Abstract
The lightning-competent Pseudomonas sp. strain N3, recently isolated from soil, has been used to study the extent of natural electrotransformation (NET) or lightning transformation as a horizontal gene transfer mechanism in soil. The variation of electrical fields applied to the soil with a laboratory-scale lightning system provides an estimate of the volume of soil affected by NET. Based on the range of the electric field that induces NET of Pseudomonas strain N3, the volume of soil, where NET could occur, ranges from 2 to 950 m3 per lightning strike. The influence of DNA parameters (amount, size, and purity) and DNA soil residence time were also investigated. NET frequencies (electrotransformants/recipient cells) ranged from 10−8 for cell lysate after 1 day of residence in soil to 4 × 10−7 with a purified plasmid added immediately before the lightning. The electrical field gradient (in kilovolts per cm) also played a role as NET frequencies ranging from 1 × 10−5 at 2.3 kV/cm to 1.7 × 10−4 at 6.5 kV/cm.
Horizontal gene transfer (HGT) is one of the main mechanisms that contribute to microbial evolution and adaptation based on the analysis and comparison of numerous complete prokaryotic genome sequences (8, 11, 19, 21, 25). About 14% of the open reading frames in more than 110 prokaryotic genomes could have been acquired from other bacterial species by horizontal DNA transfer (19). Given that intraspecies gene exchange is still more frequent than interspecies transfer, bacteria probably transfer genes at even higher frequencies. However, the clear discrepancy between HGT frequencies deduced from in silico analysis and those resulting from in situ experiments in which transduction, conjugation, and natural genetic transformation were studied led to the hypothesis that other, and not necessarily genetically encoded, mechanisms are involved in HGT (4, 15).
Among examples of environmental HGT mechanisms is the salt-dependent transformation of an Escherichia coli strain, which was not competent in the absence of salt, and underwent chemical transformation in calcareous freshwater (2). E. coli transformants were also detected after incubation in foodstuffs (1) and on agar plates (17, 24). In soil, bacteria can be subjected to a passive entry of DNA when reversible pores are created in the cell wall after an electrical perturbation. The electrical effect of a lightning discharge could produce this electrical stress based on the isolation of E. coli transformants after these bacteria and plasmids were subjected to simulated natural lightning discharge in soil (5). Moreover, two soil isolates belonging to the genus Pseudomonas were electrotransformed in soil at a frequency several orders of magnitude higher than that for E. coli (3). These results confirm that natural electrotransformation (NET) could be involved in the horizontal gene transfer process among soil bacteria. On the other hand, the extent of the proper conditions for NET in soil during a lightning strike has implications concerning the potential importance of this HGT mechanism.
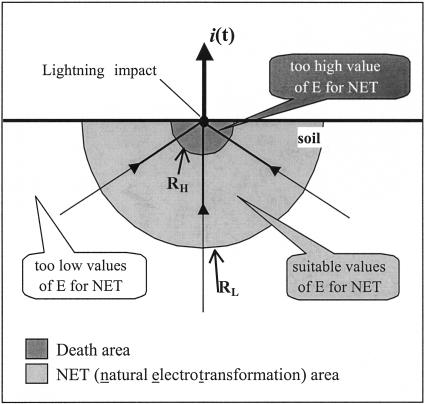
Lightning is due to the flow of a high potential electrical current into an ionized channel, which connects the ground to the cloud. At the ground level, these currents penetrate the soil from a small surface area (a few square centimeters) and then spread more or less uniformly depending on the soil homogeneity over a soil volume up to several cubic meters (Fig. 1). From the lightning impact point downward, the current density decreases with distance so that electrotransformation conditions would not be identical over the entire soil volume subjected to the lightning discharge. Moreover, NET frequencies (transformants/recipients) are most likely going to depend on the density of potential recipients and the localization of bacteria in internal or external soil compartments, as well as the concentration and availability of extracellular DNA.
FIG. 1.
