Abstract
Due to the high Lyme borreliosis incidence in Alsace, in northeastern France, we investigated in 2003-2004 three cantons in this region in order to determine the density of Ixodes ricinus ticks infected by Borrelia burgdorferi sensu lato and Anaplasmataceae. The peak density of nymphs infected by B. burgdorferi sensu lato at Munster and Guebwiller, where the disease incidence was high, was among the highest reported in Europe (105 and 114 per 100 m2, respectively). In contrast, the peak density of infected nymphs was low in the canton of Dannemarie (5/100 m2), where the disease incidence was low. The two main species detected in ticks were Borrelia afzelii, more frequent in nymphs, and Borrelia garinii, more frequent in adult ticks. The rates of tick infection by Anaplasma phagocytophilum were 0.4% and 1.2% in nymphs and adults, respectively.
Lyme borreliosis (LB) is a worldwide disease due to bacteria belonging to the complex Borrelia burgdorferi sensu lato (31) and transmitted in Europe by the bite of Ixodes ricinus ticks (10), which is also involved in the transmission of other pathogens, particularly members of the Anaplasmataceae family (7). A phytoecological mapping of I. ricinus in France revealed a very large distribution of this tick (12). Moreover, the infestation of ticks by B. burgdorferi sensu lato has been reported in the whole French territory (11) and more precisely in restricted areas (1, 29, 30, 32, 39). A single study in Alsace revealed the presence of B. burgdorferi sensu lato in 11% of 2,223 nymphs collected in August 1989 (6). This northeastern region of France is reported as a region where LB is highly endemic (24). Moreover, a prospective study conducted in Alsace from 2001 to 2003 (18) estimated the LB incidence between 180 and 232 cases per 100,000 inhabitants per year and recorded high local variations between cantons (administrative divisions of a department in France). On the basis of this study (18), we selected two cantons in the Haut-Rhin department where the LB incidence was high and one canton where the incidence was low, in order to establish a correlation between the LB incidence and the density of infected ticks. We conducted a two-year survey in 2003 and 2004 of the I. ricinus population density and the B. burgdorferi sensu lato infection rate. Because Anaplasma phagocytophilum is maintained in the same tick, the infection rate of I. ricinus ticks by members of Anaplasmataceae was also determined.
The study was carried out in the Parc Régional des Ballons des Vosges, in northeastern France. By the drawing of lots, five and three sampling sites were designated, respectively, in the north part of the Parc (Munster canton) and in the south part (Guebwiller canton), where a high LB incidence of 250 cases/100,000 inhabitants was recorded (18). These sites are at an altitude of 400 to 700 m, largely covered by dense and continuous forests, and the climate is defined as continental. One sampling site was designated a “negative control site” in the Dannemarie canton, where the LB incidence was 36 cases/100,000 inhabitants. This site is at a lower level (altitude 230 m), the woods are small and individualized, interspersed with fields, and the climate is typical of hills in the area.
Questing ticks were collected monthly by three or four collectors, by dragging a 1-m2 white cotton cloth on the vegetation and litter. Each 10 m, designated a subsampling, ticks attached on the cloth were removed, identified, and counted. A minimum of 64, 48, and 16 subsamplings were investigated at Munster, Guebwiller, and Dannemarie, respectively. Adult and nymph ticks were transported still alive to the laboratory, where they were analyzed for infection by B. burgdorferi sensu lato. We proceeded using either culture or PCR on DNA directly extracted from ticks as described previously (31, 38), after having checked the concordance between the two methods. Identification at the species level was assessed by PCR-restriction fragment length polymorphism of the intergenic spacer as described previously (31, 38). For the detection of members of Anaplasmataceae, we used primers Ehr521 and Ehr747 (27) to amplify 247 bp of the rrs gene. The identification was systematically confirmed by sequencing of PCR products. For all analyses, ticks were processed individually. To monitor any contamination during the extraction process, one extraction control (distilled water) was added in each extraction experiment for every 20 ticks.
