Abstract
The abundance, identities, and degradation abilities of indigenous polychlorinated biphenyl (PCB)-degrading bacteria associated with five species of mature trees growing naturally in a contaminated site were investigated to identify plants that enhance the microbial PCB degradation potential in soil. Culturable PCB degraders were associated with every plant species examined in both the rhizosphere and root zone, which was defined as the bulk soil in which the plant was rooted. Significantly higher numbers of PCB degraders (2.7- to 56.7-fold-higher means) were detected in the root zones of Austrian pine (Pinus nigra) and goat willow (Salix caprea) than in the root zones of other plants or non-root-containing soil in certain seasons and at certain soil depths. The majority of culturable PCB degraders throughout the site and the majority of culturable PCB degraders associated with plants were identified as members of the genus Rhodococcus by 16S rRNA gene sequence analysis. Other taxa of PCB-degrading bacteria included members of the genera Luteibacter and Williamsia, which have not previously been shown to include PCB degraders. PCB degradation assays revealed that some isolates from the site have broad congener specificities; these isolates included one Rhodococcus strain that exhibited degradation abilities similar to those of Burkholderia xenovorans LB400. Isolates with broad congener specificity were widespread at the site, including in the biostimulated root zone of willow. The apparent association of certain plant species with increased abundance of indigenous PCB degraders, including organisms with outstanding degradation abilities, throughout the root zone supports the notion that biostimulation through rhizoremediation is a promising strategy for enhancing PCB degradation in situ.
Polychlorinated biphenyls (PCBs) are toxic, persistent pollutants of worldwide concern whose cleanup using conventional methods like incineration or relocation to specialized landfills is often prohibitively expensive. An alternative strategy for in situ PCB removal is biodegradation by microorganisms capable of metabolizing PCBs. Although bioaugmentation of soil with degradative bacteria has been largely unsuccessful in achieving significant aerobic PCB degradation in the field (29), efforts to biostimulate indigenous PCB-degrading bacteria have been promising. Analogue enrichment with biphenyl has been shown to increase the numbers of aerobic PCB-degrading bacteria in soil microcosms (12, 51) and to enhance PCB degradation rates in soils (6, 12) and in situ sediments (18). Unfortunately, as a field remedial strategy, addition of biphenyl is problematic due to the low water solubility of biphenyl, the necessity of repeated application, and concerns about biphenyl toxicity. By capitalizing on the innate ability of plants to alter soil microbial community structure, rhizoremediation offers an attractive and affordable alternative means for long-term biostimulation of aerobic PCB degradation in situ.
Rhizostimulation of aromatic pollutant-degrading bacteria is postulated to occur via several different mechanisms (43), including analogue enrichment and/or induction of degradative genes by secondary plant metabolites (11, 45) or by less specific stimulation due to increased availability of simple substrates (sugars, amino acids, organic acids) (22, 36), improved soil aeration, or other processes (43). The analogue enrichment hypothesis is supported by a growing body of evidence indicating that plant compounds such as flavonoids, coumarins, terpenes, and resin acids can function as growth substrates and/or inducers of PCB-degrading bacteria (45). Plant secondary compounds may be released into soil via exudation from living roots (35) or via lysis of dead fine roots as a result of seasonal root turnover (26). Because the quantity and variety of secondary plant compounds vary widely for different plant species (15), it is expected that not all plant species are equally effective at rhizostimulating PCB-degrading bacteria. Thus, screening studies designed to identify plant species that are most capable of increasing the PCB degradation potential of the soil microbial community would potentially benefit the development of successful rhizoremediation technology. However, conventional screening studies with young plants grown in pots under greenhouse conditions may not reflect the true rhizosphere features of mature trees, which affect large volumes of soil through many years of fine root exploration and turnover (50). An alternative method is to investigate microbial populations associated with the naturally established vegetation at a contaminated site. This forensic field approach provides a view of the long-term influence of a diverse array of plant species, including mature trees, on the degradation potential of the soil microbial community and in some cases on contaminant disappearance (25, 34).
The success of biostimulation approaches, including rhizoremediation, lies not only in successfully increasing the size of the degradative microbial population but also in the specific catabolic abilities of the indigenous bacteria present. This is particularly relevant for PCB degradation, since even closely related PCB-degrading bacteria vary widely in the ability to aerobically catabolize the array of congeners that comprise commercial PCB mixtures (3, 13). Thus, when the PCB degradation potential of a microbial community is assessed, both the population size and the congener specificities of individual organisms are important factors.
In this study, we investigated the abundance, identities, and degradation abilities of indigenous PCB-degrading bacteria associated with different species of mature trees that naturally colonized a PCB-contaminated site in the Czech Republic. In this work we aimed to identify plants capable of increasing the PCB degradation potential of aerobic microorganisms in the root zone or the rhizosphere and to understand the composition and catabolic potential of the microbial community in a long-term rhizoremediation setting. To our knowledge, this is the first report of indigenous PCB-degrading microbial populations associated with mature perennial plants growing in PCB-contaminated soil.
MATERIALS AND METHODS
Site description.
The study site was located on the grounds of the Colorlak paint production plant in Staré Město near Uherské Hradiště in South Moravia, Czech Republic. Empty barrels that had contained the commercial PCB mixture Delor 106 (analogous to Aroclor 1260) were stored in the area from the 1950s to 1988, during which time inadvertent spillage of residual PCBs occurred, resulting in an approximately 65-m2 contaminated area.
The site was naturally vegetated with 25 different plant species, including five tree species, growing in the presence of soil PCB concentrations that ranged from 8 to 500 mg/kg. All of the established trees at the site were studied. These trees included a 24-year-old Austrian pine (Pinus nigra), a 14-year-old ash (Fraxinus excelsior), two weeping birch trees (Betula pendula) that were 11 and 13 years old growing immediately adjacent to each other and sharing the same root zone, a 5-year-old goat willow (Salix caprea), and a 3-year-old black locust (Robinia pseudoacacia). The PCB concentrations beneath the trees at a depth of 20 to 40 cm were as follows: Austrian pine, 8 to 15 mg/kg; ash, 15 to 16 mg/kg; weeping birch, 153 mg/kg; black locust, 13 to 153 mg/kg; and willow, 471 to 500 mg/kg. The ground was covered with a variety of grasses (Agropyron repens, Agrostis alba [Agrostis stolonifera], Calamogrostis epigeois, Lolium perenne) and forbs (predominantly Achillea millefolium). The PCB concentration in non-root-containing control soil collected at a depth of 20 to 40 cm beneath grasses and forbs was 50 mg/kg. The PCB concentration in the soil beneath a control birch tree located approximately 50 m from the contaminated area was 0.365 mg/kg. A complete list of the plant species and a map of the vegetation with soil contamination levels have been published previously (27).
Soil nutrient and texture analyses were performed with hexane-washed soil samples from seven locations across the site associated with each different tree species, non-root-containing soil, and the control birch tree (depth, 20 to 40 cm). Soil samples were analyzed to determine the pH (1:1), the nitrate N, P, and K contents (Mehlich 3 method), and the soil texture (hydrometer) by the Oklahoma Cooperative Extension Service Soil, Water and Forage Analytical Laboratory (Stillwater, OK). The Ca and Mg contents and the oxidizable carbon content (Mehlich 3 method) were determined by the Czech Agricultural University Department of Agricultural Chemistry (Prague, Czech Republic). The soil pH ranged from 7.5 to 8.0 across the site, and the average pH was 7.7. The nitrate nitrogen content ranged from 2 to 24 mg/kg, and the average was 8 mg/kg. The nutrient contents (averages ± standard deviations) were as follows: 40 ± 14 mg/kg P, 256 ± 159 mg/kg K, 4,436 ± 614 mg/kg Ca, and 223 ± 60 mg/kg Mg. The soil texture was 61% ± 14% sand, 16% ± 8% silt, and 22% ± 6% clay, and the oxidizable carbon content was 1.7% ± 0.6%.
Sampling methods.
