels in cell extracts of E. cloacae strain 99 were low under all growth conditions. This suggested that allophanate hydrolase is constitutively expressed at low levels and may be at an earlier stage of evolution than the physiologically controlled cyanuric acid operon observed in Pseudomonas sp. strain ADP. Moreover, Pseudomonas sp. strain ADP has a separate functional biuret hydrolase, AtzE, that is contiguous on plasmid pADP-1 (21). An analogous protein has never been identified in E. cloacae strain 99. The low biuret hydrolase activity for the allophanate hydrolases observed in this study may be relevant to the evolution of the cyanuric acid catabolic pathway in E. cloacae strain 99 and other bacteria.
The observation that homologous enzymes catalyze consecutive reactions and act on structurally analogous substrates is reminiscent of the retrograde model of catabolic pathway evolution originally proposed in 1945 (16). This model has not been shown to explain the genetic composition underlying most metabolic pathways; rather, a patchwork model is more commonly invoked (1, 6). Still, enzyme catalytic promiscuity is increasingly recognized as a key element for evolving new enzymatic activities (2, 7, 22), which can contribute to metabolic pathway evolution. In this context, the observation here that allophanate hydrolase has activity with biuret shows that it can catalyze the two terminal reactions in the cyanuric acid catabolic pathway (Fig. 1 and 2). Since these are the two reactions that liberate ammonia, allophanate hydrolase can act in concert with TrzD or AtzD and allow bacterial growth on cyanuric acid. This was demonstrated here with a recombinant E. coli strain expressing AtzD and AtzF. Control experiments with E. coli containing the atzD gene alone showed that no indigenous biuret hydrolase was present as this would have allowed growth in the absence of atzF, which was not observed. Moreover, biuret hydrolase activity was observed with highly purified TrzF, obtained from a recombinant bacterium thought not to contain an indigenous biuret hydrolase activity. It is interesting to consider the possibility that TrzF contributes to biuret hydrolysis in E. cloacae strain 99, promoting growth on cyanuric acid. Thus, it might be another example of catalytic promiscuity providing a selectable enzyme activity with evolutionary pressure that could act over time to improve activity. Although a biuret hydrolase has yet to be purified to homogeneity, the sequence of AtzE indicates that it is also a member of the amidase signature family.
Acknowledgments
We thank Gil Johnson for the synthesis of allophanate and José Carrascosa for the GroEL GroES construct.
This work was supported in part by U.S. Department of Agriculture grant 2002-35107-12508 and by a grant from the University of Minnesota Biocatalysis Initiative.
REFERENCES
- 1.Babbitt, P. C., and J. A. Gerlt. 1997. Understanding enzyme superfamilies: chemistry as the fundamental determinant in the evolution of new catalytic activities. J. Biol. Chem. 272:30591-30594. [DOI] [PubMed] [Google Scholar]
- 2.Bornsheur, U., and R. J. Kazluaskas. 2004. Catalytic plasticity in biocatalysis: Using old enzymes to form new bonds and follow new pathways. Angew. Chem. Int. Ed. Engl. 43:6032-6040. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, G., N. Shapir, M. J. Sadowsky, and L. P. Wackett. 2005. Allophanate hydrolase, not urease, functions in bacterial cyanuric acid metabolism. Appl. Environ. Microbiol. 71:4437-4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook, A. M. 1987. Biodegradation of s-triazine xenobiotics. FEMS Microbiol. Rev. 46:93-116. [Google Scholar]
- 5.Cook, A. M., P. Beilstein, H. Grossenbacher, and R. Hütter. 1985. Ring cleavage and degradative pathway of cyanuric acid in bacteria. Biochem. J. 231:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copley, S. D. 2002. Evolution of a metabolic pathway for degradation of a toxic xenobiotic: the patchwork approach. Trends Biochem. Sci. 25:261-265. [DOI] [PubMed] [Google Scholar]
- 7.Copley, S. D. 2003. Enzymes with extra talents: moonlighting functions and catalytic promiscuity. Curr. Opin. Chem. Biol. 7:265-272. [DOI] [PubMed] [Google Scholar]
