Abstract
The ability of potentially probiotic strains of Lactobacillus plantarum and Lactobacillus paracasei to survive on artichokes for at least 90 days was shown. The anchorage of bacterial strains to artichokes improved their survival in simulated gastrointestinal digestion. L. paracasei IMPC2.1 was further used in an artichoke human feeding study involving four volunteers, and it was shown that the organism could be recovered from stools.
Commercial interest in functional foods containing probiotic strains has consistently increased due to the awareness of the benefits for gut health and disease prevention and therapy. Research in this area is focusing on the development of new health-promoting foods as well as on selecting new cultures with an enhanced ability to colonize the human gut (1, 13). To exert their beneficial effect after human consumption, probiotic bacteria need to survive first the manufacturing process of the carrier food and then the gastrointestinal ecosystem. The ability of probiotic strains to survive passage through the gastrointestinal tract can be mainly attributed to their acid and bile tolerance. This is an intrinsic characteristic of the strains, which can be improved by the protective action of carrier foods (3) and/or by the presence of nutrients such as metabolizable sugars (5).
The most common foods used as vehicles for probiotics able to enhance the transit tolerance of bacteria are dairy products. Some strains of Lactobacillus and Bifidobacterium species can tolerate acidic stress when ingested with milk products (4, 12), and the high fat content of cheeses protects probiotic populations during passage through the gastrointestinal tract (18). Current research is mainly focused on developing new probiotic nondairy foods that can contribute to the regular consumption of beneficial microorganisms. Since probiotic bacteria are only transient in the intestinal tract and do not become part of the host's gut microflora, their regular consumption is required for the maintenance of positive effects. Therefore, probiotic strains must be ingested in large quantities and on a daily basis. Procedures that may enhance the viability of probiotic populations during processing, storage, and transit through the gastrointestinal tract have recently been investigated (1, 6, 13, 14, 19). Apart from dairy products, other foods investigated as carriers for probiotic cultures include meat- and fish-based products, confectionery, table olives, soy- and cereal-based products, edible spreads, plant seed extracts, etc. (1, 10). Cereal products have been shown to be suitable substrates for the growth of potentially probiotic lactobacilli, also due to the protective effects of soluble sugars (2, 3, 5, 15, 16). Bacterial strains have recently been selected for their potential probiotic characteristics and the ability to survive on table olives (10). Table olives enriched with these microorganisms have been validated as a vehicle for incorporating probiotic bacterial species and delivering them into the human gastrointestinal tract (10). The strains have also been shown to adhere to the surfaces of artichokes and other vegetables and to contribute to product preservation in brine (P. Lavermicocca et al., patent application EP2005/0104138 October 2004, Italian Patent Office).
The aim of the current work was to establish whether artichokes are a good substrate for the survival of potentially probiotic strains and whether they can act as a carrier for the transport and the release of viable bacterial cells in the gut. Artichokes were also shown to protect bacterial strains during simulated gastric and intestinal digestion.