Elementary model of the current density distribution in soil. i(t), lightning intensity. If we assume that NET is possible when the electrical field E is in the range [EL − EH], two radii, RL and RH, which define the volume V in which NET occurs, can be determined. R, which is the distance to the impact point of the lightning, is connected to E by the relation R2 = ρi/(2πE). The volume of soil between RH and RL is V = 2/3π(RL3 − RH3 ) = 1/3√(ip)3/(2π) [(1/EL)3/2 − (1/EH)3/2].
We sought to determine the influence of various abiotic and biotic conditions on the occurrence of NET of soil bacteria. Experiments were conducted in sterile soil microcosms inoculated with the Pseudomonas sp. strain N3, a soil isolate that exhibits a high electrocompetence potential (3).
MATERIALS AND METHODS
Bacterial strains, plasmids and culture conditions.
Pseudomonas sp. strain N3 and E. coli DH5α were grown on modified Luria-Bertani (LB) medium (0.5% NaCl) at 28 and 37°C, respectively (18). Pseudomonas sp. strain N3 cells were harvested at the late exponential phase and concentrated by centrifugation to reach approximately 2 × 1010 CFU/ml. Pseudomonas sp. strain N3 pBHC transformants were selected on tetracycline (Tc) 20 (Boehringer Mannheim France SA, Meylan, France) and spectinomycin (Sp) 25 (Sigma-Aldrich, Saint-Quentin Fallavier, France) to counterselect E. coli pBHC when cell lysate was used as DNA source. The plasmids used are listed in Table 1. The pBBR1MCS-3, pBHCRec, and pBCal plasmids were used as circular DNA sources of different sizes.
TABLE 1.
Plasmids examined in this study
| Plasmid | Characteristics | Resistance(s) | Source or reference |
|---|---|---|---|
| pLEPO1 | 7 kb, plastid (accD-rbcL) sequences flanking aadA gene | Ap | 12 |
| pUClinA | Lindane-degrading linA gene | Ap | 10 |
| pLEPO1linA | pLEPO1 containing linA gene | Ap | This study |
| pBBR1MCS-3 | 5 kb, broad host range | Tc | 14 |
| pBHC | 8.1 kb, pBBR1MC-S3 derivative containing accD-rbcL genes | Tc | 3 |
| pBHCRec | 9.7 kb, pBBR1MC-S3 derivative, containing accD-rbcL genes, aadA gene | Tc, Sp, Sm | 3 |
| pBcal | 11.2 kb, pBBR1MC-S3 derivative containing accD-rbcL genes, linA gene | Tc, Sp, Sm | This study |
DNA manipulations.
The 1.2-kb HindII fragment of pUClinA was cloned into the single EcoRV site of pLEPO1 plasmid in order to construct the pLEPO1linA plasmid. The PvuII fragment (6.0 kb) of pLEPO1linA plasmid has been cloned into the single SmaI restriction site of pBBR1MCS-3 to produce the pBcal plasmid. All plasmids were isolated from E. coli strain DH5α with a miniprep extraction kit (QIAGEN, Courtaboeuf, France). To check the occurrence of NET, the Chloro1 and Chloro2 primers (12) were used to amplify chloroplastic sequences carried by the pBHC plasmid. The thermal cycling parameters were 3 min at 94°C, followed by 30 cycles of denaturation for 60 s at 94°C, annealing for 60 s at 55°C, and elongation for 70 s, with a final extension of 7 min at 72°C.
Preparation of bacterial cell lysate.
In order to simulate plasmid release from dead bacteria, E. coli strain DH5α pBHC cells were frozen and boiled for 5 min. Based on an estimated 10 copies of pBHC per cell, the plasmid quantity was evaluated at 4.4 ng of pBHC/μl of lysate for 5 × 1010 CFU/ml. The absence of viable cells was checked by inoculating 10 μl of cell lysate in 10 ml of LB liquid medium twice, followed by incubation overnight at 37°C.
Soil microcosm.
