Abstract
Transmission ratio distortion (TRD) is defined as a significant departure from expected Mendelian ratios of inheritance of an allele or chromosome. TRD is observed among specific regions of the mouse and human genome and is frequently associated with chromosome rearrangements such as Robertsonian (Rb) chromosomes. We intercrossed mice heterozygous for a (7.18) Rb translocation and genotyped chromosomes 7 and 18 in 1812 individuals, 47% of which were informative for chromosome segregation. We substantiated previous findings that females were less likely than expected to transmit the Rb chromosome to their offspring. Surprisingly, however, we report that heterozygous males transmitted the Rb translocation chromosome significantly more frequently than the acrocentrics. The transmission of the Rb chromosome was not significantly influenced by either the sex of the Rb grandparent or the strain of the Rb.
MENDEL's law of segregation mandates the equal transmission of each allele at each locus of a chromosome, ensuring the maintenance of the proper chromosome number during meiosis. Transmission ratio distortion (TRD) is the unequal representation of alleles or chromosomes (Pardo-Manuel de Villena and Sapienza 2001a). The resulting deviations from a 1:1 Mendelian transmission ratio have been observed in both human and mouse, but the mechanisms involved are not well understood.
TRD is frequently associated with chromosomal rearrangements such as Robertsonian (Rb) chromosomes (Gropp and Winking 1981; Aranha and Martin-DeLeon 1991). Rb translocations are metacentric chromosomes that result from the fusion of two acrocentric chromosomes (Davisson and Akeson 1993; Beechey and Evans 1996). Wild populations of Rb translocation mice exist mostly in Western Europe and North Africa (Redi and Capanna 1988) and many strains have been collected and characterized that involve a variety of mouse chromosomes (Gropp and Winking 1981; Davisson and Akeson 1993). Human Rb translocations occur among the acrocentric chromosomes 13–15, 21, and 22 (Bandyopadhyay et al. 2002). Rb translocations are the most common form of structural rearrangement in humans (Hamerton et al. 1975; Nicolaidis and Peterson 1998; Pardo-Manuel de Villena and Sapienza 2001c) with Rb (13q14q) and (14q21q) being the most frequent (Therman et al. 1989).
Several genomic regions in the mouse have been associated with TRD, including the Om locus on mouse chromosome 11 in the offspring of both sexes and on the X chromosome in female offspring (Pardo-Manuel de Villena et al. 2000). In humans, non-Mendelian transmission of chromosome 13 and the X chromosome has been documented (Naumova and Sapienza 1994; Naumova et al. 1998). Several models have been suggested to explain TRD. One well-established mechanism involves the nonrandom segregation of chromosomes in female meiosis (Pardo-Manuel de Villena and Sapienza 2001a) where different efficiencies of the meiotic spindle are thought to account for preferences in acrocentric vs. metacentric chromosome centromere capture by the egg and polar body. A second model that differs in mechanism but makes similar predictions of outcome is based on stochastic errors in imprinting (Croteau et al. 2002). Studies of imprinted regions of the human (Naumova et al. 2001) and mouse genome (Croteau et al. 2002) suggest that problems associated with resetting of imprinting may be a cause of allele preference. The imprinted Meg3/Gtl2-Dlk1 region of mouse chromosome 12 elicits a TRD resulting from postimplantation loss of embryos that inherit distal chromosome 12 alleles from maternal grandfathers. The mechanism is not understood but imprinting of one or more genes may not be properly maintained or appropriately recognized (Croteau et al. 2003).
The mouse is a good model in which to study the transmission of Rb chromosomes because these chromosome anomalies are present on a defined genetic background and the parent of origin of specific chromosomes can be determined in the progeny by several methods including molecular genotyping. Genotyping can be performed at any stage during development and is not dependent on the location of a phenotypic marker in the region or chromosome of interest since molecular markers are available throughout the mouse genome.
We have studied the transmission of the Robertsonian (7.18) chromosome in ∼1812 embryos (equivalent to 3624 meioses) of which 854 embryos had balanced karyotypes that were evaluated statistically to ascertain evidence for Mendelian inheritance vs. TRD in this cross.