In order to evaluate the survival of bacteria adhering to artichokes, two strains, Lactobacillus plantarum ITM21B (strain LMG P-22033 in the Belgian Coordinated Collection of Microorganisms, Ghent, Belgium) and Lactobacillus paracasei IMPC2.1 (LMG P-22043), previously selected for their potential probiotic characteristics (10) (P. Lavermicocca et al., patent application EP2005/010413), were used in this study. Artichokes were preserved in brine containing citric acid (5% [wt/vol] NaCl, pH 4.2) and heat sterilized in 15-liter cans (Copaim, Albinia, Italy). Artichokes (about 240 g) were placed in 500-ml sterile glass jars and covered with their own brine diluted with sterile water (4% final concentration). Bacterial inocula were prepared by growing L. plantarum ITM21B and L. paracasei IMPC2.1 in MRS broth (Difco, Detroit, Mich.) for 24 h at 37°C (10). Strains were separately added to artichokes (two jars each) in order to obtain cell concentrations of approximately 108 to 109 CFU ml−1 of brine. Noninoculated jars were used as controls. Jars were stored at room temperature for 3 months. For microbiological analysis, half of an artichoke (20 g) was taken from each jar at inoculation time and after 30, 60, and 90 days and homogenized in 180 ml of sterile Bacto peptone (Difco) (0.1%, wt/vol) solution using a Stomacher laboratory blender (Seward, London, United Kingdom) for 4 min. The resulting suspensions were serially diluted and plated in duplicate on Rogosa SL agar (Difco) (10) for counting purposes. In order to assess the ability of artichokes to transport bacterial cells into the human gastrointestinal tract, two artichoke heads, carrying about 3 × 1010 CFU of L. paracasei IMPC2.1, were administered daily (intervention period, 10 days) to four healthy volunteers (one male and three female) aged 39 to 64 years. Microbiological analysis of fecal samples was performed as previously described (10) by plating sample dilutions on Rogosa SL agar with 12 μg of vancomycin ml−1 (Sigma). Vancomycin-resistant lactobacilli, including L. paracasei, were enumerated, and the genetic identification of the strain was performed. Ten to twenty percent of total colonies randomly selected from countable agar plates were isolated and checked for purity. DNA was extracted using an FTA Starter Pack (Whatman, Maidstone, United Kingdom) and analyzed by repetitive extragenic palindromic-PCR (REP-PCR) according to the method of Hyytiä-Trees et al. (9) as previously described (10). Simulated gastrointestinal digestion was performed on cells anchored on artichokes in comparison with cells suspended in saline solution or skim milk or anchored on olives. In the case of saline solution or skim milk, cells of each strain from an MRS culture incubated at 37°C for 24 h under the appropriate conditions were washed in sterile saline solution (0.9% [wt/vol] NaCl), centrifuged, and suspended in 2 ml of sterile saline solution or skim milk (Difco) to obtain a concentration of 1 × 1011 CFU ml−1. The resulting suspensions were treated with artificial gastric and intestinal juices prepared as reported in Fernández et al. (7). For olive experiments, about 240 g of table olives (pitted, Spanish black olives) placed in 500-ml sterile glass jars and covered with their own brine were inoculated as previously reported (10) in order to obtain a cell concentration of about 108 to 109 CFU ml−1 of brine. Half an artichoke or four olives carrying about 1 × 109 CFU of Lactobacillus strains were taken from each jar after 30 days of incubation, drained, finely chopped, and treated with the gastric and intestinal juices. Twenty milliliters of artificial gastric juice was added to 2 ml of cells suspended in saline solution or in skim milk or to the chopped vegetables carrying the bacterial cells. Samples were shaken (200 revolutions min−1 at 37°C) to simulate peristalsis. Aliquots were taken and plated on MRS agar for the enumeration of viable cells at 0 and 180 min of simulated gastric digestion at both pH 2.0 and pH 7.0. Samples were centrifuged (5,000 × g for 10 min at 4°C), and after the supernatant was discarded, cells were suspended in artificial intestinal juice. Samples for total viable counts were taken at time zero and after 180 min of intestinal digestion.
For microbiological analyses, log10 mean values ± standard error of CFU were calculated for each experiment. Data were analyzed by one-way analysis of variance, followed by the Fisher test using STATISTICA 6.0 software (StatSoft software package; Tulsa, OK). A P value of <0.05 was accepted as statistically significant.