- 8.Eaton, R. W., and J. S. Karns. 1991. Cloning and analysis of s-triazine catabolic genes from Pseudomonas sp. strain NRRLB-12227. J. Bacteriol. 173:1215-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton, R. W., and J. S. Karns. 1991. Cloning and comparison of the DNA encoding ammelide aminohydrolase and cyanuric acid amidohydrolase from three s-triazine-degrading bacterial strains. J. Bacteriol. 173:1363-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton, R. W., and D. W. Ribbons. 1982. Metabolism of dibutylphthalate and phthalate by Micrococcus sp. strain 12B. J. Bacteriol. 151:48-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fruchey, I., N. Shapir, M. J. Sadowsky, and L. P. Wackett. 2003. On the origins of cyanuric acid hydrolase: purification, substrates, and prevalence of AtzD from Pseudomonas sp. strain ADP. Appl. Environ. Microbiol. 69:3653-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Gonzalez, V., F. Govantes, O. Porrua, and E. Santero. 2005. Regulation of the Pseudomonas sp. strain ADP cyanuric acid degradation operon. J. Bacteriol. 187:155-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gopalakrishna, K. N., B. H. Stewart, M. M. Kneen, A. D. Andricopulo, G. L. Kenyon, and M. J. McLeish. 2004. Mandelamide hydrolase from Pseudomonas putida: characterization of a new member of the amidase signature family. Biochemistry 43:7725-7735. [DOI] [PubMed] [Google Scholar]
- 14.Hausinger, R. P. 2004. Metabolic versatility of prokaryotes for urea decomposition. J. Bacteriol. 186:2520-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodson, R. C., S. K. Williams II, and W. R. Davidson, Jr. 1975. Metabolic control of urea catabolism in Chlamydomonas reinhardi and Chlorella pyrenoidosa. J. Bacteriol. 121:1022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horowitz, N. H. 1945. On the evolution of biochemical syntheses. Proc. Natl. Acad. Sci. USA 31:153-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanamori, T., N. Kanou, H. Atomi, and T. Imanake. 2004. Enzymatic characterization of a prokaryotic urea carboxylase. J. Bacteriol. 186:2532-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanamori, T., N. Kanou, S. Kusakabe, H. Atomi, and T. Imanaka. 2005. Allophanate hydrolase of Oleomonas sagaranensis involved in an ATP-dependent degradation pathway specific to urea. FEMS Microbiol. Lett. 245:61-65. [DOI] [PubMed] [Google Scholar]
- 19.Karns, J. S., and R. W. Eaton. 1997. Genes encoding s-triazine degradation are plasmid-borne in Klebsiella pneumonia strain 99. J. Agric. Food Chem. 45:1017-1022. [Google Scholar]
- 20.Koo, H. M., S. O. Choi, H. M. Kim, and Y. S. Kim. 2000. Identification of active-site residues in Bradyrhizobium japonicum molonamidase E2. Biochem. J. 349:501-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez, B., J. Tomkins, L. P. Wackett, R. Wing, and M. J. Sadowsky. 2001. Complete nucleotide sequence and organization of the atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. J. Bacteriol. 183:5684-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien, P. J., and D. Herschlag. 1999. Catalytic promiscuity and the evolution of new enzymatic activities. Chem. Biol. 6:R91-R105. [DOI] [PubMed] [Google Scholar]
- 23.Patricelli, M. P., and B. F. Cravatt. 2000. Clarifying the catalytic roles of conserved residues in the amidase signature family. J. Biol. Chem. 275:19177-19184. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Pérez, J., and J. Gutiérrez. 1995. An arabinose-inducible expression vector, pAR3, compatible with ColE1-derived plasmids. Gene 158:141-142. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Segel, I. H. 1975. Other methods of plotting enzyme kinetics data, p. 208-224. In I. H. Segal (ed.), Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. Wiley-Interscience, New York, N.Y.
- 27.Shapir, N., M. J. Sadowsky, and L. P. Wackett. 2005. Purification and characterization of allophanate hydrolase (AtzF) from Pseudomonas sp. strain ADP. J. Bacteriol. 187:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin, S., T. H. Lee, C. N. Ha, H. M. Koo, S. Y. Kim, H. S. Lee, Y. S. Kim, and B. H. Oh. 2002. Structure of malonamidase E2 reveals a novel Ser-cisSer-Lys catalytic triad in a new serine hydrolase fold that is prevalent in nature. EMBO J. 21:2509-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumrada, R. A., and T. G. Cooper. 1982. Urea carboxylase and allophanate hydrolase are components of a multifunctional protein in yeast. J. Biol. Chem. 257:9119-9127. [PubMed] [Google Scholar]
- 30.Weatherburn, M. W. 1967. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39:791-794. [Google Scholar]
- 31.Whitney, P. A., and T. G. Cooper. 1972. Urea carboxylase and allophanate hydrolase. Two components of adenosine triphosphate:urea amido-lyase in Saccharomyces cerevisiae. J. Biol. Chem. 247:1349-1353. [PubMed] [Google Scholar]
- 32.Zeyer, J., J. Bodmer, and R. Hütter. 1981. Microbial degradation of ammeline. Zentbl. Bakteriol. Hyg. Abt. 1 Orig. C 2:289-298. [Google Scholar]