For the survey, we used a two-level sampling design with forest sampling sites as the first level and the 10-m-long subsamplings as the second level, both randomly selected. Tick density (d) was estimated from the total number of ticks collected in each forest as follows:
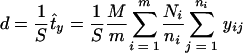 |
where S is the surface of the forest, t̂y the estimated total number of ticks, m the number of sampling sites randomly selected, M the total number of sampling sites, ni the number of subsamplings randomly selected in the sampling site i, Ni the total number of subsamplings in the sampling site i, and yij the number of ticks collected in subsampling j in the sampling site i. A 95% confidence interval was calculated for each collection. The chi-square test was used for between-group comparisons. The Spearman rank correlation coefficient (rS) was calculated between the tick density and the density of infected ticks and between the tick density and the infection rate. A P value less than 0.05 was considered significant.
Densities of I. ricinus ticks, expressed as the number of ticks per 100 m2, are shown in Table 1. The seasonal pattern of tick activity was unimodal in Alsace, where nymphs and adults reached a peak at the end of spring. However, the questing period was spread over a large period of the year. Nymph activity drastically declined from September and disappeared in November. The adult activity showed a drop as early as June in 2004, and the density remained more or less stable until November, when adults also disappeared (Table 1). Seasonal patterns of nymph and adult ticks were very similar in the two cantons of Munster and Guebwiller. The mean nymph density deduced from the whole tick collection at Munster and Guebwiller from April to October 2004 (201/100 m2) was two times higher than in 2003 (100 nymphs/100 m2). Such a distribution has been previously observed in a British woodland (5). Annual variations in tick density, as observed in Alsace between 2003 and 2004, have been reported by other authors (22, 35, 36).
TABLE 1.
Density of I. ricinus ticks collected in three cantons of Alsace in 2003-2004 and prevalence of B. burgdorferi sensu lato infection of ticks
| Month or parameter | Density per 100 m2 (CI) and infection rate (%) for indicated ticks or parameter valuea
|
|||||
|---|---|---|---|---|---|---|
| Nymphs
|
Adults
|
|||||
| Munster | Guebwiller | Dannemarie | Munster | Guebwiller | Dannemarie | |
| 2003 | ||||||
| April | 51 (34-69), 10.3 | NC | NC | 4 (0-9), 27.6 | NC | NC |
| May | 103 (78-129), 21.1 | 248 (54-442), 8.2 | NC | 7 (5-9), 29.8 | 18 (5-31), 28.3 | NC |
| June | 110 (56-164), 13.3 | 308 (53-563), 9.3 | NC | 12 (5-18), 16.2 | 19 (17-22), 17.6 | NC |
| July | 117 (84-150), 17.3 | 203 (97-308), 6.9 | 29 (28-31), 5.1 | 12 (7-17), 21.6 | 8 (6-11), 13.3 | 0 |
| September | 31 (4-58), 21.2 | 39 (20-58), 11.7 | 6 (5-8), 33.3 | 3 (0-6), 25 | 6 (3-8), 35.7 | 0 |
| October | 30 (24-35), 20.3 | 53 (9-97), 15.9 | NC | 5 (1-8), 27.6 | 6 (6-6), 21.7 | NC |
| Total no. of infected | 119/697 (17.1) | 47/487 (9.6) | 5/48 (10.4) | 129/557 (23.2) | 44/209 (21) | |
| ticks/examined | ||||||
| ticks (%) | ||||||
| 2004 | ||||||
| March | 80 (12-149), 21.6 | 109 (38-180), 14.7 | 4 (1-6), 0 | 5 (1-9), 18.4 | 16 (4-29), 31.6 | 0 |
| April | 246 (38-455), 30.9 | 308 (156-459), 20 | 40 (29-51), 9.5 | 17 (3-30), 23.7 | 33 (7-58), 36 | 6 (2-10), 0 |
| May | 398 (26-770), 26.2 | 488 (303-673), 23.3 | 41 (24-58), 10 | 34 (5-63), 24.3 | 51 (14-89), 39.6 | 6 (2-10), 0 |
| June | 177 (0-384), 23.7 | 238 (101-375), 21.7 | 35 (19-51), 15 | 11 (3-18), 23.5 | 8 (2-13), 29.7 | 2 (0-4), 0 |
| July | 215 (0-471), 20 | 255 (63-447), 25 | 34 (21-47), 15 | 14 (0-29), 20 | 14 (1-28), 40 | 2 (0-4), 0 |
| September | 34 (3-65), 17.8 | 59 (10-107), 22.8 | 21 (12-31), 15 | 4 (0-8), 30.8 | 8 (1-15), 42.1 | 0 |
| October | 14 (0-28), 28.6 | 16 (0-31), 30.2 | NC | 4 (1-6), 8.3 | 7 (0-14), 32.3 | NC |
| November | 0 | 1 (0-2) | NC | 0 | 1 (0-2) | NC |
| Total no. of infected | 130/540 (24.1) | 92/420 (21.9) | 13/104 (12.5) | 82/376 (21.8) | 106/291 (36.4) | 0/26 |
| ticks/examined | ||||||
| ticks (%) | ||||||
NC, no tick collection was carried out during this month.