The soil used was a sandy loam prairie soil (La Côte Saint-André, France) that was dried before sterilization by γ-irradiation (25 kGy; Ionizos, Dagneux, France). The water-holding capacity of this soil was ca. 28%. The day before the lightning current injection, 35 g of dry soil was hydrated with distilled water in order to have between 13 and 15% (wt/wt) humidity. This humidity was dependent on the electrical parameters required. A drier soil led to lightning breakdown, and a more humid soil led to a decrease in electrical potential due to a decrease in the soil resistivity. The experimental system (a 50-mm-diameter petri dish containing a round disk of aluminum foil [electrode] at the bottom) was filled with soil (depth, 1.2 cm). The soil was seeded on the surface with the recipient bacterium Pseudomonas sp. strain N3 (40 μl, ca. 108 cells). The DNA source used as purified DNA or E. coli DH5α cell lysate was either coinoculated with Pseudomonas sp. strain N3 or preincubated 24 h in soil at 28°C (50 ng/g of dry soil). The amount of DNA varied from 0 to 400 ng equivalents of pBHC plasmid.
Lightning transformation.
Once the bacteria, with or without DNA, were deposited on the surface of the soil, the second electrode was placed on the top of the microcosm, and the soil samples were immediately subjected to electrical discharges. The electric field applied was about 5 kV/cm when we tested the influence of nucleic parameters and between 0 to 6.3 kV/cm when we evaluated the volume of soil affected by the NET process. After exposure to the electrical discharge, a subsample of the inoculated soil (deposit area) was then placed in 50 ml of LB medium, followed by incubation for 2 h at 28°C to allow bacterial cells to recover from the electrical shock. This soil suspension was then plated onto LB plates supplemented with Tc (electrotransformant counts) and spectinomycin when cell lysate was used as the DNA source. The number of recipients was determined by the most-probable-number method after incubation in 96-well microplates containing 50 μl of diluted soil suspension in 150 μl of LB liquid medium. Both plates and microplates were incubated for 2 days at 28°C. Randomly picked Tcr colonies were tested by PCR for plasmid detection based on the chloroplastic sequences. Negative controls corresponded to the cell-DNA mixture without electrical discharge and to cell lysate alone with electrical discharge. NET frequencies were determined after incubation for 2 h at 28°C as the number of Tcr colonies divided by the number of recipient cells. All of the transformation frequencies reported in this article represent the means of data from three independent experiments.
Electrical data.
Ohm's law can be expressed locally as E = ρJ or, for a system, u = ri. E is the electric field (unit = V/m), ρ is the electrical resistivity of the material (Ω · m), and J is the current density which corresponds to the current intensity per surface unit. For a given system, i is the electrical current flowing through the system, u is the difference of potential between the two leads, and r is the resistance of the system. The current (i) is related to the current density by the following relation:
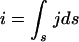 |
RESULTS AND DISCUSSION
Volume of soil affected by NET.
The biological role that lightning-induced transformation or NET could play in soil is directly related to the frequency of lightning discharges and the quantity of bacteria exposed to the appropriate electrical conditions during each discharge. Lightning frequency and intensity can be estimated at a worldwide scale, but the soil volume subjected to the appropriate conditions for NET of bacteria cannot be estimated by field experiments.
When lightning strikes the ground in a homogenous soil, the current lines converge to the impact point, with the current density decreasing gradually from the lightning impact point (Fig. 1). Based on the current conservation law, the current density decreases with the square of the inverse of the distance (R) to the impact point. Therefore, the electric field, which is related to the current density by Ohm's law through the soil resistivity (ρsoil), decreases in the same way. Thus, it provides a means for defining the theoretical distance values to the impact point, RH and RL, where the electric field E is too high (EH) and too low (EL) to allow NET process. Indeed, between the impact point and the point at RH, the electric field (>EH) is expected to have a lethal or sublethal effect on bacteria colonizing these areas. On the other hand, the electric field (<EL) in soil located outside the RL value would be too low to induce pore formation (Fig. 1). The EH and EL values can be determined experimentally by testing the range of values that induce NET. The volume of soil affected by NET can then be deduced from the values EH and EL based on a simple model of current density distribution in soil (Fig. 1) at a given ρsoil and crest value (maximum) of the lightning current Icrest (Table 2).
TABLE 2.