Root zone and rhizosphere samples were collected beneath each tree and the grass-forb mixture on 22 June, 23 and 24 August, and 21 and 22 November 1999 and on 28 May 2000. On each date, one large hole (diameter, >0.5 m; depth, 0.6 m) was excavated beneath each tree within 1.5 m of the trunk and in the grass-forb area. Samples were collected at three depths (0 to 20 cm, 20 to 40 cm, and 40 to 60 cm) using an ethanol-disinfected shovel. For rhizosphere samples, fine roots (diameter, approximately 1 mm) were carefully collected from each depth, loose soil was removed by shaking, and then the roots with tightly bound rhizosphere soil were stored in plastic bags. Root zone soil, which was defined as the bulk soil beneath the plant, was collected from each of the three depths and homogenized in separate mounds, and then approximately 50 g soil from each depth was placed in plastic bags for storage prior to microbial analyses and approximately 20 g was stored in aluminum foil for determination of the PCB concentration. Samples were kept on ice during transport to the laboratory and were stored at 4°C for 1 to 12 months prior to analysis. Although multiple excavations beneath each tree on each date would have been preferable, single large-diameter excavations were necessary to collect adequate quantities of fine root material at all depths while we worked around shallow buried electrical cables and ensured that undisturbed root zone soil was available for sampling in multiple seasons.
Several non-root-containing control samples were collected at depths of 20 to 40 and 40 to 60 cm from areas inhabited by grasses, whose roots extended only to a depth of approximately 15 cm. A noncontaminated control sample was collected from beneath a large birch tree growing elsewhere on the Colorlak factory grounds approximately 50 m from the contaminated area. Samples could not be obtained beneath birch or locust trees in August, because the soil was too dry and hardened to permit manual excavation. In May 2000, samples were collected only from the pine, willow, and birch soil because of time restrictions related to site access. Samples from a depth of 40 to 60 cm could not be obtained beneath the pine tree and in some grassy areas due to the presence of buried electrical cables.
Microbial enumeration.
The numbers of aerobic PCB degraders and total heterotrophs in soil and rhizosphere samples were determined using direct plate count methods modified from the method of Kastner et al. (24). Microorganisms in subsamples of soil (1.0 g) or fine roots with attached rhizosphere soil (0.1 g) were suspended by shaking the subsamples or roots in a 1% (wt/vol) sodium pyrophosphate (Na4P2O7) solution for 1 h in the presence of glass beads (only for soil). Following standing for 30 min, serial dilutions of the suspension were prepared in basal mineral (BM) liquid (2.13 g/liter Na2HPO4, 1.3 g/liter KH2PO4, 0.5 g/liter NH4Cl, 0.2 g/liter MgSO4 · 7H2O) and spread plated in duplicate onto agar plates. Total culturable heterotrophs were grown on 1/8-strength plate count agar (2.94 g plate count agar [Difco, Detroit, MI] and 13.12 g Bacto agar [Difco, Detroit, MI] in 1 liter of BM liquid) for 1 week at room temperature (21 to 25°C). Biphenyl utilizers were cultivated on basal mineral agar (15 g/liter Noble agar [Difco, Detroit, MI] in BM liquid) with biphenyl vapor provided as the sole carbon source by sprinkling crystals in the lid of an inverted petri dish. Following growth for 3 weeks at 21 to 25°C, biphenyl-utilizing colonies were further screened for halogenated biphenyl-degrading ability by subjecting them to a clearing zone assay with 4-bromobiphenyl (47). Colonies that formed zones of clearing within 1 to 2 weeks were enumerated. Numbers of microbes were calculated based on soil dry weight and root dry weight, as determined by oven drying separate aliquots of the same soil sample and by drying roots recovered following microbial suspension, respectively. For each sample, microbial suspension and plate counting were performed with two to eight replicate subsamples. For all sampling dates combined, the total number of replicate samples plated for each plant species on each medium ranged from 30 to 61 for root zone soil and from 10 to 30 for rhizosphere samples.
Phylogenetic identification of PCB-degrading bacteria.
For each plant species, several agar plates representing different sampling dates and dilutions were examined together, and several of each morphological type of 4-bromobiphenyl-degrading colonies were selected for isolation. Bacteria were isolated by repeated streaking on 1/8-strength plate count agar with biphenyl vapor provided, and then degradation abilities were confirmed by the clearing zone assay. Isolates were Gram stained, and gram-positive bacteria were subjected to acid-fast staining. Isolates from all plant species were pooled and then assigned to separate groups based on colony and cell morphology. One to seven representatives of each morphological group were selected for taxonomic identification using 16S rRNA gene sequence analysis. Altogether, sequence analyses were performed for 26 isolates.
DNA was extracted from isolates using the FastDNA SPIN kit for soil (Bio 101 Systems, Qbiogene, Carlsbad, CA). 16S rRNA genes were then PCR amplified using primers 27F and 1522R (21) to generate an approximately 1,495-bp product. The presence, size, and purity of amplicons were confirmed by agarose gel electrophoresis, and then the remaining PCR product was purified with Wizard PCR Preps (Promega, Madison, Wis.). Cycle sequencing was performed directly with the purified PCR product using an ABI Prism Big Dye Terminator Ready Reaction kit (Applied Biosystems, Foster City, Calif.), forward primers 27F and 530F, reverse primer 1522R, and either reverse primer 1100R for gram-positive bacteria or reverse primer 1069R for gram-negative isolates (21). The 20-μl cycle sequencing products were purified by ethanol precipitation and then were analyzed with an ABI Prism 377 automated sequencer (PE Biosystems, Foster City, Calif.). Electropherograms were assembled and edited with the Sequencher 3.1 software (GeneCodes, Ann Arbor, Mich.). Contiguous 16S rRNA gene sequences were subjected to sequence match searches with the Ribosomal Database Project II (RDP II) database (7) to determine the taxonomic identities of the isolates. Levels of similarity between isolates and database sequences were determined by using the Similarity Matrix program in RDP I.
Characterization of PCB degradation abilities.
The aerobic PCB degradation abilities of the isolates were characterized by performing a 24-h resting cell assay in the presence of two different defined mixtures of PCB congeners, using the method of Bedard et al. (4). The well-characterized PCB-degrading control strains Burkholderia xenovorans LB400 (4, 16) and Rhodococcus sp. strain RHA1 (41) were assayed with isolates for comparison. Most cells were grown in liquid PAS medium with biphenyl crystals as the sole carbon source (4); the exceptions were isolates 46 and 70, which did not grow in PAS medium and instead were grown in 1/8-strength plate count broth (0.63 g/liter tryptone, 0.31 g/liter yeast extract, 0.13 g/liter dextrose) in the presence of biphenyl crystals. Live cells and heat-inactivated cells (70°C for 20 min) were assayed in duplicate. After incubation with shaking at room temperature (21 to 25°C) for 24 h, PCBs were recovered by hexane extraction (4). The disappearance of PCBs was determined by gas chromatography using a Varian 3300 gas chromatograph equipped with an electron capture detector and a glass column (6 ft [1.83 m] by 4 mm) packed with 1.5% SP-2250 and 1.95% SP-2401 on 100/120 Supelcoport (Supelco, Inc., Bellefonte, PA). Samples were run isothermally at 185°C with injector and detector temperatures of 250°C and 300°C, respectively, using nitrogen as the carrier gas at a flow rate of approximately 60 ml/min. One microliter of each hexane extract was analyzed in duplicate, peaks were normalized to the internal standard peak (2,4,6,2′,4′-pentachlorobiphenyl), and the values were averaged and compared to the values for the heat-inactivated controls in order to calculate the percent disappearance of each congener.
Statistical analyses.
Statistical analyses of microbial counts were performed using SPSS software (SPSS, Inc., Chicago, IL). The distribution of each data set was first tested for normality using the one-sample Kolmogorov-Smirnov test, which indicates whether data sets are distributed normally (P > 0.05) or nonparametrically (P < 0.05). Normally distributed data were analyzed using analysis of variance (ANOVA) with Tukey's post hoc test or Pearson's product-moment correlation test. Nonparametric data sets were analyzed using the Kruskal-Wallis test for several independent samples, followed by the Mann-Whitney U test to determine pairwise differences among groups; correlations were tested using the Spearman's rank-order correlation. These tests are the nonparametric equivalents of ANOVA, the paired t test, and Pearson's product-moment correlation, respectively, and are considered to be more conservative (9). The significance level for all comparison and correlation tests was set at a P value of <0.05 unless indicated otherwise.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences determined in this study have been deposited in the GenBank database under accession numbers AY785730 through AY785751.
RESULTS
Root zone microbial population size.