MATERIALS AND METHODS
Mice:
All mouse strains were obtained from The Jackson Laboratory. The Rb(2.8)2Lub(7.18)9Lub or RB92.82Lub (W) and C57BL/6JEi-Rb(7.18)9Lub (B) strains were obtained from the Cytogenetic Models Resource at The Jackson Laboratory. The W strain has been maintained on its own inbred background, a combination of ∼50% wild-derived Mus musculus domesticus and 50% laboratory mouse strain background (Davisson and Akeson 1993). The B strain was derived by repeated backcrossing to a C57BL/6J-Ei background. For analysis of TRD, W mice were crossed to DBA/2J (D) and B mice were crossed either to C3H/HeJ (3) or to M. musculus castaneus (C) to generate progeny heterozygous for the (7.18) Robertsonian chromosome. C3H/HeJ mice were substituted for M. m. castaneus in some crosses because the B × C F1 males were not fertile and at the outset of this study we wished to test all cross combinations (for example, B × C as well as C × B progeny) to take potential strain differences into account. Four out of five of the parental strains were inbred laboratory mice (a combination of M. musculus × M. m. domesticus) and one strain was a more genetically distant subspecies of M. m. castaneus (Beck et al. 2000). Rb(7.18) heterozygotes of each strain combination were intercrossed and produced 1812 F2 progeny that were harvested at 8.5 days post-coitum (dpc) in 364 timed matings. This time point was chosen because this is the latest time at which phenotypically normal embryos with uniparental disomy (UPD) of 7 or 18 survive to and we wished to survey balanced and unbalanced products including UPD in the first instance in this cross (Oakey et al. 1995).
We were interested in scoring the incidence of balanced progeny from Rb heterozygote intercrosses and in determining the parental origin of transmission of the Rb chromosome in these offspring. A total of 854 balanced embryos were included in our statistical analyses. Aneuploid and UPD embryos as well as embryos with equivocal genotypes (of which there were only a few resulting from genotyping errors) were excluded from the analysis.
DNA preparation:
A small tissue sample was dissected from the tail region of each embryo and frozen for DNA preparations. DNA was isolated as in Oakey et al. (1995).
Genotyping:
Simple sequence repeat (SSR) polymorphism primers (Map Pairs) were purchased from Research Genetics (now Invitrogen Life Technologies; http://mp.invitrogen.com/resources/apps/mappairs/). The chromosome 7 marker used was D7Mit25 and D18Mit14 was used for chromosome 18 genotyping. Genotyping was performed as in Oakey et al. (1995). Two independent individuals scored the genotypes. The embryos were scored as chromosomally balanced, trisomic, or UPD for chromosomes 7 and 18. Equivocal genotypes were confirmed with a second marker, D7MIT222 for chromosome 7 and D18MIT87 for chromosome 18.
Data acquisition:
Data from 854 mice from 364 litters were characterized with respect to the parental and grandparental strain, sex of the Rb-transmitting grandparent and parent, and embryonic genotype. Each chromosomally balanced embryo was scored as having no Rb chromosome, only a maternally derived Rb chromosome, only a paternally derived Rb chromosome, or both a maternally and paternally derived Rb chromosome (Table 1).
TABLE 1.
The numbers of balanced progeny produced from the eight combinations of mouse crosses including parental and grandparental origin of the Robertsonian (Rb) chromosomes
| No. of embryosa
|
|||||||
|---|---|---|---|---|---|---|---|
| Maternal Rb grandparentb |
Paternal Rb grandparentc |
Total no. |
MM | MP | PM | PP | No Rb chr. |
| Bd | W | ||||||
| MGM | PGF | 88 | 40 | 0 | 0 | 45 | 28 |
| MGM | PGM | 74 | 36 | 0 | 43 | 0 | 16 |
| MGF | PGF | 143 | 0 | 73 | 0 | 83 | 26 |
| MGF | PGM | 113 | 0 | 46 | 57 | 0 | 26 |
| W | B | ||||||
| MGF | PGM | 150 | 0 | 68 | 71 | 0 | 40 |
| MGF | PGF | 111 | 0 | 54 | 0 | 68 | 30 |
| MGM | PGM | 126 | 57 | 0 | 77 | 0 | 32 |
| MGM | PGF | 49 | 19 | 0 | 0 | 18 | 15 |
| Total | 854 | ||||||
Rb, Robertsonian chromosome.