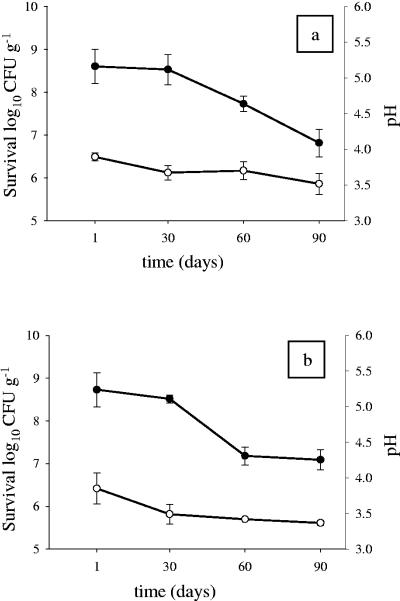
Artichokes preserved in brine enriched with L. plantarum ITM21B or L. paracasei IMPC2.1 did not show any change in appearance, texture, and organoleptic quality (data not shown). Both strains adhering to artichokes survived over a 3-month storage period, as shown in Fig. 1. No significant reduction in the bacterial population was observed for either strain after 30 days of storage (from 8.60 log10 to 8.52 log10 CFU g−1, P > 0.05, for L. plantarum ITM21B and from 8.72 log10 to 8.52 log10 CFU g−1, P > 0.05, for L. paracasei IMPC2.1), and a slight decrease was observed after 90 days (6.8 log10 CFU g−1, P < 0.05, for ITM21B and 7.08 log10 CFU g−1, P < 0.05, for IMPC2.1). The addition of the cell suspension lowered the pH from 4.2 to 3.9 during the first 48 h for both strains. After 30 days pH values decreased to 3.4 for L. paracasei IMPC2.1 and to 3.7 for L. plantarum ITM21B and remained without variation (Fig. 1). These results demonstrated that artichokes are a suitable food carrier that allow the survival of potentially probiotic strains, as previously shown for olives (10). Artichokes provide nutrients to enable bacterial survival during shelf storage. The high survival rate observed for both strains on artichokes implies that a two-head portion of artichokes (corresponding to about 80 g) allows the ingestion of about 100 million to 10 billion live L. paracasei IMPC2.1 or L. plantarum ITM21B cells. The high cell viability shown in this study can also be explained by the roughness of the vegetable structure, which may offer protection to the bacterial population in the acid environment.
FIG. 1.
Survival of L. plantarum ITM21B (a) and L. paracasei IMPC2.1 (b) on artichokes (•) and the pH value of their brines (○). Data, expressed as means ± standard errors, are from three independent experiments with two replicates each (n = 6).
L. paracasei IMPC2.1 was recovered from fecal samples of the volunteers fed with artichoke heads carrying the strain, as confirmed by the REP-PCR results (Table 1), indicating that artichokes are suitable carriers for transporting bacterial cells into the gastrointestinal tract. Moreover, a relevant increase in total vancomycin-resistant Lactobacillus spp. populations was observed: an increase of more than 2 logs was observed for subjects 1 and 3, while for subjects 2 and 4 an increase of more than 1 log was observed after 10 days of consumption (Table 1). The presence of prebiotic substances carried by artichokes, such as inulin, contributes to an efficient implantation of advantageous bacterial cells. In particular, inulin has been shown to favor growth of indigenous lactobacilli and/or bifidobacteria and to improve the functionality of the gastrointestinal system (8, 11, 17).
TABLE 1.
Total vancomycin-resistant Lactobacillus count in the feces of four healthy volunteers fed with L. paracasei IMPC2.1-containing artichokes and recovery of the strain identified by REP-PCR
| Day of samplinga | Subject no. | Lactobacillus spp. (CFU/g of feces [wet wt])b | No. of L. paracasei IMPC2.1 colonies/no. of colonies analyzed | L. paracasei IMPC2.1 (CFU/g of feces [wet wt])c |
|---|---|---|---|---|
| 0 | 1 | 1.0 × 105 | 0/10 | 0 |
| 2 | 8.9 × 105 | 0/10 | 0 | |
| 3 | 2.7 × 107 | 0/20 | 0 | |
| 4 | 1.2 × 106 | 0/20 | 0 | |
| 10 | 1 | 1.7 × 107 | 11/18 | 1.1 × 107 |
| 2 | 1.2 × 107 | 4/20 | 2.5 × 105 | |
| 3 | 1.0 × 1010 | 15/15 | 1.0 × 1010 | |
| 4 | 5.1 × 107 | 8/15 | 5.1 × 107 |
Day 0, before consumption; day 10, end of consumption. The daily dose (total CFU) was 3.1 × 1010 CFU.
Vancomycin-resistant lactobacilli.
Counts were confirmed by REP-PCR.