PCB degraders were present in soil throughout the contaminated site and in association with every plant species examined.
Root zone (bulk) soil at a depth of 0 to 20 cm was colonized by the roots of grasses and forbs throughout the site, which coexisted with tree roots when they were present, whereas deeper soil (20 to 40 cm and 40 to 60 cm) was influenced solely by tree roots. The numbers of heterotrophs and PCB degraders at a depth of 20 to 40 cm did not differ significantly among tree species in June or August; however, species-dependent differences were observed in November and the following May, when there were significantly higher numbers of PCB metabolizers in the pine root zone than in the root zones of all other trees (P < 0.02, as determined by ANOVA) (Fig. 1a and Table 1).
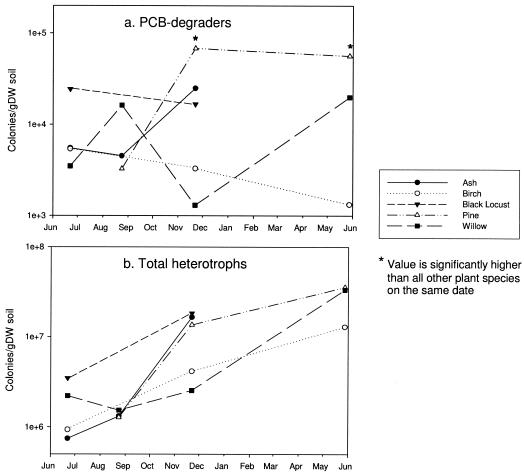
FIG. 1.
Seasonal trends in the mean numbers of PCB degraders (a) and total heterotrophs (b) at a depth of 20 to 40 cm. The mean values are based on four to six replicate plate counts for each plant species on each date. gDW, gram (dry weight).
TABLE 1.
Numbers of organisms in the root zone in relation to plant species and soil depth (November)
| Microorganisms | Plant species | No. of subsamples analyzed
|
Mean no. of colonies/g (dry wt) of soil (SD)
|
||||
|---|---|---|---|---|---|---|---|
| 0-20 cm | 20-40 cm | 40-60 cm | 0-20 cma | 20-40 cm | 40-60 cm | ||
| PCB degraders (103) | Ash | 4 | 4 | 4 | 7.3 (5.2) Aab | 25.0 (27.0) Aa | 27.0 (43.0) ABa |
| Birch | 2 | 4 | 0 | 6.7 (0.01) Aa | 3.3 (2.0) Aa | NDc | |
| Black locust | 4 | 4 | 4 | 8.1 (5.2) Aa | 17.0 (15.0) Aa | 2.6 (1.0) ABa | |
| Pine | 2 | 4 | 0 | 8.0 (0.3) Aa | 68.0 (27.0) Bb | ND | |
| Willow | 6 | 4 | 5 | 2.2 (1.8) Aa | 1.3 (0.8) Aab | 120.0 (96.0) Ab | |
| Grass or no roots | 8 | 4 | 8 | 3.3 (3.5) Aa | 1.2 (1.2) Aa | 3.9 (4.9) Ba | |
| Total heterotrophs (106) | Ash | 4 | 4 | 4 | 5.7 (5.1) DEd | 17.0 (7.8) Dd | 8.9 (5.0) DEd |
| Birch | 2 | 4 | 0 | 1.4 (0.1) DEd | 4.1 (3.6) EFd | ND | |
| Black locust | 4 | 4 | 4 | 9.8 (6.2) DEd | 18.0 (1.6) De | 11.0 (2.7) Dde | |
| Pine | 2 | 4 | 0 | 12.0 (12.0) Dd | 14.0 (7.2) DFd | ND | |
| Willow | 6 | 4 | 5 | 3.0 (2.2) DEd | 2.5 (1.2) Ed | 5.4 (2.9) Ed | |
| Grass or no rootsd | 8 | 4 | 8 | 2.5 (1.9) Ed | 1.5 (0.4) Ede | 0.5 (0.2) Fe | |
Beneath all trees there were grass and forb roots at a depth of 0 to 20 cm.
Uppercase letters indicate significant differences in the same column, and lowercase letters indicate differences in the same row (P < 0.05, as determined by ANOVA).
ND, not determined because a sample was not available.
Grass roots were limited to the upper 0 to 20 cm of soil; hence, the soil obtained from 20 to 40 and 40 to 60 cm was considered non-root-containing control soil.
The November samples were selected for a detailed examination of microbial numbers in relation to plant species and depth (Table 1). Differences in the numbers of PCB metabolizers between plant species were not detected at a depth of 0 to 20 cm, where the roots of individual trees occupied the soil together with grass and forb roots. However, at a depth of 20 to 40 cm, there were significantly higher numbers of PCB metabolizers in the pine root zone than in the root zones of all other trees and non-root-containing soil; 2.7- to 56.7-fold differences in mean numbers were found. Overall, the highest mean number of PCB metabolizers (1.2 × 105 colonies/g) was found in the willow root zone at a depth of 40 to 60 cm. This value was significantly higher (30.7-fold higher) than the value for non-root-containing soil at the same depth and was statistically similar to the high value for the soil beneath the pine tree at a depth of 20 to 40 cm (P = 0.582, as determined by a t test for independent samples).
The numbers of total culturable heterotrophs declined significantly with depth beneath grasses, whose roots occupied only the upper 15 cm of soil, a trend that was not observed beneath trees, perhaps due to the stimulatory effect of roots (Table 1). The numbers of PCB degraders did not decline with depth beneath any tree species and actually increased beneath the pine and willow trees, which could be attributed to the fact that at greater depths the root zone soil was affected solely by these species and not by grasses and forbs.
The seasonal fluctuations in the number of microbes beneath each tree at a depth of 20 to 40 cm are shown in Fig. 1. The number of PCB degraders in the pine root zone increased significantly between August and November and remained high the following May (Fig. 1a). Over the course of the experiment, the number of degraders decreased significantly beneath the birch trees (P < 0.05, as determined by ANOVA), and no significant seasonal differences were detected beneath the other plant species. The seasonal patterns for the numbers of PCB degraders did not parallel the seasonal patterns for the numbers of total heterotrophs, which increased approximately 10-fold during the study period beneath all plant species examined (Fig. 1b), a phenomenon that was potentially attributable to seasonal differences in soil moisture content at the site, as described below (Fig. 2).
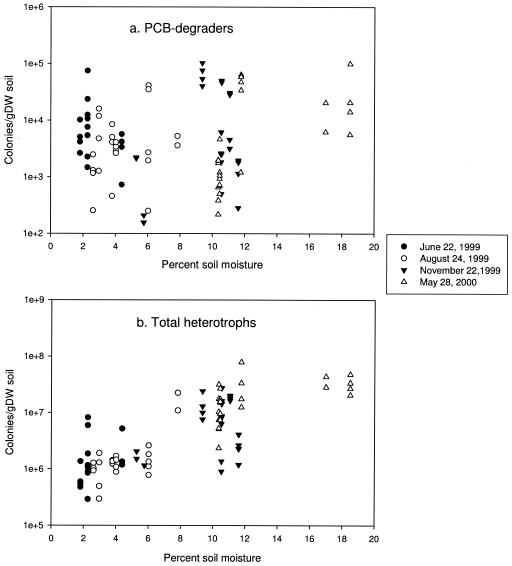
FIG. 2.
Relationship between soil moisture and numbers of PCB degraders (a) and total heterotrophs (b) (n = 88). gDW, gram (dry weight).
At 20 to 40 cm beneath different plant species, the average percentage of members of the total heterotrophic community that were PCB metabolizers ranged from 0.02% (birch, May 2000) to 1.4% (black locust, June 1999). PCB degraders in the pine root zone comprised 0.59% and 0.23% of the total culturable community in November and May, respectively. In the deep root zone (depth, 40 to 60 cm) of the willow tree in November, where the highest numbers of PCB degraders were detected, these organisms comprised 2.11% of the culturable community.
Influence of abiotic factors on soil microbial population size.
The influence of soil moisture content and the influence of PCB concentration on microbial numbers were evaluated statistically in order to distinguish plant-mediated effects from the effects of abiotic heterogeneity at the site and to investigate seasonal fluctuations in populations. Additionally, the possibility that sample storage time introduced artifacts was investigated, and no correlation was found between the number of heterotrophic or PCB degraders and sample storage time ranging from 1 to 12 months, the time frame in which all samples were analyzed.