MM indicates inheritance of the Rb chromosome from the mother and grandmother; MP, from the mother and her father; PM, from the father and his mother; and PP, from the father and his father. No Rb chromosome indicates inheritance only of acrocentric chromosomes.
MGM, maternal grandmother; MGF, maternal grandfather.
PGF, paternal grandfather; PGM, paternal grandmother.
C57BL/6JEi-Rb(7.18)9Lub; W, Rb(2.8)2Lub(7.18)9Lub.
Statistical methods:
Differences in the transmission of the Rb and normal chromosomes from heterozygous parents to their offspring were assessed using the chi-square test statistics,
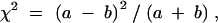 |
where a is the number of times that heterozygous parents transmit the Rb chromosome to their offspring and b is the number of times that heterozygous parents transmit the normal chromosome to their offspring. This test has 1 d.f. The null hypothesis that is tested using this statistic is that of Mendelian (i.e., equal) transmission of the Robertsonian and normal chromosomes. A significant test result (i.e., P < 0.05) indicates that one of the chromosomes is preferentially transmitted to offspring. Since this test is based on parental transmission of chromosomes, each evaluated embryo contributes information on two (maternal and paternal) transmissions.
Chi-square analyses were used to determine whether the transmission of chromosomes from Rb heterozygous mice is influenced by: (1) sex of the transmitting parent, (2) sex of the transmitting grandparent, or (3) parental strain (W or B). These tests assess the null hypothesis that transmission of the Rb and normal chromosomes from heterozygous mice is not influenced by the variable (i.e., parent sex, grandparent sex, or parental strain) of interest. A significant test result (i.e., P < 0.05) indicates that the transmission of the Rb chromosome by heterozygotes is significantly associated with the variable of interest. Since these tests are based on parental transmission of chromosomes, the available sample is twice the number of embryos. All statistical analyses were performed using SAS release 8.02.
RESULTS
Offspring with an unbalanced karyotype that included the Rb chromosome and either chromosome 7 or 18 are not informative for the evaluation of TRD because they result from meiotic divisions where paired chromosomes do not segregate. Consequently, the transmission of chromosomes 7 and 18 both as part of the Rb and as the free acrocentrics was considered in balanced offspring only, so that segregation bias cannot be explained by postfertilization selection against aneuploid offspring. The eight combinations of intercrosses resulted in 854 balanced progeny (Table 1). As some embryos inherited both a maternal and paternal Rb chromosome, the total number of Rb chromosomes is greater than the total number of embryos.
The transmission of Rb and normal chromosomes from Rb heterozygous parents is summarized in Table 2. For example, the first data row of Table 2 summarizes the data for mating type 1, which involved a strain W dam with a maternally inherited Rb chromosome and a strain B sire with a maternally inherited Rb chromosome. The counts of Rb and normal chromosomes transmitted from these dams to their offspring (maternal transmissions) are summarized in columns 6 and 7. Column 8 provides the chi-square test statistic comparing the transmission of Rb and normal chromosomes from these dams to their offspring and its associated P-value. Likewise the counts of Rb and normal chromosomes transmitted from these sires to their offspring (paternal transmissions) are summarized in columns 9 and 10, and column 11 provides the chi-square test statistic and its associated P-value. Analysis of the transmissions from all Rb heterozygous females showed that the Rb chromosome was transmitted significantly less frequently (0.46) than the normal chromosome (P = 0.02). In contrast, when transmissions from all Rb heterozygous males were considered, the Rb chromosome was transmitted significantly more frequently (0.54) than the normal chromosomes (P = 0.02). In general, similar trends were observed within the eight mating types. However, the observed differences in the transmission of the Rb and normal chromosomes often did not achieve statistical significance within these groups (Table 2).
TABLE 2.