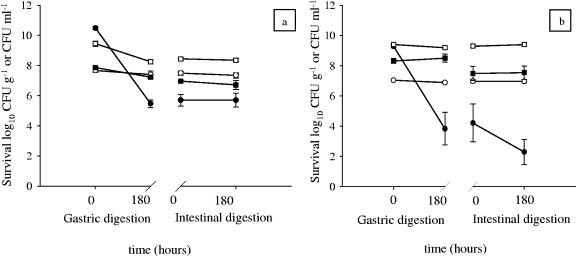
The presence of prebiotic substances and the physical structure of the vegetable could also be reasons for the successful survival of bacteria during the simulated digestion tests. Experiments carried out to demonstrate the protective action of artichokes showed that more than 85% of the total bacterial population anchored to artichokes survived during simulated gastric and intestinal digestion. The viability of L. plantarum ITM21B and L. paracasei IMPC2.1 cells during simulated gastric and intestinal digestion in the presence of artichokes is presented in Fig. 2 in comparison with saline solution or other protective matrices, such as olives and milk. At pH 2.0, in the absence of a protective matrix, such as vegetables or milk, a significant decrease in viability was observed during simulated gastric and intestinal digestion for both strains (from 10.48 log10 to 5.70 log10 CFU ml−1 for L. plantarum ITM21B and from 9.29 log10 to 2.29 log10 CFU ml−1 for L. paracasei IMPC2.1). With artichokes and milk, a slight decrease was observed for L. plantarum ITM21B during gastric passage (from 7.86 log10 to 7.22 log10 CFU g−1, P < 0.05, for artichokes; from 9.45 log10 to 8.24 log10 CFU ml−1, P < 0.05, for milk), whereas the population remained stable in the intestinal juice (from 7.22 log10 to 6.71 log10 CFU g−1, P > 0.05, for artichokes; from 8.24 log10 to 8.35 log10 CFU ml−1, P > 0.05, for milk) (Fig. 2a). The population of L. paracasei IMPC2.1 on artichokes (from 8.32 log10 to 7.54 log10 CFU g−1, P > 0.05) and milk (from 9.40 log10 to 9.40 log10 CFU ml−1, P > 0.05) remained without variation during the entire simulated digestion (Fig. 2b). In the presence of olives both strains showed high survival (from 7.66 log10 to 7.34 log10 CFU g−1, P > 0.05, for L. plantarum ITM21B and from 7.04 log10 to 6.96 log10 CFU g−1, P > 0.05, for L. paracasei IMPC2.1) (Fig. 2). The high recovery rate of the total bacterial population anchored to artichokes or olives or suspended in skim milk could be explained by the protective action of the fat content for olives and milk or to the presence of prebiotic carbohydrates for artichokes.
FIG. 2.
Survival of L. plantarum ITM21B (a) and L. paracasei IMPC2.1 (b) during simulated gastric (pH 2.0) and intestinal digestion (pH 8.0) in the presence of saline solution (•), skim milk (□), olives (○), and artichokes (▪). Data, expressed as means ± standard errors, are from three independent experiments with two replicates each (n = 6).
In conclusion, these results demonstrate that artichokes, similar to olives, are a suitable substrate for producing vegetables “fortified” with selected bacterial strains that may ensure a regular consumption of probiotics in foods that are part of a daily diet. Artichokes act as a food vector by sustaining adequate populations of viable bacteria, and the anchorage of strains to artichokes improves their survival in gastrointestinal digestion. The development of new probiotic foods should consider not only the intrinsic characteristics of effective bacterial strains but also the ability of the food matrix to protect bacterial cells through the gastrointestinal tract.
Acknowledgments
This work has been supported in part by “Fondazione Cassa di Risparmio di Puglia” (Bari, Italy) and Copaim (Albinia, Italy).