The median soil moisture contents on different sampling dates were 2.3% in June 1999, 3.8% in August 1999, 10.5% in November 1999, and 10.4% in May 2000, and the soil moisture content was significantly influenced by the sampling date (P = 8 × 10−15, as determined by the Kruskal-Wallis test). There was not a significant correlation between soil moisture content and the number of PCB metabolizers (P = 0.265; Spearman's rho = 0.115; n = 96) (Fig. 2a). Alternatively, the number of total heterotrophic bacteria correlated significantly and positively with the soil moisture content (P = 0.000001; Spearman's rho = 0.766; n = 96) (Fig. 2b). The sampling date significantly influenced the total heterotroph population size (P = 0.0000001, as determined by the Kruskal-Wallis test); the total numbers significantly increased from the dry summer months (June and August 1999) to the wetter fall and spring months (November 1999 and May 2000) (P < 0.0005, as determined by the Mann-Whitney U test) (Fig. 2b). A temporal difference in populations was not observed for PCB degraders (P = 0.587, as determined by the Kruskal-Wallis test) when data from across the site were analyzed collectively (Fig. 2a).
There was no correlation between PCB concentration (see Materials and Methods) and the number of PCB metabolizers at the 95% confidence level; however, a slightly negative correlation was significant at the 90% confidence level (P = 0.078; Spearman's rho = −0.254; n = 49), and high levels of contamination were associated with lower numbers of PCB degraders (data not shown). The number of total heterotrophs did not correlate significantly with soil PCB concentration (P = 0.219; Spearman's rho = −0.179; n = 49). Likewise, the soil from the birch tree growing in uncontaminated soil (<1 mg PCBs/kg soil) contained numbers of total heterotrophs (median, 5.59 × 106 colonies/g [dry weight]) that were statistically similar to the numbers obtained for the birch tree growing in soil with 153 mg PCBs/kg soil (median, 1.02 × 107 colonies/g [dry weight]) (P = 0.077, as determined by the Kruskal-Wallis test). In contrast, the numbers of PCB degraders in the birch root zone were significantly higher in uncontaminated soil (5.56 × 103 colonies/g [dry weight] soil) than in contaminated soil (1.06 × 103 colonies/g [dry weight] soil) (P = 0.001, as determined by the Kruskal-Wallis test). Several factors may have contributed to this difference; the most notable was the fact that the uncontaminated soil had a very different composition and contained more organic matter than the soil in the contaminated area.
Rhizosphere microbial population size.
PCB-degrading microorganisms were detected in the rhizosphere of each plant species examined, which was defined as the soil that tightly adhered to the root following shaking. The abundance in relation to plant species and depth in November is shown in Table 2. Although the differences are not significant, the grass rhizosphere contained 30- to 1,600-fold more PCB degraders than the rhizospheres of other plants contained, which may have been attributable to the fact that the very small grass root diameter resulted in a surface area per gram (dry weight) that was greater than that of the tree roots. The pine rhizosphere contained a significantly higher number of PCB degraders than black locust rhizosphere contained at a depth of 0 to 20 cm. At a depth of 40 to 60 cm, the willow rhizosphere possessed higher numbers of PCB metabolizers than the ash or black locust rhizosphere contained. When the rhizospheres of pine (depth, 20 to 40 cm) and willow (depth, 40 to 60 cm) were compared, the numbers of PCB metabolizers were found to be statistically similar (P = 0.32, as determined by an independent sample t test,).
TABLE 2.
Numbers of organisms in the rhizosphere in relation to plant species and soil depth (November)
| Microorganisms | Plant species | No. of subsamples
|
Mean no. of colonies/g (dry wt) of roots (SD)
|
||||
|---|---|---|---|---|---|---|---|
| 0-20 cm | 20-40 cm | 40-60 cm | 0-20 cm | 20-40 cm | 40-60 cm | ||
| PCB degraders (106) | Ash | 4 | 6 | 4 | 1.6 (0.9) Aaa | 1.0 (0.9) ABa | 0.3 (0.2) Aa |
| Birch | 0 | 4 | 0 | NDb | 2.0 (1.5) AB | ND | |
| Black locust | 4 | 3 | 4 | 5.2 (5.1) Aa | 0.1 (0.1) Aa | 0.7 (0.4) Aa | |
| Pine | 6 | 6 | 0 | 2.4 (1.8) Aa | 3.0 (1.8) Ba | ND | |
| Willow | 4 | 4 | 3 | 0.95 (0.9) Aa | 1.4 (0.3) ABa | 5.3 (3.1) Bb | |
| Grass | 8 | 0 | 0 | 160.0 (210.0) A | ND | ND | |
| Total heterotrophs (109) | Ash | 4 | 6 | 4 | 2.8 (1.3) Dd | 2.6 (2.1) DEd | 4.0 (1.3) Dd |
| Birch | 0 | 4 | 0 | ND | 1.0 (0.5) D | ND | |
| Black locust | 4 | 3 | 4 | 8.6 (7.2) DEd | 0.4 (0.3) Dd | 1.4 (0.6) Ed | |
| Pine | 6 | 6 | 0 | 3.1 (3.5) Dd | 5.8 (3.1) Ee | ND | |
| Willow | 4 | 4 | 3 | 2.2 (1.3) Dd | 2.8 (1.2) DEde | 2.9 (0.3) DEe | |
| Grass | 8 | 0 | 0 | 17.0 (7.5) E | ND | ND | |
Uppercase letters indicate significant differences in the same column, and lowercase letters indicate differences in the same row (P < 0.05, as determined by ANOVA).
ND, not determined because a sample was not available.
The numbers of total culturable heterotrophs were significantly higher in the grass rhizosphere than in the ash, willow, and pine rhizospheres, which again may have been due to a surface area per gram that was greater for grass roots than for tree roots. At a depth of 20 to 40 cm, the pine rhizosphere contained the highest number of total heterotrophs, and the number was significantly higher than the number in the birch or black locust rhizosphere. The numbers of PCB metabolizers correlated significantly and positively with the numbers of total heterotrophs in rhizosphere samples at a depth of 20 to 40 cm (P = 0.00005; Pearson's correlation coefficient = 0.744; n = 23), indicating that the numbers of PCB degraders in the rhizosphere closely paralleled the numbers of total heterotrophs.
Identities of PCB-metabolizing bacteria.
Colonies were selected for isolation from agar plates to represent the range of morphotypes associated with each plant species. A total of 163 aerobic PCB-metabolizing bacteria were isolated from root zone soil samples, and 80 bacteria were isolated from the rhizospheres associated with six plant species. The majority of PCB-metabolizing bacteria isolated from both types of samples were gram positive (93% of the root zone isolates and 100% of the rhizosphere isolates). Colonies with gram-positive morphotypes were the most abundant PCB degraders observed on agar plates from each plant species, comprising approximately >90% of the root zone colonies and 100% of the rhizosphere colonies.
Isolates were assigned to 19 groups based on their colony and cell morphologies; these groups included 15 groups of gram-positive bacteria and 4 groups of gram-negative bacteria. The majority of gram-positive organisms were acid-fast cocci that grew in chains, while acid-fast cocci that grew in clusters (isolate OUCZ22) and non-acid-fast cocci that grew in large clusters (isolates OUCZ45 and OUCZ212) were also isolated. The gram-negative isolates were all very short rods that occurred either in chains (OUCZ24) or in pairs (OUCZ52A, OUCZ70, and OUCZ118). One gram-variable long rod was also isolated (OUCZ63). Many isolates released a bright yellow compound during growth in the presence of biphenyl vapor; this compound presumably was the biphenyl ring cleavage product 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid (33).
Twenty-six isolates were selected for 16S rRNA sequence analysis; these isolates included one isolate of each of the 19 morphotypes plus additional representatives of large groups. Twenty-two different 16S rRNA gene sequences were obtained and were subjected to Sequence Match searches of the RDP II database (Table 3). All isolates exhibited 99 to 100% sequence homology with their nearest matches. The cell morphologies, as well as the Gram and acid-fast staining properties, of the isolates were consistent with known characteristics of each genus (20, 23).
TABLE 3.