Transmissions of Robertsonian and normal chromosomes from Robertsonian heterozygous parents
| Maternal characteristicsa
|
Paternal characteristicsb
|
Maternal transmissionsc
|
Paternal transmissionsd
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mating type |
Origin of Rb chromosome |
Strain | Origin of Rb chromosome |
Strain | Rb (%) | Normal (%) |
χ2 (P-value) |
Rb (%) | Normal (%) |
χ2 (P-value) |
| 1 | Mother | W | Mother | B | 36 (0.49) |
38 (0.51) |
0.05 (0.82) |
43 (0.58) |
31 (0.42) |
1.94 (0.16) |
| 2 | Mother | W | Father | B | 40 (0.45) |
48 (0.55) |
0.73 (0.39) |
45 (0.51) |
43 (0.49) |
0.04 (0.83) |
| 3 | Father | W | Mother | B | 46 (0.41) |
67 (0.59) |
3.90 (0.05) |
57 (0.50) |
56 (0.50) |
0.01 (0.92) |
| 4 | Father | W | Father | B | 73 (0.51) |
70 (0.49) |
0.06 (0.80) |
83 (0.58) |
60 (0.42) |
3.70 (0.05) |
| 5 | Mother | B | Mother | W | 57 (0.45) |
69 (0.55) |
1.14 (0.28) |
77 (0.61) |
49 (0.39) |
6.22 (0.01) |
| 6 | Mother | B | Father | W | 19 (0.39) |
30 (0.61) |
2.46 (0.11) |
18 (0.37) |
31 (0.63) |
3.45 (0.06) |
| 7 | Father | B | Mother | W | 68 (0.45) |
82 (0.55) |
1.31 (0.25) |
71 (0.47) |
79 (0.53) |
0.43 (0.51) |
| 8 | Father | B | Father | W | 54 (0.49) |
57 (0.51) |
0.08 (0.78) |
68 (0.61) |
43 (0.39) |
5.63 (0.02) |
| Total | — | — | — | — | 393 (0.46) |
461 (0.54) |
5.41 (0.02) |
462 (0.54) |
392 (0.46) |
5.74 (0.02) |
Maternal characteristics include the strain of the dams and the parental origin (maternal/paternal) of the dams' Rb chromosome.
Paternal characteristics include the strain of the sires and the parental origin (maternal/paternal) of the sires' Rb chromosome.
Maternal transmissions provide the count of offspring that inherited the Rb or the normal chromosome from a Rb heterozygous dam.
Paternal transmissions provide the count of offspring that inherited the Rb or the normal chromosome from a Rb heterozygous sire.
Considering transmissions from all heterozygous parents, females were significantly less likely than males to transmit the Rb chromosome (0.46 vs. 0.54, 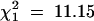 , P = 0.0008). Because of this difference, the impact of sex of the transmitting grandparent and strain of the transmitting parent was examined separately for maternal and paternal transmissions. The sex of the transmitting grandparent was not significantly related to transmission of the Rb chromosome in either female
, P = 0.0008). Because of this difference, the impact of sex of the transmitting grandparent and strain of the transmitting parent was examined separately for maternal and paternal transmissions. The sex of the transmitting grandparent was not significantly related to transmission of the Rb chromosome in either female 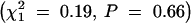 or male
or male 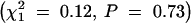 Rb heterozygotes. The strain of the transmitting parent also was not significantly related to transmission of the Rb chromosome in either female
Rb heterozygotes. The strain of the transmitting parent also was not significantly related to transmission of the Rb chromosome in either female 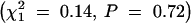 or male
or male 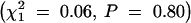 Rb heterozygotes.
Rb heterozygotes.
The effect of the paternal strain on transmission of the Rb chromosome from heterozygous females was examined, since the genotype of the sire can influence nonrandom segregation of maternal chromosomes (Pardo-Manuel de Villena and Sapienza 2001b). However, there was no evidence that paternal strain (B vs. W) was significantly associated with transmission of the Rb chromosome in heterozygous females 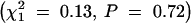 .
.
DISCUSSION
Female meiosis is characterized by asymmetrical divisions that produce a single functional gamete per primary oocyte. Conversely, male meiosis is characterized by symmetrical divisions that generate four functionally equivalent gametes. Therefore, in females but not males, nonrandom segregation of chromosomes may result from preferential transmission of homologous chromosomes into either the polar body or functional gamete (Pardo-Manuel de Villena and Sapienza 2001b), which is the case for most multicellular eukaryotic organisms.