High tick infection rates were recorded (Table 1) in Munster and Guebwiller. Overall 18% of nymphs (406/2,296) and 25% of adults (361/1,459) were infected. Similar infection rates were found in male (26% [88/334]) and female (30% [100/333]) ticks in 2004. Percentages of infected ticks differed widely, ranging from 7 to 33% for nymphs and from 8 to 42% for adult ticks. We did not find any seasonal variation in the prevalence of infection in I. ricinus (Table 1). Despite monthly variations, the infection prevalences were regularly and often significantly higher in Munster and Guebwiller than in Dannemarie, where ticks were less abundant (Table 1). Particularly, significantly more nymphs were infected in Munster (P = 0.01) and Guebwiller (P = 0.03) than in Dannemarie (Table 1). In contrast with the large continuous forests of Munster and Guebwiller, the smallness of the investigated Gildwiller wood in Dannemarie, which is interspersed with open fields, could hamper the maintenance of an abundant wild fauna which could be used as hosts for ticks. Consequently, the likelihood of acquiring an infected tick bite in this forest is lower than in neighboring forests, as was confirmed by the low incidence of the disease at Dannemarie.
The human threat is linked to the frequency of encountering an infected tick and therefore is correlated to the density of infected ticks (Fig. 1), the most important parameter for evaluating the infection risk for humans. Our results confirm that in Alsace, the LB incidence is correlated to the density of infected nymphs, as previously shown in Europe as in the United States (14, 33). Due to their abundance, their smallness, the fact that they are not easily visible on the skin, and their more pronounced anthropophily, nymphs represent the tick stage the most threatening for humans. However, usually, less nymphs are infected than adult ticks, which have ingested a supplementary potentially infected meal. In Alsace, nymphs are a particular risk for humans because of their high density and especially because of their high infection rate, often very close to that of adult ticks (Table 1). We failed to quantitatively correlate the climatic parameters and the density of infected ticks observed in Alsace (Fig. 1). High temperatures during the summer of 2003 (Fig. 1A) could have contributed to a loss of water by ticks and an interruption of their questing behavior (28). Moreover, higher hygrometry values and abundant rainfalls in January (Fig. 1A) could be one of the reasons of the rise of density in 2004. Otherwise, it has been shown that climatic parameters of the last year or the year before last had more influence on tick density (15).
FIG. 1.
(A) Climatic parameters provided by Météo France at the Munster meteorological station, monthly average temperature (line) and maximum temperatures (vertical bars). (B) Density of adult I. ricinus ticks infected by B. burgdorferi sensu lato (obtained by multiplying the tick density/100 m2 by the infection rate) in the three sampling sites. (C) Density of nymph ticks infected by B. burgdorferi sensu lato.