Identification of PCB-metabolizing bacteria isolated from various plant species by 16S rRNA gene sequence analysis using RDP II
| Isolates
|
Nearest sequence match in RDP II
|
Association with plant speciesa
|
||||||
|---|---|---|---|---|---|---|---|---|
| Designation | GenBank accession no. | Strain or species | GenBank accession no. | Reference | No. of base pairs compared | % Similarity | Root zone | Rhizosphere |
| Gram-positive High-G+C-content bacteria | ||||||||
| Arthrobacter subdivision | ||||||||
| OUCZ22 | AY785732 | Arthrobacter oxidans type strain | X83408 | 24 | 1,275 | 99.9 | L | |
| OUCZ46 | AY785738 | Microbacterium oxydans type strain | Y17227 | 43 | 1,204 | 100.0 | A, B | B, G, P |
| OUCZ212 | AY785751 | Microbacterium sp. strain VKM Ac-2016 | AB042081 | Unpublished | 740 | 100.0 | P | |
| Mycobacterium subdivision | ||||||||
| OUCZ100 | AY785746 | Rhodococcus erythreus type strain | X79289 | 39 | 1,296 | 99.8 | A, (B), (G), L | (A), (B), (G), L |
| OUCZ108 | AY785747 | Rhodococcus erythreus type strain | X79289 | 39 | 1,296 | 99.8 | A, (B), (G), L | (A), (B), (G), L |
| OUCZ211 | AY785750 | Rhodococcus erythropolis | AF532870 | 49 | 1,296 | 100.0 | L | |
| OUCZ20 | AY785731 | Rhodococcus erythropolis isolate EK5 | AJ237967 | 22 | 1,257 | 100.0 | (A), L, (W) | |
| OUCZ91B | AY785745 | Rhodococcus ruber | AF350248 | Unpublished | 1,320 | 100.0 | B, G, W | |
| OUCZ16 | AY785730 | Rhodococcus sp. strain IFO16253 | AB023374 | Unpublished | 1,274 | 99.3 | A | |
| OUCZ35 | AY785735 | Rhodococcus sp. strain 871-AN040 | AF420421 | 7 | 1,275 | 99.9 | P | |
| OUCZ26 | AY785734 | Rhodococcus sp. strain 871-AN040 | AF420421 | 7 | 1,267 | 100.0 | A | |
| OUCZ44 | AY785736 | Rhodococcus sp. strain 871-AN040 | AF420421 | 7 | 1,358 | 100.0 | A, (L), W | A, (B), G, W |
| OUCZ58 | AY785741 | Rhodococcus sp. strain 871-AN040 | AF420421 | 7 | 1,383 | 100.0 | A, (L), W | A, (B), (G), W |
| OUCZ204 | AY785749 | Rhodococcus sp. strain 871-AN040 | AF420421 | 7 | 1,374 | 99.9 | A | |
| OUCZ48 | AY785739 | Rhodococcus wratislaviensis type strain | Z37138 | Unpublished | 1,438 | 100.0 | A, B, G, L, NR, P, W | P |
| OUCZ59 | AY785742 | Rhodococcus wratislaviensis type strain | Z37138 | Unpublished | 1,434 | 99.9 | A | |
| OUCZ45 | AY785737 | Williamsia murale type strain | Y17384 | 21 | 1,443 | 99.0 | G | |
| Low-G+C-content bacterium | ||||||||
| OUCZ63 | AY785743 | Bacillus sp. strain LMG 20240 | AJ316310 | 16 | 1,444 | 100.0 | G | |
| Gram-negative Gammaproteobacteria | ||||||||
| OUCZ70 | AY785744 | Luteibacter rhizovicina type strain LJ96T | AJ580498 | Unpublished | 1,357 | 99.0 | W | |
| OUCZ118 | AY785748 | Pseudomonas fluorescens strain Biotype F | AJ308306 | Unpublished | 1,354 | 99.9 | P | |
| OUCZ24 | AY785733 | Pseudomonas frederiksbergensis strain JAJ28 | AJ249382 | 1 | 1,258 | 100.0 | L, W | L |
| OUCZ52A | AY785740 | Pseudomonas sp. strain SMCC B0361 | AF500621 | 51 | 1,342 | 99.9 | G, W | |
The data indicate that a bacterium with same the 16S rRNA gene sequence was isolated from the root zone or rhizosphere of other plant species at the site and sequenced. A, ash; B, birch; G, Grass-forb mixture; L, Black locust; NR, non-root-containing soil; P, pine; W, willow. Parentheses indicate that a bacterium with the same cell and colony morphology was isolated but not sequenced; thus, the identity is tentative. Underlining indicates the isolation source.
The association of PCB-degrading bacterial morphotypes with different plant species is shown in Table 3. PCB-degrading rhodococci were ubiquitous and were isolated from the root zones of every plant investigated, as well as from non-root-containing soil. OUCZ48 (nearest match, Rhodococcus wratislaviensis) represented the most frequently isolated PCB-metabolizing morphotype and was the only strain that was isolated from the root zone of all plants. Gram-negative PCB-degrading morphotypes were isolated only from the root zones of grass, locust, pine, and willow. The results for the association of microorganisms with plants should be considered conservative since certain bacteria may have been present beneath some plants but were not isolated or identified because of financial and time constraints.
PCB degradation abilities.
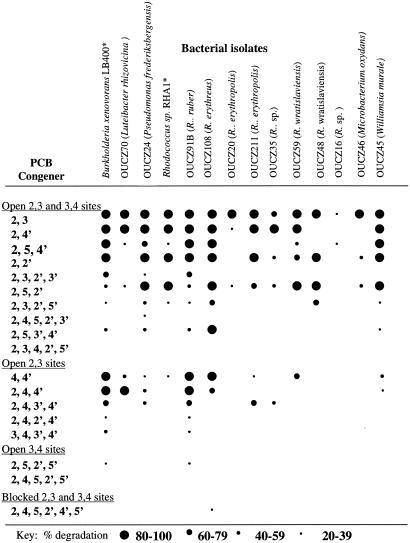
The aerobic PCB degradation abilities of 12 isolates and two well-characterized reference strains, B. xenovorans LB400 and Rhodococcus sp. strain RHA1, are shown in Fig. 3. All isolates were capable of degrading some congeners with both the 2,3 and 3,4 sites open. Many of the isolates displayed 2,3-dioxygenase activity, as shown by their metabolism of congeners with open 2,3 sites and their inability to metabolize congeners with only the 3,4 sites open. One isolate, OUCZ91B (100% match with Rhodococcus ruber), exhibited both 2,3 and 3,4-dioxygenase activities almost exactly like those of the reference strain B. xenovorans LB400 (3). Following the degradation assay, Gram staining confirmed that OUCZ91B was a pure gram-positive culture and not contaminated with LB400. Isolate OUCZ108 (nearest match, Rhodococcus erythreus) was capable of metabolizing the highly recalcitrant congener 2,4,5,2′,4′,5′-chlorobiphenyl, in which both the 2,3 and 3,4 sites are blocked. This congener is degraded only by exceptional strains, including LB400, which has been reported to catalyze 40 to 59% disappearance of this congener (4) but in our hands degraded only up to 7% of it. Isolates OUCZ48 and OUCZ59 exhibited different PCB degradation abilities despite the fact that they were very closely related rhodococci and there was only one base pair difference in the 16S rRNA gene sequences analyzed. Overall, the isolate with the broadest congener specificity and most extensive degradation as determined by the 24-h resting cell assay was OUCZ91B (100% match with R. ruber), whose degradative properties surpassed the degradative properties of Rhodococcus sp. strain RHA1 in our experiments and closely resembled the activity of LB400 with the mixtures of PCBs examined.
FIG. 3.
PCB degradation abilities of bacteria isolated from the root zones or rhizospheres of plants growing in PCB-contaminated soil. Species names in parentheses indicate the nearest matches in the RDP II database. The previously characterized strains B. xenovorans LB400 and Rhodococcus sp. strain RHA1 are included for reference.
The degradative abilities of several isolates, including the outstanding degrader OUCZ91B, were reduced or completely lost following storage at either −20°C in 16% glycerol or at 4°C on slants prepared from 1/8-strength plate count agar with biphenyl vapor provided. Loss of PCB degradation activity during cultivation and storage has been observed previously for other PCB-degrading bacteria (28). In some cases, the loss of activity occurred before PCB degradation assays could be conducted, and thus not all representative strains were assayed.