Robertsonian translocations and TRD in females:
Rb chromosomes can be passed either to normal heterozygous balanced progeny (via alternate segregation) or to chromosomally unbalanced progeny (via adjacent segregation). Among the balanced progeny, Rb translocations from various mouse chromosomes have been shown to experience TRD through the female line (Gropp and Winking 1981). Unequal segregation in Robertsonian mice has been observed via karyotyping of oocytes and screening metaphase II spermatocytes (Tease and Fisher 1991) and first cleavage metaphase zygotes (Aranha and Martin-DeLeon 1994). These studies also show preferential segregation of the Rb metacenteric to the polar body, leading to a deficiency of cells propagating the Rb translocation. Thus female mice heterozygous for Rb chromosomes display distortion of segregation with a prevalence of progeny with normal chromosomes rather than with the translocation. Differences between male and female Rb heterozygotes can be explained if the TRD in females originates at meiosis (Pardo-Manuel de Villena and Sapienza 2001a,c). This is postulated to be due to the preferential distribution of the Robertsonian metacentric to the first polar body.
Our data support the findings in mice that the Rb chromosome is transmitted significantly less frequently than the normal chromosomes in heterozygous females. A model to explain this distortion involves functional heterozygosity at the centromere structure that may result in different efficiencies of chromosome segregation (Pardo-Manuel de Villena and Sapienza 2001a). In the mouse, the functional heterozygosity of the centromeres derives from two active centromeres in the acrocentric chromosomes but only a single active centromere in the rearranged Rb chromosome (Pardo-Manuel de Villena and Sapienza 2001a). This may lead to nonrandom segregation at meiosis I (Pardo-Manuel de Villena and Sapienza 2001c). Since nonrandom segregation appears to be chromosome independent in mouse (Gropp and Winking 1981; Tease and Fisher 1991), the egg pole must be more efficient at capturing centromeres. This proposed mechanism of TRD resulting from chromosome segregation inequalities is consistent with our data in females where there is a preference for acrocentrics to be passed on compared with the Rb chromosome. The egg pole is more efficient in capturing the two active centromeres or the polar body is more efficient at capturing chromosomes with single active centromeres of the metacentric structure. Differences in the frequency of nondisjunction between males and females for chromosome 7 (Underkoffler et al. 2002) might suggest a correlation between centromere capturing efficiency and the influence of the centromere on nondisjunction. Both Rb lines used here have been well integrated into laboratory mouse strains via many crosses; however, the Rb centromere may be of “feral origin” and the acrocentrics will be of laboratory origin, also possibly introducing a source of centromere inequality.
Nonrandom chromosome segregation plays an important role in karyotype evolution (Pardo-Manuel de Villena and Sapienza 2001b). Acrocentric chromosomes are predicted to be favored in species such as the mouse where the most efficient pole of the spindle is on the egg side of a meiotic division. Conversely in humans the metacentric chromosomes should be favored where the most efficient pole of the meiotic spindle is on the polar body side of a meiotic division. Over the course of evolution, nonrandom segregation can result in species with either predominantly acrocentric or metacentric chromosomes with few species having equal numbers of both (Pardo-Manuel de Villena and Sapienza 2001b). The feral Rb mouse populations that exist in western Europe and North Africa are an interesting in that they harbor stable inherited metacentric fusions.