There was a positive correlation between the nymph density and the density of infected nymphs (rS = 1, P < 0.001) or adult ticks (rS = 0.97, P < 0.001) in 2004, while in 2003, there was a positive significant correlation for adults (rS = 0.94, P = 0.005) but not for nymphs. In contrast, there was no correlation between the tick density and the B. burgdorferi sensu lato infection rate in 2003 as in 2004. Although the density of nymphs was higher in Guebwiller than in Munster, the high infection rate in the latter canton resulted in similar values for the density of infected nymphs in the two cantons (Fig. 1C). The tick density and the density of infected ticks observed in Alsace are among the highest reported in Europe, where considerable variations have been recorded (16). In foci of endemicity of Switzerland, the highest density of infected ticks was 30/100 m2 (20), values lower than those found in Alsace where the peak of infected ticks reached near 120 ticks/100 m2. One could argue that the large confidence interval of estimated density values in Alsace (Table 1) could be at least partly related to different performances of different collectors. However, no significant difference was recorded, and our observations showed that large differences in the numbers of ticks collected occurred for the same operator from one subsampling to another one, even in the same sampling site. Instead, the very heterogeneous distribution of ticks, both in time and space, should account for this large confidence interval. Various B. burgdorferi sensu lato infection rates were reported from different European countries (3, 8, 17, 19, 21). In the Lyon region of France, the global infection prevalence was 13%, without any significant difference in the infection rate of nymphs and adults (32). In contrast in the Ile de France region, nymphs were significantly more infected than adult ticks (39). A high variability of infection levels occurred according to sites (even those very close together) and time of collection but also between batches of ticks. Therefore, a large vegetation surface should be sampled and a large number of ticks should be included in infection studies in order to obtain more robust results of the density of infected ticks.
The prevalences of different B. burgdorferi sensu lato species detected in I. ricinus ticks vary widely. However, as usually reported in Europe, Borrelia afzelii and B. garinii were by far the species most frequently encountered in Alsace, each infecting 36% (275/767) of ticks tested. Prevalence of B. burgdorferi sensu stricto was rather low (8%) in Alsace, although it was highly variable in different studies (19, 21, 34). Borrelia valaisiana infected 16% of ticks (125/767) collected in Alsace. A similar species distribution was reported in the region of Lyon (32), France. “Borrelia spielmanii” (which is still awaiting standing in nomenclature) was identified in only one nymph collected in Munster in 2004. No Borrelia lusitaniae was found in Alsace during the two-year survey, while it is the most prevalent species found in I. ricinus ticks in Portugal (2) and North Africa (38). We did not observe any difference in the distribution of B. burgdorferi sensu lato species in ticks according to site or time of tick collection. In contrast, the distribution of B. burgdorferi sensu lato species varied widely according to the tick stages. B. afzelii was significantly more common in nymphs (212/406 infected nymphs, i.e., 52%) than in adults (63/361 infected adults, i.e., 17%). In contrast, B. garinii was more common in adults (164/361, i.e., 46%) than in nymphs (112/406, i.e., 28%). In both cases, the difference was highly significant (P < 0.001). Similarly B. valaisiana was more common in adults (71/361, i.e., 20%) than in nymphs (54/406, i.e., 13%) (P = 0.02). This fact could be due to different feeding preferences of ticks according to their development stages. Nymphs are issued from larvae, which frequently feed on small rodents, which have been identified as a major reservoir of B. afzelii (23). In contrast, adults are issued from nymphs, which frequently feed on birds, a major reservoir of B. garinii and B. valaisiana (9). The most frequent mixture of species found in ticks, which associated B. garinii and B. valaisiana (17 ticks/30 ticks infected by two or three species), is consistent with this hypothesis.
Few data are available concerning the incidence of anaplasmosis in Europe, mostly documented on serological evidence (4, 26). Therefore, 1,065 nymphs and 171 adult ticks, collected in the three cantons of Alsace, were investigated in 2004 for infection by Anaplasmataceae. DNA from A. phagocytophilum was detected in four nymphs and two adults collected at Munster and Dannemarie, yielding very low infection rates of 0.4% in nymphs and 1.2% in adult ticks, in the range usually reported in Europe (13, 25, 37). Additionally, DNA of Ehrlichia sp. was amplified from 44 nymphs and 1 adult tick, whereas DNA of Wolbachia sp. was amplified from 30 nymphs. No DNA from Ehrlichia chaffeensis was ever amplified from ticks collected in Alsace.