Theoretical calculation of electrotransformed-soil volumes according to the current distribution model in soila
| Lightning amplitude
|
Vol of soil (m3)
|
||
|---|---|---|---|
| Icrest (kA) | Probabilityb (%) | ρsoil = 50 Ω · mc | ρsoil = 500 Ω · m |
| 26 | 50 | 2 | 50 |
| 73 | 10 | 8 | 250 |
| 180 | 1 | 30 | 950 |
Volumes are given for two soil resistivity values and between electric fields: EL = 2.3 kV/cm and EH = 13 kV/cm.
That is, the probability of exceeding a given Icrest.
Soil resistivity.
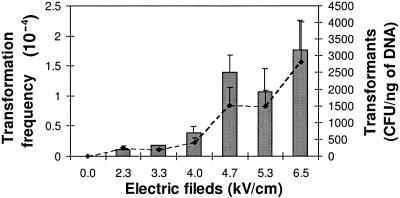
Experiments were carried out with sterilized soil microcosms seeded with the recipient bacterium Pseudomonas sp. strain N3 and the pBHC plasmid and submitted to six artificial electric fields ranging from 0 to 6.3 kV/cm. Transformants were not detected in the negative-control experiments for inoculated microcosms that were not subjected to lightning discharges. NET was effective for all of the voltage values tested and increased with increasing the electric field potential (Fig. 2). The minimum voltage (EL) under which NET can occur might be difficult to accurately ascertain due to both the electrical equipment limits and the transformation frequency detection limit. The lowest voltage tested (2.3 kV/cm) still yielded measurable transformants. At this voltage, NET occurred at a relatively high frequency (1.1 × 10−5) compared to our detection limit. Application of a higher electric field in the experimental system was limited by the soil resistivity leading to an electrical breakdown and the disappearance of a uniform electric field. Based on the results from electroporation experiments conducted in vitro in which the Pseudomonas N3 strain was efficiently electrotransformed under an electric field of 12.5 kV/cm (3), the theoretical EH value might be higher than 12.5 kV/cm.
FIG. 2.
Influence of electrical parameters on the NET of Pseudomonas sp. strain N3 using plasmid pBHC (200 ng). The histograms represent the NET frequencies, and the curves correspond to the number of electrotransformants per nanogram of pBHC plasmid.
The fact that neither EH or EL could be determined from the voltage values tested indicates that NET can be induced by a wide range of electric fields, suggesting that the soil volume in which bacteria could be electrotransformed is larger than was previously thought (5). The natural lightning discharges electric currents into the soil in a range between 20 and 200 kA depending on the soil topography and latitude. Table 2 provides a rough estimation of the soil volume affected by NET-compatible electric parameters based on 2.3 and 13 kV/cm for EL and EH, respectively, and for 3 lightning intensities and a medium (50 Ω · m) and a high (500 Ω · m) value of soil resistivity. Half of the lightning discharges that strike the ground worldwide exhibit an Icrest value of greater than 26 kA (9). Thus, under average conditions, the soil volume undergoing appropriate electrical conditions for NET of Pseudomonas sp. strain N3 would be between 2 and 50 m3. Finally, very large soil volumes (from 30 to up to 950 cubic meters) are affected when the rarest (ca. 1%), but the strongest (Icrest = 180), lightning strikes the ground.
With a soil density of 1.5 kg/liter and 4.2 × 1010 bacteria/g of soil (23), at least 6.3 × 1016 bacteria per m3 of soil would be exposed to the appropriate electrical potential for NET based on these results with Pseudomonas. Thus, a single lightning discharge would expose up to 6 × 1019 bacteria under optimum conditions. The number of lightning strikes per year in France is estimated to be 106 (Meteorage). In addition, when the percentage of indigenous bacteria that could undergo NET is estimated, a competence state is not required as in the case of natural transformation (3). NET would be much less affected by either the physiological state of bacteria or their cultivability status and might, therefore, involve a majority of the bacteria subjected to the transforming electric fields. Another interesting point was revealed by the range of the electric field values inducing NET in soil. The comparison of the electric field values inducing electrotransformation in vitro and in soil showed that NET could be reached at relatively low voltages. Classical electric fields for in vitro electrotransformation range from 6.25 to 16 kV/cm for 18 tested bacterial genera (16).