Robertsonian translocations and TRD in males:
We observed increased paternal transmission of the Rb chromosome. Nonrandom segregation in males is unlikely to account for the observed TRD due to symmetrical divisions that give rise to sperm. Other possible explanations include prezygotic selection of karyotypically different sperm (Aranha and Martin-DeLeon 1991; Aranha and Martin-DeLeon 1994) or effects of a locus that mediates attachment of a chromosome to a spindle (Pardo-Manuel de Villena and Sapienza 2001b). The putative locus that is subject to a slight selective advantage may interact with the spindle or modulate the kinetochore action. Imprinting also offers an explanation for the TRD observed in males. A region of distal mouse chromosome 12 has previously been shown to elicit a grandparental origin-dependent TRD (Croteau et al. 2002) attributable to a genomic imprinting-related mechanism. Mouse chromosome 7 in particular, but also chromosome 18 are known to harbor imprinted genes and it is possible that some imprinted gene(s) may fail to be expressed properly and thus affect segregation of the Rb chromosome. Mouse chromosome 7 harbors regions with imprinting phenotypes and a wealth of known paternally and maternally imprinted genes. A single imprinted gene has been identified thus far on mouse chromosome 18 but there may be as yet unidentified imprinted genes that could affect chromosome segregation if misregulated on this chromosome. The mechanism of failure to reset the imprint is not known but may be due to minor structural differences between the Rb chromosome and the acrocentric homologs that arise as a result of Rb formation (Davisson and Akeson 1993). Alternatively, alterations in chromatin as a result of the structural changes in Rb formation may cause epigenetic alterations that affect imprinting mechanisms. Since we detect preferential inheritance of the metacentric chromosome compared to the acrocentrics, misexpression of putative imprinted genes on the translocation would be conferring a segregation advantage.
Nonrandom segregation, genetic background, and paternal origins:
We investigated the influence of strain background on the segregation of the Rb chromosome by examining transmission from the various intercrosses. The strain of the transmitting parent for maternal and paternal transmissions was not significantly related to transmission of the Rb chromosome in either female or male Rb heterozygotes. Similarly since the genotype of the sire can influence nonrandom segregation of maternal chromosomes (Pardo-Manuel de Villena and Sapienza 2001b) we investigated it in our data set. In these crosses there was no evidence that the paternal strain (B vs. W) was significantly associated with transmission of the Rb chromosome in heterozygous females.
This analysis of the segregation in a large number of progeny genotyped for parental origin of a (7.18) Rb chromosome produced some expected and some unexpected results. These data support previous observations in mice that Rb chromosomes are preferentially excluded from the egg and a TRD in offspring from female Rb heterozygotes is observed. The observation that the Rb chromosome was transmitted significantly more frequently from heterozygous males was somewhat unexpected. This TRD must have resulted from a different mechanism from that for female Rb heterozygotes, possibly from prezygotic selection of karyotypically different sperm or from imprinting problems.
Acknowledgments
We thank C. V. Beechey, E. P. Evans, M. H. Malim, and T. R. Menheniott for their critical reading of this article. This work was supported by Public Health Service grant GM58759 from the National Institutes of Health (R.J.O.) and The Wellcome Trust (R.J.O.) and L.E.M. is supported by grants HD39195 and HD39081 from the National Institutes of Health.
References
- Aranha, I. P., and P. A. Martin-DeLeon, 1991. The murine Rb(6.16) translocation: evidence for sperm selection and a modulating effect of aging. Hum. Genet. 87: 278–284. [DOI] [PubMed] [Google Scholar]
- Aranha, I. P., and P. A. Martin-DeLeon, 1994. Segregation analysis of the mouse Rb (6.16) translocation in zygotes produced by heterozygous female carriers. Cytogenet. Cell Genet. 66: 51–53. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay, R., A. Heller, C. Knox-DuBois, C. McCaskill, S. A. Berend et al., 2002. Parental origin and timing of de novo Robertsonian translocation formation. Am. J. Hum. Genet. 71: 1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, J. A., S. Lloyd, M. Hafezparast, M. Lennon-Pierce, J. T. Eppig et al., 2000. Genealogies of mouse inbred strains. Nat. Genet. 24: 23–25. [DOI] [PubMed] [Google Scholar]
- Beechey, C. V., and E. P. Evans, 1996 Numerical variants and structural rearrangements, pp. 1452–1505 in Genetic Variants and Strains of the Laboratory Mouse, edited by M. F. Lyon, S. Rastan and S. D. M. Brown. Oxford University Press, Oxford.