REFERENCES
- 1.Champagne, C. P., and N. J. Gardner. 2005. Challenges in the addition of probiotic cultures to foods. Crit. Rev. Food Sci. Nutr. 45:61-84. [DOI] [PubMed] [Google Scholar]
- 2.Charalampopoulos, D., S. S. Pandiella, and C. Webb. 2002. Growth studies of potentially probiotic lactic acid bacteria in cereal-based substrates. J. Appl. Microbiol. 92:851-859. [DOI] [PubMed] [Google Scholar]
- 3.Charalampopoulos, D., S. S. Pandiella, and C. Webb. 2003. Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int. J. Food Microbiol. 82:133-141. [DOI] [PubMed] [Google Scholar]
- 4.Charteris, W. P., P. M. Kelly, L. Morelli, and J. K. Collins. 1998. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 84:759-768. [DOI] [PubMed] [Google Scholar]
- 5.Corcoran, B. M., C. Stanton, G. F. Fitzgerald, and R. P. Ross. 2005. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 71:3060-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crittenden, R., A. Laitila, P. Forssell, J. Mättö, M. Saarela, T. Mattila-Sandholm, and P. Myllärinen. 2001. Adhesion of bifidobacteria to granular starch and its implications in probiotic technologies. Appl. Environ. Microbiol. 67:3469-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández, M. F., S. Boris, and C. Barbés. 2003. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 94:449-455. [DOI] [PubMed] [Google Scholar]
- 8.Guarner, F. 2005. Inulin and oligofructose: impact on intestinal diseases and disorders. Br. J. Nutr. 93(Suppl.):61S-65S. [DOI] [PubMed] [Google Scholar]
- 9.Hyytiä-Trees, E., U. Lyhs, H. Korkeala, and J. Björkroth. 1999. Characterisation of ropy slime-producing Lactobacillus sakei using repetitive element sequence-based PCR. Int. J. Food Microbiol. 50:215-219. [Google Scholar]
- 10.Lavermicocca, P., F. Valerio, S. L. Lonigro, M. De Angelis, L. Morelli, M. L. Callegari, C. G. Rizzello, and A. Visconti. 2005. Study of adhesion and survival of lactobacilli and bifidobacteria on table olives with the aim of formulating a new probiotic food. Appl. Environ. Microbiol. 71:4233-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Molina, D., M. D. Navarro-Martinez, F. R. Melgarejo, A. N. P. Hiner, S. Chazarra, and J. N. Rodriguez-López. 2005. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 66:1476-1484. [DOI] [PubMed] [Google Scholar]
- 12.Mater, D. D. G., L. Bretigny, O. Firmesse, M. J. Flores, A. Mogenet, J. L. Bresson, and G. Corthier. 2005. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiol. Lett. 250:185-187. [DOI] [PubMed] [Google Scholar]
- 13.Mattila-Sandholm, T., P. Myllärinen, R. Crittenden, G. Mogensen, R. Fondén, and M. Saarela. 2002. Technological challenges for future probiotic foods. Int. Dairy J. 12:173-182. [Google Scholar]
- 14.McMaster, L. D., S. A. Kokott, S. J. Reid, and V. R. Abratt. 2005. Use of traditional African beverages as delivery vehicles for Bifidobacterium lactis DSM 10140. Int. J. Food Microbiol. 102:231-237. [DOI] [PubMed] [Google Scholar]
- 15.Michida, H., S. Tamalampudi, S. S. Pandiella, C. Webb, H. Fukuda, and A. Kondo. 2006. Effect of cereal extracts and cereal fiber on viability of Lactobacillus plantarum under gastrointestinal tract conditions. Biochem. Eng. J. 28:73-78. [Google Scholar]
- 16.Ouwehand, A. C., T. Kurvinen, and P. Rissanen. 2004. Use of probiotic Bifidobacterium in a dry food matrix, an in vivo study. Int. J. Food Microbiol. 95:103-106. [DOI] [PubMed] [Google Scholar]
- 17.Roberfroid, M. B. 2002. Functional foods: concepts and application to inulin and oligofructose. Br. J. Nutr. 87(Suppl.):139S-143S. [DOI] [PubMed] [Google Scholar]
- 18.Stanton, C., G. Gardiner, P. B. Lynch, J. K. Collins, G. Fitzgerald, and R. P. Ross. 1998. Probiotic cheese. Int. Dairy J. 8:491-496. [Google Scholar]
- 19.Truelstrup Hansen, L., P. M. Allan-Wojtas, Y.-L. Jin, and A. T. Paulson. 2002. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol. 18:35-45. [Google Scholar]