NET of Pseudomonas sp. strain N3 based on nucleic parameters.
The influence of nucleic parameters (amount, size, purity, and soil residence) on the NET of Pseudomonas sp. strain N3 constituted the second part of the present study.
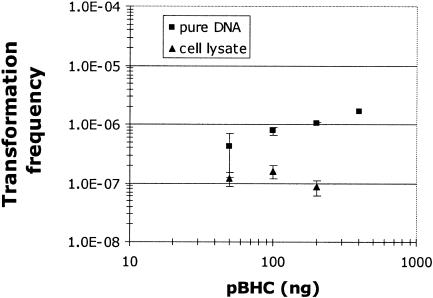
Pseudomonas sp. strain N3 strain was coinoculated in soil with the plasmid pBHC (50 to 400 ng of plasmid). The results indicate that the electrotransformation frequency expressed as the ratio between electrotransformants and recipient cells increased linearly when the amount of plasmid increased (Fig. 3). A similar experiment performed on naturally competent Pseudomonas stutzeri in natural soil with the same range of plasmid DNA amounts gave strongly comparable frequencies (22). The highest number of transformants was obtained for the greatest amount of DNA, which suggests that DNA was not saturating NET under our experimental conditions. In order to determine whether the plasmid size could affect NET efficiency, tests were carried out with four different plasmids, including pBBR1MCS-3 (5 kb), pBHC (8.1 kb), pBHCRec (9.7 kb), and pBcal (11.2 kb). The results indicate that the frequencies of these four replicons were of the same order of magnitude (Table 3), with the 11.2-kb plasmid transforming with the same efficiency as plasmids two times smaller when standardized to a similar number of plasmid molecules.
FIG. 3.
NET frequencies of Pseudomonas sp. strain N3 with increasing pure plasmid DNA and cell lysate amounts. The cell lysate was obtained after freezing and boiling of an E. coli DH5α/pBHC culture. The electric field was in the range of 5 kV/cm.
TABLE 3.
Influence of circular DNA size on the NET of Pseudomonas sp. strain N3 using pBBR1MCS3 derivative plasmids (0.1 pmol)
| Plasmida | Size (kb) | NET frequency (SD) | Transformants/ pmol (SD) |
|---|---|---|---|
| pBBR1MCS-3 | 5 | 1.8 × 10−4 (1.3 × 10−5) | 6 × 103 (1.7 × 103) |
| pBHC* | 8.1 | 9.3 × 10−5 (1.4 × 10−5) | 3.7 × 103 (4 × 102) |
| pBHCRec* | 9.7 | 2.7 × 10−4 (2.2 × 10−5) | 5.9 × 103 (2.5 × 102) |
| pBCal* | 11.2 | 3.7 × 10−4 (1.2 × 10−4) | 9.7 × 103 (1.8 × 103) |
*, pBBR1MCS-3 derivatives.
These results obtained in situ confirm previous in vitro electroporation data that demonstrated that bacterial electrotransformation was not affected by plasmid size within the range tested (6, 13) and yet increased in frequency and number of transformants with the amount of DNA (6). Consequently, NET occurs despite the soil complexity, with features comparable to the in vitro process.
Although the amount of extracellular DNA can be easily determined in soil (7), there is little information available about the biological potential of this DNA. The highly purified state of the DNA used in most experiments does not correspond to the state of DNA released by soil organisms in situ. In the present study, the natural state of DNA in soil was simulated by inoculating dead cells containing plasmids as donors instead of pure plasmid DNA. The results show that a killed bacterial suspension could electrotransform the Pseudomonas sp. strain N3 in sterile soil (Fig. 3). NET frequencies based on cell lysate DNA was around 1 log less than that obtained with pure DNA injected in the soil. This difference is consistent with results from natural transformation experiments under sterile conditions (20). Doubling the bacterial inoculum did not double the transformation frequency. These results indicate that an increase in the bacterial material can reduce the electrotransformation efficiency. A direct lethal effect of cell debris on the recipient Pseudomonas population can be excluded based on the number of Pseudomonas colonies that remained constant during the experiment (data not shown). A similar experiment involving the naturally competent bacterium Acinetobacter sp. strain BD413 indicated that natural transformation in which DNA was specifically taken up by the cell wall proteins was not affected by the cell debris accumulation (20). The difference between the two transformation processes could be a consequence of the uptake mechanism: DNA specific for natural transformation and nonspecific (pore) for NET. The hypothesis that the pore transportation of some bacterial components or complex between cell debris and soil particles in addition to DNA could inhibit the NET process or be lethal to the transformed cell needs to be investigated further.