- Croteau, S., M. Freitas Nadrade, F. Huang, C. M. T. Greenwood, K. Morgan et al., 2002. Inheritance of maternal alleles in imprinted regions of the mouse genome at different stages of development. Mamm. Genome 13: 24–29. [DOI] [PubMed] [Google Scholar]
- Croteau, S., M.-C. Charron, K. E. Latham and A. K. Naumova, 2003. Alternative splicing and imprinting control of the Meg3/Gtl2-Dlk locus in mouse embryos. Mamm. Genome 14: 231–241. [DOI] [PubMed] [Google Scholar]
- Davisson, M. T., and E. C. Akeson, 1993. Recombination suppression by heterozygous Robertsonian chromosomes in the mouse. Genetics 133: 649–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp, A., and H. Winking, 1981 Robertsonian translocations: cytology, meiosis, segregation pattern and biological consequences of heterozygosity, pp. 141–181 in Biology of the House Mouse, edited by R. J. Berry. Academic Press, New York/London.
- Hamerton, J. L., N. Canning, M. Ray and S. Smith, 1975. A cytogenetic survey of 14,069 newborn infants. I. Incidence of chromosome abnormalities. Clin. Genet. 8: 223–243. [DOI] [PubMed] [Google Scholar]
- Naumova, A. K., and C. Sapienza, 1994. The genetics of retinoblastoma, revisited. Am. J. Hum. Genet. 54: 264–273. [PMC free article] [PubMed] [Google Scholar]
- Naumova, A. K., M. Leppert, K. Barker, K. Morgan and C. Sapienza, 1998. Parental origin-dependent, male offspring-specific transmission-ration distortion on the human X chromosome. Am. J. Hum. Genet. 62: 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova, A. K., C. M. T. Greenwood and K. Morgan, 2001. Imprinting and deviation from Mendelian transmission ratios. Genome 44: 311–320. [DOI] [PubMed] [Google Scholar]
- Nicolaidis, P., and M. B. Peterson, 1998. Origin and mechanisms of non-disjunction in human autosomal trisomies. Hum. Reprod. 13: 313–319. [DOI] [PubMed] [Google Scholar]
- Oakey, R. J., P. G. Matteson, S. Litwin, S. M. Tilghman and R. L. Nussbaum, 1995. Nondisjunction rates and abnormal embryonic development in a mouse cross between heterozygotes carrying a (7,18) Robertsonian translocation chromosome. Genetics 141: 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Manuel de Villena, F., and C. Sapienza, 2001. a Female meiosis drives karyotypic evolution in mammals. Genetics 159: 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Manuel de Villena, F., and C. Sapienza, 2001. b Nonrandom segregation during meiosis: the unfairness of females. Mamm. Genome 12: 331–339. [DOI] [PubMed] [Google Scholar]
- Pardo-Manuel de Villena, F., and C. Sapienza, 2001. c Transmission ratio distortion in offspring of heterozygous female carriers of Robertsonian translocation. Hum. Genet. 108: 31–36. [DOI] [PubMed] [Google Scholar]
- Pardo-Manuel de Villena, F., E. de la Casa-Esperon, T. L. Briscore, J.-M. Malette and C. Sapienza, 2000. Male-offspring-specific, haplotype-dependent, nonrandom cosegregation of alleles at loci on two mouse chromosomes. Genetics 154: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redi, C. A., and E. Capanna, 1988 Robertsonian heterozygotes in the house mouse and the fate of their germ cells, pp. 315–359 in The Cytogenetics of Mammalian Autosomal Rearrangements, edited by A. Daniel. Alan R. Liss, New York.
- Tease, C., and G. Fisher, 1991. Two new X-autosome Robertsonian translocations in the mouse. Meiotic chromosome segregation in male hemizygotes and female heterozygotes. Genet. Res. 58: 115–121. [DOI] [PubMed] [Google Scholar]
- Therman, E., B. Susman and C. Denniston, 1989. The nonrandom participation of human acrocentric chromosomes in Robertsonian translocations. Ann. Hum. Genet. 53: 49–65. [DOI] [PubMed] [Google Scholar]
- Underkoffler, L. A., L. E. Mitchell, A. R. Localio, S. M. Marchegiani, J. Morabito et al., 2002. Molecular analysis of nondisjunction in mice heterozygous for a Robertsonian translocation. Genetics 161: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]