DISCUSSION
The findings of this study strongly suggest that certain tree species are associated with increased numbers of PCB-metabolizing bacteria in the root zone, including organisms with broad congener specificities, following long-term growth at a contaminated site. Austrian pine (P. nigra) and willow (S. caprea) trees were identified as rhizoremediation candidates because of their association with increased numbers of PCB degraders in the root zone at certain times of the year compared with the numbers found for three other tree species and non-root-containing soil. The culturable PCB degraders throughout the site and in association with all plants were predominantly rhodococci, and the degradation abilities exhibited by isolates from the site revealed that the indigenous microbial community had strong catabolic potential for PCB degradation. This work was the first investigation of plant-associated populations of PCB-degrading microorganisms and provided a view of the long-term influence of mature trees on degradative populations under actual field conditions.
Pine and willow trees were found to positively affect the size of the PCB-degrading populations in the root zone, which was defined as the large volume of bulk soil in which the plants were rooted. These findings are consistent with previous reports that plant species differentially affect microbial community structure and composition in the bulk soil (31, 38). Higher numbers of polycyclic aromatic hydrocarbon-degrading organisms and higher degradation rates have been detected in the bulk soil collected from planted treatments than in the bulk soil collected from unplanted treatments in rhizoremediation studies (30, 44). These findings are promising indications that certain trees may positively affect the number of degrading microorganisms in a large volume of soil, beyond the immediate vicinity of the roots traditionally defined as the rhizosphere.
Seasonal fluctuations in the sizes of the PCB-degrading populations in the root zones of trees indicated that rhizostimulation is not continuous but instead is periodic. The periodicity may be attributable to root turnover events that release substrates to soil microorganisms, including plant secondary compounds that promote the growth of PCB degraders (26). The natural process of fine root turnover in perennial plants occurs in seasonal cycles. Major root mortality events typically occur in the autumn and sometimes also in the spring, and the timing varies among plant species (50). Likewise, in the root zone of the pine tree, the numbers of PCB degraders increased significantly between the August and November sampling dates, while beneath the willow tree the numbers appeared to increase between autumn and spring (Fig. 1a).
Attributing spatial and temporal differences in the sizes of PCB-degrading populations to the influence of plants at a contaminated site should be done cautiously due to the potentially confounding effects of abiotic heterogeneities. In this study the effect of soil PCB concentration, which varied from 8 to 500 mg/kg beneath different plant species, was ruled out by statistical analyses, indicating that there was no significant correlation between the number of PCB degraders and the soil PCB concentration. This finding is consistent with previous reports which showed that most PCB congeners are not growth substrates for aerobic PCB-degrading bacteria (3). Both the pH and the composition of the soil also varied somewhat throughout the site (see above). However, both the pH and the soil composition, as well as the observed root density, were identical in the soil beneath the neighboring ash and pine trees, which contained significantly different numbers of PCB degraders at certain times of year. Soil moisture content, which varied spatially and temporally, also did not correlate with the number of PCB degraders. The possibility of analytical artifacts introduced by sample storage time was evaluated, and no correlation was found between the number of heterotrophs or PCB degraders and sample storage time between 1 to 12 months, the time frame in which all samples were analyzed. While it would be impossible to rule out every potentially confounding variable, our results strongly suggest that plants have differential effects on PCB-degrading microbial populations in the root zone.
The identification of pine and willow as rhizoremediation candidates is consistent with hypotheses that plant secondary metabolites play a role in biostimulation (45). Both of these plants produce compounds that have previously been demonstrated to be significant for the degradation of aromatic contaminants. P. nigra produces terpenoids (1), simple phenolic compounds (17), and tannins (14). Some terpenoids are known to serve as substrates for PCB-degrading bacteria and/or to induce the PCB degradation pathway (45) and to enhance survival of PCB degraders bioaugmented in soil (32). Yu et al. (53) reported that biphenyl-utilizing bacteria were capable of growth on two tricyclic diterpenoids (resin acids) that P. nigra produces in abundance (1). Willows (Salix spp.) are widely recognized for their production of salicylic acid, which induces transcription of genes involved in polycyclic aromatic hydrocarbon degradation (39) and also functions as a growth substrate and inducer for a PCB-degrading bacterium (45). Salicylate-degrading bacteria were previously found to be enriched beneath willows in comparison to other vegetation (40). Although S. caprea does not produce salicylate, it does contain two derivatives of this compound, saligenin (salicyl alcohol) and salicin (a glucoside of saligenin), in the leaves and bark (46). Other aromatic compounds of S. caprea have not been characterized, but related willows are also known to produce flavonoids and condensed tannins (15), including two flavonoids found by Donnelly et al. (8) to serve as growth substrates for PCB-degrading bacteria.
The populations of culturable PCB-degrading bacteria at the site and in association with plants included members of the Arthrobacter and Mycobacterium subdivisions, as well as Gammaproteobacteria. PCB degradation abilities have previously been observed in species of Arthrobacter (13), Bacillus (42), Microbacterium (37), Pseudomonas (13, 49), and Rhodococcus, including strains previously known as Corynebacterium spp. (49, 52). This is the first report that members of the genera Luteibacter and Williamsia are capable of PCB metabolism, although a member of the genus Williamsia was previously isolated from soil that degrades a nitroamine explosive (48). Isolate OUCZ70 exhibited 99% similarity to Luteibacter rhizovicina, which was previously isolated from the rhizosphere of barley and is a member of the family Xanthomonadaceae.
Rhodococci were the most diverse and abundant group of culturable PCB degraders present at the contaminated site, and they were associated with all plants examined and were present in non-root-containing soil. PCB-degrading rhodococci have previously been isolated from several different PCB-contaminated sites (49, 51). However, the relative abundances of different taxa of PCB-degrading bacteria in contaminated soils have not been investigated previously since PCB-degrading bacteria are routinely isolated using enrichment methods. Using direct agar plates with biphenyl as the sole carbon source, followed by a clearing zone assay, we observed that colony morphotypes associated with rhodococci were the most abundant culturable degraders throughout the site investigated, comprising at least 90% of the colonies in the soil and 100% of the colonies in the rhizosphere at this site.
Rhodococci are nocardioform actinomycetes that contain mycolic acids in the cell wall, which are known to confer resistance to dehydration (2). This property may explain the stability of populations of PCB degraders observed at the site with soil moisture contents ranging from 18.5 to 1.8% (Fig. 2), while the numbers of total heterotrophs fluctuated with the soil moisture. For this reason, rhodococci may be valuable organisms for long-term low-maintenance bioremediation approaches, including rhizoremediation, since their survival does not require carefully controlled moisture conditions. In the future, studies of the relative abundance of different PCB-degrading taxa at other contaminated sites may provide insight into whether rhodococci predominate elsewhere and what role environmental or biogeographic factors play in the phylogenetic composition of degradative populations.
Rhodococci have been observed previously in the rhizospheres or root zones of various annual and perennial plant species (5, 10); however, this is the first report of PCB-degrading rhodococci associated with plants. Rhodococci capable of degrading PCBs have previously been shown to respond to biostimulation with compounds analogous to PCBs, including secondary plant metabolites. In PCB-contaminated sediment microcosms, rhodococci were enriched over the course of 6 months by addition of biphenyl (51). Plant phenolics and flavonoids have been demonstrated to support the growth and degradation activities of the PCB-degrading bacterium Rhodococcus sp. strain MB1 in culture (8, 53). In a microcosm study of soil spiked with PCBs, the addition of terpene-rich plant residues (orange peel, pine needles, etc.) increased the numbers of PCB degraders, including Rhodococcus species (19). The reports that rhodococci both utilize plant secondary compounds and degrade PCBs, together with the finding in this study that these organisms are found in the root zones of plants known to produce these compounds suggest that rhodococci are well adapted for PCB rhizoremediation.