In conclusion, the results of our study show that the density of I. ricinus infected by B. burgdorferi sensu lato in Alsace is among the highest reported in Europe. Therefore, these results are in total accordance with the known high endemicity of LB in Alsace. The demonstration of the infection of ticks by A. phagocytophilum reveals their possible role in human infections. However, further investigations, particularly epidemiological studies, are needed to provide evidence of human anaplasmosis in Alsace and to know the actual significance, in terms of public health, of symbionts in I. ricinus ticks.
Acknowledgments
We thank Didier Guillemot for his expertise in statistical analysis.
REFERENCES
- 1.Anderson, J. F., J. M. Doby, A. Courtarmanac'h, F. W. Hyde, and R. C. Johnson. 1986. Antigenically different Borrelia burgdorferi strains from Ixodes ricinus in Brittany, France. Méd. Mal. Infect. 3:171-175. [Google Scholar]
- 2.Baptista, S., A. Quaresma, T. Aires, K. Kurtenbach, M. Santos-Reis, M. Nicholson, and M. Collares-Pereira. 2004. Lyme borreliosis spirochetes in questing ticks from mainland Portugal. Int. J. Med. Microbiol. 293:109-116. [DOI] [PubMed] [Google Scholar]
- 3.Barral, M., A. L. Garcia-Perez, R. A. Juste, A. Hurtado, R. Escudero, R. E. Sellek, and P. Anda. 2002. Distribution of Borrelia burgdorferi sensu lato in Ixodes ricinus (Acari: Ixodidae) ticks from the Basque Country, Spain. J. Med. Entomol. 39:177-184. [DOI] [PubMed] [Google Scholar]
- 4.Brouqui, P., J. S. Dumler, R. Lienhard, M. Brossard, and D. Raoult. 1995. Human granulocytic ehrlichiosis in Europe. Lancet 346:782-783. [DOI] [PubMed] [Google Scholar]
- 5.Craine, N. G., S. E. Randolf, and P. A. Nutall. 1995. Seasonal variation in the role of grey squirrels as hosts of Ixodes ricinus, the tick vector of the Lyme disease spirochaete, in a British woodland. Folia Parasitol. 42:73-80. [PubMed] [Google Scholar]
- 6.Doby, J. M., C. Lemble, G. Bigaignon, M. Kremer, C. Rolland, and M. C. Lambert. 1990. Borrelia burgdorferi, agent of tick-spirochetosis (Lyme disease and other clinical forms) in Ixodes ricinus (Acari: Ixodidae) in Alsace. Examination of 2.223 ticks. Bull. Soc. Franc. Parasitol. 8:339-350. [Google Scholar]
- 7.Dumler, J. S., and J. S. Bakken. 1998. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu. Rev. Med. 49:201-213. [DOI] [PubMed] [Google Scholar]
- 8.Gern, L., C. M. Hu, E. Kocianova, V. Vyrostekova, and J. Rehacek. 1999. Genetic diversity of Borrelia burgdorferi sensu lato isolates obtained from Ixodes ricinus ticks collected in Slovakia. Eur. J. Epidemiol. 15:665-669. [DOI] [PubMed] [Google Scholar]
- 9.Gern, L., and P.-F. Humair. 2002. Ecology of Borrelia burgdorferi sensu lato in Europe, p. 149-174. In J. Gray, O. Kahl, R. S. Lane, and G. Stanek (ed.), Lyme borreliosis: biology, epidemiology and control. CABI Publishing, Wallingford, Oxfordshire, United Kingdom.
- 10.Gern, L., and P. F. Humair. 1998. Natural history of Borrelia burgdorferi sensu lato. Wien. Klin. Wochenschr. 110:856-858. [PubMed] [Google Scholar]
- 11.Gilot, B., B. Degeilh, J. Pichot, B. Doche, C. Guiguen, L. Boero, C. Pawlack, and S. Lebourdeles. 1996. Prevalence of Borrelia burgdorferi (sensu lato) in Ixodes ricinus (L.) populations in France, according to a phytoecological zoning of the territory. Eur. J. Epidemiol. 12:395-401. [DOI] [PubMed] [Google Scholar]
- 12.Gilot, B., C. Guiguen, B. Degeilh, B. Doche, J. Pichot, and J. C. Beaucournu. 1994. Phytoecological mapping of Ixodes ricinus as an approach to the distribution of Lyme borreliosis in France, p. 105-112. In J. S. Axford and D. H. E. Rees (ed.), Lyme borreliosis. Lenum Press, New York, N.Y.