The effect of residence time was also investigated in order to determine the influence of the interaction between soil and DNA on NET. Here, the electric discharge was applied after the plasmid solution was incubated for 24 h in soil as pure DNA or as killed bacterial cells. Although the pure DNA solution did not yield detectable transformants, the bacterial cell lysate yielded transformants at a frequency that was reduced only by 1 order of magnitude compared to the control (no incubation in soil) (Table 4). Spontaneous tetracycline mutation was not detected at the tetracycline concentration used. The natural transformation of Acinetobacter sp. strain BD413 by a bacterial lysate (20) was affected in the same proportion as in the NET of Pseudomonas sp., confirming that DNA protection by cell debris contributed to the high transformation frequency compared to that of pure DNA.
TABLE 4.
NET of Pseudomonas sp. strain N3 with pure plasmid or cell lysate, with or without (sterile) soil incubation
| pBHCa | Without soil incubation
|
One day of incubation in soil
|
||
|---|---|---|---|---|
| Frequency (SD) | Transformants/ng (SD) | Frequency (SD) | Transformants/ng (SD) | |
| Plasmid | 4.1 × 10−7 (1.3 × 10−7) | 14.7 (2.4) | 0 | 0 |
| Cell lysate | 1.2 × 10−7 (3.4 × 10−8) | 4.4 (1.3) | 1.2 × 10−8 (7.7 × 10−9) | 0.6 (0.4) |
Equivalent to 50 ng.
The results obtained in the present study indicate that lightning discharge allows the NET of Pseudomonas sp. strain N3 with small amounts of DNA originating from dead bacteria after 1 day in sterile soil and that NET might affect a large volume of soil. Moreover, based on the electroporation data, the lightning effects on bacterial cells could influence not only the uptake of extracellular DNA but also the increase in the extracellular DNA pool. Indeed, some bacteria would be lysed by a given electric field, whereas others might be electrotransformed or unaffected (16). This bacterial sensitivity to an electric field is likely to be dependent on size, species, and soil. The contribution of lightning to increasing extracellular DNA in soil needs to be investigated further.
Acknowledgments
The European Community funded this research through the fifth RTD program Quality of Life and Management of Living Resources, project TRANSBAC QLK3-CT-2001-02242, and additional funding came from Impact des Plantes OGM sur les Bactéries du Sol: Effet Direct du Transgène ou Après Transfert dans une Bactérie (PloBen; Projet 2005-07 du Programme National de Recherches sur les OGM [ANR-INRA]).
REFERENCES
- 1.Bauer, F., C. Hertel, and W. P. Hammes. 1999. Transformation of Escherichia coli in foodstuffs. Syst. Appl. Microbiol. 22:161-168. [DOI] [PubMed] [Google Scholar]
- 2.Baur, B., K. Hanselmann, W. Schlimme, and B. Jenni. 1996. Genetic transformation in freshwater: Escherichia coli is able to develop natural competence. Appl. Environ. Microbiol. 62:3673-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cérémonie, H., F. Buret, P. Simonet, and T. M. Vogel. 2004. Isolation of lightning-competent soil bacteria. Appl. Environ. Microbiol. 70:6342-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davison, J. 1999. Genetic exchange between bacteria in the environment. Plasmid 42:73-91. [DOI] [PubMed] [Google Scholar]
- 5.Demanèche, S., F. Bertolla, F. Buret, R. Nalin, A. Sailland, P. Auriol, T. M. Vogel, and P. Simonet. 2001. Laboratory-scale evidence for lightning-mediated gene transfer in soil. Appl. Environ. Microbiol. 67:3440-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dower, W. J., J. F. Hiller, and C. W. Ragsdale. 1988. High efficiency transformation of Escherichia coli by high-voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frostegard, A., S. Courtois, V. Ramisse, S. Clerc, D. Bernillon, F. Le Gall, P. Jeannin, X. Nesme, and P. Simonet. 1999. Quantification of bias related to the extraction of DNA directly from soils. App. Environ. Microbiol. 65:5409-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta, S., and M. C. Maiden. 2001. Exploring the evolution of diversity in pathogen populations. Trends Microbiol. 9:181-185. [DOI] [PubMed] [Google Scholar]
- 9.Ianoz, A. 1987. “Haute tension”: traité d'electricité de l'Ecole Polytechnique Fédérale de Lausanne. Presses Polytechniques et Universitaires Romandes, Lausanne, Switzerland.