Biostimulation approaches to PCB degradation, including rhizoremediation, are dependent upon the presence of indigenous microorganisms with broad congener specificities. One organism, OUCZ91B (100% match with R. ruber), exhibited degradation abilities comparable to those of the best-known aerobic PCB degrader, B. xenovorans LB400. Two other bacteria, OUCZ108 (99.8% match with R. erythreus) and OUCZ24 (100% match with Pseudomonas frederiksbergensis), were also impressive. The best three degraders were found to be widespread at the site, and they were isolated from the biostimulated root zone of willow and were associated with several other plant species (Table 3). Although they were not isolated from soil obtained beneath the pine tree, their presence cannot been ruled out since the number of isolations and identifications performed for each plant was limited. The presence of organisms with broad congener specificities indicates that bioaugmentation is unnecessary to ensure the presence of strong catabolic ability. The fact that outstanding degraders were present in the root zone of willow, which contained 30-fold-higher numbers of PCB degraders than non-root-containing soil from an equivalent depth contained, indicates that both an increased abundance of PCB-degrading microbes and strong catabolic potential can be achieved with rhizoremediation technology.
The observation that the long-term growth of certain plant species increases the degradation potential of the microbial community in the bulk soil supports the notion that rhizoremediation is a promising strategy for increasing PCB degradation rates in situ. The findings of this study indicate that future investigations of the actual rates of PCB degradation in soils influenced by plants and/or plant secondary compounds are warranted in order to evaluate whether rhizostimulation of degraders results in increased degradation rates. Additionally, the secondary chemistry of pine and willow and the temporal fluctuations in the sizes of their root zone PCB degrader populations have implications for the mechanisms of rhizostimulation that invite future study.
Because in this study we relied on culture-based measurement of microbial numbers, we likely detected only a fraction of the microorganisms in the environment. Molecular ecological tools, including quantitative PCR and stable isotope probing methods, have the potential to significantly improve the detection of PCB degraders by circumventing biases associated with culture-based methods. Stable isotope probing studies are currently under way to identify microorganisms actively involved in PCB degradation at this site and other contaminated areas and to investigate the role of plant secondary compounds in biostimulation of PCB-degrading microorganisms.
Acknowledgments
This work was supported in part by two David L. Boren Graduate International Fellowships from the U.S. National Security Education Program (NSEP), by research project Z40550506, GACR 203/06/0563, the Oklahoma Center for the Advancement of Science and Technology and the International Petroleum Environmental Consortium.
We thank Colorlak for providing access to the research area, and we especially thank Ivo Bětak and Alena Hradilová for on-site assistance and Vítek Matějuů (Envisan GEM spol. s.r.o., Prague, Czech Republic) for arranging site access. We also thank Kateřina Demnerová and Jiří Burkhard for support at the Institute for Chemical Technology and Nathan Stewart for field assistance. At the University of Oklahoma, we are grateful to Michael D. Kyle for methodological and statistical advice and Aaron White, Gary Schooler, Kelley Davis, and Becky Schuler for lab assistance.
REFERENCES
- 1.Bardyshev, I. I., G. Ya, and R. I. Zen'ko. 1970. Properties and chemical composition of colophony and turpentine produced from Bulgarian oleoresin from Pinus sylvestris and Pinus nigra. Biologiya 8:113-120. [Google Scholar]
- 2.Barry, C. E. I., R. E. Lee, K. Mdluli, A. E. Sampson, B. G. Schroeder, R. A. Slayden, and Y. Yuan. 1998. Mycolic acids: structure, biosynthesis and physiological functions. Prog. Lipid Res. 37:143-179. [DOI] [PubMed] [Google Scholar]
- 3.Bedard, D. L., and M. L. Haberl. 1990. Influence of chlorine substitution pattern on the degradation of polychlorinated biphenyls by eight bacterial strains. Microb. Ecol. 20:87-102. [DOI] [PubMed] [Google Scholar]
- 4.Bedard, D. L., R. Unterman, L. H. Bopp, M. J. Brennan, M. L. Haberl, and C. Johnson. 1986. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl. Environ. Microbiol. 51:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belimov, A. A., V. I. Sfronova, T. A. Sergeyeva, T. N. Egorova, V. A. Matveyeva, V. E. Tsyganov, A. Y. Borisov, I. A. Tikhonovich, C. Kluge, A. Preisfeld, K.-J. Dietz, and V. V. Stepanok. 2001. Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 47:642-652. [DOI] [PubMed] [Google Scholar]
- 6.Brunner, W., F. H. Sutherland, and D. D. Focht. 1985. Enhanced biodegradation of polychlorinated biphenyls in soil by analog enrichment and bacterial inoculation. J. Environ. Qual. 14:324-328. [Google Scholar]
- 7.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly, P. K., R. S. Hegde, and J. S. Fletcher. 1994. Growth of PCB-degrading bacteria on compounds from photosynthetic plants. Chemosphere 28:981-988. [Google Scholar]
- 9.Dytham, C. 1999. Choosing and using statistics: a biologist's guide. Blackwell Science Ltd., Oxford, United Kingdom.
- 10.Elo, S., L. Maunuksela, M. Salkinoja-Salonen, A. Smolander, and K. Haahtela. 2000. Humus bacteria of Norway spruce stands: plant growth promoting properties and birch, red fescue and alder colonizing capacity. FEMS Microbiol. Ecol. 31:143-152. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher, J. S., P. K. Donnelly, and R. S. Hegde. 1995. Biostimulation of PCB-degrading bacteria by compounds released from plant roots, p. 131-136. In R. E. Hoeppel (ed.), Bioremediation of recalcitrant organics. Battelle Press, Columbus, Ohio.
- 12.Focht, D. D., and W. Brunner. 1985. Kinetics of biphenyl and polychlorinated biphenyl metabolism in soil. Appl. Environ. Microbiol. 50:1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa, K. 1994. Molecular genetics and evolutionary relationship of PCB-degrading bacteria. Biodegradation 5:289-300. [DOI] [PubMed] [Google Scholar]
- 14.Gerngross, O., and E. Gulbaran. 1952. Analytical studies of tannin content of the bark of the Turkish pines, Pinus nigra, Pinus sylvestris, Pinus pinea and Pinus brutia. Leder 3:169-174. [Google Scholar]
- 15.Glasby, J. S. 1991. Dictionary of plants containing secondary metabolites. Taylor & Francis, Ltd., London, United Kingdom.
- 16.Goris, J., P. de Vos, J. Caballero-Mellado, J. Park, E. Falsen, J. F. Quensen III, J. M. Tiedje, and P. Vandamme. 2004. Classification of the biphenyl- and polychlorinated biphenyl-degrading strain LB400T and relatives as Burkholderia xenovorans sp. nov. Int. J. Syst. Evol. Microbiol. 54:1677-1681. [DOI] [PubMed] [Google Scholar]
- 17.Hafizoglu, H., B. Harzemsah, B. Holmbom, and M. Reunanen. 2002. Chemical composition of lipophilic and phenolic constituents of barks from Pinus nigra, Abies bornmulleriana and Castanea sativa. Holzforschung 56:257-260. [Google Scholar]
- 18.Harkness, M. R., J. B. McDermott, D. A. Abramowicz, J. J. Salvo, W. P. Flanagan, M. L. Stephens, F. J. Mondello, R. J. May, J. H. Lobos, K. M. Carroll, M. J. Brennan, A. A. Bracco, K. M. Fish, G. L. Warner, P. R. Wilson, D. K. Dietrich, D. T. Lin, C. B. Morgan, and W. L. Gately. 1993. In situ stimulation of aerobic PCB biodegradation in Hudson River sediments. Science 259:503-507. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez, B. S., S. C. Koh, M. Chial, and D. D. Focht. 1997. Terpene-utilizing isolates and their relevance to enhanced biotransformation of polychlorinated biphenyls in soil. Biodegradation 8:153-158. [Google Scholar]
- 20.Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, and S. T. Williams. 1994. Bergey's manual of determinative bacteriology, 9th ed. Williams & Wilkins, Baltimore, Md.