- 13.Hildebrandt, A., K. H. Schmidt, V. Fingerle, B. Wilske, and E. Straube. 2002. Prevalence of granulocytic Ehrlichiae in Ixodes ricinus ticks in Middle Germany (Thuringia) detected by PCR and sequencing of a 16S ribosomal DNA fragment. FEMS Microbiol. Lett. 211:225-230. [DOI] [PubMed] [Google Scholar]
- 14.Hubálek, Z., J. Halouzka, and Z. Juricová. 2003. Longitudinal surveillance of the tick Ixodes ricinus for Borreliae. Med. Vet. Entomol. 17:46-51. [DOI] [PubMed] [Google Scholar]
- 15.Hubalek, Z. 2005. North Atlantic weather oscillation and human infectious diseases in the Czech Republic. Eur. J. Epidemiol. 20:263-270. [DOI] [PubMed] [Google Scholar]
- 16.Hubalek, Z., and J. Halouzka. 1998. Prevalence rates of Borrelia burgdorferi sensu lato in host-seeking Ixodes ricinus ticks in Europe. Parasitol. Res. 84:167-172. [DOI] [PubMed] [Google Scholar]
- 17.Hubalek, Z., J. Halouzka, and Z. Juricova. 1991. A comparison of the occurrence of Borreliae in nymphal and adult Ixodes ricinus ticks. Zentbl. Bakteriol. Hyg. A 275:133-137. [DOI] [PubMed] [Google Scholar]
- 18.Institut de Veille Sanitaire. 2005. La maladie de Lyme. Données du réseau de surveillance de la maladie en Alsace. Mars 2001-Février 2003. Institut de Veille Sanitaire, Saint-Maurice, France. [Online.] http://www.invs.sante.fr/recherche/index2.asp?txtQuery=Lyme.
- 19.Janouskovcova, E. 2004. Occurrence of Borrelia afzelii and Borrelia garinii in Ixodes ricinus ticks from Southern Moravia, Czech Republic. Vector Borne Zoonotic Dis. 4:43-52. [DOI] [PubMed] [Google Scholar]
- 20.Jouda, F., J. L. Perret, and L. Gern. 2004. Density of questing Ixodes ricinus nymphs and adults infected by Borrelia burgdorferi sensu lato in Switzerland: spatio-temporal pattern at a regional scale. Vector Borne Zoonotic Dis. 4:23-32. [DOI] [PubMed] [Google Scholar]
- 21.Junttila, J., M. Peltomaa, H. Soini, M. Marjamäki, and M. K. Viljanen. 1999. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in Urban recreational areas of Helsinki. J. Clin. Microbiol. 37:1361-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kampen, H., D. C. Rotzel, K. Kurtenbach, W. A. Maier, and H. M. Seitz. 2004. Substantial rise in the prevalence of Lyme borreliosis spirochetes in a region of western Germany over a 10-year period. Appl. Environ. Microbiol. 70:1576-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtenbach, K., H. Kampen, A. Dizij, S. Arndt, H. M. Seitz, U. E. Schaible, and M. M. Simon. 1995. Infestation of rodents with larval Ixodes ricinus (Acari: Ixodidae) is an important factor in the transmission cycle of Borrelia burgdorferi s. l. in German woodlands. J. Med. Entomol. 32:807-817. [DOI] [PubMed] [Google Scholar]
- 24.Letrilliart, L., B. Ragon, T. Hanslik, and A. Flahault. 2005. Lyme disease in France: a primary care-based prospective study. Epidemiol. Infect. 133:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leutenegger, C. M., N. Pusterla, C. N. Mislin, R. Weber, and H. Lutz. 1999. Molecular evidence of coinfection of ticks with Borrelia burgdorferi sensu lato and the human granulocytic ehrlichiosis agent in Switzerland. J. Clin. Microbiol. 37:3390-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotric-Furlan, S., M. Petrovec, T. Avsic-Zupanc, W. L. Nicholson, J. W. Sumner, J. E. Childs, and F. Strle. 1998. Human erlichiosis in Central Europe. Wien. Klin. Wochenschr. 110:894-897. [PubMed] [Google Scholar]