- 10.Imai, R., Y. Nagata, M. Fukuda, M. Takagi, and K. Yano. 1991. Molecular cloning of a Pseudomonas paucimobilis gene encoding a 17-kilodalton polypeptide that eliminates HCl molecules from γ-hexachlorocyclohexane. J. Bacteriol. 173:6811-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain, R., M. C. Rivera, J. E. Moore, and J. A. Lake. 2002. Horizontal gene transfer microbial genome evolution. Theor. Popul. Biol. 61:489-495. [DOI] [PubMed] [Google Scholar]
- 12.Kay, E., T. M. Vogel, F. Bertolla, R. Nalin, and P. Simonet. 2002. In situ transfer of antibiotic resistance genes from transgenic (transplastomic) tobacco plants to bacteria. Appl. Environ. Microbiol. 68:3345-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koranyi, P., K. Burg, and M. Berenyi. 1998. Stable electrotransformation of symbiont candidate diazotrophic bacterium with plasmids carrying selectable and screenable marker genes. Res. Microbiol. 149:361-372. [DOI] [PubMed] [Google Scholar]
- 14.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, M. R. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz, M. G., and W. Wackernagel. 1994. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 58:563-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lurquin, P. F. 1997. Gene transfer by electroporation. Mol. Biotechnol. 7:5-35. [DOI] [PubMed] [Google Scholar]
- 17.Maeda, S., A. Sawamura, and A. Matsuda. 2004. Transformation of colonial Escherichia coli on solid media. FEMS Microbiol. Lett. 236:61-64. [DOI] [PubMed] [Google Scholar]
- 18.Maniatis, T. E., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Nakamura, Y., T. Itoh, H. Matsuda, and T. Gojobori. 2004. Biased biological functions of horizontally transferred genes in prokaryotic genomes. Nat. Genet. 36:760-766. (Erratum, 36:1126.) [DOI] [PubMed] [Google Scholar]
- 20.Nielsen, K. M., K. Smalla, and J. D. van Elsas. 2000. Natural transformation of Acinetobacter sp. strain BD413 with cell lysates of Acinetobacter sp., Pseudomonas fluorescens, and Burkholderia cepacia in soil microcosms. Appl. Environ. Microbiol. 66:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 22.Sikorski, J., S. Graupner, M. G. Lorenz, and W. Wackernagel. 1998. Natural genetic transformation of Pseudomonas stutzeri in a non-sterile soil. Microbiology 144:569-576. [DOI] [PubMed] [Google Scholar]
- 23.Torsvik, V., and L. Ovreas. 2002. Microbial diversity and function in soil: from genes to ecosystems. Curr. Opin. Microbiol. 5:240-245. [DOI] [PubMed] [Google Scholar]
- 24.Tsen, S. D., S. S. Fang, M. J. Chen, J. Y. Chien, C. C. Lee, and D. H. Tsen. 2002. Natural plasmid transformation in Escherichia coli. J. Biomed. Sci. 9:246-252. [DOI] [PubMed] [Google Scholar]
- 25.Ziebuhr, W., K. Ohlsen, H. Karch, T. Korhonen, and J. Hacker. 1999. Evolution of bacterial pathogenesis. Cell Mol. Life Sci. 56:719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]