- 21.Johnson, J. L. 1994. Similarity analyses of rRNAs, p. 683-700. In N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 22.Jordahl, J. L., L. Foster, J. L. Schnoor, and P. J. J. Alvarez. 1997. Effect of hybrid poplar trees on microbial populations important to hazardous waste bioremediation. Environ. Toxicol. Chem. 16:1318-1321. [Google Scholar]
- 23.Kampfer, P., M. A. Anderson, F. A. Rainey, R. M. Kroppenstedt, and M. Salkinoja-Salonen. 1999. Williamsia muralis gen. nov., sp. nov., isolated from the indoor environment of a children's day care center. Int. J. Syst. Bacteriol. 49:681-687. [DOI] [PubMed] [Google Scholar]
- 24.Kastner, M., M. B. Jammali, and B. Mahro. 1994. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons (PAH). Appl. Microbiol. Biotechnol. 41:267-273. [Google Scholar]
- 25.Leigh, M. B. 2006. Methods for rhizoremediation research, p. 32-55. In M. Mackova, T. Macek, and D. Dowling (ed.), Phytoremediation and rhizoremediation, focus on biotechnology, vol. 9A. Springer, Berlin, Germany. [Google Scholar]
- 26.Leigh, M. B., J. S. Fletcher, X. O. Fu, and F. J. Schmitz. 2002. Root turnover: an important source of microbial substrates in rhizosphere remediation of recalcitrant contaminants. Environ. Sci. Technol. 36:1579-1583. [DOI] [PubMed] [Google Scholar]
- 27.Leigh, M. B., J. S. Fletcher, D. P. Nagle, M. Mackova, and T. Macek. 2001. Vegetation and fungi at Czech PCB-contaminated sites as bioremediation candidates, p. 61-68. In A. Leeson, E. A. Foote, M. K. Banks, and V. Magar (ed.), Phytoremediation, wetlands and sediments, vol. 5. 6th International In Situ and On-Site Bioremediation Symposium, San Diego, Calif. Battelle Press, Columbus, Ohio. [Google Scholar]
- 28.Masai, E., K. Sugiyama, N. Iwashita, S. Shimizu, J. E. Hauschild, T. Hatta, K. Kimbara, K. Yano, and M. Fukuda. 1997. The bphDEF meta-cleavage pathway genes involved in biphenyl/polychlorinated biphenyl degradation are located on a linear plasmid and separated from the initial bphACB genes in Rhodococcus sp. strain RHA1. Gene 187:147-149. [DOI] [PubMed] [Google Scholar]
- 29.McDermott, J. B., R. Unterman, M. J. Brennan, R. E. Brooks, D. P. Mobley, C. C. Schwartz, and D. Dietrich. 1989. Two strategies for PCB soil remediation: biodegradation and surfactant extraction. Environ. Prog. 8:46-51. [Google Scholar]
- 30.Miya, R. K., and M. K. Firestone. 2000. Phenanthrene-degrader community dynamics in rhizosphere soil from a common annual grass. J. Environ. Qual. 29:584-592. [Google Scholar]
- 31.Nusslein, K., and J. M. Tiedje. 1999. Soil bacterial community shift correlated with change from forest to pasture vegetation in a tropical soil. Appl. Environ. Microbiol. 65:3622-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh, E. T., S.-C. Koh, E. Kim, Y.-H. Ahn, and J.-S. So. 2003. Plant terpenes enhance survivability of polychlorinated biphenyl (PCB) degrading Pseudomonas pseudoalcaligenes KF707 labeled with gfp in microcosms contaminated with PCB. J. Microbiol. Biotechnol. 13:463-468. [Google Scholar]
- 33.Ohtsubo, Y., Y. Nagata, K. Kimbara, M. Takagi, and A. Ohta. 2000. Expression of the bph genes involved in biphenyl/PCB degradation in Pseudomonas sp. KKS102 induced by the biphenyl degradation intermediate, 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid. Gene 256:223-228. [DOI] [PubMed] [Google Scholar]
- 34.Olson, P. E., J. S. Fletcher, and P. R. Philp. 2001. Natural attenuation/phytoremediation in the vadose zone of a former industrial sludge basin. Environ. Sci. Pollut. Res. 8:243-249. [DOI] [PubMed] [Google Scholar]
- 35.Rao, A. S. 1990. Root flavonoids. Bot. Rev. 56:1-84. [Google Scholar]
- 36.Rentz, J. A., P. J. J. Alvarez, and J. L. Schnoor. 2004. Repression of Pseudomonas putida phenanthrene-degrading activity by plant root extracts and exudates. Environ. Microbiol. 6:574-583. [DOI] [PubMed] [Google Scholar]
- 37.Rybkina, D. O., E. G. Plotnikova, L. V. Dorofeeva, I. L. Mironenko, and V. A. Demakov. 2003. A new aerobic gram-positive bacterium with a unique ability to degrade ortho- and para-chlorinated biphenyl(s). Microbiology (New York) 72:672-677. [PubMed] [Google Scholar]
- 38.Saetre, P., and E. Baath. 2000. Spatial variation and patterns of soil microbial community structure in a mixed spruce-birch stand. Soil Biol. Biochem. 32:909-917. [Google Scholar]
- 39.Schell, M. A. 1985. Transcriptional control of the nah and sal hydrocarbon-degradation genes from plasmid NAH7. J. Bacteriol. 153:822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt, S. K., D. A. Lipson, and T. K. Raab. 2000. Effects of willows (Salix brachycarpa) on populations of salicylate-mineralizing microorganisms in alpine soils. J. Chem. Ecol. 26:2049-2057. [Google Scholar]
- 41.Seto, M., K. Kimbara, M. Shimura, T. Hatta, M. Fukuda, and K. Yano. 1995. A novel transformation of polychlorinated biphenyls by Rhodococcus sp. strain RHA1. Appl. Environ. Microbiol. 61:3353-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimura, M., G. Mukerjee-Dhar, K. Kimbara, H. Nagato, H. Kiyohara, and T. Hatta. 1999. Isolation and characterization of a thermophilic Bacillus sp. JF8 capable of degrading polychlorinated biphenyls and naphthalene. FEMS Microbiol. Lett. 178:87-93. [DOI] [PubMed] [Google Scholar]
- 43.Siciliano, S. D., and J. J. Germida. 1998. Mechanisms of phytoremediation: biochemical and ecological interactions between plants and bacteria. Environ. Rev. 6:65-79. [Google Scholar]
- 44.Siciliano, S. D., J. J. Germida, K. Banks, and C. W. Greer. 2003. Changes in microbial community composition and function during a polyaromatic hydrocarbon phytoremediation field trial. Appl. Environ. Microbiol. 69:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer, A. C., D. E. Crowley, and I. P. Thompson. 2003. Secondary plant metabolites in phytoremediation and biotransformation. Trends Biotechnol. 21:123-130. [DOI] [PubMed] [Google Scholar]
- 46.Soto, M., and A. Larque-Saavedra. 1982. Study on salicylic acid of Salix caprea. Rev. Soc. Quim. Mex. 26:125-126, 128-129. [Google Scholar]
- 47.Sylvestre, M. 1980. Isolation method for bacterial isolates capable of growth on p-chlorobiphenyl. Appl. Environ. Microbiol. 39:1223-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson, K. T., F. H. Crocker, and H. L. Frederickson. 2005. Mineralization of the cyclic nitramine explosive hexahydro-1,3,5-trinitro-1,3,5-triazine by Gordonia and Williamsia spp. Appl. Environ. Microbiol. 71:8265-8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unterman, R., D. L. Bedard, M. J. Brennan, L. H. Bopp, F. J. Mondello, R. E. Brooks, D. P. Mobley, J. B. McDermott, C. C. Schwartz, and D. K. Dietrich. 1988. Biological approaches for PCB degradation, p. 253-269. In G. S. Osman et al. (ed.), Reducing risks from environmental chemicals through biotechnology. Plenum Press, New York, N.Y.
- 50.Vogt, K. A., and J. Bloomfield. 1991. Tree root turnover and senescence, p. 297-306. In U. Kafkafi (ed.), Plant roots, the hidden half, 1st ed. Marcel Dekker, New York, N.Y.
- 51.Wagner-Dobler, I., A. Bennasar, M. Vancanneyt, C. Strompl, I. Brummer, C. Eichner, I. Grammel, and E. R. B. Moore. 1998. Microcosm enrichment of biphenyl-degrading microbial communities from soils and sediments. Appl. Environ. Microbiol. 64:3014-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams, W. A., J. H. Lobos, and W. E. Cheetham. 1997. A phylogenetic analysis of aerobic polychlorinated biphenyl-degrading bacteria. Int. J. Syst. Bacteriol. 47:207-210. [DOI] [PubMed] [Google Scholar]
- 53.Yu, Z., G. R. Stewart, and W. W. Mohn. 2000. Apparent contradiction: psychrotolerant bacteria from hydrocarbon-contaminated arctic tundra soils that degrade diterpenoids synthesized by trees. Appl. Environ. Microbiol. 66:5148-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]