- 27.Pancholi, P., C. P. Kolbert, P. D. Mitchell, K. D. Reed, J. S. Dumler, J. S. Bakken, S. R. Telford, and D. H. Persing. 1995. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J. Infect. Dis. 172:1007-1012. [DOI] [PubMed] [Google Scholar]
- 28.Perret, J.-L., E. Guigoz, O. Rais, and L. Gern. 2000. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 86:554-557. [DOI] [PubMed] [Google Scholar]
- 29.Pichon, B., L. Mousson, C. Figureau, F. Rodhain, and C. Perez-Eid. 1999. Density of deer in relation to the prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus nymphs in Rambouillet forest, France. Exp. Appl. Acarol. 23:267-275. [DOI] [PubMed] [Google Scholar]
- 30.Pichot, J., B. Gilot, N. Almire, K. Polette, and B. Degeilh. 1997. Ixodes populations (Ixodes ricinus Linné, 1758; Ixodes hexagonus Leach, 1815) in the city of Lyon (France) and its outskirts: preliminary results. Parasite 2:167-171. [Google Scholar]
- 31.Postic, D., M. V. Assous, P. A. D. Grimont, and G. Baranton. 1994. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S) rrl (23S) intergenic spacer amplicons. Int. J. Syst. Bacteriol. 44:743-752. [DOI] [PubMed] [Google Scholar]
- 32.Quessada, T., F. Martial-Convert, S. Arnaud, H. Leudet de la Valllee, B. Gilot, and J. Pichot. 2003. Prevalence of Borrelia burgdorferi species and identification of Borrelia valaisiana in questing Ixodes ricinus in the Lyon region of France as determined by polymerase chain reaction-restriction fragment length polymorphism. Eur. J. Clin. Microbiol. Infect. Dis. 22:165-173. [DOI] [PubMed] [Google Scholar]
- 33.Stafford, K. C., III, M. L. Cartter, L. A. Magnarelli, S. H. Ertel, and P. A. Mshar. 1998. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J. Clin. Microbiol. 36:1240-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanczak, J., B. Kubica-Biernat, M. Racewicz, W. Kruminis-Lozowska, and J. Kur. 2000. Detection of three genospecies of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in different regions of Poland. Int. J. Med. Microbiol. 290:559-566. [DOI] [PubMed] [Google Scholar]
- 35.Stepánová-Tresová, G., B. Pet'ko, A. Stefancíková, and D. Nadzamova. 2000. Occurrence of Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii in the Ixodes ricinus ticks from Eastern Slovakia. Eur. J. Epidemiol. 16:105-109. [DOI] [PubMed] [Google Scholar]
- 36.Tälleklint, L., and T. G. T. Jaenson. 1998. Increasing geographical distribution and density of Ixodes ricinus (Acari: Ixodidae) in central and northern Sweden. J. Med. Entomol. 35:521-526. [DOI] [PubMed] [Google Scholar]
- 37.Von Loewenich, F. D., G. Stumpf, B. U. Baumgarten, M. Röollinghoff, J. S. Dumler, and C. Bogdan. 2003. Human granulocytic ehrlichiosis in Germany—evidence from serological studies, tick analyses, and a case of equine ehrlichiosis. Ann. N. Y. Acad. Sci. 990:116-117. [DOI] [PubMed] [Google Scholar]
- 38.Younsi, H., M. Sarih, F. Jouda, E. Godfroid, L. Gern, A. Bouattour, G. Baranton, and D. Postic. 2005. Characterization of Borrelia lusitaniae isolates collected in Tunisia and Morocco. J. Clin. Microbiol. 44:1587-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhioua, E., D. Postic, F. Rodhain, and C. Perez-Eid. 1996. Infection of Ixodes ricinus (Acari: Ixodidae) by Borrelia burgdorferi in Ile de France. J. Med. Entomol. 33:694-697. [DOI] [PubMed] [Google Scholar]



